Understanding the Heterogeneity of Human Pericyte Subsets in Blood–Brain Barrier Homeostasis and Neurological Diseases
Abstract
1. Introduction
2. Distinguishing PCs from Other Perivascular Cell Types in the Human Brain
2.1. Distinguishing PCs from Perivascular Macrophages
2.2. Distinguishing PCs from ECs
2.3. Distinguishing PCs from Vascular Smooth Muscle Cells
2.4. Summary
3. Pathological PC Subsets in the Human Brain
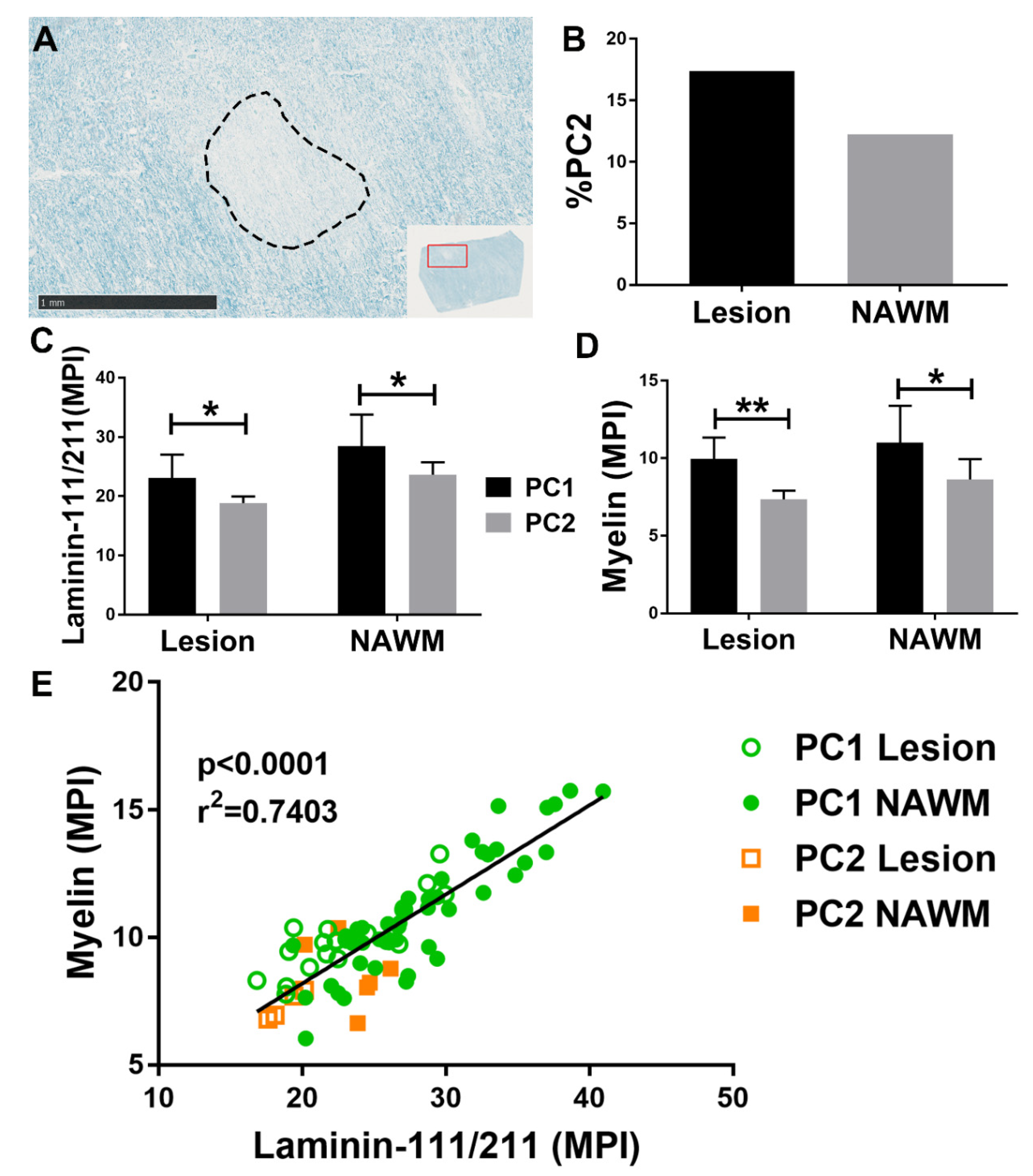
3.1. Origin of PC2
3.2. Identifying PC2
3.3. Functional Differences between PC1 and PC2
3.4. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood–brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef]
- Barone, F.C.; Knudsen, D.J.; Nelson, A.H.; Feuerstein, G.Z.; Willette, R.N. Mouse Strain Differences in Susceptibility to Cerebral Ischemia are Related to Cerebral Vascular Anatomy. Br. J. Pharmacol. 1993, 13, 683–692. [Google Scholar] [CrossRef]
- Gautam, J.; Zhang, X.; Yao, Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci. Rep. 2016, 6, 36450. [Google Scholar] [CrossRef]
- Gautam, J.; Cao, Y.; Yao, Y. Pericytic Laminin Maintains Blood-Brain Barrier Integrity in an Age-Dependent Manner. Transl. Stroke Res. 2019, 11, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. PLoS ONE 2015, 10, e0134765. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Heo, J.-I.; Kim, J.H.; Cho, C.-H. Interaction between pericytes and endothelial cells leads to formation of tight junction in hyaloid vessels. Mol. Cells 2013, 36, 465–471. [Google Scholar] [CrossRef]
- Bohannon, D.G.; Ko, A.; Filipowicz, A.R.; Kuroda, M.J.; Kim, W.-K. Dysregulation of sonic hedgehog pathway and pericytes in the brain after lentiviral infection. J. Neuroinflammation 2019, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Podjaski, C.; Alvarez, J.I.; Bourbonniere, L.; Larouche, S.; Terouz, S.; Bin, J.M.; Lécuyer, M.-A.; Saint-Laurent, O.; LaRochelle, C.; Darlington, P.J.; et al. Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain 2015, 138, 1598–1612. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Ding, Q.; Que, J.; Liu, K.; Wang, H.; Liao, S. Netrin-1 Ameliorates Blood-Brain Barrier Impairment Secondary to Ischemic Stroke via the Activation of PI3K Pathway. Front. Neurosci. 2017, 11, 700. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood–brain barrier. Nat. Cell Biol. 2010, 468, 557–561. [Google Scholar] [CrossRef]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; Van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nikolakopoulou, A.M.; Zhao, Z.; Sagare, A.P.; Si, G.; Lazic, D.; Barnes, S.R.; Daianu, M.; Ramanathan, A.; Go, A.; et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 2018, 24, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mato, M.; Ookawara, S.; Kurihara, K. Uptake of exogenous substances and marked infoldings of the fluorescent granular pericyte in cerebral fine vessels. Am. J. Anat. 1980, 157, 329–332. [Google Scholar] [CrossRef]
- Ookawara, S.; Mitsuhashi, U.; Suminaga, Y.; Mato, M. Study on distribution of pericyte and fluorescent granular perithelial (FGP) cell in the transitional region between arteriole and capillary in rat cerebral cortex. Anat. Rec. 1996, 244, 257–264. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J.; Büringer, D.; Sparks, D.L.; Kreutzberg, G.W. Ultrastructural Location of Major Histocompatibility Complex (MHC) Class II Positive Perivascular Cells in Histologically Normal Human Brain. J. Neuropathol. Exp. Neurol. 1992, 51, 303–311. [Google Scholar] [CrossRef]
- Angelov, D.N.; Walther, M.; Neiss, W.F.; Streppel, M.; Guntinas-Lichius, O. The cerebral perivascular cells. Met. Norm. Cancer Cells 1998, 147, 1–87. [Google Scholar] [CrossRef]
- Balabanov, R.; Washington, R.; Wagnerova, J.; Dore-Duffy, P. CNS Microvascular Pericytes Express Macrophage-like Function, Cell Surface Integrin αM, and Macrophage Marker ED-2. Microvasc. Res. 1996, 52, 127–142. [Google Scholar] [CrossRef]
- Bandopadhyay, R.; Orte, C.; Lawrenson, J.; Reid, A.; De Silva, S.; Allt, G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J. Neurocytol. 2001, 30, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Streit, W.J.; Kreutzberg, G.W. The microglial cytoskeleton: Vimentin is localized within activated cellsin situ. J. Neurocytol. 1988, 17, 573–580. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Kim, D.H.; Kim, K.-W. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J. Neurosci. Res. 2008, 87, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Alvarez, X.; Fisher, J.; Bronfin, B.; Westmoreland, S.; McLaurin, J.; Williams, K. CD163 Identifies Perivascular Macrophages in Normal and Viral Encephalitic Brains and Potential Precursors to Perivascular Macrophages in Blood. Am. J. Pathol. 2006, 168, 822–834. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Van Haastert, E.S.; Galea, I.; Polfliet, M.M.; Döpp, E.D.; Heuvel, M.M.V.D.; Berg, T.K.V.D.; De Groot, C.J.; Van Der Valk, P.; Dijkstra, C.D. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 2005, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Akhtar, N.; Nishiyama, A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia 2001, 34, 296–310. [Google Scholar] [CrossRef]
- Kida, S.; Steart, P.V.; Zhang, E.-T.; Weller, R.O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993, 85, 646–652. [Google Scholar] [CrossRef]
- Moransard, M.; Dann, A.; Staszewski, O.; Fontana, A.; Prinz, M.; Suter, T. NG2 expressed by macrophages and oligodendrocyte precursor cells is dispensable in experimental autoimmune encephalomyelitis. Brain 2011, 134, 1315–1330. [Google Scholar] [CrossRef]
- Smyth, L.C.; Rustenhoven, J.; Scotter, E.L.; Schweder, P.; Faull, R.L.; Park, T.I.; Dragunow, M. Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 2018, 92, 48–60. [Google Scholar] [CrossRef]
- Alliot, F.; Rutin, J.; Leenen, P.J.; Pessac, B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J. Neurosci Res. 1999, 58, 367–378. [Google Scholar] [CrossRef]
- Ramsauer, M.; Kunz, J.; Krause, D.; Dermietzel, R. Regulation of a Blood-Brain Barrier—Specific Enzyme Expressed by Cerebral Pericytes (Pericytic Aminopeptidase N/pAPN) under Cell Culture Conditions. Br. J. Pharmacol. 1998, 18, 1270–1281. [Google Scholar] [CrossRef]
- Maxwell, K.; Berliner, J.A.; Cancilla, P.A. Induction of γ-glutamyl transpeptidase in cultured cerebral endothelial cells by a product released by astrocytes. Brain Res. 1987, 410, 309–314. [Google Scholar] [CrossRef]
- Orlowski, M.; Sessa, G.; Green, J.P. ggr-Glutamyl Transpeptidase in Brain Capillaries: Possible Site of a Blood-Brain Barrier for Amino Acids. Science 1974, 184, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Dingler, A.; Albrecht, U.; Dehouck, M.-P.; Cecchelli, R. Blood–Brain Barrier Pericytes Are the Main Source of γ-Glutamyltranspeptidase Activity in Brain Capillaries. J. Neurochem. 1992, 58, 667–672. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Dymecki, S.M. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev. Biol. 2010, 340, 430–437. [Google Scholar] [CrossRef]
- Ly, N.P.; Komatsuzaki, K.; Fraser, I.P.; Tseng, A.A.; Prodhan, P.; Moore, K.J.; Kinane, T.B. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14729–14734. [Google Scholar] [CrossRef]
- Yamazaki, T.; Nalbandian, A.; Uchida, Y.; Li, W.; Arnold, T.D.; Kubota, Y.; Yamamoto, S.; Ema, M.; Mukouyama, Y.-S. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-β Signaling in Developing Skin Vasculature. Cell Rep. 2017, 18, 2991–3004. [Google Scholar] [CrossRef]
- Korn, J.; Christ, B.; Kurz, H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J. Comp. Neurol. 2001, 442, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.C.; Vincent, C.; Le Douarin, N.M.; Couly, G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128, 1059–1068. [Google Scholar]
- Grant, R.I.; Hartmann, D.A.; Underly, R.G.; Berthiaume, A.-A.; Bhat, N.R.; Shih, A.Y. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. Br. J. Pharmacol. 2017, 39, 411–425. [Google Scholar] [CrossRef]
- Nehls, V.; Drenckhahn, D.; Harnik-Ort, V.; Prakash, K.; Marcantonio, E.; Colman; Rosenfeld, M.; Adesnik, M.; Sabatini, D.; Kreibich, G. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J. Cell Biol. 1991, 113, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Hui, H.; Cai, T.; Huang, H.; Yang, F.; Feng, J.; Zhang, J.; Yan, X. CD146 coordinates brain endothelial cell–pericyte communication for blood–brain barrier development. Proc. Natl. Acad. Sci. USA 2017, 114, E7622–E7631. [Google Scholar] [CrossRef]
- Hasumi, Y.; Kłosowska-Wardęga, A.; Furuhashi, M.; Östman, A.; Heldin, C.-H.; Hellberg, C. Identification of a subset of pericytes that respond to combination therapy targeting PDGF and VEGF signaling. Int. J. Cancer 2007, 121, 2606–2614. [Google Scholar] [CrossRef]
- Girolamo, F.; Errede, M.; Longo, G.; Annese, T.; Alias, C.; Ferrara, G.; Morando, S.; Trojano, M.; De Rosbo, N.K.; Uccelli, A.; et al. Defining the role of NG2-expressing cells in experimental models of multiple sclerosis. A biofunctional analysis of the neurovascular unit in wild type and NG2 null mice. PLoS ONE 2019, 14, e0213508. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359.e5. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; D’Souza, S.S.; Moskvin, O.V.; Toh, H.; Wang, B.; Zhang, J.; Swanson, S.; Guo, L.-W.; Thomson, J.A.; Slukvin, I.I. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017, 19, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Rieske, P.; Azizi, S.A.; Augelli, B.; Gaughan, J.; Krynska, B. A population of human brain parenchymal cells express markers of glial, neuronal and early neural cells and differentiate into cells of neuronal and glial lineages. Eur. J. Neurosci. 2007, 25, 31–37. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; Döpp, E.A.; Joling, P.; Kraal, G. The Heterogeneity of Mononuclear Phagocytes in Lymphoid Organs: Distinct Macrophage Subpopulations in Rat Recognized by Monoclonal Antibodies ED1, ED2 and ED3. Microenviron. Lymphoid Syst. 1985, 186, 409–419. [Google Scholar] [CrossRef]
- Proteinatlas. The Human Protein Atlas. Available online: https://www.proteinatlas.org (accessed on 19 December 2019).
- Graeber, M.B.; López-Redondo, F.; Ikoma, E.; Ishikawa, M.; Imai, Y.; Nakajima, K.; Kreutzberg, G.W.; Kohsaka, S. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res. 1998, 813, 241–253. [Google Scholar] [CrossRef]
- Herman, I.M.; D’Amore, P.A. Microvascular pericytes contain muscle and nonmuscle actins. J. Cell Biol. 1985, 101, 43–52. [Google Scholar] [CrossRef]
- Skalli, O.; Pelte, M.F.; Peclet, M.C.; Gabbiani, G.; Gugliotta, P.; Bussolati, G.; Ravazzola, M.; Orci, L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J. Histochem. Cytochem. 1989, 37, 315–321. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Otte-Höller, I.; Wesseling, P.; Ruiter, D.J.; De Waal, R.M. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta. Am. J. Pathol. 1994, 144, 372–382. [Google Scholar]
- Bohannon, D.G.; Okhravi, H.R.; Kim, J.; Kuroda, M.J.; Didier, E.S.; Kim, W.-K. A subtype of cerebrovascular pericytes is associated with blood-brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol. Aging 2020, 96, 128–136. [Google Scholar] [CrossRef]
- Damisah, E.C.; Hill, R.A.; Tong, L.; Murray, K.N.; Grutzendler, J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci. 2017, 20, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Sabins, N.C.; Taylor, J.L.; Fabian, K.P.L.; Appleman, L.J.; Maranchie, J.K.; Stolz, D.B.; Storkus, W.J. DLK1: A Novel Target for Immunotherapeutic Remodeling of the Tumor Blood Vasculature. Mol. Ther. 2013, 21, 1958–1968. [Google Scholar] [CrossRef]
- He, P.; Staufenbiel, M.; Li, R.; Shen, Y. Deficiency of Patched 1-induced Gli1 signal transduction results in astrogenesis in Swedish mutated APP transgenic mice. Hum. Mol. Genet. 2014, 23, 6512–6527. [Google Scholar] [CrossRef]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in Pericytes on Blood Vessels and Endothelial Sprouts in Tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Sieczkiewicz, G.J.; Herman, I.M. TGF-β1 signaling controls retinal pericyte contractile protein expression. Microvasc. Res. 2003, 66, 190–196. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Katychev, A.; Wang, X.; Van Buren, E. CNS Microvascular Pericytes Exhibit Multipotential Stem Cell Activity. Br. J. Pharmacol. 2005, 26, 613–624. [Google Scholar] [CrossRef]
- Park, T.I.-H.; Feisst, V.; Brooks, A.E.S.; Rustenhoven, J.; Monzo, H.J.; Feng, S.X.; Mee, E.W.; Bergin, P.S.; Oldfield, R.; Graham, E.S.; et al. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci. Rep. 2016, 6, 26587. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Olson, J.D.; Mintz, A.; Delbono, O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Physiol. 2014, 307, C25–C38. [Google Scholar] [CrossRef]
- Peters, A.; Sethares, C. Age-related changes in the morphology of cerebral capillaries do not correlate with cognitive decline. J. Comp. Neurol. 2012, 520, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, H.M.; Pellowe, A.S.; Ramanathan, A.; Liu, R.; Miller-Jensen, K.; McNiff, J.M.; Pober, J.S.; Gonzalez, A.L. Tumor Necrosis Factor-α and IL-17A Activation Induces Pericyte-Mediated Basement Membrane Remodeling in Human Neutrophilic Dermatoses. Am. J. Pathol. 2017, 187, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef] [PubMed]
| Marker | Pericytes | Vascular Smooth Muscle Cells | Endothelial Cells | Perivascular Macrophages | Source |
|---|---|---|---|---|---|
| CD163 | − | − | − | + | [24,25,27,48] |
| CD11b | − | − | − | + | [49] |
| Vimentin | − | − | +/− | + | [18,22,50] |
| NG2 | +/− | − | − | +/− | [26,28] |
| CD206 | − | − | − | +/− | [24,27] |
| CD68 | − | − | − | + | [24,27] |
| CD13 | + | + | − | − | [29,46] |
| CD31 | − | − | + | − | [29,46] |
| GGTP | + | − | − | − | [34] |
| Netrin-1 | + | + | − | − | [8] |
| PDGFRB | + | + | − | − | [29,40,41,46] |
| SMA | +/− | + | − | − | [21,46,51,52,53] |
| Desmin | +/− | +/− | − | − | [46] |
| CD146 | + | + | +/− | − | [42,46] |
| Nestin | +/− | − | − | − | [46] |
| Tbx18 | + | + | − | − | [45] |
| MYH11 | − | + | − | − | [46,54] |
| Neuro Trace 500/525 | + | − | − | − | [55] |
| DLK1 | +/− | − | − | − | [46,49,56] |
| RGS5 | + | + | − | − | [49,57] |
| KIR6.1 | + | + | + | − | [49,57] |
| CD274 | +/− | + | + | + | [46,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohannon, D.G.; Long, D.; Kim, W.-K. Understanding the Heterogeneity of Human Pericyte Subsets in Blood–Brain Barrier Homeostasis and Neurological Diseases. Cells 2021, 10, 890. https://doi.org/10.3390/cells10040890
Bohannon DG, Long D, Kim W-K. Understanding the Heterogeneity of Human Pericyte Subsets in Blood–Brain Barrier Homeostasis and Neurological Diseases. Cells. 2021; 10(4):890. https://doi.org/10.3390/cells10040890
Chicago/Turabian StyleBohannon, Diana G., Danielle Long, and Woong-Ki Kim. 2021. "Understanding the Heterogeneity of Human Pericyte Subsets in Blood–Brain Barrier Homeostasis and Neurological Diseases" Cells 10, no. 4: 890. https://doi.org/10.3390/cells10040890
APA StyleBohannon, D. G., Long, D., & Kim, W.-K. (2021). Understanding the Heterogeneity of Human Pericyte Subsets in Blood–Brain Barrier Homeostasis and Neurological Diseases. Cells, 10(4), 890. https://doi.org/10.3390/cells10040890






