Hypothalamic GHR—SIRT1 Axis in Fasting
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Perfusion and Histology
2.3. Stereotaxic Trichostatin A Intracerebroventricular Injection
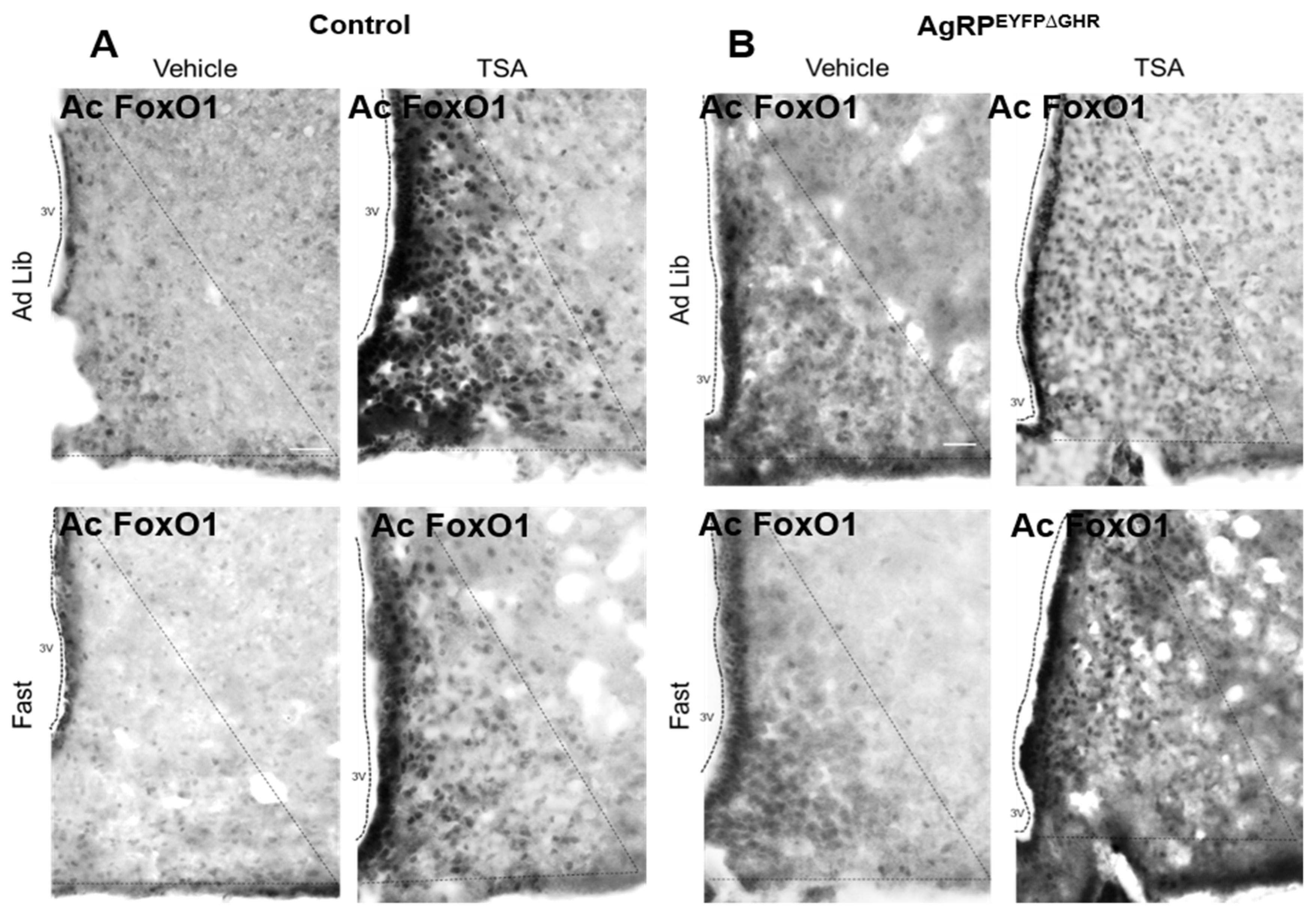
2.4. Ac-Forkhead Transcription Factor (FoxO1) Staining
2.5. Statistical Analysis
3. Results
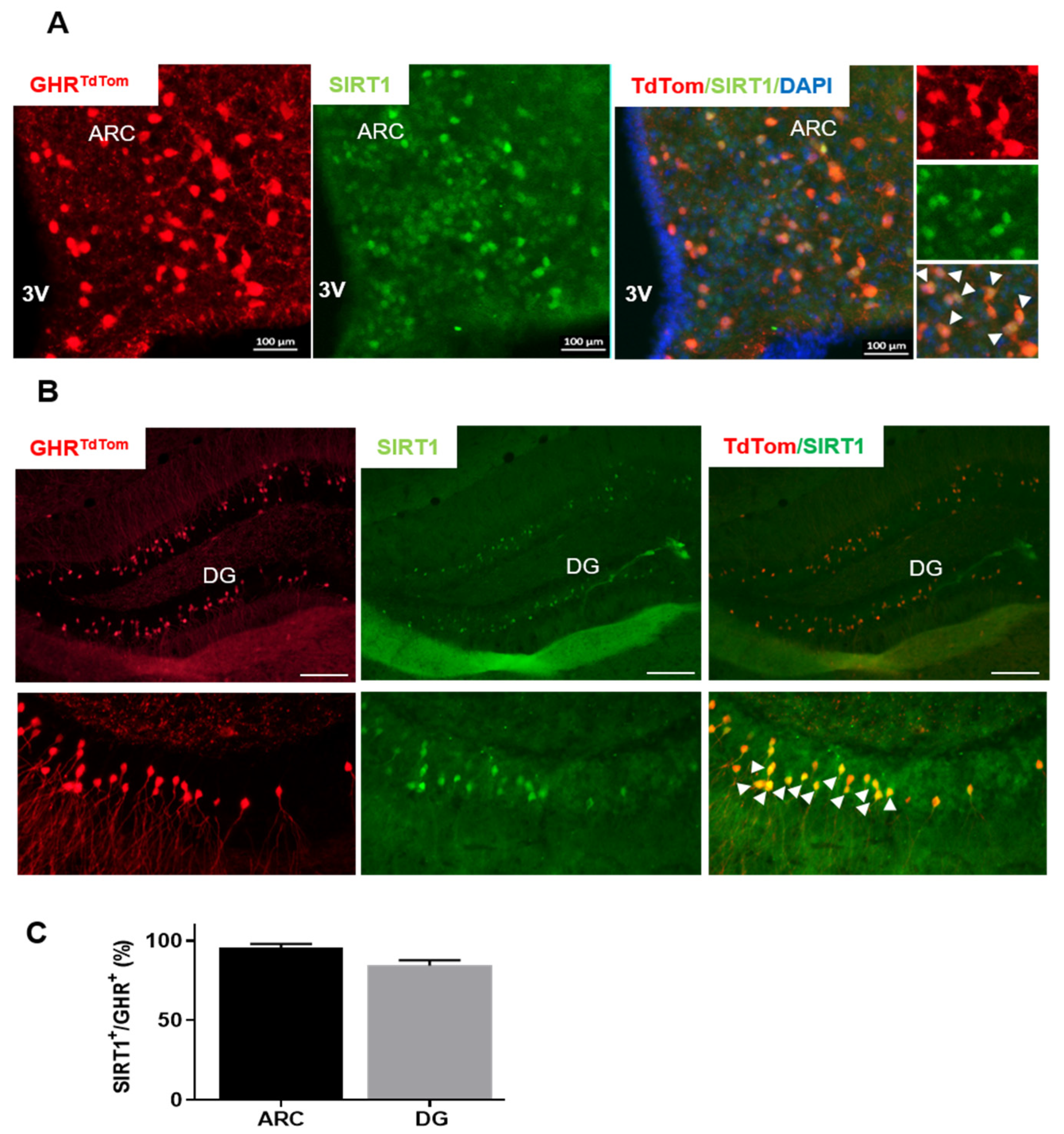
3.1. GHR-Expressing Neurons Co-Localize with SIRT1
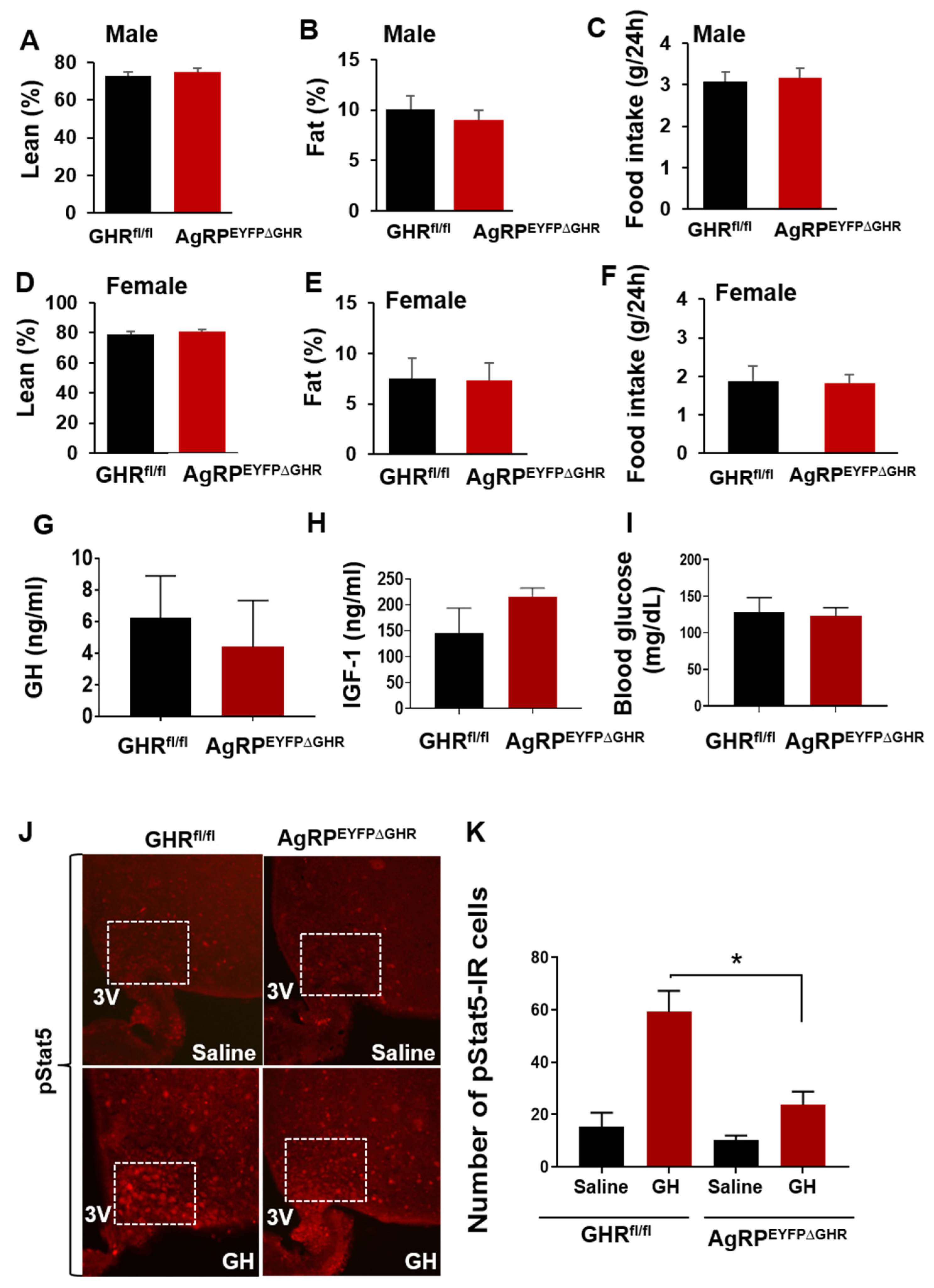
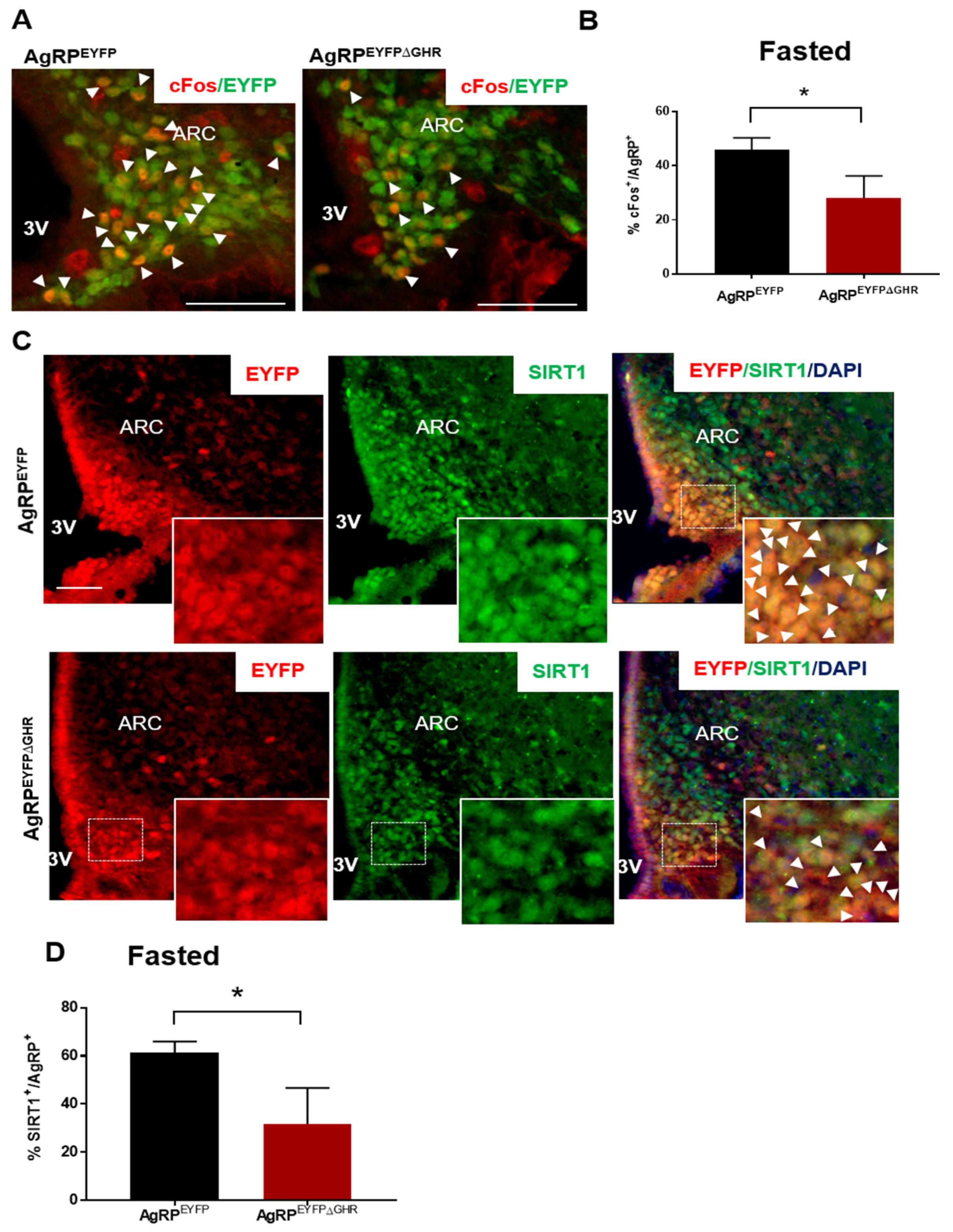
3.2. GHR Signaling in AgRP Neurons Regulates Adaptation to Fasting via SIRT1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| GH | growth hormone |
| GHR | growth hormone receptor |
| Lepr | leptin receptor |
| STAT3 | signal transducer and activator of transcription 3 |
| STAT5 | signal transducer and activator of transcription 5 |
| POMC | proopiomelanocortin |
| ARC | arcuate nucleus of the hypothalamus |
| DMH | dorsomedial hypothalamic nucleus |
| LHA | lateral hypothalamus |
| PVH | paraventricular hypothalamic nucleus |
| SIRT1 | sirtuin 1 |
References
- Barsh, G.; Gunn, T.; He, L.; Wilson, B.; Lu, X.; Gantz, I.; Watson, S. Neuroendocrine regulation by the Agouti/Agrp-melanocortin system. Endocr. Res. 2000, 26, 571. [Google Scholar] [CrossRef]
- Williams, G.; Bing, C.; Cai, X.J.; Harrold, J.A.; King, P.J.; Liu, X.H. The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiol. Behav. 2001, 74, 683–701. [Google Scholar] [CrossRef]
- Timper, K.; Bruning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Cady, G.; Landeryou, T.; Garratt, M.; Kopchick, J.J.; Qi, N.; Garcia-Galiano, D.; Elias, C.F.; Myers, M.G., Jr.; Miller, R.A.; Sandoval, D.A.; et al. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRb) expressing neurons. Mol. Metab. 2017, 6, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perello, M.; Frazao, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y. The Essential Role of SIRT1 in Hypothalamic-Pituitary Axis. Front. Endocrinol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical distribution and regulation by energy availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front. Neurosci. 2018, 12, 778. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Antunes, C.; Geliang, G.; Liu, Z.W.; Borok, E.; Nie, Y.; Xu, A.W.; Souza, D.O.; Gao, Q.; Diano, S.; et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: Roles for Sirt1 in neuronal firing and synaptic plasticity. J. Neurosci. 2010, 30, 11815–11825. [Google Scholar] [CrossRef]
- Cakir, I.; Perello, M.; Lansari, O.; Messier, N.J.; Vaslet, C.A.; Nillni, E.A. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE 2009, 4, e8322. [Google Scholar] [CrossRef]
- Sasaki, T.; Kikuchi, O.; Shimpuku, M.; Susanti, V.Y.; Yokota-Hashimoto, H.; Taguchi, R.; Shibusawa, N.; Sato, T.; Tang, L.; Amano, K.; et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 2014, 57, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E.; Supinski, A.M.; Bonkowski, M.S.; Donmez, G.; Guarente, L.P. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009, 23, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; McBurney, M.W.; Longo, V.D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008, 8, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Iguchi, G.; Fukuoka, H.; Suda, K.; Bando, H.; Takahashi, M.; Nishizawa, H.; Seino, S.; Takahashi, Y. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proc. Natl. Acad. Sci. USA 2013, 110, 14948–14953. [Google Scholar] [CrossRef]
- Li, H.; Rajendran, G.K.; Liu, N.; Ware, C.; Rubin, B.P.; Gu, Y. SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res. 2007, 9, R1. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef]
- Clasen, B.F.; Poulsen, M.M.; Escande, C.; Pedersen, S.B.; Møller, N.; Chini, E.N.; Jessen, N.; Jørgensen, J.O.L. Growth Hormone Signaling in Muscle and Adipose Tissue of Obese Human Subjects: Associations With Measures of Body Composition and Interaction With Resveratrol Treatment. J. Clin. Endocrinol. Metab. 2014, 99, E2565–E2573. [Google Scholar] [CrossRef][Green Version]
- List, E.O.; Berryman, D.E.; Funk, K.; Jara, A.; Kelder, B.; Wang, F.; Stout, M.B.; Zhi, X.; Sun, L.; White, T.A.; et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 2014, 155, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.M.; Bouret, S.G.; Park, S.; Irani, B.G.; Dunn-Meynell, A.A.; Levin, B.E. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 2010, 151, 4270–4279. [Google Scholar] [CrossRef]
- Sadagurski, M.; Landeryou, T.; Cady, G.; Kopchick, J.J.; List, E.O.; Berryman, D.E.; Bartke, A.; Miller, R.A. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell 2015, 14, 1045–1054. [Google Scholar] [CrossRef]
- Qiang, M.; Li, J.G.; Denny, A.D.; Yao, J.M.; Lieu, M.; Zhang, K.; Carreon, S. Epigenetic mechanisms are involved in the regulation of ethanol consumption in mice. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef]
- Yakar, S. Meeting abstracts from the 2020 International Meeting on GH/IGF: Actions in the shadow of COVID19. Pituitary 2020. [Google Scholar] [CrossRef]
- Lima, J.; Debarba, L.K.; Khan, M.; Ubah, C.; Ayyar, I.; Koch, M.; Sadagurski, M. Growth hormone receptor (GHR)-expressing neurons in the hypothalamic arcuate nucleus regulate glucose metabolism and energy homeostasis. BioRxiv 2020. [Google Scholar] [CrossRef]
- Matsui, S.; Sasaki, T.; Kohno, D.; Yaku, K.; Inutsuka, A.; Yokota-Hashimoto, H.; Kikuchi, O.; Suga, T.; Kobayashi, M.; Yamanaka, A.; et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat. Commun. 2018, 9, 4604. [Google Scholar] [CrossRef]
- Dobbin, M.M.; Madabhushi, R.; Pan, L.; Chen, Y.; Kim, D.; Gao, J.; Ahanonu, B.; Pao, P.C.; Qiu, Y.; Zhao, Y.; et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 2013, 16, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.A.; Ma, C.; D’Mello, S.R. Catalytic-independent neuroprotection by SIRT1 is mediated through interaction with HDAC1. PLoS ONE 2019, 14, e0215208. [Google Scholar]
- Solomon, J.M.; Pasupuleti, R.; Xu, L.; McDonagh, T.; Curtis, R.; DiStefano, P.S.; Huber, L.J. Inhibition of SIRT1 Catalytic Activity Increases p53 Acetylation but Does Not Alter Cell Survival following DNA Damage. Mol. Cell. Biol. 2006, 26, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.T.; Bonomi, R.E.; Gelovani, D.J.; Llaniguez, J.; Lu, X.; Mangner, T.; Gelovani, J.G. Noninvasive quantification of SIRT1 expression–activity and pharmacologic inhibition in a rat model of intracerebral glioma using 2-[18F]BzAHA PET/CT/MRI. Neuro Oncol. Adv. 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, R.; Popov, V.; Laws, M.T.; Gelovani, D.; Majhi, A.; Shavrin, A.; Lu, X.; Muzik, O.; Turkman, N.; Liu, R.; et al. Molecular Imaging of Sirtuin1 Expression-Activity in Rat Brain Using Positron-Emission Tomography-Magnetic-Resonance Imaging with [(18)F]-2-Fluorobenzoylaminohexanoicanilide. J. Med. Chem. 2018, 61, 7116–7130. [Google Scholar] [CrossRef] [PubMed]
- Monteserin-Garcia, J.; Al-Massadi, O.; Seoane, L.M.; Alvarez, C.V.; Shan, B.; Stalla, J.; Paez-Pereda, M.; Casanueva, F.F.; Stalla, G.K.; Theodoropoulou, M. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB J. 2013, 27, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, R.; Giorgio, M.; Pelicci, P.G. Insights into the beneficial effect of caloric/ dietary restriction for a healthy and prolonged life. Front. Physiol. 2012, 3, 318. [Google Scholar] [CrossRef]
- Anisimov, V.N. Mutant and genetically modified mice as models for studying the relationship between aging and carcinogenesis. Mech. Ageing Dev. 2001, 122, 1221–1255. [Google Scholar] [CrossRef]
- Argente, J.; Caballo, N.; Barrios, V.; Munoz, M.T.; Pozo, J.; Chowen, J.A.; Morande, G.; Hernandez, M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: Effect of short- and long-term weight recuperation. J. Clin. Endocrinol. Metab. 1997, 82, 2084–2092. [Google Scholar] [PubMed]
- Scacchi, M.; Pincelli, A.I.; Caumo, A.; Tomasi, P.; Delitala, G.; Baldi, G.; Cavagnini, F. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J. Clin. Endocrinol. Metab. 1997, 82, 3225–3229. [Google Scholar] [CrossRef][Green Version]
- Jin, Q.; Yan, T.; Ge, X.; Sun, C.; Shi, X.; Zhai, Q. Cytoplasm-localized SIRT1 enhances apoptosis. J. Cell. Physiol. 2007, 213, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kim, H.J.; Kobayashi, M.; Kitamura, Y.I.; Yokota-Hashimoto, H.; Shiuchi, T.; Minokoshi, Y.; Kitamura, T. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology 2010, 151, 2556–2566. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.; He, L.; Wu, X.; Yin, Y. Long-Term l-Serine Administration Reduces Food Intake and Improves Oxidative Stress and Sirt1/NFkappaB Signaling in the Hypothalamus of Aging Mice. Front. Endocrinol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Scarpace, P.J.; Sakarya, Y.; Kirichenko, N.; Matheny, M.; Bruce, E.B.; Carter, C.S.; Morgan, D.; Tumer, N. Intracerebroventricular tempol administration in older rats reduces oxidative stress in the hypothalamus but does not change STAT3 signalling or SIRT1/AMPK pathway. Appl. Physiol. Nutr. Metab. 2017, 42, 59–67. [Google Scholar] [CrossRef]
- Banks, A.S.; Kim-Muller, J.Y.; Mastracci, T.L.; Kofler, N.M.; Qiang, L.; Haeusler, R.A.; Jurczak, M.J.; Laznik, D.; Heinrich, G.; Samuel, V.T.; et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011, 14, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Daitoku, H.; Sakamaki, J.; Fukamizu, A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim. Biophys. Acta 2011, 1813, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, J.B.M.; Ubah, C.; Debarba, L.K.; Ayyar, I.; Didyuk, O.; Sadagurski, M. Hypothalamic GHR—SIRT1 Axis in Fasting. Cells 2021, 10, 891. https://doi.org/10.3390/cells10040891
de Lima JBM, Ubah C, Debarba LK, Ayyar I, Didyuk O, Sadagurski M. Hypothalamic GHR—SIRT1 Axis in Fasting. Cells. 2021; 10(4):891. https://doi.org/10.3390/cells10040891
Chicago/Turabian Stylede Lima, Juliana Bezerra Medeiros, Chidera Ubah, Lucas Kniess Debarba, Iven Ayyar, Olesya Didyuk, and Marianna Sadagurski. 2021. "Hypothalamic GHR—SIRT1 Axis in Fasting" Cells 10, no. 4: 891. https://doi.org/10.3390/cells10040891
APA Stylede Lima, J. B. M., Ubah, C., Debarba, L. K., Ayyar, I., Didyuk, O., & Sadagurski, M. (2021). Hypothalamic GHR—SIRT1 Axis in Fasting. Cells, 10(4), 891. https://doi.org/10.3390/cells10040891






