Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sampling and Microbial Endophytes Isolation
2.2. Molecular Identification of Microbial Endophytes
2.3. Screening of Plant Growth Promotion Potential of Microbial Endophytes
2.3.1. Colorimetric Determination of Indole-3-Acetic Acid (IAA)
2.3.2. Ammonia Production
2.3.3. Phosphate Solubilization
2.3.4. Extracellular Enzymatic Activities
2.3.5. In Vitro Evaluation of Antagonistic Activity of Endophytic Isolates against Selected Phytopathogenic Fungi
2.4. Field Experiment Using Phaseolus vulgaris L. Plant
2.4.1. Experimental Design
2.4.2. Culture Conditions and Extraction of Endophytic Secondary Metabolites
2.4.3. Foliar Spraying of Phaseolus Vulgaris Plants with Endophytic Metabolites and Exogenously Applied Hormone
2.4.4. Growth and Vegetative Parameters Measurement
2.4.5. Physiological Parameters Measurements
Determination of Photosynthetic Pigments
Determination of Total Soluble Carbohydrates and Proteins
Determination of Amylase and Protease Enzymes Activity
Determination of Antioxidant Enzymes Activity
Extraction, Purification, and Determination of Endogenous Hormones
Yield Parameters Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Bacterial and Fungal Endophytes Isolated from Root of Common Bean Plant
3.2. In Vitro Assessment of the Plant Growth-Promoting Activities of Bacterial and Fungal Endophytes
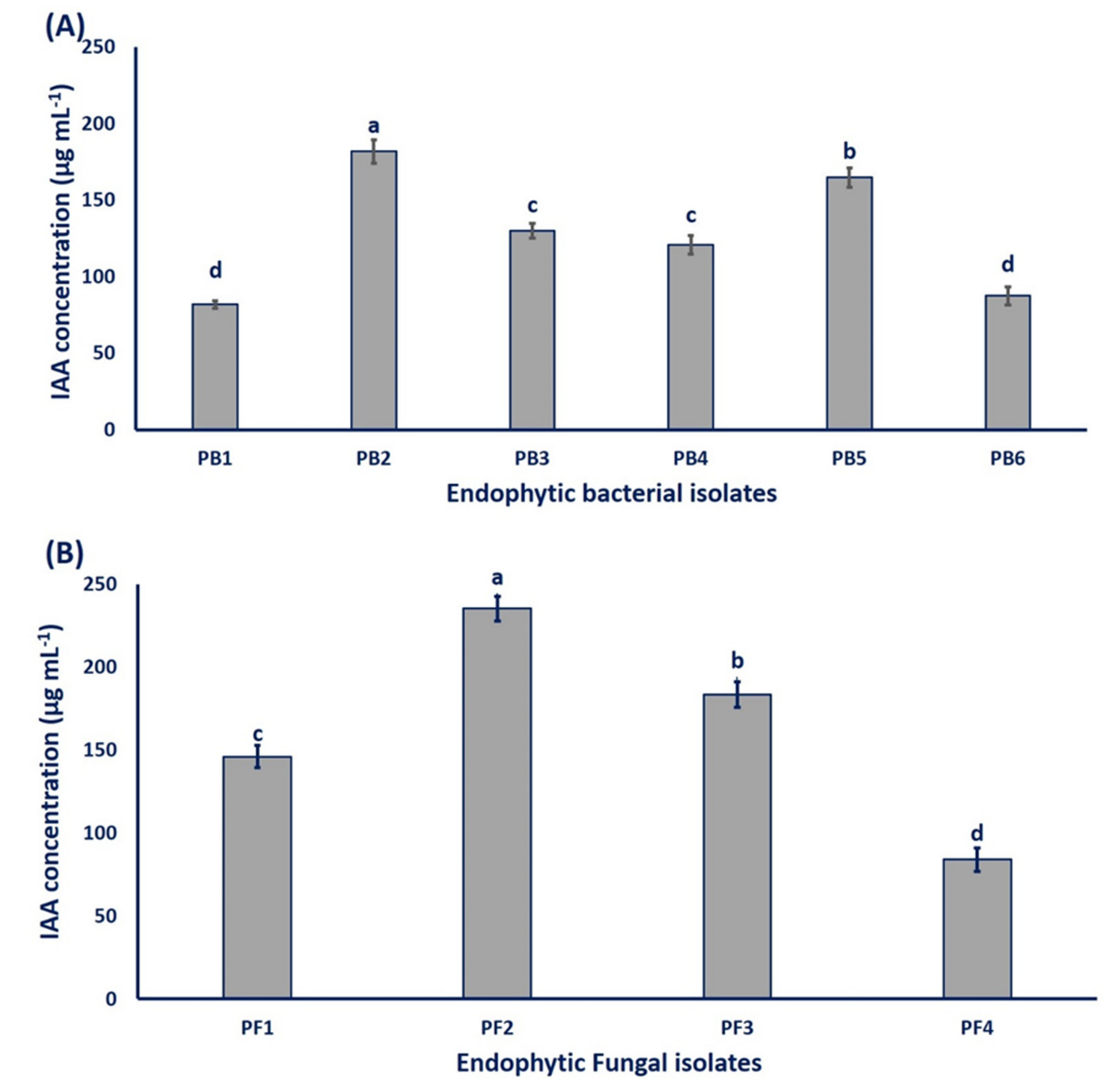
3.2.1. Colorimetric Determination of IAA
3.2.2. Ammonia Production and Phosphate Solubilization
3.2.3. Extracellular Enzymatic Activities
3.2.4. In Vitro Antagonistic Activities
3.3. Evaluation of the Promotion Effect of the Most Potent Bacterial and Fungal Endophytic Strains on the Growth and Metabolic Activities of the Phaseolus vulgaris Plant under Field Conditions
3.3.1. Effect of the Foliar Spraying of Exogenously Applied Hormone and Microbial Endophyte Metabolites on the Vegetative Growth Traits of Common Bean Plants
3.3.2. Effect of the Foliar Spraying of Exogenously Applied Hormones and Microbial Endophyte Metabolites on the Chlorophyll and Carotene Contents of Common Bean Plants
3.3.3. Effect of the Foliar Spraying of Exogenously Applied Hormones and Microbial Endophyte Metabolites on the Total Soluble Carbohydrate and Protein Contents of Common Bean Plants
3.3.4. Effect of the Foliar Spraying of Exogenously Applied Hormones and Microbial Endophyte Metabolites on the Amylase, Protease, Catalase, and Peroxidase Activities of Common Bean Plants
3.3.5. Effect of the Foliar Spraying of Exogenously Applied Hormones and Microbial Endophyte Metabolites on the Endogenous Hormones of Common Bean Plants
3.3.6. Effect of the Foliar Spraying of Exogenously Applied Hormones and Microbial Endophyte Metabolites on the Yield Parameters of Common Bean Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassary, E.K.; Baijukya, F.; Ndakidemi, P.A. Assessing the Productivity of Common Bean in Intercrop with Maize across Agro-Ecological Zones of Smallholder Farms in the Northern Highlands of Tanzania. Agriculture 2020, 10, 117. [Google Scholar] [CrossRef]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; Valdés-López, O. Common Bean: A Legume Model on the Rise for Unraveling Responses and Adaptations to Iron, Zinc, and Phosphate Deficiencies. Front. Plant Sci. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.R.; Kmiecik, K. Common Bean: Economic Importance and Relevance to Biological Science Research. In The Common Bean Genome; Pérez de la Vega, M., Santalla, M., Marsolais, F., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.M.; Hansen, J.W.; Rosenstock, T.; Wollenberg, E. Food and Earth Systems: Priorities for Climate Change Adaptation and Mitigation for Agriculture and Food Systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef]
- Trewavas, A. How do plant growth substances work? Plant Cell Environ. 1981, 4, 203–228. [Google Scholar] [CrossRef]
- Gan, L.; Wei, Z.; Yang, Z.; Li, F.; Wang, Z. Updated Mechanisms of GCN5—The Monkey King of the Plant Kingdom in Plant Development and Resistance to Abiotic Stresses. Cells 2021, 10, 979. [Google Scholar] [CrossRef]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.M.; El-Far, A.H.; Abd El-Hack, M.E.; Noreldin, A.E.; El-Naggar, K.; Abdelnour, S.A.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental Toxicity of Carbon Nanoparticles during Embryogenesis in Chicken. Environ Sci. Pollut. Res. 2020, 27, 19058–19072. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Lin, L.; Liao, M.; Lv, X.; Tang, Y.; Wang, X.; Xia, H.; Liang, D.; Ren, W.; et al. Effects of mutual grafting on cadmium accumulation characteristics of first post-generations of Bidens pilosa L. and Galinsoga parviflora Cav. Environ. Sci. Pollut. Res. Int. 2019, 26, 33228–33235. [Google Scholar] [CrossRef]
- Dewitte, W.; Chiappetta, A.; Azmi, A.; Witters, E.; Strnad, M.; Rembur, J.; Noin, M.; Chriqui, D.; Van Onckelen, H. Dynamics of Cytokinins in Apical Shoot Meristems of a Day-Neutral Tobacco during Floral Transition and Flower Formation. Plant Physiol. 1999, 119, 111. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium Availability Regulates Antioxidant System, Physio-Biochemical Activities and Alleviates Salinity Stress Mediated Oxidative Damage in Soybean Seedlings. J. Appl. Bot. Food Qual 2019, 92, 258–266. [Google Scholar]
- Çakmakçı, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid Seed Priming with Nitrogen Supplementation Improves Salt Tolerance in Soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef]
- Collenburg, L.; Beyersdorf, N.; Wiese, T.; Arenz, C.; Saied, E.M.; Becker-Flegler, K.A.; Schneider-Schaulies, S.; Avota, E. The Activity of the Neutral Sphingomyelinase Is Important in T Cell Recruitment and Directional Migration. Front. Immunol. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Hassan, S.E.L.D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Refat, M.S.; Ibrahim, H.K.; Sowellim, S.Z.A.; Soliman, M.H.; Saeed, E.M. Spectroscopic and Thermal Studies of Mn(II), Fe(III), Cr(III) and Zn(II) Complexes Derived from the Ligand Resulted by the Reaction between 4-Acetyl Pyridine and Thiosemicarbazide. J. Inorg. Organomet. Polym. Mater. 2009, 19, 521–531. [Google Scholar] [CrossRef]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem. 2015, 7, 1971–1980. [Google Scholar] [CrossRef]
- Hassan, S.E.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii Mediated Green Synthesis of Ag-NPs with Antibacterial and Anticancer Properties for Developing Functional Textile Fabric Properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Passari, A.K.; Mishra, V.K.; Leo, V.V.; Gupta, V.K.; Singh, B.P. Phytohormone production endowed with antagonistic potential and plant growth promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol. Res. 2016, 193, 57–73. [Google Scholar] [CrossRef]
- Molina, G.; Pimentel, M.R.; Bertucci, T.C.; Pastore, G.M. Application of fungal endophytes in biotechnological processes. Chem. Eng. Trans. 2012, 27. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elements Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Miller, D.N.; Bryant, J.E.; Madsen, E.L.; Ghiorse, W.C. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 1999, 65, 4715–4724. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Eid, A.M.; Barghoth, M.G.; El-Sadany, M.A.-H. Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 2019, 26, 6583–6597. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M. Indole Acetic Acid Production by the Indigenous Isolates of Azotobacter and Fluorescent Pseudomonas in the Presence and Absence of Tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- ALKahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Da Silva Ribeiro, A.; Polonio, J.C.; Costa, A.T.; Dos Santos, C.M.; Rhoden, S.A.; Azevedo, J.L.; Pamphile, J.A. Bioprospection of Culturable Endophytic Fungi Associated with the Ornamental Plant Pachystachys lutea. Curr. Microbiol. 2018, 75, 588–596. [Google Scholar] [CrossRef]
- Fouda, A.H.; Hassan, S.E.-D.; Eid, A.M.; Ewais, E.E.-D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann. Agric. Sci. 2015, 60, 95–104. [Google Scholar] [CrossRef]
- Ghildiyal, A.; Pandey, A. Isolation of cold tolerant antifungal strains of Trichoderma sp. from glacial sites of Indian Himalayan region. Res. J. Microbiol. 2008, 3, 559–564. [Google Scholar]
- Kim, H.Y.; Choi, G.J.; Lee, H.B.; Lee, S.W.; Lim, H.K.; Jang, K.S.; Son, S.W.; Lee, S.O.; Cho, K.Y.; Sung, N.D.; et al. Some fungal endophytes from vegetable crops and their anti-oomycete activities against tomato late blight. Lett. Appl. Microbiol. 2007, 44, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Vernon, L.P.; Seely, G.R. The Chlorophylls; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Umbreit, W.W.; Burris, R.H.; Stauffer, J.F. Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism; Burgess: Minneapolis, MN, USA, 1964. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Afifi, W.; Ahmed, M.; Moussa, Z.; Abd El-Hamid, M. Effect of gamma irradiation and GA3 on amylase activity of pea seedlings. Ann. Agric. Sci. Moshtohor. 1986, 24, 2047–2057. [Google Scholar]
- Ong, P.S.; Gaucher, G.M. Protease production by thermophilic fungi. Can. J. Microbiol. 1973, 19, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, R.; Owuor, E.D.; Kong, A.N. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res. 2000, 23, 605–612. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B.; et al. Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. PPB 2014, 77, 23–34. [Google Scholar] [CrossRef]
- López-López, A.; Rogel, M.A.; Ormeño-Orrillo, E.; Martínez-Romero, J.; Martínez-Romero, E. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. Syst. Appl. Microbiol. 2010, 33, 322–327. [Google Scholar] [CrossRef]
- Sendi, Y.; Pfeiffer, T.; Koch, E.; Mhadhbi, H.; Mrabet, M. Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Prot. 2020, 127, 453–462. [Google Scholar] [CrossRef]
- De Oliveira Costa, L.E.; de Queiroz, M.V.; Borges, A.C.; de Moraes, C.A.; de Araújo, E.F. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz. J. Microbiol. 2012, 43, 1562–1575. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.-D. Plant Growth-Promoting Endophytic Bacterial Community Inhabiting the Leaves of Pulicaria incisa (Lam.) DC Inherent to Arid Regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Hassan, S.E.-D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.-D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Numponsak, T.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola CMU-A109. PLoS ONE 2018, 13, e0205070. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Liu, Q.; Li, Z.; Li, R.; Zhang, H.; Liu, L.; Emelchenko, G.A.; Wang, J. A graphene oxide/amidoxime hydrogel for enhanced uranium capture. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Szilagyi-Zecchin, V.J.; Ikeda, A.C.; Hungria, M.; Adamoski, D.; Kava-Cordeiro, V.; Glienke, C.; Galli-Terasawa, L.V. Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. Amb Express 2014, 4, 26. [Google Scholar] [CrossRef]
- Brígido, C.; Singh, S.; Menéndez, E.; Tavares, M.J.; Glick, B.R.; Félix, M.D.R.; Oliveira, S.; Carvalho, M. Diversity and Functionality of Culturable Endophytic Bacterial Communities in Chickpea Plants. Plants 2019, 8, 42. [Google Scholar] [CrossRef]
- Ripa, F.A.; Cao, W.-D.; Tong, S.; Sun, J.-G. Assessment of Plant Growth Promoting and Abiotic Stress Tolerance Properties of Wheat Endophytic Fungi. BioMed Res. Int. 2019, 2019, 6105865. [Google Scholar] [CrossRef]
- Miller, S.H.; Browne, P.; Prigent-Combaret, C.; Combes-Meynet, E.; Morrissey, J.P.; O’Gara, F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ. Microbiol. Rep. 2010, 2, 403–411. [Google Scholar] [CrossRef]
- Haidar, B.; Ferdous, M.; Fatema, B.; Ferdous, A.S.; Islam, M.R.; Khan, H. Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiol Res. 2018, 208, 43–53. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Chapter 11—Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In Advances in Biological Science Research; Meena, S.N., Naik, M.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 161–176. [Google Scholar]
- Khan, A.L.; Shahzad, R.; Al-Harrasi, A.; Lee, I.-J. Endophytic Microbes: A Resource for Producing Extracellular Enzymes. In Endophytes: Crop Productivity and Protection: Volume 2; Maheshwari, D.K., Annapurna, K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 95–110. [Google Scholar]
- Sopalun, K.; Iamtham, S. Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. S. Afr. J. Bot. 2020, 134, 273–279. [Google Scholar] [CrossRef]
- Sunitha, V.; Nirmala Devi, D.; Srinivas, C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J. Agric. Sci. 2013, 9, 1–9. [Google Scholar]
- Naik, S.; Abrar, D.S.; Krishnappa, M. Industrially important enzymes from fungal endophytes. Recent Adv. White Biotechnol. Fungi 2019, 263–280. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microbiol. 2018, 49, 269–278. [Google Scholar] [CrossRef]
- Chowdhary, K.; Sharma, S. Plant Growth Promotion and Biocontrol Potential of Fungal Endophytes in the Inflorescence of Aloe vera L. Proc. Natl. Acad. Sci. USA 2020, 90, 1045–1055. [Google Scholar] [CrossRef]
- Hamzah, T.N.T.; Lee, S.Y.; Hidayat, A.; Terhem, R.; Faridah-Hanum, I.; Mohamed, R. Diversity and Characterization of Endophytic Fungi Isolated From the Tropical Mangrove Species, Rhizophora mucronata, and Identification of Potential Antagonists Against the Soil-Borne Fungus, Fusarium solani. Front. Microbiol. 2018, 9, 1707. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
- Sarkar, D.; Mandal, B.; Kundu, M.C. Increasing use efficiency of boron fertilisers by rescheduling the time and methods of application for crops in India. Plant Soil 2007, 301, 77–85. [Google Scholar] [CrossRef]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.; Škrbić, B.D.; Jigjiddorj, E.-A.; Vágvölgyi, C.; et al. Characterization of the Plant Growth-Promoting Activities of Endophytic Fungi Isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.T.; Gunatilaka, M.K.; Wijeratne, K.; Gunatilaka, L.; Arnold, A.E. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 2013, 8, e73132. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Tumangger, B.; Nadilla, F.; Baiduri, N.; Fitriani; Mardina, V. In vitro screening of endophytic fungi associated with mangroveas biofertilizer on the growth of black rice (Oryza sativa L. “Cempo Ireng”). IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012080. [Google Scholar] [CrossRef]
- Sosnowski, J.; Malinowska, E.; Jankowski, K.; Król, J.; Redzik, P. An estimation of the effects of synthetic auxin and cytokinin and the time of their application on some morphological and physiological characteristics of Medicago x varia T. Martyn. Saudi J. Biol. Sci. 2019, 26, 66–73. [Google Scholar] [CrossRef]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Abdallah, R.A.B.; Mejdoub-Trabelsi, B.; Nefzi, A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Isolation of endophytic bacteria from Withania somnifera and assessment of their ability to suppress Fusarium wilt disease in tomato and to promote plant growth. J. Plant Pathol. Microbiol. 2016, 7, 5. [Google Scholar] [CrossRef]
- Sukhov, V.; Sukhova, E.; Sinitsyna, Y.; Gromova, E.; Mshenskaya, N.; Ryabkova, A.; Ilin, N.; Vodeneev, V.; Mareev, E.; Price, C. Influence of Magnetic Field with Schumann Resonance Frequencies on Photosynthetic Light Reactions in Wheat and Pea. Cells 2021, 10, 149. [Google Scholar] [CrossRef]
- Hanaa, H.; Safaa, A. In Foliar application Foliar application of IAA at different growth stages and their influenced on growth and productivity of bread Wheat (triticum aestivum L.). J. Phys. Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kudaka, R.; Inaba, H.; Furuya, T.; Kitamura, M.; Kitaya, Y.; Yamamoto, R.; Yahata, M.; Matsumoto, H.; et al. Exogenous Application of ABA and NAA Alleviates the Delayed Coloring Caused by Puffing Inhibitor in Citrus Fruit. Cells 2021, 10, 308. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Kumar, A.; Bahadur, D.; Maurya, B.; Raghuwanshi, R.; Meena, V.; Singh, S.; Dixit, J. Does a plant growth promoting rhizobacteria enhance agricultural sustainability? J. Pure Appl. Microbiol. 2015, 9, 715–724. [Google Scholar]
- Leopold, A.C.; Noodén, L.D. Hormonal Regulatory Systems in Plants. In Hormonal Regulation of Development II: The Functions of Hormones from the Level of the Cell to the Whole Plant; Scott, T.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 4–22. [Google Scholar]
- Naeem, M.; Bhatti, I.R.A.M.; Ahmad, R.H.; Ashraf, M.Y. Effect of some growth hormones (GA3, IAA and Kintin) on the Morphology and early or delayed iniation of bud of lintil (Lens culinaris Medik). Pak. J. Bot. 2004, 36, 801–809. [Google Scholar]
- Di Benedetto, A.; Galmarini, C.; Tognetti, J. Effects of combined or single exogenous auxin and/or cytokinin applications on growth and leaf area development in Epipremnum aureum. J. Hortic. Sci. Biotechnol. 2015, 90, 643–654. [Google Scholar] [CrossRef]
- Chu, E.; Tavares, A.; Kanashiro, S.; Giampaoli, P.; Yokota, E. Effects of auxins on soluble carbohydrates, starch and soluble protein content in Aechmea blanchetiana (Bromeliaceae) cultured in vitro. Sci. Hortic. 2010, 125, 451–455. [Google Scholar] [CrossRef]
- Farrar, J.; Pollock, C.; Gallagher, J. Sucrose and the integration of metabolism in vascular plants. Plant Sci. 2000, 154, 1–11. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic Fungus Aspergillus japonicus Mediates Host Plant Growth under Normal and Heat Stress Conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar] [CrossRef]
- Dietrich, J.; Kaminek, M.; Blevins, D.; Reinbott, T.; Morris, R. Changes in cytokinins and cytokinin oxidase activity in developingmaize kernels and the effects of exogenous cytokinin on kernel development. Plant Physiol. Biochem. 1995, 33, 327–336. [Google Scholar]
- Bhatia, S.; Singh, R. Phytohormone-mediated transformation of sugars to starch in relation to the activities of amylases, sucrose-metabolising enzymes in sorghum grain. Plant Growth Regul. 2002, 36, 97–104. [Google Scholar] [CrossRef]
- Sadar, P.; Jadhav, N.; Kulkarni, N.; Pachori, R. Diversity and enzymatic activity of endophytic fungi isolated from pine apple (Ananasa comosus). Eur. J. Biomed. Pharm. Sci. 2017, 4, 647–652. [Google Scholar]
- Naliwajski, M.; Skłodowska, M. The Relationship between the Antioxidant System and Proline Metabolism in the Leaves of Cucumber Plants Acclimated to Salt Stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Zluhan-Martínez, E.; Sánchez, M.D.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. Interplay between Hormones and Several Abiotic Stress Conditions on Arabidopsis thaliana Primary Root Development. Cells 2020, 9, 2576. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Abd El-Wahed, M.S.A. Effect of exogenous salicylic acid or indole acetic acid on their endogenous levels, germination, and growth in maize. Bull. Natl. Res. Cent. 2020, 44, 167. [Google Scholar] [CrossRef]
- Szalai, G.; Horgosi, S.; Soós, V.; Majláth, I.; Balázs, E.; Janda, T. Salicylic acid treatment of pea seeds induces its de novo synthesis. J. Plant Physiol. 2011, 168, 213–219. [Google Scholar] [CrossRef]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.-J.; Hussain, A. Plant growth promoting endophytic fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Polisetty, R.; Paul, V.; Deveshwar, J.J.; Khetarpal, S.; Suresh, K.; Chandra, R. Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicer arietinum L.). Plant Cell Rep. 1997, 16, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.; Lindow, S.E. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl. Environ. Microbiol. 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castro, R.; Díaz-Pérez, C.; Martínez-Trujillo, M.; del Río, R.E.; Campos-García, J.; López-Bucio, J. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 7253–7258. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Groenhagen, U.; Schulz, S.; Geisler, M.; Eberl, L.; Weisskopf, L. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J. Cell Mol. Biol. 2014, 80, 758–771. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins Producing Endophytic Fungus Porostereum spadiceum AGH786 Rescues Growth of Salt Affected Soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef]
- Giannakoula, A.E.; Ilias, I.F.; Dragišić Maksimović, J.J.; Maksimović, V.M.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. J. Food Compos. Anal. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Bagde, U.S.; Prasad, R.; Varma, A. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis 2011, 53, 83–88. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]
- Straub, D.; Yang, H.; Liu, Y.; Tsap, T.; Ludewig, U. Root ethylene signalling is involved in Miscanthus sinensis growth promotion by the bacterial endophyte Herbaspirillum frisingense GSF30(T). J. Exp. Bot. 2013, 64, 4603–4615. [Google Scholar] [CrossRef]
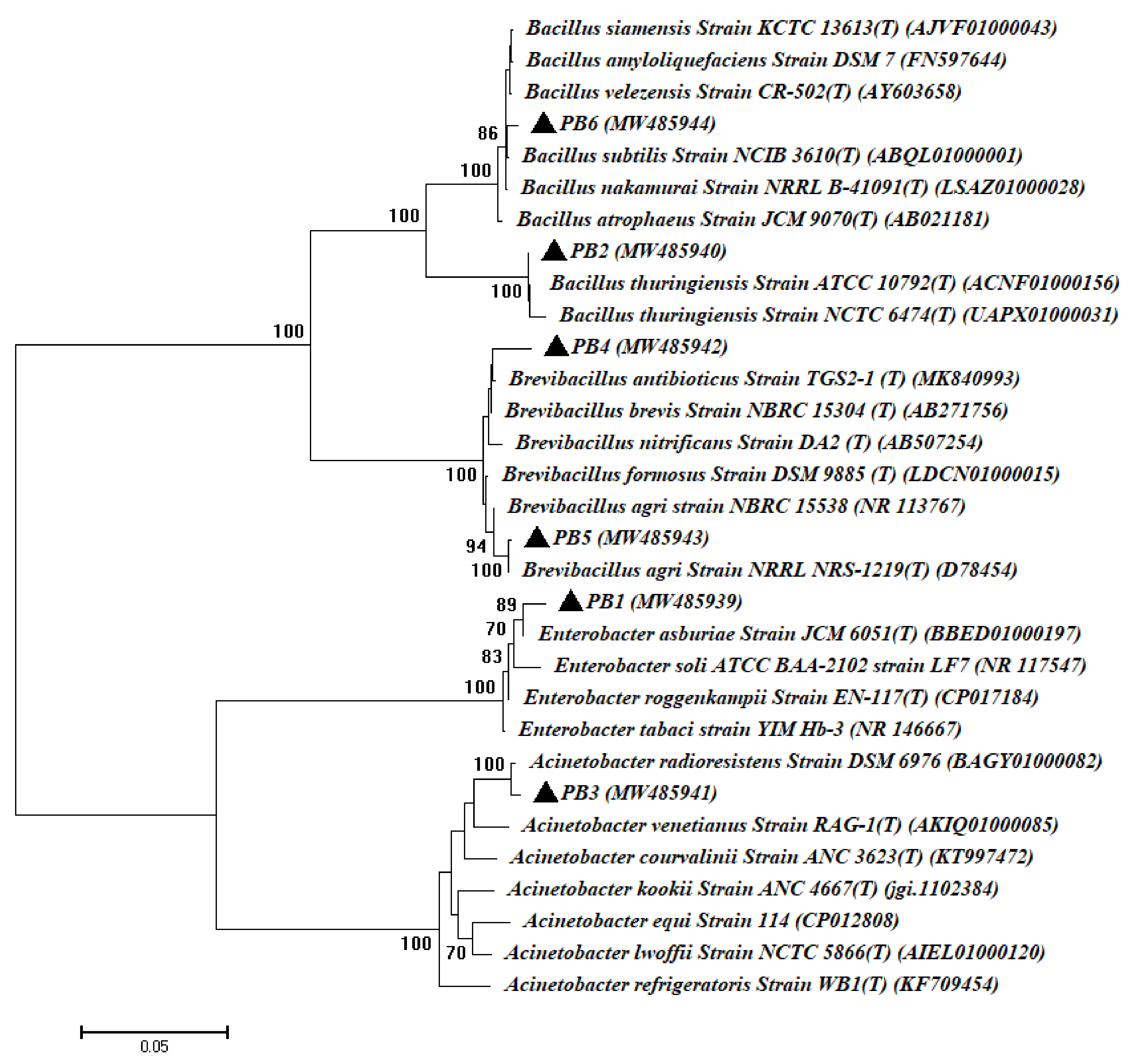
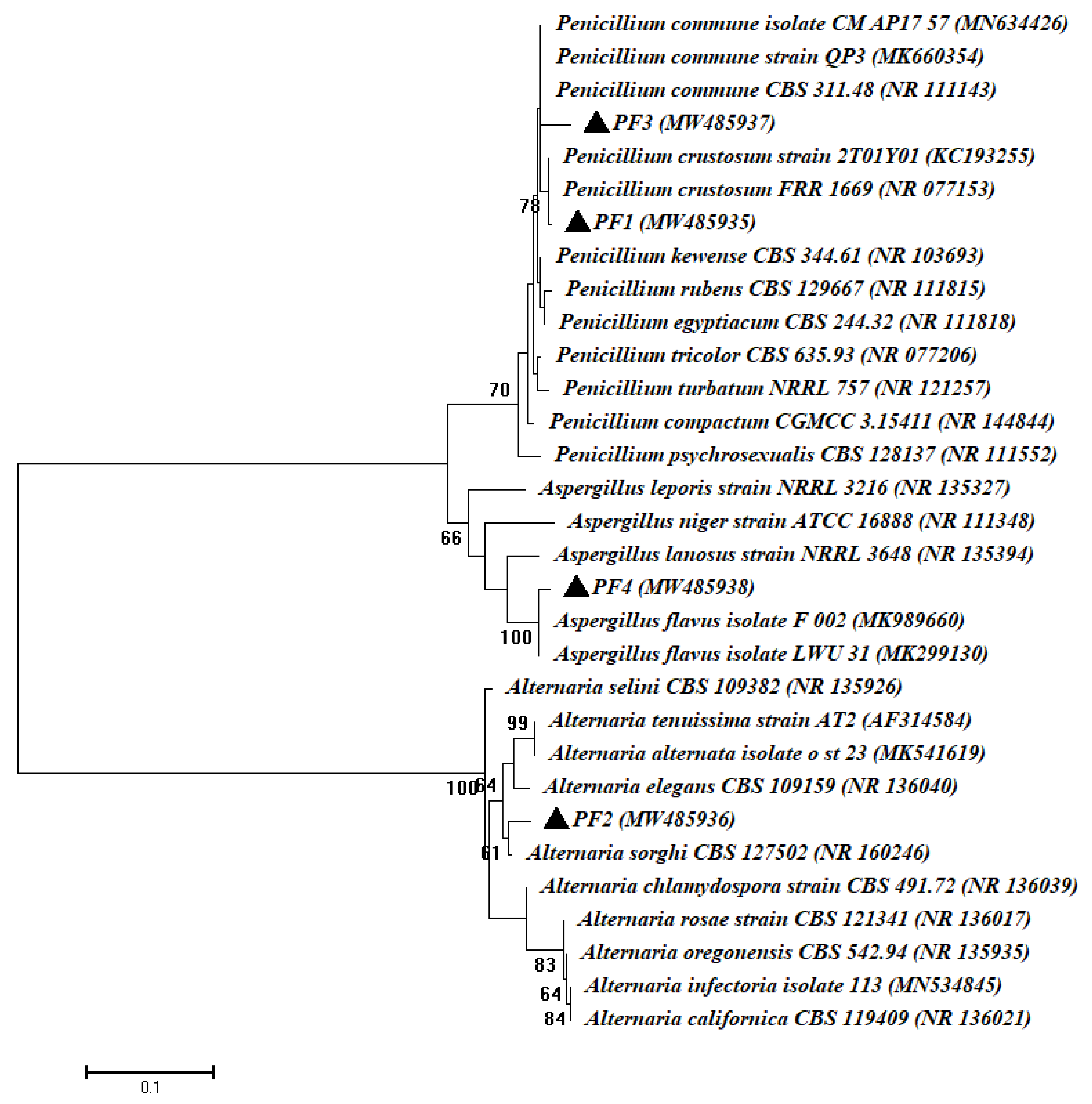
| Microbial Strain Code | GenBank Accession Number | Homolog Sequences | Sequence Identity (%) | Closest Accession Number |
|---|---|---|---|---|
| PB1 | MW485939 | Enterobacter asburiae | 99.16 | BBED01000197 |
| PB2 | MW485940 | Bacillus thuringiensis | 99.69 | ACNF01000156 |
| PB3 | MW485941 | Acinetobacter radioresistens | 99.70 | BAGY01000082 |
| PB4 | MW485942 | Brevibacillus brevis | 98.88 | AB271756 |
| PB5 | MW485943 | Brevibacillus agri | 99.84 | D78454 |
| PB6 | MW485944 | Bacillus subtilis | 98.99 | ABQL01000001 |
| PF1 | MW485935 | Penicillium crustosum | 99.62 | NR077153 |
| PF2 | MW485936 | Alternaria sorghi | 98.63 | NR160246 |
| PF3 | MW485937 | Penicillium commune | 98.69 | NR111143 |
| PF4 | MW485938 | Aspergillus flavus | 98.84 | NR111041 |
| Endophytic Isolates | Percentage of Growth Inhibition (%) | |||
|---|---|---|---|---|
| F. oxysporum | A. alternata | V. dahlia | P. ultimum | |
| Control | 0 i | 0 h | 0 h | 0 h |
| PB1 | 31.6 ± 1.4 g | 45.6 ± 0.8 c | 28.6 ± 0.3 f | 12.3 ± 0.8 f |
| PB2 | 54 ± 0.57 c | 49 ± 1.1 b | 57.6 ± 2 b | 24.3 ± 1.4 d |
| PB3 | 24.6 ± 0.33 h | 34.6 ± 0.8 f | 22.6 ± 1.4 g | 13.3 ± 1.4 ef |
| PB4 | 41.6 ± 1.4 e | 44.6 ± 0.8 c | 33.6 ± 0.8 e | 15 ± 1.1 e |
| PB5 | 58.6 ± 0.6 ab | 51.6 ± 0.5 a | 62.6 ± 2 a | 22.6 ± 2.6 d |
| PB6 | 26.6 ± 2 h | 21 ± 0.57 g | 36.3 ± 0.3 d | 9.6 ± 0.3 g |
| FP1 | 46.6 ± 1.45 d | 37.3 ± 1.2 e | 21.6 ± 1.3 g | 41 ± 1.7 c |
| FB2 | 57.3 ± 0.6 b | 42.3 ± 0.8 d | 38.6 ± 0.8 c | 55.3 ± 2.6 a |
| FB3 | 61.3 ± 2.3 a | 45.6 ± 1.4 c | 31.6 ± 1.4 e | 48.3 ± 3.1 b |
| FB4 | 35.6 ± 1.4 f | 33.3 ± 0.8 f | 26.6 ± 0.8 f | 24.6 ± 0.8 d |
| Treatments | Shoot Length (cm) | Root Length (cm) | Leaf Numbers | ||||
|---|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | ||
| Control | 22.4 ± 0.5 b | 27.4 ± 0.3 ab | 9.3 ± 0.6 ab | 8.98 ± 0.3 b | 5.0 ± 0.0 a | 5.67 ± 0.2 a | |
| Exogenously Applied Hormones | IAA | 23.3 ± 0.5 b | 29.3 ± 0.9 ab | 8.0 ± 0.4 b | 9.8 ± 0.7 ab | 4.6 ± 0.3 a | 6.0 ± 0.0 a |
| BA | 25.3 ± 0.4 a | 28.2 ± 0.7 ab | 9.7 ± 0.4 ab | 8.9 ± 0.4 b | 4.4 ± 0.2 a | 6.0 ± 0.0 a | |
| Bacterial Endophytes | PB2 | 26.9 ± 0.8 a | 34.0 ± 0.9 a | 10.03 ± 0.3 ab | 11.5 ± 0.6 a | 4.6 ± 0.2 a | 6.0 ± 0.0 a |
| PB5 | 28.7 ± 0.9 a | 33.8 ± 1.6 a | 9.4 ± 0.4 ab | 10.5 ± 0.9 ab | 4.6 ± 0.3 a | 6.0 ± 0.0 a | |
| BM | 25.1 ± 0.8 a | 25.5 ± 0.7 b | 9.5 ± 0.2 ab | 11.5 ± 0.4 a | 4.1 ± 0.1 a | 6.0 ± 0.0 a | |
| Fungal Endophytes | PF2 | 26.1 ± 0.4 a | 29.2 ± 0.7 ab | 8.4 ± 0.3 b | 12.7 ± 0.7 a | 4.0 ± 0.0 a | 6.0 ± 0.0 a |
| PF3 | 26.1 ± 0.7 a | 25.5 ± 0.3 b | 10.2 ± 0.5 ab | 12.5 ± 0.5 a | 4.1 ± 0.1 a | 6.0 ± 0.0 a | |
| FM | 25.3 ± 0.7 a | 27.2 ± 0.8 ab | 10.4 ± 0.3 ab | 11.0 ± 0.5 a | 4.0 ± 0.0 a | 6.0 ± 0.0 a | |
| Treatments | Root Carbohydrate (mg g−1 DW) | Root Protein (mg g−1 DW) | Shoot Carbohydrate (mg g−1 DW) | Shoot Protein (mg g−1 DW) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | ||
| Control | 90.7 ± 0.4 c | 70.4 ± 0.7 e | 58.8 ± 0.6 b | 60.9 ± 0.9 b | 75.7 ± 1.2 d | 77.2 ± 1.5 d | 49.8 ± 0.3 d | 56.4 ± 0.7 b | |
| Exogenously Applied Hormones | IAA | 108.4 ± 1.7 b | 86.6 ± 1.1 d | 59.8 ± 0.7 b | 64.2 ± 0.9 b | 98.7 ± 0.6 c | 101.9 ± 2.9 c | 53.7 ± 0.4 c | 59.3 ± 0.8 a |
| BA | 113.2 ± 3.2 ab | 90.6 ± 4.2 cd | 61.2 ± 0.8 b | 67.4 ± 1.5 ab | 115.6 ± 3.5 ab | 112.4 ± 1.4 ab | 65.5 ± 0.3 a | 59.3 ± 0.4 a | |
| Bacterial Endophytes | PB2 | 108.4 ± 1.7 bb | 99.5 ± 0.7 bc | 61.1 ± 0.7 b | 64.6 ± 0.9 b | 113.2 ± 4.5 ab | 107.6 ± 4.9 bc | 58.6 ± 0.7 b | 60.7 ± 1.1 a |
| PB5 | 118.2 ± 1.7 a | 95.5 ± 1.4 c | 64.8 ± 0.1 a | 69.3 ± 1.3 a | 110.0 ± 5.5 b | 117.3 ± 2.4 ab | 58.9 ± 0.6 b | 59.8 ± 0.2 a | |
| BM | 107.6 ± 3.7 b | 105.9 ± 3.5 b | 64.9 ± 0.6 a | 70.8 ± 0.9 a | 122.9 ± 2.02 a | 116.5 ± 1.6 ab | 59.0 ± 0.5 b | 59.3 ± 0.6 a | |
| Fungal Endophytes | PF2 | 103.5 ± 4.5 b | 121.4 ± 4.2 a | 61.5 ± 0.8 b | 66.2 ± 0.8 b | 119.8 ± 6.9 a | 111.2 ± 1.2 ab | 58.3 ± 0.9 b | 59.8 ± 0.3 a |
| PF3 | 104.4 ± 2.1 b | 106.1 ± 2.9 b | 64.01 ± 0.8 a | 69.85 ± 1.21 a | 103.6 ± 2.9 b | 107.6 ± 4.9 bc | 59.3 ± 0.4 b | 60.3 ± 0.2 a | |
| FM | 109.9 ± 2.8 b | 110.03 ± 2.8 ab | 63.9 ± 0.8 a | 70.7 ± 1.5 a | 113.2 ± 4.5 ab | 120.5 ± 0.7 a | 58.9 ± 0.6 b | 60.3 ± 0.4 a | |
| Treatments | Amylase (Unit/µg FW) | Protease (Unit/µg FW) | Catalase (Unit/µg FW) | Peroxidase (Unit/µg FW) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | ||
| Control | 1.2 ± 0.1 b | 1.3 ± 0.2 c | 0.14 ± 0.01 a | 0.15 ± 0.0 b | 66.7 ± 1.4 a | 95.0 ± 2.9 c | 58.33 ± 1.7 d | 58.33 ± 1.7 c | |
| Exogenously Applied Hormones | IAA | 1.7 ± 0.1 b | 1.9 ± 0.02 b | 0.15 ± 0.01 a | 0.18 ± 0.0 ab | 82.0 ± 1.7 b | 106.6 ± 1.7 b | 95.00 ± 5.0 ab | 96.67 ± 3.3 b |
| BA | 1.8 ± 0.1 b | 1.9 ± 0.1 b | 0.14 ± 0.0 a | 0.16 ± 0.01 b | 63.3 ± 1.3 a | 63.3 ± 3.3 d | 101.6 ± 1.7 a | 105.0 ± 2.9 a | |
| Bacterial Endophytes | PB2 | 2.7 ± 0.2 a | 2.9 ± 0.1 a | 0.16 ± 0.01 a | 0.15 ± 0.01 b | 83.3 ± 0.4 b | 123.3 ± 3.3 b | 86.7 ± 3.3 b | 103.3 ± 1.7 a |
| PB5 | 2.7 ± 0.1 a | 3.1 ± 0.1 a | 0.17 ± 0.0 a | 0.19 ± 0.0 a | 88.3 ± 1.7 b | 155.0 ± 5.0 a | 101.7 ± 1.7 a | 111.6 ± 4.4 a | |
| BM | 1.7 ± 0.1 b | 2.8 ± 0.3 a | 0.15 ± 0.0 a | 0.19 ± 0.0 a | 83.3 ± 1.4 b | 113.3 ± 3.3 b | 103.3 ± 1.7 a | 96.67 ± 3.3 b | |
| Fungal Endophytes | PF2 | 2.13 ± 0.2 a | 1.9 ± 0.1 b | 0.18 ± 0.0 a | 0.18 ± 0.01 ab | 86.7 ± 0.3 b | 111.6 ± 4.4 b | 76.67 ± 1.7 c | 103.3 ± 3.3 ab |
| PF3 | 1.7 ± 0.1 b | 2.2 ± 0.1 b | 0.18 ± 0.0 a | 0.18 ± 0.01 ab | 75.0 ± 0.4 c | 83.33 ± 4.4 c | 98.33 ± 4.4 a | 91.67 ± 1.7 b | |
| FM | 1.76 ± 0.1 b | 2.3 ± 0.1 b | 0.15 ± 0.0 a | 0.18 ± 0.0 ab | 85.0 ± 0.9 b | 91.67 ± 1.7 c | 38.33 ± 4.4 e | 66.67 ± 4.4 c | |
| Treatments | IAA (mg/100 g FW) | BA (mg/100 g FW) | GA3 (mg/100 g FW) | ABA (µg/100 g FW) | |
|---|---|---|---|---|---|
| Control | 0.3 ± 0.02 b | 1.3 ± 0.7 cd | 2.6 ± 0.9 c | 1.02 ± 0.7 c | |
| Exogenously Applied Hormones | IAA | 0.3 ± 0.05 b | 0.85 ± 0.6 d | 14.8 ± 2.4 b | 2.1 ± 0.3 c |
| BA | 0.4 ± 0.05 ab | 1.9 ± 0.7 bc | 3.2 ± 1.7 c | 1.6 ± 0.6 c | |
| Bacterial Endophytes | PB2 | 0.8 ± 0.5 a | 3.4 ± 0.1 a | 18.4 ± 1.3 a | 2.2 ± 0.5 c |
| PB5 | 0.4 ± 0.1 b | 2.0 ± 0.8 b | 8.3 ± 1.9 d | 2.7 ± 0.2 c | |
| BM | 0.31 ± 0.06 b | 1.4 ± 0.5 c | 3.8 ± 1.9 c | 4.1 ± 0.3 b | |
| Fungal Endophytes | PF2 | 0.4 ± 0.04 b | 2.6 ± 0.6 ab | 16.9 ± 1.02 ab | 6.2 ± 0.5 a |
| PF3 | 0.34 ± 0.04 b | 1.8 ± 0.1 bc | 17.1 ± 1.7 a | 5.7 ± 0.7 ab | |
| FM | 0.6 ± 0.05 a | 3.0 ± 0.9 a | 2.9 ± 1.5 c | 4.3 ± 0.8 b | |
| Treatments | Pods/Plant | Seeds/Pod | 100 Seeds Weight | Seed Yield Components | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Weight (g) | Number | Weight (g) | Weight (g) | Carbohydrate (mg/g DW) | Protein (mg/g DW) | ||
| Control | 2.0 ± 0.02 c | 7.3 ± 0.1 b | 6.3 ± 0.3 d | 6.0 ± 0.0 b | 45.2 ± 4.8 b | 81.5 ± 0.9 c | 54.6 ± 0.2 b | |
| Exogenously applied Hormones | IAA | 3.0 ± 0.6 b | 8.8 ± 0.4 ab | 11.3 ± 2.03 c | 7.7 ± 0.3 ab | 71.2 ± 9.9 a | 110.8 ± 3.5 b | 60.1 ± 1.0 a |
| BA | 3.7 ± 0.3 ab | 7.7 ± 0.3 b | 13.7 ± 0.9 b | 6.7 ± 0.3 b | 49.3 ± 4.7 b | 123.1 ± 4.3 ab | 63.5 ± 1.9 a | |
| Bacterial Endophytes | PB2 | 4.0 ± 0.1 a | 8.5 ± 0.3 ab | 15.0 ± 0.6 b | 7.1 ± 0.1 ab | 47.6 ± 1.2 b | 134.6 ± 2.1 a | 64.9 ± 1.2 a |
| PB5 | 4.7 ± 0.3 a | 10.4 ± 0.2 a | 18.0 ± 0.6 a | 9.0 ± 0.1 a | 50.1 ± 1.3 b | 138.9 ± 1.9 a | 62.8 ± 0.1 a | |
| BM | 4.7 ± 0.3 a | 8.7 ± 0.3 ab | 16.3 ± 2.2 ab | 7.7 ± 0.3 ab | 48.3 ± 5.1 b | 120.7 ± 0.7 b | 64.9 ± 1.2 a | |
| Fungal Endophytes | PF2 | 5.0 ± 1.0 a | 8.9 ± 0.2 ab | 18.7 ± 0.9 a | 7.7 ± 0.3 ab | 41.3 ± 3.02 b | 118.4 ± 1.2 b | 61.2 ± 0.8 a |
| PF3 | 5.3 ± 0.3 a | 9.5 ± 0.4 a | 17.0 ± 2.7 ab | 8.3 ± 0.3 a | 51.6 ± 8.3 b | 127.5 ± 4.5 ab | 65.9 ± 0.5 a | |
| FM | 5.4 ± 0.3 a | 9.9 ± 0.3 a | 20.3 ± 0.3 a | 8.7 ± 0.3 a | 42.7 ± 2.3 b | 107.6 ± 3.7 b | 67.1 ± 1.3 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.-D.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. https://doi.org/10.3390/cells10051059
Ismail MA, Amin MA, Eid AM, Hassan SE-D, Mahgoub HAM, Lashin I, Abdelwahab AT, Azab E, Gobouri AA, Elkelish A, et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells. 2021; 10(5):1059. https://doi.org/10.3390/cells10051059
Chicago/Turabian StyleIsmail, Mohamed A., Mohamed A. Amin, Ahmed M. Eid, Saad El-Din Hassan, Hany A. M. Mahgoub, Islam Lashin, Abdelrhman T. Abdelwahab, Ehab Azab, Adil A. Gobouri, Amr Elkelish, and et al. 2021. "Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L." Cells 10, no. 5: 1059. https://doi.org/10.3390/cells10051059
APA StyleIsmail, M. A., Amin, M. A., Eid, A. M., Hassan, S. E.-D., Mahgoub, H. A. M., Lashin, I., Abdelwahab, A. T., Azab, E., Gobouri, A. A., Elkelish, A., & Fouda, A. (2021). Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells, 10(5), 1059. https://doi.org/10.3390/cells10051059











