Central Regulation of Metabolism by Growth Hormone
Abstract
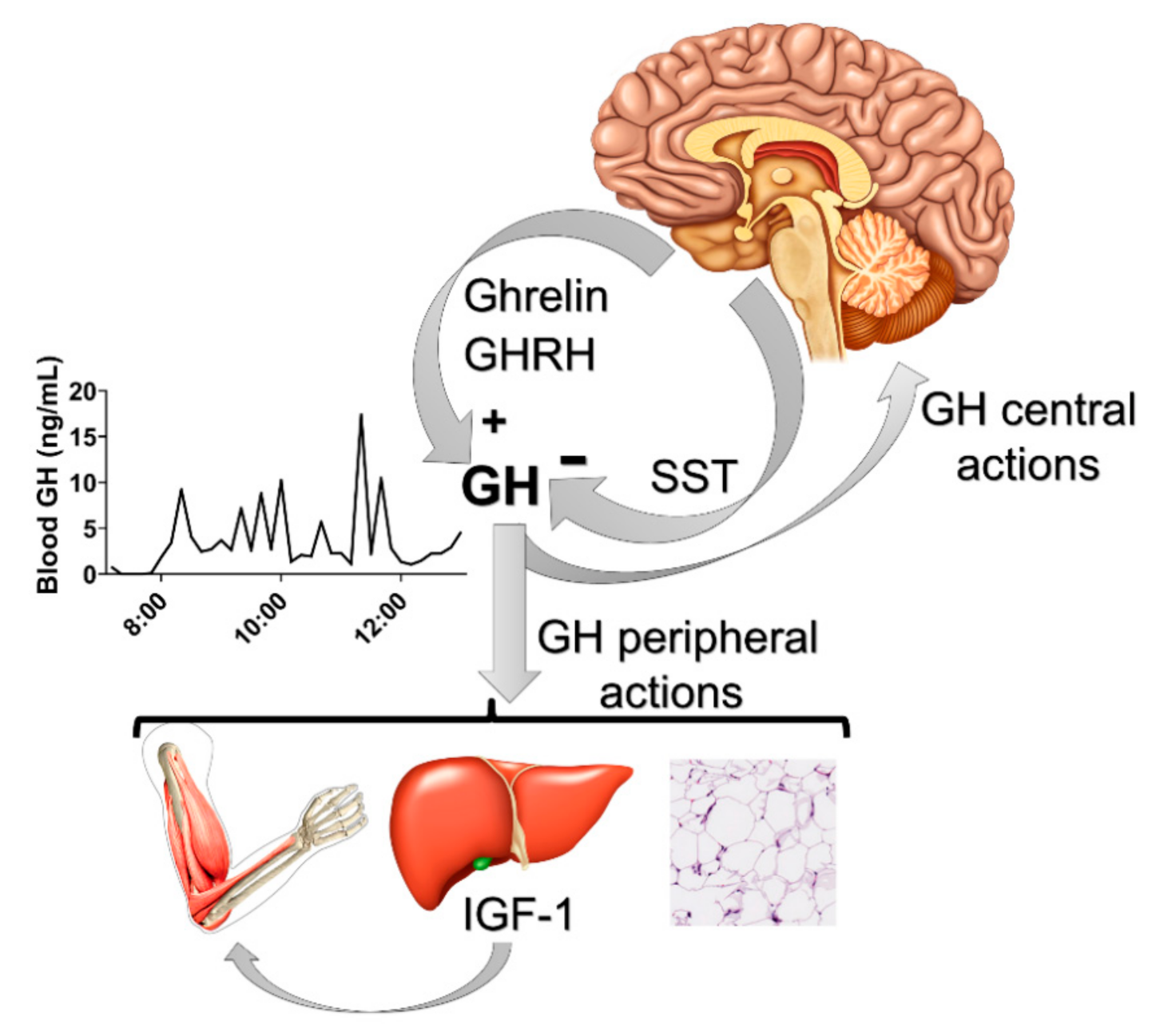
1. Introduction
2. GH Action in the Brain
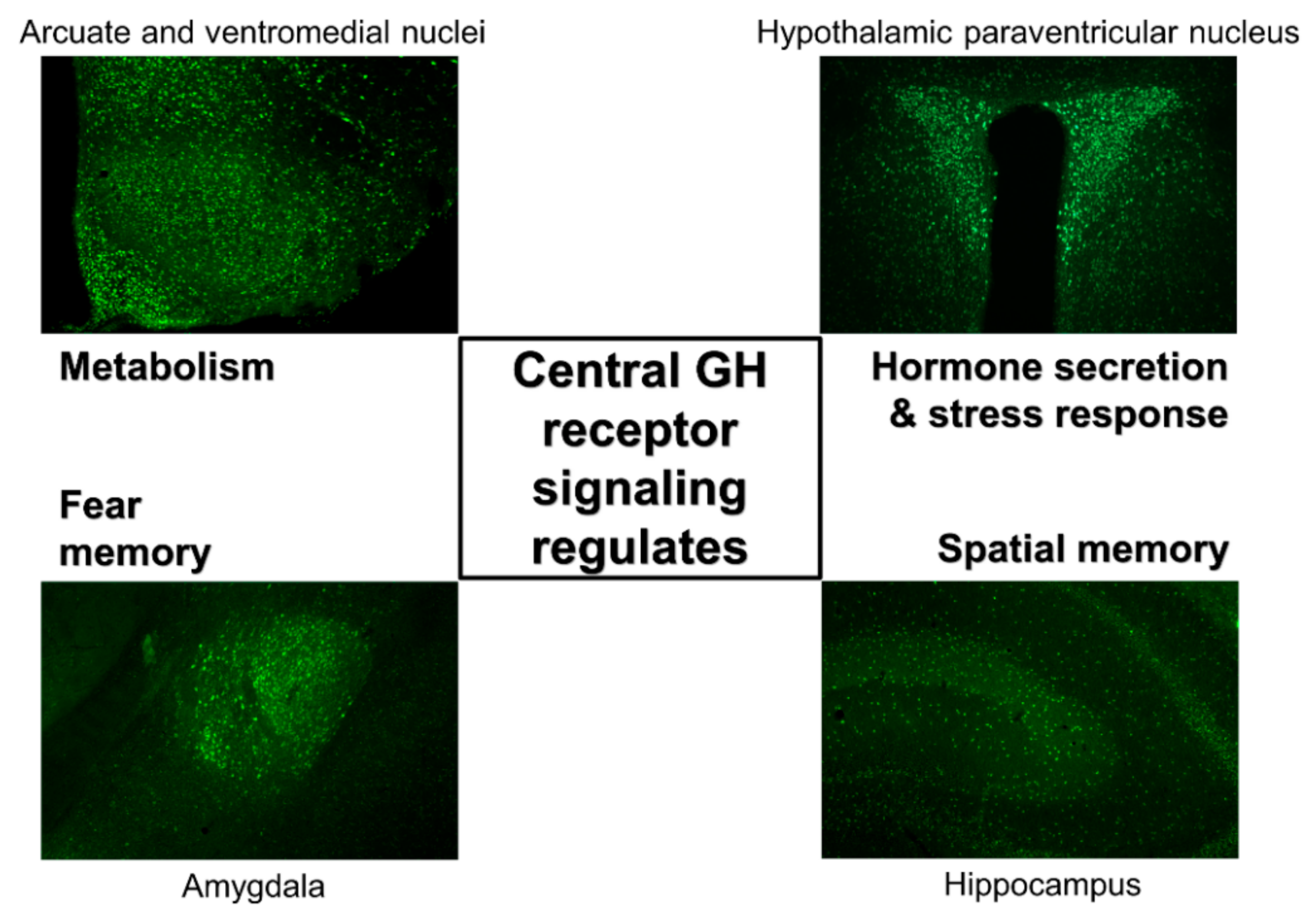
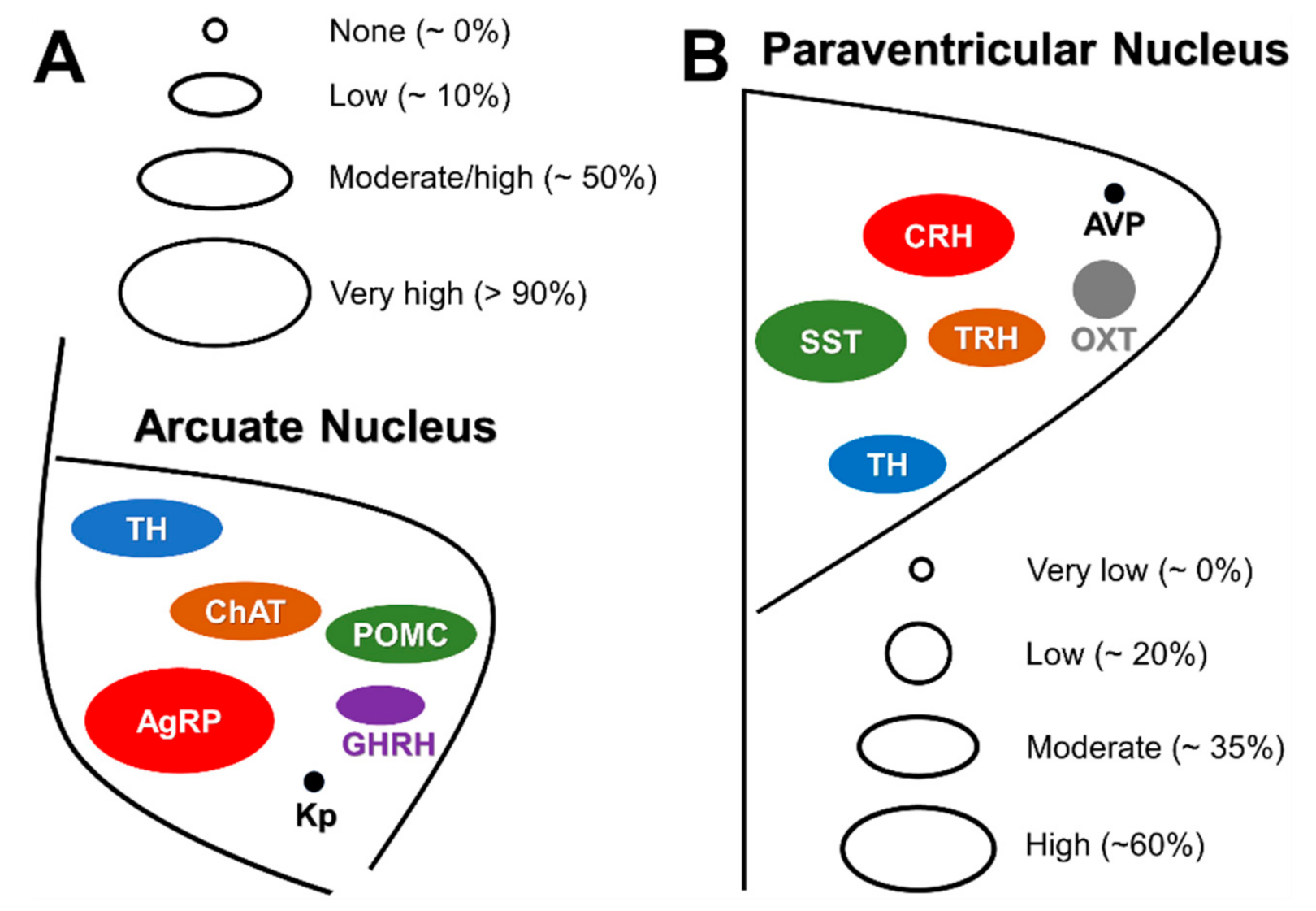
Distribution of GH-Responsive Neurons in Mouse and Rat Brains
3. Central Regulation of Metabolism by GH
3.1. GH Regulates Food Intake
3.2. GH Action in the Brain Modulates Insulin Sensitivity and Glucose Homeostasis
3.3. Central GH Action Regulates the Metabolic Responses to Calorie Restriction
3.3.1. Central GHR Signaling Modulates Calorie Restriction-Induced Changes in Energy Expenditure
3.3.2. Central GH Action Is Necessary to Maintain Blood Glucose Levels During Food Restriction
3.4. Adaptation Capacity to Aerobic Exercise Is Affected by Central GHR Signaling
4. Neurotropic Effects of GH on ARH Neurons
5. Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steyn, F.J.; Tolle, V.; Chen, C.; Epelbaum, J. Neuroendocrine regulation of growth hormone secretion. Compr. Physiol. 2016, 6, 687–735. [Google Scholar] [PubMed]
- Murray, P.G.; Higham, C.E.; Clayton, P.E. 60 years of neuroendocrinology: The hypothalamo-GH axis: The past 60 years. J. Endocrinol. 2015, 226, T123–T140. [Google Scholar] [CrossRef] [PubMed]
- Donahue, L.R.; Beamer, W.G. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J. Endocrinol. 1993, 136, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Peino, R.; Baldelli, R.; Rodriguez-Garcia, J.; Rodriguez-Segade, S.; Kojima, M.; Kangawa, K.; Arvat, E.; Ghigo, E.; Dieguez, C.; Casanueva, F.F. Ghrelin-induced growth hormone secretion in humans. Eur. J. Endocrinol. 2000, 143, R11–R14. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- Seoane, L.M.; Tovar, S.; Baldelli, R.; Arvat, E.; Ghigo, E.; Casanueva, F.F.; Dieguez, C. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. Eur. J. Endocrinol. 2000, 143, R7–R9. [Google Scholar] [CrossRef]
- Reichenbach, A.; Steyn, F.J.; Sleeman, M.W.; Andrews, Z.B. Ghrelin receptor expression and colocalization with anterior pituitary hormones using a GHSR-GFP mouse line. Endocrinology 2012, 153, 5452–5466. [Google Scholar] [CrossRef]
- Mano-Otagiri, A.; Nemoto, T.; Sekino, A.; Yamauchi, N.; Shuto, Y.; Sugihara, H.; Oikawa, S.; Shibasaki, T. Growth hormone-releasing hormone (GHRH) neurons in the arcuate nucleus (Arc) of the hypothalamus are decreased in transgenic rats whose expression of ghrelin receptor is attenuated: Evidence that ghrelin receptor is involved in the up-regulation of GHRH expression in the arc. Endocrinology 2006, 147, 4093–4103. [Google Scholar]
- Rabkin, R.; Fervenza, F.C.; Maidment, H.; Ike, J.; Hintz, R.; Liu, F.; Bloedow, D.C.; Hoffman, A.R.; Gesundheit, N. Pharmacokinetics of insulin-like growth factor-1 in advanced chronic renal failure. Kidney Int. 1996, 49, 1134–1140. [Google Scholar] [CrossRef]
- List, E.O.; Berryman, D.E.; Funk, K.; Jara, A.; Kelder, B.; Wang, F.; Stout, M.B.; Zhi, X.; Sun, L.; White, T.A.; et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGH-I, increased local IGH-I, and altered body size, body composition, and adipokine profiles. Endocrinology 2014, 155, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Menon, R.K.; Cohen, P.; Hwang, D.; Clemens, T.; DiGirolamo, D.J.; Kopchick, J.J.; Le Roith, D.; Trucco, M.; Sperling, M.A. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 2009, 284, 19937–19944. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J. Lessons learned from studies with the growth hormone receptor. Growth Horm. IGF Res. 2016, 28, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kineman, R.D.; Del Rio-Moreno, M.; Sarmento-Cabral, A. 40 years of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- List, E.O.; Berryman, D.E.; Jensen, E.A.; Kulkarni, P.; McKenna, S.; Kopchick, J.J. New insights of growth hormone (GH) actions from tissue-specific GH receptor knockouts in mice. Arch. Endocrinol. Metab. 2019, 63, 557–567. [Google Scholar] [CrossRef] [PubMed]
- List, E.O.; Duran-Ortiz, S.; Kopchick, J.J. Effects of tissue-specific GH receptor knockouts in mice. Mol. Cell. Endocrinol. 2020, 515, 110919. [Google Scholar] [CrossRef]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perello, M.; Frazao, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef]
- Hussain, M.A.; Schmitz, O.; Mengel, A.; Glatz, Y.; Christiansen, J.S.; Zapf, J.; Froesch, E.R. Comparison of the effects of growth hormone and insulin-like growth factor I on substrate oxidation and on insulin sensitivity in growth hormone-deficient humans. J. Clin. Invest. 1994, 94, 1126–1133. [Google Scholar] [CrossRef][Green Version]
- Nielsen, S.; Moller, N.; Christiansen, J.S.; Jorgensen, J.O. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 2001, 50, 2301–2308. [Google Scholar] [CrossRef]
- Sakharova, A.A.; Horowitz, J.F.; Surya, S.; Goldenberg, N.; Harber, M.P.; Symons, K.; Barkan, A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 2008, 93, 2755–2759. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef]
- Donato, J., Jr. The central nervous system as a promising target to treat diabetes mellitus. Curr. Top. Med. Chem. 2012, 12, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Vianna, C.R.; Donato, J., Jr.; Kim, M.H.; Chuang, J.C.; Lee, C.E.; Lauzon, D.A.; Lin, P.; Brule, L.J.; Scott, M.M.; et al. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012, 122, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Zhao, C.; Cai, X.; Montez, J.M.; Rohani, S.C.; Feinstein, P.; Mombaerts, P.; Friedman, J.M. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 2001, 108, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Ferno, J.; Gonzalez, F.; Dieguez, C.; Leis, R.; Nogueiras, R.; Lopez, M. Hypothalamic-autonomic control of energy homeostasis. Endocrine 2015, 50, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Kabigting, E.B.; Clifton, D.K.; Steiner, R.A. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology 1992, 131, 958–963. [Google Scholar]
- Walsh, R.J.; Mangurian, L.P.; Posner, B.I. The distribution of lactogen receptors in the mammalian hypothalamus: An in vitro autoradiographic analysis of the rabbit and rat. Brain Res. 1990, 530, 1–11. [Google Scholar] [CrossRef]
- Pellegrini, E.; Bluet-Pajot, M.T.; Mounier, F.; Bennett, P.; Kordon, C.; Epelbaum, J. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J. Neurosci. 1996, 16, 8140–8148. [Google Scholar] [CrossRef]
- Kastrup, Y.; Le Greves, M.; Nyberg, F.; Blomqvist, A. Distribution of growth hormone receptor mRNA in the brain stem and spinal cord of the rat. Neuroscience 2005, 130, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Kabigting, E.B.; Steiner, R.A.; Clifton, D.K. Identification of target cells for growth hormone’s action in the arcuate nucleus. Am. J. Physiol. 1995, 269, E716–E722. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Pedroso, J.A.B.; Dos Santos, W.O.; Furigo, I.C.; Garcia-Galiano, D.; Elias, C.F.; List, E.O.; Kopchick, J.J.; Szawka, R.E.; Donato, J., Jr. Tyrosine hydroxylase neurons regulate growth hormone secretion via short-loop negative feedback. J. Neurosci. 2020, 40, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Steiner, R.; Clifton, D. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology 1996, 137, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kamegai, J.; Minami, S.; Sugihara, H.; Hasegawa, O.; Higuchi, H.; Wakabayashi, I. Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 1996, 137, 2109–2112. [Google Scholar] [CrossRef]
- Furigo, I.C.; Metzger, M.; Teixeira, P.D.; Soares, C.R.; Donato, J., Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct. Funct. 2017, 222, 341–363. [Google Scholar] [CrossRef]
- Wasinski, F.; Klein, M.O.; Bittencourt, J.C.; Metzger, M.; Donato, J., Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res. 2021, 1751, 147189. [Google Scholar] [CrossRef]
- Wasinski, F.; Frazão, R.; Donato, J.J. Effects of growth hormone in the central nervous system. Arch. Endocrinol. Metab. 2019, 63, 549–556. [Google Scholar] [CrossRef]
- Gisabella, B.; Farah, S.; Peng, X.; Burgos-Robles, A.; Lim, S.H.; Goosens, K.A. Growth hormone biases amygdala network activation after fear learning. Transl. Psychiatry 2016, 6, e960. [Google Scholar] [CrossRef]
- Meyer, R.M.; Burgos-Robles, A.; Liu, E.; Correia, S.S.; Goosens, K.A. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry 2014, 19, 1284–1294. [Google Scholar] [CrossRef]
- Deijen, J.B.; de Boer, H.; van der Veen, E.A. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology 1998, 23, 45–55. [Google Scholar] [CrossRef]
- Maruff, P.; Falleti, M. Cognitive function in growth hormone deficiency and growth hormone replacement. Horm. Res. 2005, 64 (Suppl. 3), 100–108. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.S.; Grover, L.M. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J. Neurophysiol. 2006, 95, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.P.; Ariwodola, O.J.; Linville, C.; Sonntag, W.E.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone modulates hippocampal excitatory synaptic transmission and plasticity in old rats. Neurobiol. Aging 2012, 33, 1938–1949. [Google Scholar] [CrossRef]
- Ramis, M.; Sarubbo, F.; Sola, J.; Aparicio, S.; Garau, C.; Miralles, A.; Esteban, S. Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 11–20. [Google Scholar] [CrossRef]
- Furigo, I.C.; Melo, H.M.; Lyra, E.S.N.M.; Ramos-Lobo, A.M.; Teixeira, P.D.S.; Buonfiglio, D.C.; Wasinski, F.; Lima, E.R.; Higuti, E.; Peroni, C.N.; et al. Brain STAT5 signaling modulates learning and memory formation. Brain Struct. Funct. 2018, 223, 2229–2241. [Google Scholar] [CrossRef]
- Kinney, B.A.; Coschigano, K.T.; Kopchick, J.J.; Steger, R.W.; Bartke, A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol. Behav. 2001, 72, 653–660. [Google Scholar] [CrossRef]
- Kinney-Forshee, B.A.; Kinney, N.E.; Steger, R.W.; Bartke, A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol. Behav. 2004, 80, 589–594. [Google Scholar] [CrossRef]
- Basu, A.; McFarlane, H.G.; Kopchick, J.J. Spatial learning and memory in male mice with altered growth hormone action. Horm. Behav. 2017, 93, 18–30. [Google Scholar] [CrossRef]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef]
- Schioth, H.B.; Frey, W.H.; Brooks, S.J.; Benedict, C. Insulin to treat Alzheimer’s disease: Just follow your nose? Expert Rev. Clin. Pharmacol. 2012, 5, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed]
- Duran-Ortiz, S.; Noboa, V.; Kopchick, J.J. Disruption of the GH receptor gene in adult mice and in insulin sensitive tissues. Growth Horm. IGF Res. 2018, 38, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocrinol. 2000, 21, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Bohlooly, Y.M.; Olsson, B.; Bruder, C.E.; Linden, D.; Sjogren, K.; Bjursell, M.; Egecioglu, E.; Svensson, L.; Brodin, P.; Waterton, J.C.; et al. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 2005, 54, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Song, Y.; Wang, Y.; Zhang, T.; Duan, M.; Li, Y.; Liao, L.; Zhu, Z.; Hu, W. Increased food intake in growth hormone-transgenic common carp (Cyprinus carpio L.) may be mediated by upregulating agouti-related protein (AgRP). Gen. Comp. Endocrinol. 2013, 192, 81–88. [Google Scholar] [CrossRef]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AgRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Kim, J.H.; Leggatt, R.A.; Chan, M.; Volkoff, H.; Devlin, R.H. Effects of chronic growth hormone overexpression on appetite-regulating brain gene expression in coho salmon. Mol. Cell. Endocrinol. 2015, 413, 178–188. [Google Scholar] [CrossRef]
- Freda, P.U.; Reyes-Vidal, C.; Jin, Z.; Pugh, M.; Panigrahi, S.K.; Bruce, J.N.; Wardlaw, S.L. Plasma agouti-related protein levels in acromegaly and effects of surgical or pegvisomant therapy. J. Clin. Endocrinol. Metab. 2019, 104, 5453–5461. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Donato, J., Jr. The role of leptin in health and disease. Temperature 2017, 4, 258–291. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.G.F.; Teixeira, P.D.S.; Furigo, I.C.; Wasinski, F.; Couto, G.C.; Frazao, R.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Growth hormone/STAT5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol. Cell. Endocrinol. 2019, 498, 110574. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.I.; Zagon, I.S.; McLaughlin, P.J. Hypophagia follows the initial hyperphagia produced by 2-deoxy-D-glucose in rats. Physiol. Behav. 1979, 23, 187–190. [Google Scholar] [CrossRef]
- Luquet, S.; Phillips, C.T.; Palmiter, R.D. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 2007, 28, 214–225. [Google Scholar] [CrossRef]
- Ozawa, Y.; Arima, H.; Watanabe, M.; Shimizu, H.; Ito, Y.; Banno, R.; Sugimura, Y.; Ozaki, N.; Nagasaki, H.; Oiso, Y. Repeated glucoprivation delayed hyperphagic responses while activating neuropeptide Y neurons in rats. Peptides 2011, 32, 763–769. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.K.; Jo, Y.H. Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol. Metab. 2017, 6, 306–312. [Google Scholar] [CrossRef]
- Herman, A.M.; Ortiz-Guzman, J.; Kochukov, M.; Herman, I.; Quast, K.B.; Patel, J.M.; Tepe, B.; Carlson, J.C.; Ung, K.; Selever, J.; et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 2016, 538, 253–256. [Google Scholar] [CrossRef]
- Meister, B.; Gomuc, B.; Suarez, E.; Ishii, Y.; Durr, K.; Gillberg, L. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur. J. Neurosci. 2006, 24, 2731–2740. [Google Scholar] [CrossRef]
- Jeong, J.H.; Woo, Y.J.; Chua, S., Jr.; Jo, Y.H. Single-cell gene expression analysis of cholinergic neurons in the arcuate nucleus of the hypothalamus. PLoS ONE 2016, 11, e0162839. [Google Scholar] [CrossRef]
- Quaresma, P.G.F.; Teixeira, P.D.S.; Wasinski, F.; Campos, A.M.P.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Cholinergic neurons in the hypothalamus and dorsal motor nucleus of the vagus are directly responsive to growth hormone. Life Sci. 2020, 259, 118229. [Google Scholar] [CrossRef]
- Tschop, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Tovar, S.; Mitchell, S.E.; Rayner, D.V.; Archer, Z.A.; Dieguez, C.; Williams, L.M. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 2004, 53, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Bjursell, M.; Ljungberg, A.; Dickson, S.L.; Kopchick, J.J.; Bergstrom, G.; Svensson, L.; Oscarsson, J.; Tornell, J.; Bohlooly, Y.M. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E317–E325. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, S.R.; Augustine, R.A.; Grattan, D.R. Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. 2010, 22, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, T.T.; Ramos-Lobo, A.M.; Furigo, I.C.; Pedroso, J.A.; Buonfiglio, D.C.; Donato, J., Jr. Socs3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol. Metab 2015, 4, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.D.S.; Couto, G.C.; Furigo, I.C.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Central growth hormone action regulates metabolism during pregnancy. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E925–E940. [Google Scholar] [CrossRef]
- Verberne, A.J.; Sabetghadam, A.; Korim, W.S. Neural pathways that control the glucose counterregulatory response. Front. Neurosci. 2014, 8, 38. [Google Scholar] [CrossRef]
- Meek, T.H.; Nelson, J.T.; Matsen, M.E.; Dorfman, M.D.; Guyenet, S.J.; Damian, V.; Allison, M.B.; Scarlett, J.M.; Nguyen, H.T.; Thaler, J.P.; et al. Functional identification of a neurocircuit regulating blood glucose. Proc. Natl. Acad. Sci. USA 2016, 113, E2073–E2082. [Google Scholar] [CrossRef]
- Cady, G.; Landeryou, T.; Garratt, M.; Kopchick, J.J.; Qi, N.; Garcia-Galiano, D.; Elias, C.F.; Myers, M.G., Jr.; Miller, R.A.; Sandoval, D.A.; et al. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRB) expressing neurons. Mol. Metab. 2017, 6, 393–405. [Google Scholar] [CrossRef]
- Furigo, I.C.; de Souza, G.O.; Teixeira, P.D.S.; Guadagnini, D.; Frazao, R.; List, E.O.; Kopchick, J.J.; Prada, P.O.; Donato, J., Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019, 33, 11909–11924. [Google Scholar] [CrossRef]
- Sorenson, R.L.; Brelje, T.C. Adaptation of islets of langerhans to pregnancy: Beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.R.; Cyphert, H.A.; Walker, E.M.; Chakravarthy, H.; Peiris, H.; Gu, X.; Liu, Y.; Conrad, E.; Goodrich, L.; Stein, R.W.; et al. Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet β-cells. Diabetes 2016, 65, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Snider, F.; Cross, J.C. Prolactin receptor is required for normal glucose homeostasis and modulation of β-cell mass during pregnancy. Endocrinology 2009, 150, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Muhlhausler, B.S.; Huang, L.; Sim, P.S.; Roberts, C.T.; Velhuis, J.D.; Chen, C. Rising maternal circulating GH during murine pregnancy suggests placental regulation. Endocr. Connect. 2017, 6, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Glick, S.M.; Yalow, R.S.; Berson, S.A. Hypoglycemia: A potent stimulus to secretion of growth hormone. Science 1963, 140, 987–988. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Cho, J.H.; Bae, J.Y.; O’Leary, T.P.; Johnson, J.D.; Bae, Y.C.; Kim, E.K. Insulin synthesized in the paraventricular nucleus of the hypothalamus regulates pituitary growth hormone production. JCI Insight 2020, 5, e135412. [Google Scholar] [CrossRef]
- Hussain, K.; Hindmarsh, P.; Aynsley-Green, A. Spontaneous hypoglycemia in childhood is accompanied by paradoxically low serum growth hormone and appropriate cortisol counterregulatory hormonal responses. J. Clin. Endocrinol. Metab. 2003, 88, 3715–3723. [Google Scholar] [CrossRef]
- Tennese, A.A.; Wevrick, R. Impaired hypothalamic regulation of endocrine function and delayed counterregulatory response to hypoglycemia in Magel2-null mice. Endocrinology 2011, 152, 967–978. [Google Scholar] [CrossRef]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin o-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef]
- Dhillon, H.; Zigman, J.M.; Ye, C.; Lee, C.E.; McGovern, R.A.; Tang, V.; Kenny, C.D.; Christiansen, L.M.; White, R.D.; Edelstein, E.A.; et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006, 49, 191–203. [Google Scholar] [CrossRef]
- Pedroso, J.A.; Silveira, M.A.; Lima, L.B.; Furigo, I.C.; Zampieri, T.T.; Ramos-Lobo, A.M.; Buonfiglio, D.C.; Teixeira, P.D.; Frazao, R.; Donato, J., Jr. Changes in leptin signaling by SOCS3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology 2016, 157, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Teixeira, P.D.; Quaresma, P.G.F.; Mansano, N.S.; Frazao, R.; Donato, J. STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J. Mol. Endocrinol. 2020, 64, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Sherbet, D.P.; Elsbernd, B.L.; Goldstein, J.L.; Brown, M.S.; Zhao, T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012, 287, 17942–17950. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, M.R.; Brown, M.S.; Goldstein, J.L.; Zhao, T.J. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Castorena, C.M.; Pearson, M.; Kusminski, C.M.; Ahmed, N.; Battiprolu, P.K.; Kim, K.W.; Lee, S.; Hill, J.A.; Scherer, P.E.; et al. SF-1 expression in the hypothalamus is required for beneficial metabolic effects of exercise. eLife 2016, 5, e18206. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; de Mendonca, P.O.R.; Fortes, M.A.S.; Tomaz, I.; Pecorali, V.L.; Auricino, T.B.; Costa, I.C.; Lima, L.B.; Furigo, I.C.; Bueno, D.N.; et al. SOCS3 expression in SF1 cells regulates adrenal differentiation and exercise performance. J. Endocrinol 2017, 235, 207–222. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J., Jr. SOCS3 as a future target to treat metabolic disorders. Hormones (Athens) 2019, 18, 127–136. [Google Scholar] [CrossRef]
- Andreoli, M.F.; Donato, J.; Cakir, I.; Perello, M. Leptin resensitisation: A reversion of leptin-resistant states. J. Endocrinol. 2019, 241, R81–R96. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Dos Santos, L.B.P.; Furigo, I.C.; Spagnol, A.R.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Deletion of growth hormone receptor in hypothalamic neurons affects the adaptation capacity to aerobic exercise. Peptides 2021, 135, 170426. [Google Scholar] [CrossRef]
- Wasinski, F.; Furigo, I.C.; Teixeira, P.D.S.; Ramos-Lobo, A.M.; Peroni, C.N.; Bartolini, P.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Growth hormone receptor deletion reduces the density of axonal projections from hypothalamic arcuate nucleus neurons. Neuroscience 2020, 434, 136–147. [Google Scholar] [CrossRef]
- Sadagurski, M.; Landeryou, T.; Cady, G.; Kopchick, J.J.; List, E.O.; Berryman, D.E.; Bartke, A.; Miller, R.A. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell 2015, 14, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lobo, A.M.; Teixeira, P.D.; Furigo, I.C.; Melo, H.M.; e Silva, N.D.M.L.; De Felice, F.G.; Donato, J., Jr. Long-term consequences of the absence of leptin signaling in early life. eLife 2019, 8, e40970. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.G.F.; Dos Santos, W.O.; Wasinski, F.; Metzger, M.; Donato, J., Jr. Neurochemical phenotype of growth hormone-responsive cells in the mouse paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 2021. [Google Scholar] [CrossRef]
- Simmons, D.M.; Swanson, L.W. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: Toward a global 3D model. J. Comp. Neurol. 2009, 516, 423–441. [Google Scholar] [CrossRef]
- Bohlen, T.M.; Zampieri, T.T.; Furigo, I.C.; Teixeira, P.D.; List, E.O.; Kopchick, J.; Donato, J., Jr.; Frazao, R. Central growth hormone signaling is not required for the timing of puberty. J. Endocrinol. 2019, 243, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Donato, J., Jr.; Tirapegui, J.; Schneider, C.D. Growth hormone and physical exercise: Current considerations. Rev. Bras. Cienc. Farm. 2008, 44, 549–562. [Google Scholar] [CrossRef]
- Amiel, S.A.; Sherwin, R.S.; Simonson, D.C.; Lauritano, A.A.; Tamborlane, W.V. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N. Engl. J. Med. 1986, 315, 215–219. [Google Scholar] [CrossRef]
| Neuronal Population | Physiological Aspects Regulated by Central GH Receptor Signaling | Reference |
|---|---|---|
| Nestin-derived cells (entire brain) | Neuroendocrine adaptations that affect energy expenditure during food restriction | [17] |
| Regulation of GH secretion via a negative feedback loop | [33] | |
| Food intake, fat retention, as well as insulin and leptin sensitivity during pregnancy | [76] | |
| LepR-expressing cells | Hepatic glucose production and insulin sensitivity | [79] |
| Neuroendocrine adaptations that affect energy expenditure during food restriction | [17] | |
| Maintenance of glycemia during prolonged food restriction | [17] | |
| Glucoprivic hyperphagia | [17] | |
| Food intake, fat retention, as well as insulin and leptin sensitivity during pregnancy | [76] | |
| Aerobic performance and metabolic adaptations to chronic exercise | [99] | |
| Recovery from hypoglycemia and counter-regulatory response | [80] | |
| Trophic effects on the formation of POMC and AgRP axonal projections | [100] | |
| TH-expressing cells | Regulation of GH secretion via a negative feedback loop | [33] |
| Dopamine transporter-expressing cells | No function identified yet | [33] |
| Dopamine β-hydroxylase-expressing cells | No function identified yet | [33] |
| AgRP-expressing neurons | Neuroendocrine adaptations that affect energy expenditure during food restriction | [17] |
| Maintenance of glycemia during prolonged food restriction | [17] | |
| Glucoprivic hyperphagia | [17] | |
| Trophic effects on the formation of AgRP axonal projections | [100] | |
| Cholinergic cells | No function identified yet | [70] |
| Kisspeptin-expressing neurons | Regulation of the hypothalamic expression of transcripts that modulate the hypothalamic-pituitary-gonadal axis | [106] |
| POMC-expressing neurons | Glucoprivic hyperphagia | [62] |
| SF1-expressing cells (VMH neurons) | Recovery from hypoglycemia and counter-regulatory response | [80] |
| Aerobic performance and metabolic adaptations to exercise | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donato, J., Jr.; Wasinski, F.; Furigo, I.C.; Metzger, M.; Frazão, R. Central Regulation of Metabolism by Growth Hormone. Cells 2021, 10, 129. https://doi.org/10.3390/cells10010129
Donato J Jr., Wasinski F, Furigo IC, Metzger M, Frazão R. Central Regulation of Metabolism by Growth Hormone. Cells. 2021; 10(1):129. https://doi.org/10.3390/cells10010129
Chicago/Turabian StyleDonato, Jose, Jr., Frederick Wasinski, Isadora C. Furigo, Martin Metzger, and Renata Frazão. 2021. "Central Regulation of Metabolism by Growth Hormone" Cells 10, no. 1: 129. https://doi.org/10.3390/cells10010129
APA StyleDonato, J., Jr., Wasinski, F., Furigo, I. C., Metzger, M., & Frazão, R. (2021). Central Regulation of Metabolism by Growth Hormone. Cells, 10(1), 129. https://doi.org/10.3390/cells10010129






