AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review
Abstract
1. Introduction
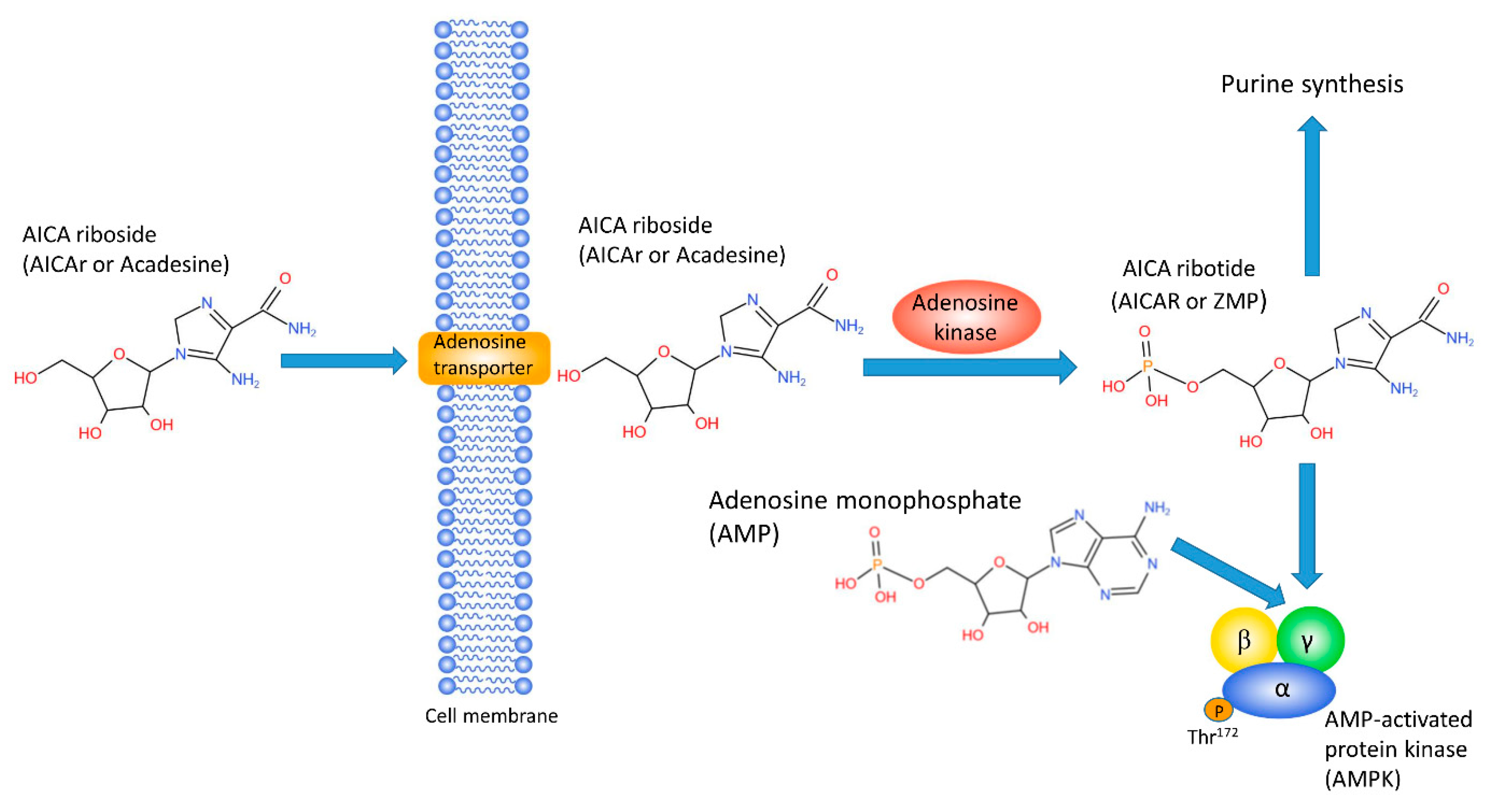
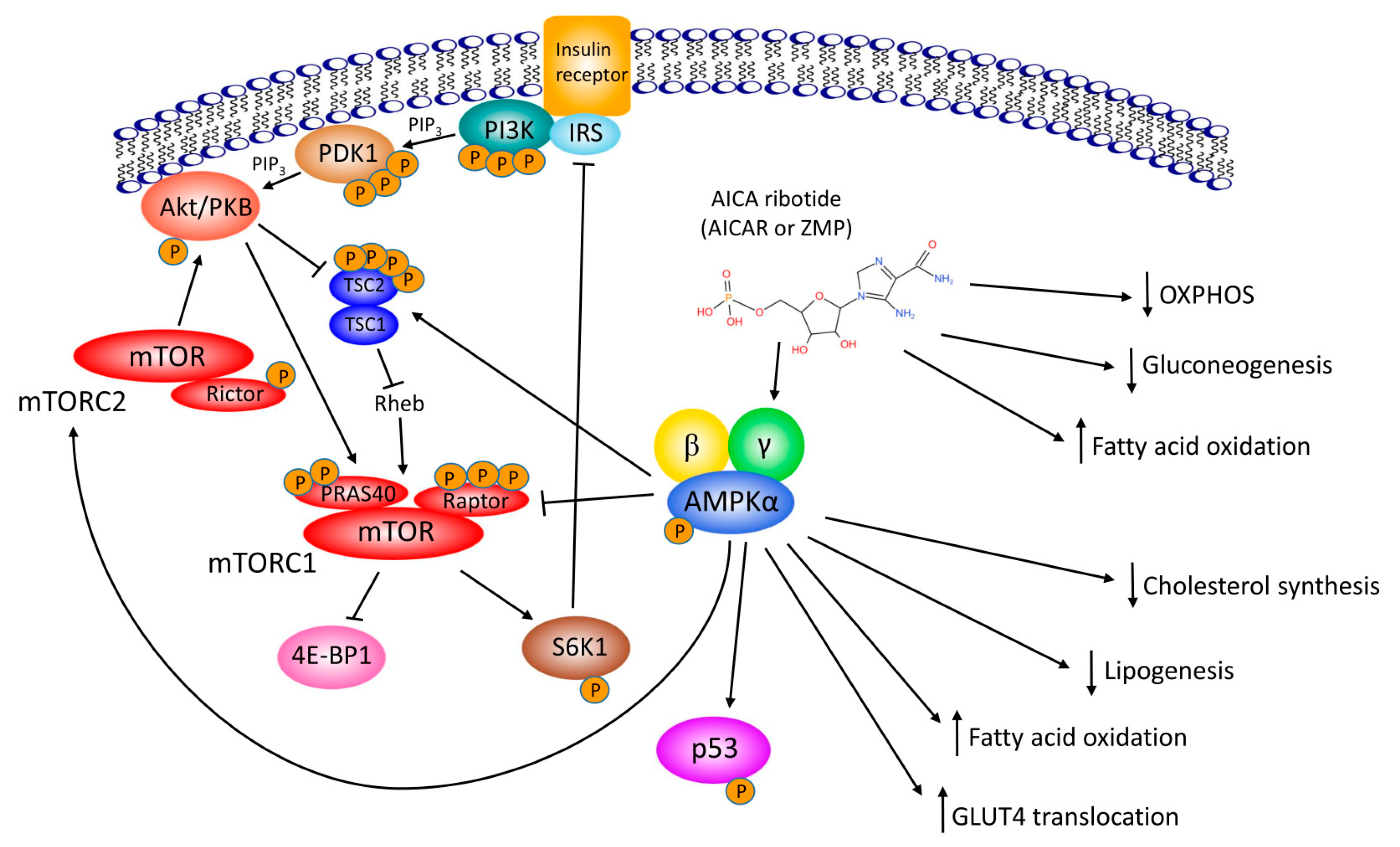
2. AICAr, Metabolism and Diabetes
| Tissue | AICAr Effect | AMPK-Dependency | Model |
|---|---|---|---|
| Skeletal muscle | Increased glucose uptake | AMPK-dependent | Skeletal and cardiac muscle-specific expression of ampkα2-KD [32] |
| Total ampkα2-KO [33] | |||
| Total ampky3-KO [34] | |||
| Muscle-specific expression of ampkα2-DN [35] | |||
| Increased FAO | AMPK-independent | Muscle-specific ampkα2-KD [50] | |
| Increased mrna HKII and (PGC)-1α | AMPK-dependent | Total ampkα2-KO [51] | |
| Liver | Decreased glucose phosphorylation | AMPK-independent | Liver-specific ampkα1α2-KO [39] |
| Decreased gluconeogenesis | AMPK-independent | Liver-specific ampkα1α2-KO [38] | |
| Inhibited OXPHOS | AMPK-independent | Liver-specific ampkα1α2-KO [40] | |
| Decreased lipogenesis | AMPK-dependent | Liver-specific ampkα1α2-KO [36] | |
| Increased FAO | AMPK-dependent | Liver-specific ampkα1α2-KO [36] | |
| Adipose tissue | Decreased FA synthesis | AMPK-dependent | Adipose tissue-specific ampkα1/α2-KO [37] |
| Decreased lipolysis | AMPK-dependent | AMPK α1-KO [52] | |
| Whole body | Acute hypoglycemia | AMPK-dependent | Skeletal and cardiac muscle-specific ampkα2-KD [32] |
| Total ampkα2-KO [33] | |||
| Muscle-specific ampkα2-DN [35] |
3. AICAr, Adenosine, and Ischemic Heart
| Year | Condition | Trial type | Doses | Toxicity | Outcome |
|---|---|---|---|---|---|
| 1991 | Healthy volunteers | Phase I | PO and IV: 10, 25, 50, and 100 mg/kg | Well tolerated, only mild and transient side effects | Poor oral bioavailability, the rapid decline of post-infusion plasma concentrations [49] |
| 1994 | Lesch-Nyhan Syndrome | Case Report | PO: 30 mg/kg/day for 4 days followed by 100 mg/kg/day for 4 days | No adverse events | No changes in plasma levels of AICAr, confirm the estimate of <5% oral bioavailability of AICAr in humans [63] |
| 1994 | Coronary artery bypass grafting | Multicenter RCT, Phase II | Continuous IV for 7 h: 0.19 and 0.38 mg/kg/min | Well tolerated, mild hyperuricemia | Limits the severity of post-bypass myocardial ischemia at higher dose [16] |
| 1994 | Exercise-induced myocardial ischemia in patients with chronic stable angina pectoris | Single center RCT, Phase II | IV: 6–48 mg/kg | Well tolerated, mild asymptomatic hyperuricemia, mild asymptomatic hypoglycemia | No significant difference in comparison to placebo [17] |
| 1995 | Coronary artery bypass grafting | Multicenter RCT, Phase II | Continuous IV for 7h: 0.1 mg/kg/min; in cardioplegic solution 5 mg/mL | No adverse events | No significant difference in MI in the overall study group; significantly reduced the incidence of Q-wave MI in high-risk patients [18] |
| 1995 | Coronary artery bypass grafting | Multicenter RCT, Phase II | Continuous IV for 7 h: 0.05 and 0.1 mg/kg/min | Well tolerated, mild hyperuricemia | No significant difference in comparison to the placebo may reduce the incidence of larger Q-wave MI [19] |
| 1997 | Coronary artery disease | Single center RCT, Phase II | Continuous IV: 5, 10, 20, 50 mg/kg | Well tolerated, hyperlactacidemia | At higher doses, minor beneficial effects on ejection fraction and myocardial lactate metabolism were observed [8] |
| 2006 | Coronary artery bypass grafting | Multicenter RCT, Phase III | Continuous IV for 7 h: 0.1 mg/kg/min | No adverse events | Reduces the severity of acute post-reperfusion MI, substantially reducing the risk of dying over the 2 years after infarction [9] |
| 2007 | Healthy volunteers | Phase I | Continuous IV for 3 h: 10 mg/kg/h | No adverse events | Acutely stimulates muscle 2-DG uptake with a minor effect on whole-body glucose disposal [43] |
| 2009 | Healthy volunteers | Phase I | Continuous intra-arterial infusion for 110 min: 1, 2, 4, or 8 mg/min/dL forearm tissue | No adverse events | Potent vasodilation in the skeletal muscle vascular bed; does not increase skeletal muscle glucose uptake [64] |
| 2012 | Coronary artery bypass grafting | Multicenter RCT, Phase III | Continuous IV for 7 h: 0.1 mg/kg/min | No adverse events | No significant reduction in the composite of all-cause mortality, nonfatal stroke, or severe left ventricular dysfunction through 28 days [15] |
| Hematologic malignancies | |||||
| 2013 | Relapsed/refractory chronic lymphocytic leukemia (CLL) | Multicenter open-label clinical study, Phase I/II | Continuous IV: single doses of 50–315 mg/kg; two doses at 210 mg/kg; five doses at 210 mg/kg | Grade ≥2 hyperuricemia (not clinically significant), transient anemia and/or thrombocytopenia (not clinically significant), renal impairment, and transient infusion-related hypotension (clinically significant) | 210 mg/kg was the MTD and OBD. Multiple-dose administrations at the OBD have an acceptable safety profile [6] |
| 2019 | Azacytidine refractory MDS/AML patients | Phase I/II | Continuous IV: 140 mg/kg or 210 mg/kg | Trial stopped after 2 to 3 cycles due to serious renal toxicities | Side effects of high doses preclude its use in patients with strong comorbidities; one patient exhibited a very strong reduction (50%) of his blast count after only 2 cycles of AICAr and more than 70% after 6 cycles [10] |
4. AICAr as an “Exercise in a Pill”
5. AICAr, AMPK, Proliferation and Cell Cycle
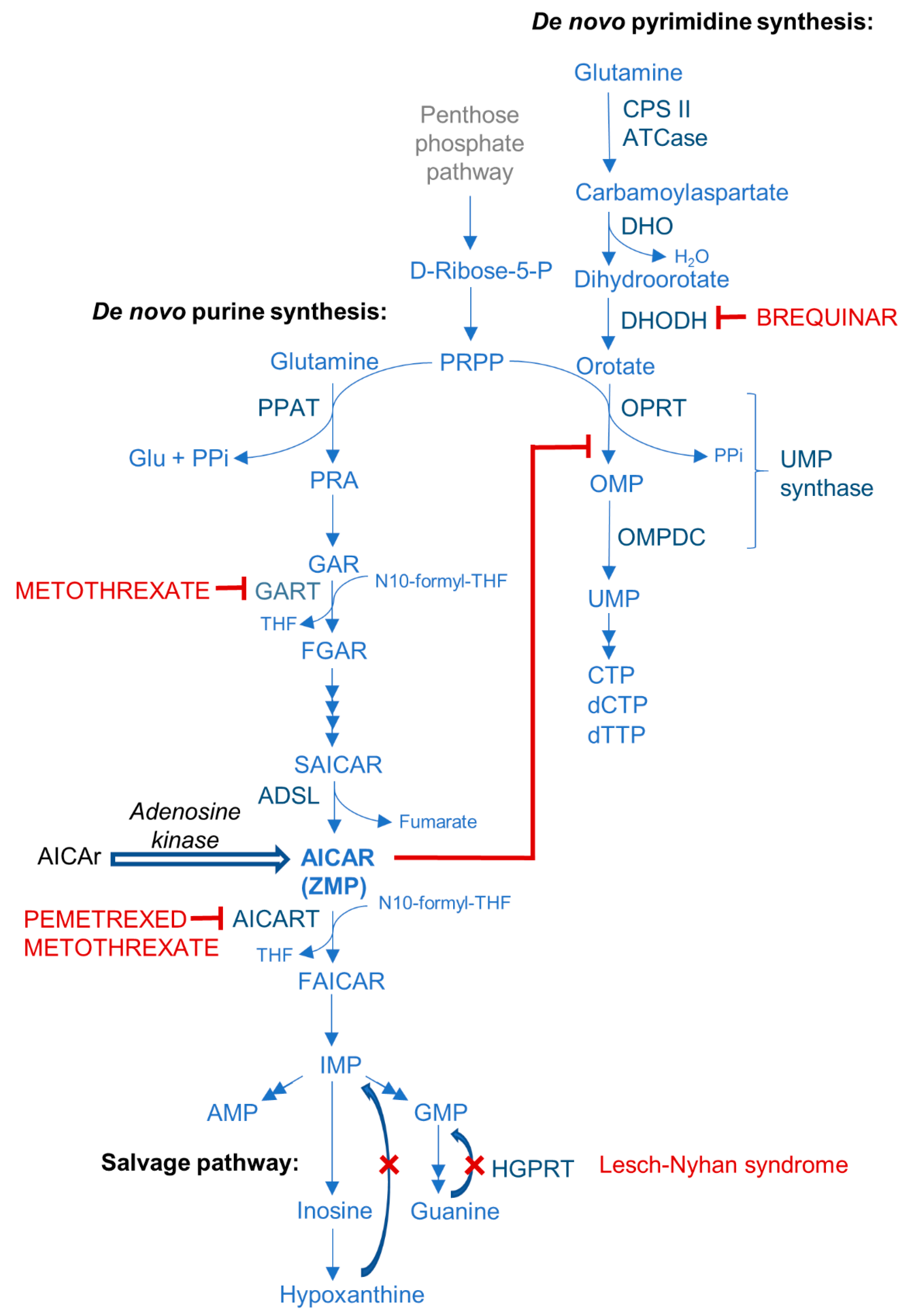
6. AICAr, ZMP, and Purine Synthesis
7. AICAr, AMPK, Cancer, and Leukemia
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-Aminoimidazole-4-Carboxamide Ribonucleoside. A Specific Method for Activating AMP-Activated Protein Kinase in Intact Cells? JBIC J. Biol. Inorg. Chem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Sabina, R.L.; Patterson, D.; Holmes, E.W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 1985, 260, 6107–6114. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Brocklehurst, K.J.; Marley, A.E.; Carey, F.; Carling, D.; Beri, R.K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994, 353, 33–36. [Google Scholar] [CrossRef]
- Neste, E.V.D.; Berghe, G.V.D.; Bontemps, F. AICA-riboside (acadesine), an activator of AMP-activated protein kinase with potential for application in hematologic malignancies. Expert Opin. Investig. Drugs 2010, 19, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Dembitz, V.; Lalic, H. The Role of AMPK/mTOR Modulators in the Therapy of Acute Myeloid Leukemia. Curr. Med. Chem. 2019, 26, 2208–2229. [Google Scholar] [CrossRef]
- Neste, E.V.D.; Cazin, B.; Janssens, A.; González-Barca, E.; Terol, M.J.; Levy, V.; De Oteyza, J.P.; Zachee, P.; Saunders, A.; De Frias, M.; et al. Acadesine for patients with relapsed/refractory chronic lymphocytic leukemia (CLL): A multicenter phase I/II study. Cancer Chemother. Pharmacol. 2013, 71, 581–591. [Google Scholar] [CrossRef]
- Campàs, C.; Santidrián, A.F.; Domingo, A.; Gil, J. Acadesine induces apoptosis in B cells from mantle cell lymphoma and splenic marginal zone lymphoma. Leukemia 2004, 19, 292–294. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, R.; MacLeod, D.C.; Suryapranata, H.; A Van Es, G.; Friedman, J.; Serruys, P.W.; De Jong, J.W. Effect of acadesine on myocardial ischaemia in patients with coronary artery disease. Eur. J. Pharmacol. 1997, 337, 41–44. [Google Scholar] [CrossRef]
- Mangano, D.T.; Miao, Y.; Tudor, I.C.; Dietzel, C. Post-Reperfusion Myocardial Infarction: Long-Term Survival Improvement Using Adenosine Regulation With Acadesine. J. Am. Coll. Cardiol. 2006, 48, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, T.; Furstoss, N.; Savy, C.; El Manaa, W.; Zerhouni, M.; Blot, L.; Calleja, A.; Dufies, M.; Dubois, A.; Ginet, C.; et al. Acadesine Circumvents Azacitidine Resistance in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Int. J. Mol. Sci. 2019, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Campàs, C.; Lopez, J.M.; Santidrián, A.F.; Barragán, M.; Bellosillo, B.; Colomer, D.; Gil, J. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood 2003, 101, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Ben Sahra, I.; Puissant, A.; Colosetti, P.; Belhacene, N.; Gounon, P.; Hofman, P.; Bost, F.; Cassuto, J.-P.; Auberger, P. Acadesine Kills Chronic Myelogenous Leukemia (CML) Cells through PKC-Dependent Induction of Autophagic Cell Death. PLoS ONE 2009, 4, e7889. [Google Scholar] [CrossRef]
- Drew, B.G.; A Kingwell, B. Acadesine, an adenosine-regulating agent with the potential for widespread indications. Expert Opin. Pharmacother. 2008, 9, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Mangano, D.T. Effects of Acadesine on Myocardial Infarction, Stroke, and Death Following SurgeryA Meta-analysis of the 5 International Randomized Trials. JAMA 1997, 277, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.F.; Ferguson, T.B.; White, J.A.; Ambrosio, G.; Koglin, J.; Nussmeier, N.A.; Pearl, R.G.; Pitt, B.; Wechsler, A.S.; Weisel, R.D.; et al. Effect of Adenosine-Regulating Agent Acadesine on Morbidity and Mortality Associated With Coronary Artery Bypass Grafting: The RED-CABG Randomized Controlled Trial. JAMA 2012, 308, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Stanley, T.; Mathew, J.; Curling, P.; Barash, P.; Salmenpera, M.; Reves, J.G.; Hollenberg, M.; Mangano, D.T. An Initial Multicenter, Randomized Controlled Trial on the Safety and Efficacy of Acadesine in Patients Undergoing Coronary Artery Bypass Graft Surgery. Anesthesia Analg. 1994, 78, 420–434. [Google Scholar] [CrossRef]
- Holdright, D.R.; Sparrow, J.L.; Wright, C.L.; Steiner, J.; Fox, K.M. Effect of acadesine, a new metabolic agent, on exercise-induced myocardial ischemia in chronic stable angina. Cardiovasc. Drugs Ther. 1994, 8, 193–197. [Google Scholar] [CrossRef]
- Menasché, P.; Jamieson, W.E.; Flameng, W.; Davies, M.K. Acadesine: A new drug that may improve myocardial protection in coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1995, 110, 1096–1106. [Google Scholar] [CrossRef]
- The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Effects of Acadesine on the Incidence of Myocardial Infarction and Adverse Cardiac Outcomes after Coronary Artery Bypass Graft Surgery. Anesthesiology 1995, 83, 658–673. [Google Scholar] [CrossRef]
- Gadalla, A.E.; Pearson, T.; Currie, A.J.; Dale, N.; Hawley, S.A.; Sheehan, M.; Hirst, W.; Michel, A.D.; Randall, A.; Hardie, D.G.; et al. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J. Neurochem. 2004, 88, 1272–1282. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Russell, F.M.; Atrih, A.; Lamont, D.J.; Hardie, D.G. Mechanism of Activation of AMPK by Cordycepin. Cell Chem. Biol. 2020, 27, 214–222.e4. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of Cells Expressing γ Subunit Variants to Identify Diverse Mechanisms of AMPK Activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.M.; Hardie, D.G. AMP-Activated Protein Kinase: Do We Need Activators or Inhibitors to Treat or Prevent Cancer? Int. J. Mol. Sci. 2021, 22, 186. [Google Scholar] [CrossRef]
- Henin, N.; Vincent, M.-F.; Gruber, H.E.; Van Den Berghe, G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995, 9, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Geelen, M.J.H.; Guzmán, M. Control of Hepatic Fatty Acid Oxidation by 5′-AMP-Activated Protein Kinase Involves a Malonyl-CoA-Dependent and a Malonyl-CoA-Independent Mechanism. Arch. Biochem. Biophys. 1997, 337, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.A.; Salt, I.P.; Walker, K.S.; Hardie, D.G.; Sutherland, C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 2000, 49, 896–903. [Google Scholar] [CrossRef]
- Young, M.E.; Radda, G.K.; Leighton, B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR—An activator of AMP-activated protein kinase. FEBS Lett. 1996, 382, 43–47. [Google Scholar] [CrossRef]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. Metab. 1997, 273, E1107–E1112. [Google Scholar] [CrossRef] [PubMed]
- Bolster, D.R.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. AMP-activated Protein Kinase Suppresses Protein Synthesis in Rat Skeletal Muscle through Down-regulated Mammalian Target of Rapamycin (mTOR) Signaling. J. Biol. Chem. 2002, 277, 23977–23980. [Google Scholar] [CrossRef]
- Longnus, S.L.; Wambolt, R.B.; Parsons, H.L.; Brownsey, R.W.; Allard, M.F. 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R936–R944. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef]
- Mu, J.; Brozinick, J.T.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A Role for AMP-Activated Protein Kinase in Contraction- and Hypoxia-Regulated Glucose Transport in Skeletal Muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Jørgensen, S.B.; Viollet, B.; Andreelli, F.; Frøsig, C.; Birk, J.B.; Schjerling, P.; Vaulont, S.; Richter, E.A.; Wojtaszewski, J.F.P. Knockout of the α2 but Not α1 5′-AMP-activated Protein Kinase Isoform Abolishes 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut Not Contraction-induced Glucose Uptake in Skeletal Muscle. J. Biol. Chem. 2004, 279, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.R.; Marklund, S.; Steiler, T.L.; Walter, M.; Göran, H.; Amarger, V.; Mahlapuu, M.; Leng, Y.; Johansson, C.; Galuska, D.; et al. The 5′-AMP-activated Protein Kinase γ3 Isoform Has a Key Role in Carbohydrate and Lipid Metabolism in Glycolytic Skeletal Muscle. J. Biol. Chem. 2004, 279, 38441–38447. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Hirshman, M.F.; Kane, E.M.; Ho, R.C.; Peter, L.E.; Seifert, M.M.; Goodyear, L.J. AMP-activated Protein Kinase α2 Activity Is Not Essential for Contraction- and Hyperosmolarity-induced Glucose Transport in Skeletal Muscle. J. Biol. Chem. 2005, 280, 39033–39041. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, N.; Marion, A.; Huet, C.; Pierre, R.; Viollet, B.; Foretz, M. AMPK Re-Activation Suppresses Hepatic Steatosis but its Downregulation Does Not Promote Fatty Liver Development. EBioMedicine 2018, 28, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.H.; McConahay, A.; Johnson, M.B.; Jeong, H.-W.; Koh, H.-J. Adipose tissue-specific knockout of AMPKα1/α2 results in normal AICAR tolerance and glucose metabolism. Biochem. Biophys. Res. Commun. 2019, 519, 633–638. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Guigas, B.; Bertrand, L.; Taleux, N.; Foretz, M.; Wiernsperger, N.; Vertommen, D.; Andreelli, F.; Viollet, B.; Hue, L. 5-Aminoimidazole-4-Carboxamide-1-β-Ribofuranoside and Metformin Inhibit Hepatic Glucose Phosphorylation by an AMP-Activated Protein Kinase–Independent Effect on Glucokinase Translocation. Diabetes 2006, 55, 865–874. [Google Scholar] [CrossRef]
- Guigas, B.; Taleux, N.; Foretz, M.; Detaille, D.; Andreelli, F.; Viollet, B.; Hue, L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem. J. 2007, 404, 499–507. [Google Scholar] [CrossRef]
- Viollet, B.; Lantier, L.; Devin-Leclerc, J.; Hebrard, S.; Amouyal, C.; Mounier, R.; Foretz, M.; Andreelli, F. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front. Biosci. (Landmark Ed.) 2009, 14, 3380–3400. [Google Scholar] [CrossRef]
- Bergeron, R.; Previs, S.F.; Cline, G.W.; Perret, P.; Russell, R.R., III; Young, L.H.; Shulman, G.I. Effect of 5-Aminoimidazole-4-Carboxamide-1-β-Ribofuranoside Infusion on In Vivo Glucose and Lipid Metabolism in Lean and Obese Zucker Rats. Diabetes 2001, 50, 1076–1082. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Babraj, J.A.; Mustard, K.J.W.; Towler, M.C.; Green, K.A.; Wackerhage, H.; Leese, G.P.; Baar, K.; Thomason-Hughes, M.; Sutherland, C.; et al. 5-Aminoimidazole-4-Carboxamide 1-β-Ribofuranoside Acutely Stimulates Skeletal Muscle 2-Deoxyglucose Uptake in Healthy Men. Diabetes 2007, 56, 2078–2084. [Google Scholar] [CrossRef]
- Song, X.M.; Fiedler, M.; Galuska, D.; Ryder, J.W.; Fernström, M.; Chibalin, A.V.; Wallberg-Henriksson, H.; Zierath, J.R. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia 2002, 45, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Buhl, E.S.; Jessen, N.; Pold, R.; Ledet, T.; Flyvbjerg, A.; Pedersen, S.B.; Pedersen, O.; Schmitz, O.; Lund, S. Long-Term AICAR Administration Reduces Metabolic Disturbances and Lowers Blood Pressure in Rats Displaying Features of the Insulin Resistance Syndrome. Diabetes 2002, 51, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.A.; Ye, J.-M.; Frangioudakis, G.; Saha, A.K.; Tomas, E.; Ruderman, N.B.; Cooney, G.J.; Kraegen, E.W. AICAR Administration Causes an Apparent Enhancement of Muscle and Liver Insulin Action in Insulin-Resistant High-Fat-Fed Rats. Diabetes 2002, 51, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Pold, R.; Jensen, L.S.; Jessen, N.; Buhl, E.S.; Schmitz, O.; Flyvbjerg, A.; Fujii, N.; Goodyear, L.J.; Gotfredsen, C.F.; Brand, C.L.; et al. Long-Term AICAR Administration and Exercise Prevents Diabetes in ZDF Rats. Diabetes 2005, 54, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Boon, H.; Bosselaar, M.; Praet, S.F.E.; Blaak, E.E.; Saris, W.H.M.; Wagenmakers, A.J.M.; McGee, S.L.; Tack, C.J.; Smits, P.; Hargreaves, M.; et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia 2008, 51, 1893. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Gourzis, J.; McDermott, D.; Fujitaki, J.; Dewland, P.; Gruber, H. AICA-Riboside: Safety, Tolerance, and Pharmacokinetics of a Novel Adenosine-Regulating Agent. J. Clin. Pharmacol. 1991, 31, 342–347. [Google Scholar] [CrossRef]
- Dzamko, N.; Schertzer, J.D.; Ryall, J.G.; Steel, R.; Macaulay, S.L.; Wee, S.; Chen, Z.-P.; Michell, B.J.; Oakhill, J.S.; Watt, M.J.; et al. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J. Physiol. 2008, 586, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.B.; Wojtaszewski, J.F.P.; Viollet, B.; Andreelli, F.; Birk, J.B.; Hellsten, Y.; Schjerling, P.; Vaulont, S.; Neufer, P.D.; Richter, E.A.; et al. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005, 19, 1146–1148. [Google Scholar] [CrossRef]
- Daval, M.; Diot-Dupuy, F.; Bazin, R.; Hainault, I.; Viollet, B.; Vaulont, S.; Hajduch, E.; Ferré, P.; Foufelle, F. Anti-lipolytic Action of AMP-activated Protein Kinase in Rodent Adipocytes. J. Biol. Chem. 2005, 280, 25250–25257. [Google Scholar] [CrossRef] [PubMed]
- Mauser, M.; Hoffmeister, H.M.; Nienaber, C.; Schaper, W. Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ. Res. 1985, 56, 220–230. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoffer, M.E.; McAllister, D.R.; Laikind, P.K.; Lane, T.A.; Schmid-Schoenbein, G.W.; Engler, R.L. Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation 1989, 80, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.R.; Bergeron, R.; Shulman, G.I.; Young, L.H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. Circ. Physiol. 1999, 277, H643–H649. [Google Scholar] [CrossRef]
- Arad, M.; Seidman, C.E.; Seidman, J. AMP-Activated Protein Kinase in the Heart. Circ. Res. 2007, 100, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, X.; Wang, Q.; Ren, S.Y.; Dong, M.; Zhang, Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J. Mol. Cell. Cardiol. 2016, 93, 18–31. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, E.; Benham, A.; Park, H.-K.; Soibam, B.; Taffet, G.E.; Kaelber, J.T.; Suh, J.H.; Taegtmeyer, H.; Entman, M.L.; et al. Transient activation of AMPK preceding left ventricular pressure overload reduces adverse remodeling and preserves left ventricular function. FASEB J. 2019, 33, 711–721. [Google Scholar] [CrossRef]
- Viglino, C.; Foglia, B.; Montessuit, C. Chronic AICAR treatment prevents metabolic changes in cardiomyocytes exposed to free fatty acids. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1219–1234. [Google Scholar] [CrossRef]
- Morrow, V.A.; Foufelle, F.; Connell, J.M.C.; Petrie, J.R.; Gould, G.W.; Salt, I.P. Direct Activation of AMP-activated Protein Kinase Stimulates Nitric-oxide Synthesis in Human Aortic Endothelial Cells. J. Biol. Chem. 2003, 278, 31629–31639. [Google Scholar] [CrossRef] [PubMed]
- Nagata, D.; Mogi, M.; Walsh, K. AMP-activated Protein Kinase (AMPK) Signaling in Endothelial Cells Is Essential for Angiogenesis in Response to Hypoxic Stress. J. Biol. Chem. 2003, 278, 31000–31006. [Google Scholar] [CrossRef]
- Ylikorkala, A.; Rossi, D.J.; Korsisaari, N.; Luukko, K.; Alitalo, K.; Henkemeyer, M.; Mäkelä, T.P. Vascular Abnormalities and Deregulation of VEGF in Lkb1-Deficient Mice. Science 2001, 293, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Page, T.; Barshop, B.; Yu, A.L.; Nyhan, W.L. Treatment of Lesch-Nyhan syndrome with AICAR. Adv. Exp. Med. Biol. 1994, 370, 353–356. [Google Scholar] [CrossRef]
- Bosselaar, M.; Boon, H.; van Loon, L.J.C.; van den Broek, P.H.H.; Smits, P.; Tack, C.J. Intra-arterial AICA-riboside administration induces NO-dependent vasodilation in vivo in human skeletal muscle. Am. J. Physiol. Metab. 2009, 297, E759–E766. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.-X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists Are Exercise Mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Pokrywka, A.; Cholbinski, P.; Kaliszewski, P.; Kowalczyk, K.; Konczak, D.; Zembron-Lacny, A. Metabolic modulators of the exercise response: Doping control analysis of an agonist of the peroxisome proliferator-activated receptor δ (GW501516) and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 469–476. [Google Scholar]
- Fan, W.; Evans, R.M. Exercise Mimetics: Impact on Health and Performance. Cell Metab. 2017, 25, 242–247. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Treebak, J.T.; Fentz, J.; Lantier, L.; Viollet, B.; Birk, J.B.; Schjerling, P.; Björnholm, M.; Zierath, J.R.; Wojtaszewski, J.F.P. Prior AICAR Stimulation Increases Insulin Sensitivity in Mouse Skeletal Muscle in an AMPK-Dependent Manner. Diabetes 2015, 64, 2042–2055. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef]
- Sajan, M.P.; Bandyopadhyay, G.; Miura, A.; Standaert, M.L.; Nimal, S.; Longnus, S.L.; Van Obberghen, E.; Hainault, I.; Foufelle, F.; Kahn, R.; et al. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am. J. Physiol. Metab. 2009, 298, E179–E192. [Google Scholar] [CrossRef] [PubMed]
- Ezagouri, S.; Zwighaft, Z.; Sobel, J.; Baillieul, S.; Doutreleau, S.; Ladeuix, B.; Golik, M.; Verges, S.; Asher, G. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab. 2019, 30, 78–91.e4. [Google Scholar] [CrossRef]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Yu, H.J.; Liu, Z.-X.; Dong, J.; Mustard, K.J.; Hawley, S.A.; et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007, 5, 151–156. [Google Scholar] [CrossRef]
- Kobilo, T.; Guerrieri, D.; Zhang, Y.; Collica, S.C.; Becker, K.G.; van Praag, H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 2014, 21, 119–126. [Google Scholar] [CrossRef]
- Pauly, M.; Chabi, B.; Favier, F.B.; Vanterpool, F.; Matecki, S.; Fouret, G.; Bonafos, B.; Vernus, B.; Feillet-Coudray, C.; Coudray, C.; et al. Combined Strategies for Maintaining Skeletal Muscle Mass and Function in Aging: Myostatin Inactivation and AICAR-Associated Oxidative Metabolism Induction. J. Gerontol. Ser. A 2015, 70, 1077–1087. [Google Scholar] [CrossRef]
- Bueno Júnior, C.R.; Pantaleão, L.C.; Voltarelli, V.A.; Bozi, L.H.M.; Brum, P.C.; Zatz, M. Combined Effect of AMPK/PPAR Agonists and Exercise Training in mdx Mice Functional Performance. PLoS ONE 2012, 7, e45699. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Daussin, F.; Burelle, Y.; Li, T.; Godin, R.; Fauconnier, J.; Koechlin-Ramonatxo, C.; Hugon, G.; Lacampagne, A.; Coisy-Quivy, M.; et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am. J. Pathol. 2012, 181, 583–592. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Kazyken, D.; Magnuson, B.; Bodur, C.; Acosta-Jaquez, H.A.; Zhang, D.; Tong, X.; Barnes, T.M.; Steinl, G.K.; Patterson, N.E.; Altheim, C.H.; et al. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Ogura, T.; Kishimoto, A.; Kaminishi, M.; Esumi, H. Cell Cycle Regulation via p53 Phosphorylation by a 5′-AMP Activated Protein Kinase Activator, 5-Aminoimidazole- 4-Carboxamide-1-β-d-Ribofuranoside, in a Human Hepatocellular Carcinoma Cell Line. Biochem. Biophys. Res. Commun. 2001, 287, 562–567. [Google Scholar] [CrossRef]
- Jones, R.G.; Plas, D.R.; Kubek, S.; Buzzai, M.; Mu, J.; Xu, Y.; Birnbaum, M.J.; Thompson, C.B. AMP-Activated Protein Kinase Induces a p53-Dependent Metabolic Checkpoint. Mol. Cell 2005, 18, 283–293. [Google Scholar] [CrossRef]
- Rattan, R.; Giri, S.; Singh, A.K.; Singh, I. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside Inhibits Cancer Cell Proliferation in Vitro and in Vivo via AMP-activated Protein Kinase. J. Biol. Chem. 2005, 280, 39582–39593. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hildebrandt, I.J.; Prins, R.M.; Soto, H.; Mazzotta, M.M.; Dang, J.; Czernin, J.; Shyy, J.Y.-J.; Watson, A.D.; Phelps, M.; et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-C.; Williams, B.R.; Siegel, J.J.; Amon, A. Identification of Aneuploidy-Selective Antiproliferation Compounds. Cell 2011, 144, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-D.; Lee, M.-R.; Broxmeyer, H.E. 5-Aminoimidazole-4-carboxyamide Ribonucleoside Induces G1/S Arrest and Nanog Downregulation via p53 and Enhances Erythroid Differentiation. Stem Cells 2012, 30, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Vincent, E.E.; Coelho, P.P.; Blagih, J.; Griss, T.; Viollet, B.; Jones, R.G. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene 2015, 34, 3627–3639. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.W.; Guan, H.-P.; Ehrhart, J.; Petrov, A.; Prahalada, S.; Tozzo, E.; Yang, X.; Kurtz, M.M.; Trujillo, M.; Gonzalez Trotter, D.; et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 2017, 357, 507–511. [Google Scholar] [CrossRef]
- Cokorinos, E.C.; Delmore, J.; Reyes, A.R.; Albuquerque, B.; Kjøbsted, R.; Jørgensen, N.O.; Tran, J.-L.; Jatkar, A.; Cialdea, K.; Esquejo, R.M.; et al. Activation of Skeletal Muscle AMPK Promotes Glucose Disposal and Glucose Lowering in Non-human Primates and Mice. Cell Metab. 2017, 25, 1147–1159.e10. [Google Scholar] [CrossRef]
- Langendorf, C.G.; Ngoei, K.R.W.; Scott, J.W.; Ling, N.X.Y.; Issa, S.M.A.; Gorman, M.A.; Parker, M.W.; Sakamoto, K.; Oakhill, J.S.; Kemp, B.E. Structural basis of allosteric and synergistic activation of AMPK by furan-2-phosphonic derivative C2 binding. Nat. Commun. 2016, 7, 10912. [Google Scholar] [CrossRef]
- Liu, X.; Chhipa, R.R.; Pooya, S.; Wortman, M.; Yachyshin, S.; Chow, L.M.L.; Kumar, A.; Zhou, X.; Sun, Y.; Quinn, B.; et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc. Natl. Acad. Sci. USA 2014, 111, E435–E444. [Google Scholar] [CrossRef] [PubMed]
- Santidrián, A.F.; González-Gironès, D.M.; Iglesias-Serret, D.; Coll-Mulet, L.; Cosialls, A.M.; de Frias, M.; Campàs, C.; González-Barca, E.; Alonso, E.; Labi, V.; et al. AICAR induces apoptosis independently of AMPK and p53 through up-regulation of the BH3-only proteins BIM and NOXA in chronic lymphocytic leukemia cells. Blood 2010, 116, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Lalic, H.; Dembitz, V.; Lukinovic-Skudar, V.; Banfic, H.; Visnjic, D. 5-Aminoimidazole-4-carboxamide ribonucleoside induces differentiation of acute myeloid leukemia cells. Leuk. Lymphoma 2014, 55, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Chatterjee, A.; Kogan, D.; Patel, D.; Foster, D.A. 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle 2015, 14, 3331–3339. [Google Scholar] [CrossRef]
- Dembitz, V.; Tomic, B.; Kodvanj, I.; Simon, J.A.; Bedalov, A.; Visnjic, D. The ribonucleoside AICAr induces differentiation of myeloid leukemia by activating the ATR/Chk1 via pyrimidine depletion. J. Biol. Chem. 2019, 294, 15257–15270. [Google Scholar] [CrossRef]
- Nyhan, W.L. Nucleotide Synthesis via Salvage Pathway. eLS 2014. [Google Scholar] [CrossRef]
- López, J.M.; Outtrim, E.L.; Fu, R.; Sutcliffe, D.J.; Torres, R.J.; Jinnah, H.A. Physiological levels of folic acid reveal purine alterations in Lesch-Nyhan disease. Proc. Natl. Acad. Sci. USA 2020, 117, 12071–12079. [Google Scholar] [CrossRef]
- Douillet, D.C.; Pinson, B.; Ceschin, J.; Hürlimann, H.C.; Saint-Marc, C.; Laporte, D.; Claverol, S.; Konrad, M.; Bonneu, M.; Daignan-Fornier, B. Metabolomics and proteomics identify the toxic form and the associated cellular binding targets of the anti-proliferative drug AICAR. J. Biol. Chem. 2019, 294, 805–815. [Google Scholar] [CrossRef]
- Ceschin, J.; Hürlimann, H.C.; Saint-Marc, C.; Albrecht, D.; Violo, T.; Moenner, M.; Daignan-Fornier, B.; Pinson, B. Disruption of Nucleotide Homeostasis by the Antiproliferative Drug 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside Monophosphate (AICAR). J. Biol. Chem. 2015, 290, 23947–23959. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Hrafnsdóttir, S.; Magnúsdóttir, M.; Fleming, R.M.T.; Thorlacius, S.; Palsson, B.Ø.; Thiele, I. Monitoring metabolites consumption and secretion in cultured cells using ultra-performance liquid chromatography quadrupole–time of flight mass spectrometry (UPLC–Q–ToF-MS). Anal. Bioanal. Chem. 2012, 402, 1183–1198. [Google Scholar] [CrossRef]
- Bardeleben, C.; Sharma, S.; Reeve, J.R.; Bassilian, S.; Frost, P.; Hoang, B.; Shi, Y.; Lichtenstein, A. Metabolomics Identifies Pyrimidine Starvation as the Mechanism of 5-Aminoimidazole-4-Carboxamide-1-β-Riboside-Induced Apoptosis in Multiple Myeloma Cells. Mol. Cancer Ther. 2013, 12, 1310–1321. [Google Scholar] [CrossRef]
- Du, L.; Yang, F.; Fang, H.; Sun, H.; Chen, Y.; Xu, Y.; Li, H.; Zheng, L.; Zhou, B.-B.S. AICAr suppresses cell proliferation by inducing NTP and dNTP pool imbalances in acute lymphoblastic leukemia cells. FASEB J. 2019, 33, 4525–4537. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.B.; Meade, J.C.; Holmes, E.W. Aminoimidazole carboxamide ribonucleoside toxicity: A model for study of pyrimidine starvation. J. Cell. Physiol. 1981, 107, 335–344. [Google Scholar] [CrossRef] [PubMed]
- ElAzzouny, M.A.; Evans, C.R.; Burant, C.F.; Kennedy, R.T. Metabolomics Analysis Reveals that AICAR Affects Glycerolipid, Ceramide and Nucleotide Synthesis Pathways in INS-1 Cells. PLoS ONE 2015, 10, e0129029. [Google Scholar] [CrossRef] [PubMed]
- Asby, D.J.; Cuda, F.; Beyaert, M.; Houghton, F.D.; Cagampang, F.R.; Tavassoli, A. AMPK Activation via Modulation of De Novo Purine Biosynthesis with an Inhibitor of ATIC Homodimerization. Chem. Biol. 2015, 22, 838–848. [Google Scholar] [CrossRef]
- Agarwal, S.; Bell, C.M.; Rothbart, S.B.; Moran, R.G. AMP-activated Protein Kinase (AMPK) Control of mTORC1 Is p53- and TSC2-independent in Pemetrexed-treated Carcinoma Cells. J. Biol. Chem. 2015, 290, 27473–27486. [Google Scholar] [CrossRef]
- Beckers, A.; Organe, S.; Timmermans, L.; Vanderhoydonc, F.; Deboel, L.; Derua, R.; Waelkens, E.; Brusselmans, K.; Verhoeven, G.; Swinnen, J. V Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol. Cancer Ther. 2006, 5, 2211–2217. [Google Scholar] [CrossRef]
- Pirkmajer, S.; Kulkarni, S.S.; Tom, R.Z.; Ross, F.A.; Hawley, S.A.; Hardie, D.G.; Zierath, J.R.; Chibalin, A. V Methotrexate Promotes Glucose Uptake and Lipid Oxidation in Skeletal Muscle via AMPK Activation. Diabetes 2015, 64, 360–369. [Google Scholar] [CrossRef]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Sengupta, T.K.; Leclerc, G.M.; Hsieh-Kinser, T.T.; Leclerc, G.J.; Singh, I.; Barredo, J.C. Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: Implication for targeted therapy. Mol. Cancer 2007, 6, 46. [Google Scholar] [CrossRef]
- Vakana, E.; Altman, J.K.; Glaser, H.; Donato, N.J.; Platanias, L.C. Antileukemic effects of AMPK activators on BCR-ABL–expressing cells. Blood 2011, 118, 6399–6402. [Google Scholar] [CrossRef]
- Dembitz, V.; Lalic, H.; Kodvanj, I.; Tomic, B.; Batinic, J.; Dubravcic, K.; Batinic, D.; Bedalov, A.; Visnjic, D. 5-aminoimidazole-4-carboxamide ribonucleoside induces differentiation in a subset of primary acute myeloid leukemia blasts. BMC Cancer 2020, 20, 1090. [Google Scholar] [CrossRef]
- Dembitz, V.; Lalic, H.; Visnjic, D. 5-Aminoimidazole-4-carboxamide ribonucleoside-induced autophagy flux during differentiation of monocytic leukemia cells. Cell Death Discov. 2017, 3, 17066. [Google Scholar] [CrossRef] [PubMed]
- De Thé, H. Differentiation therapy revisited. Nat. Rev. Cancer 2018, 18, 117–127. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Sugimoto, T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am. J. Physiol. Metab. 2009, 296, E139–E146. [Google Scholar] [CrossRef]
- Zang, Y.; Yu, L.-F.; Pang, T.; Fang, L.-P.; Feng, X.; Wen, T.-Q.; Nan, F.-J.; Feng, L.-Y.; Li, J. AICAR Induces Astroglial Differentiation of Neural Stem Cells via Activating the JAK/STAT3 Pathway Independently of AMP-activated Protein Kinase. J. Biol. Chem. 2008, 283, 6201–6208. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.B.; Kfoury, Y.S.; Mercier, F.E.; Wawer, M.J.; Law, J.M.; Haynes, M.K.; Lewis, T.A.; Schajnovitz, A.; Jain, E.; Lee, D.; et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell 2016, 167, 171–186.e15. [Google Scholar] [CrossRef]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde?†. Open Biol. 2021, 9, 190099. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Višnjić, D.; Lalić, H.; Dembitz, V.; Tomić, B.; Smoljo, T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells 2021, 10, 1095. https://doi.org/10.3390/cells10051095
Višnjić D, Lalić H, Dembitz V, Tomić B, Smoljo T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells. 2021; 10(5):1095. https://doi.org/10.3390/cells10051095
Chicago/Turabian StyleVišnjić, Dora, Hrvoje Lalić, Vilma Dembitz, Barbara Tomić, and Tomislav Smoljo. 2021. "AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review" Cells 10, no. 5: 1095. https://doi.org/10.3390/cells10051095
APA StyleVišnjić, D., Lalić, H., Dembitz, V., Tomić, B., & Smoljo, T. (2021). AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells, 10(5), 1095. https://doi.org/10.3390/cells10051095






