Beyond Il-5: Metabolic Reprogramming and Stromal Support Are Prerequisite for Generation and Survival of Long-Lived Eosinophil
Abstract
1. Introduction
2. Material and Methods
2.1. Mice and Bone Marrow Extraction
2.2. Conventional Bone Marrow-Derived Eosinophil (bmEos) Culture
2.3. IL-5 Only Culture
2.4. Long-Lived Eosinophil (llEos) Culture
2.5. “Old Stroma” Eosinophil Culture
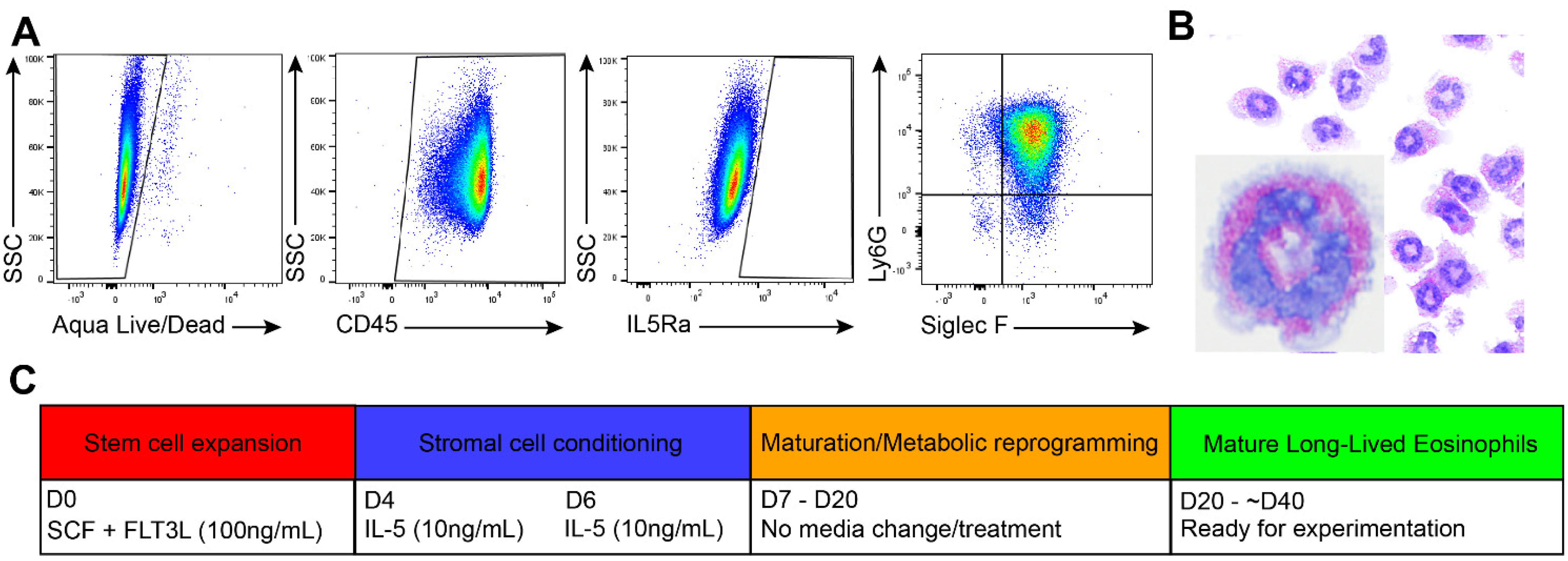
2.6. Flow Cytometry
2.7. Cytospin Preparations
2.8. Chemotaxis Assay
2.9. Fibrinogen Interaction Assay
2.10. Biolog Metabolism Assay
2.11. Statistical Analysis
3. Results
3.1. Generation of Long-Lived Eosinophils (llEos) Using Modified Bone Marrow-Derived Culture Protocol
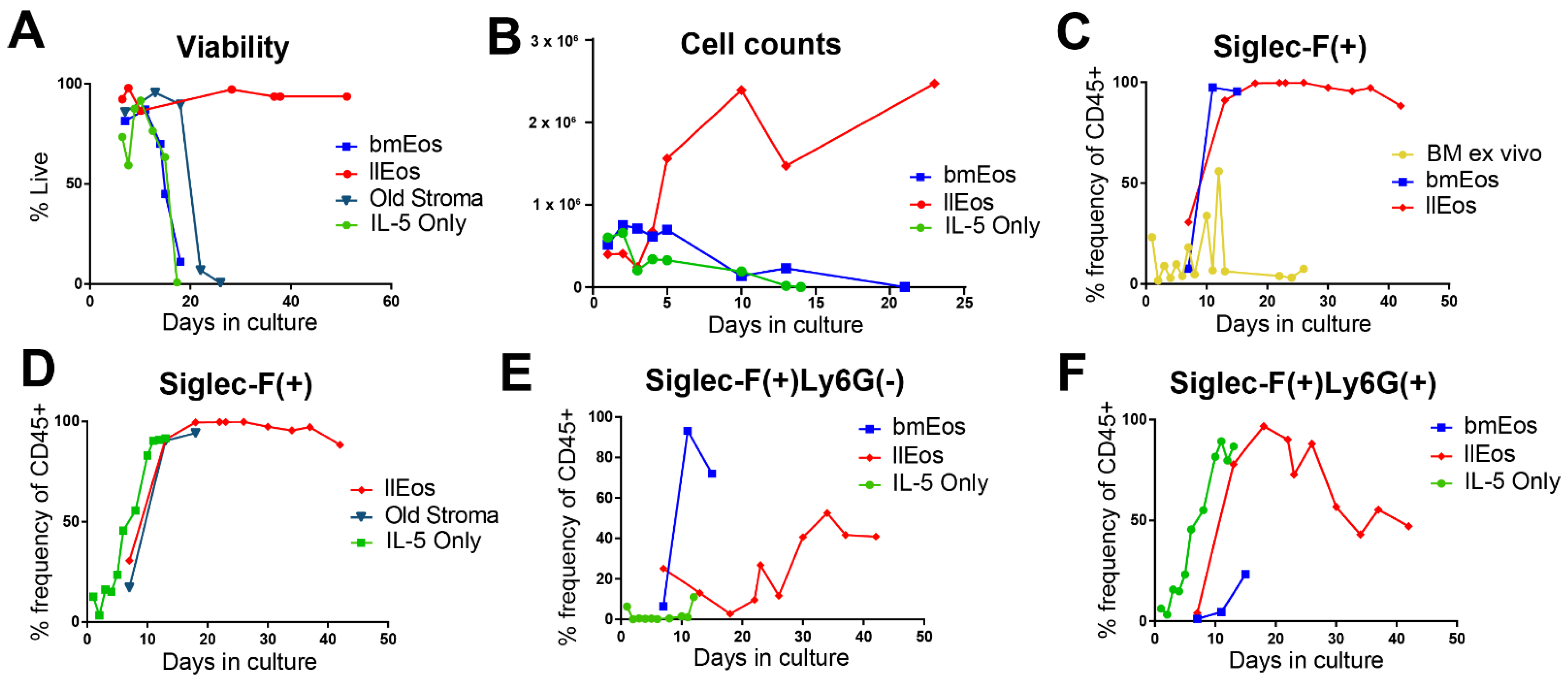
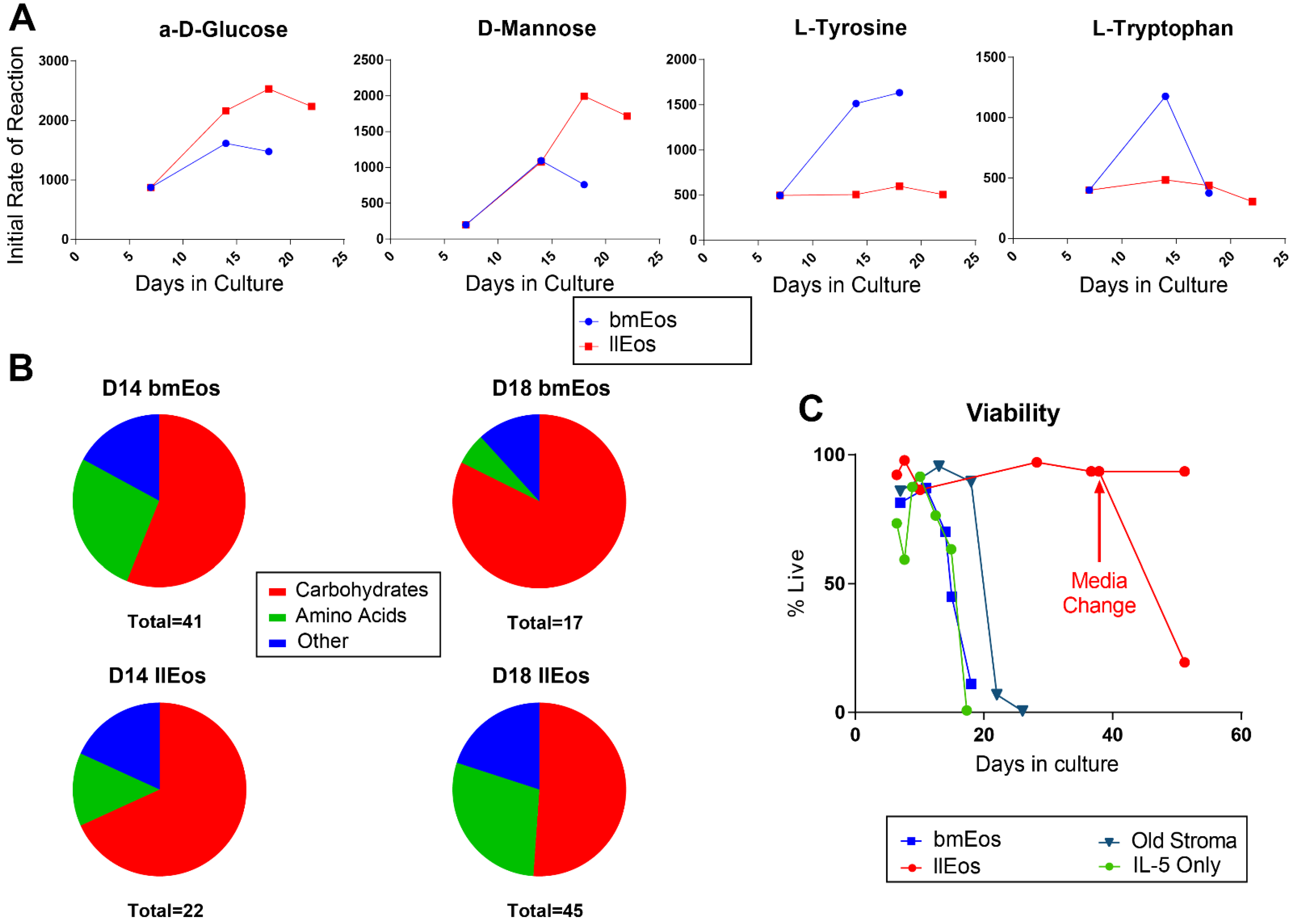
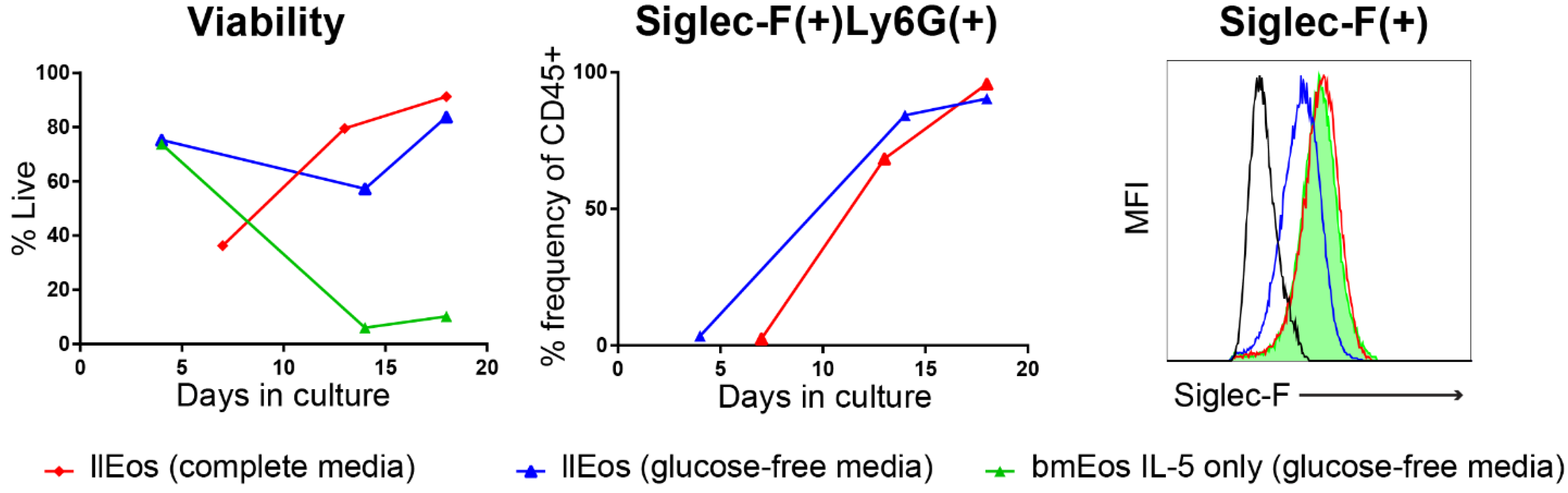
3.2. Only the llEos Protocol Supports Long Term Survival of Differentiated Eosinophils
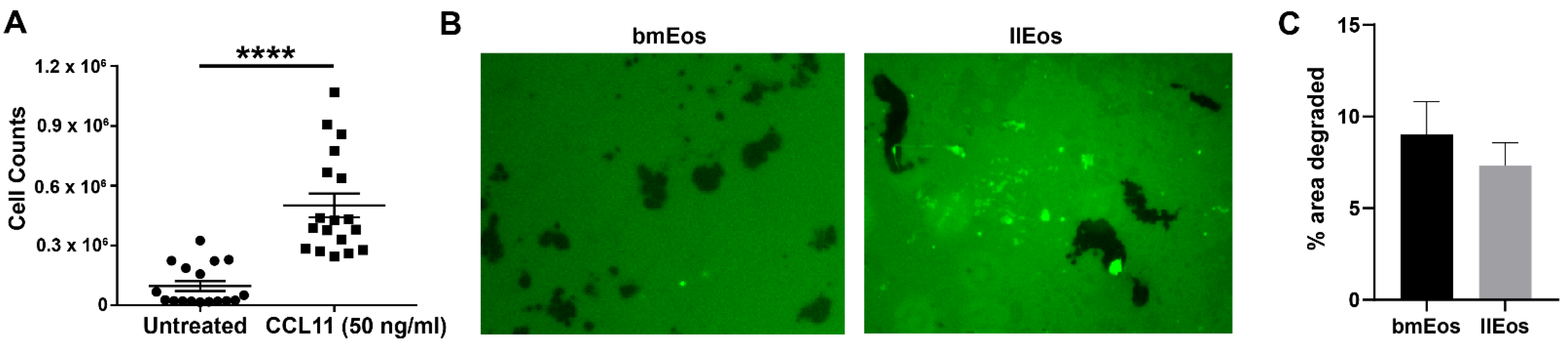
3.3. llEos Exhibit Competent Chemotaxis and Promote Fibrinogenolysis
3.4. Metabolic Reprogramming Is an Important Feature of llEos Phenotype
3.5. Glucose Is Dispensable for the Maturation and Survival of llEos but Is Necessary for Adequate Expression of Siglec-F
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdala-Valencia, H.; Coden, M.E.; Chiarella, S.E.; Jacobsen, E.A.; Bochner, B.S.; Lee, J.J.; Berdnikovs, S. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018, 104, 95–108. [Google Scholar] [CrossRef]
- Hui, C.C.; McNagny, K.M.; Denburg, J.A.; Siracusa, M.C. In situ hematopoiesis: A regulator of TH2 cytokine-mediated immunity and inflammation at mucosal surfaces. Mucosal. Immunol. 2015, 8, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Radinger, M.; Bossios, A.; Sjostrand, M.; Lu, Y.; Malmhall, C.; Dahlborn, A.K.; Lee, J.J.; Lotvall, J. Local proliferation and mobilization of CCR3(+) CD34(+) eosinophil-lineage-committed cells in the lung. Immunology 2011, 132, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Need, E.F.; Atashgaran, V.; Ingman, W.V.; Dasari, P. Hormonal regulation of the immune microenvironment in the mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 229–239. [Google Scholar] [CrossRef]
- Todd, R.; Donoff, B.R.; Chiang, T.; Chou, M.Y.; Elovic, A.; Gallagher, G.T.; Wong, D.T. The eosinophil as a cellular source of transforming growth factor alpha in healing cutaneous wounds. Am. J. Pathol. 1991, 138, 1307–1313. [Google Scholar]
- Coden, M.E.; Berdnikovs, S. Eosinophils in wound healing and epithelial remodeling: Is coagulation a missing link? J. Leukoc. Biol. 2020, 108, 93–103. [Google Scholar] [CrossRef]
- Behm, C.A.; Ovington, K.S. The role of eosinophils in parasitic helminth infections: Insights from genetically modified mice. Parasitol. Today 2000, 16, 202–209. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E. A hidden residential cell in the lung. J. Clin. Investig. 2016, 126, 3185–3187. [Google Scholar] [CrossRef]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy 2016, 71, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Kelly, E.A. Essential Mechanisms of Differential Activation of Eosinophils by IL-3 Compared to GM-CSF and IL-5. Crit. Rev. Immunol. 2016, 36, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.C.; Jeong, B.M.; Coden, M.E.; Loffredo, L.F.; Bhattacharyya, S.; Chiarella, S.E.; Varga, J.; Abdala-Valencia, H.; Berdnikovs, S. Matrix protein tenascin-C expands and reversibly blocks maturation of murine eosinophil progenitors. J. Allergy Clin. Immunol. 2018, 142, 695–698. [Google Scholar] [CrossRef]
- Loffredo, L.F.; Coden, M.E.; Jeong, B.M.; Walker, M.T.; Anekalla, K.R.; Doan, T.C.; Rodriguez, R.; Browning, M.; Nam, K.; Lee, J.J.; et al. Eosinophil accumulation in postnatal lung is specific to the primary septation phase of development. Sci. Rep. 2020, 10, 4425. [Google Scholar] [CrossRef]
- Kelly, E.A.; Esnault, S.; Liu, L.Y.; Evans, M.D.; Johansson, M.W.; Mathur, S.; Mosher, D.F.; Denlinger, L.C.; Jarjour, N.N. Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 1385–1395. [Google Scholar] [CrossRef]
- Vanderhaegen, T.; Gengler, I.; Dendooven, A.; Chenivesse, C.; Lefevre, G.; Mortuaire, G. Eosinophils in the Field of Nasal Polyposis: Towards a Better Understanding of Biologic Therapies. Clin. Rev. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Lee, L.Y.; Hew, G.S.Y.; Mehta, M.; Shukla, S.D.; Satija, S.; Khurana, N.; Anand, K.; Dureja, H.; Singh, S.K.; Mishra, V.; et al. Targeting eosinophils in respiratory diseases: Biological axis, emerging therapeutics and treatment modalities. Life Sci. 2021, 267, 118973. [Google Scholar] [CrossRef]
- Dyer, K.D.; Moser, J.M.; Czapiga, M.; Siegel, S.J.; Percopo, C.M.; Rosenberg, H.F. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008, 181, 4004–4009. [Google Scholar] [CrossRef]
- Ishihara, K.; Satoh, I.; Mue, S.; Ohuchi, K. Generation of rat eosinophils by recombinant rat interleukin-5 in vitro and in vivo. Biochim. Biophys. Acta 2000, 1501, 25–32. [Google Scholar] [CrossRef]
- Coden, M.E.; Loffredo, L.F.; Walker, M.T.; Jeong, B.M.; Nam, K.; Bochner, B.S.; Abdala-Valencia, H.; Berdnikovs, S. Fibrinogen Is a Specific Trigger for Cytolytic Eosinophil Degranulation. J. Immunol. 2020, 204, 438–448. [Google Scholar] [CrossRef]
- Dyer, K.D.; Percopo, C.M.; Rosenberg, H.F. Generation of eosinophils from unselected bone marrow progenitors: Wild-type, TLR- and eosinophil-deficient mice. Open Immunol. J. 2009, 2, 163–167. [Google Scholar] [CrossRef]
- Percopo, C.M.; Brenner, T.A.; Ma, M.; Kraemer, L.S.; Hakeem, R.M.; Lee, J.J.; Rosenberg, H.F. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J. Leukoc. Biol. 2017, 101, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Limkar, A.R.; Mai, E.; Sek, A.C.; Percopo, C.M.; Rosenberg, H.F. Frontline Science: Cytokine-mediated developmental phenotype of mouse eosinophils: IL-5-associated expression of the Ly6G/Gr1 surface Ag. J. Leukoc. Biol. 2020, 107, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, S.J.; Rattan, S.I. What determines longevity: Metabolic rate or stability? Discov. Med. 2005, 5, 359–362. [Google Scholar] [PubMed]
- Jones, N.; Vincent, E.E.; Felix, L.C.; Cronin, J.G.; Scott, L.M.; Hole, P.S.; Lacy, P.; Thornton, C.A. Interleukin-5 drives glycolysis and reactive oxygen species-dependent citric acid cycling by eosinophils. Allergy 2020, 75, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Ferreira, F.S.; Marques, E.P.; Schmitz, F.; Gassen, R.B.; Breda, R.V.; Wyse, A.T.S.; et al. Autophagy induces eosinophil extracellular traps formation and allergic airway inflammation in a murine asthma model. J. Cell Physiol. 2020, 235, 267–280. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Kachler, K.; Llerins Perez, M.; Kirchner, P.; Kolle, J.; Giessl, A.; Rauber, S.; Song, R.; Aust, O.; et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021. [Google Scholar] [CrossRef]
- Yan, K.; Da, T.T.; Bian, Z.H.; He, Y.; Liu, M.C.; Liu, Q.Z.; Long, J.; Li, L.; Gao, C.Y.; Yang, S.H.; et al. Multi-omics analysis identifies FoxO1 as a regulator of macrophage function through metabolic reprogramming. Cell Death Dis. 2020, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Griseri, T.; Arnold, I.C.; Pearson, C.; Krausgruber, T.; Schiering, C.; Franchini, F.; Schulthess, J.; McKenzie, B.S.; Crocker, P.R.; Powrie, F. Granulocyte Macrophage Colony-Stimulating Factor-Activated Eosinophils Promote Interleukin-23 Driven Chronic Colitis. Immunity 2015, 43, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Nobs, S.P.; Kayhan, M.; Kopf, M. GM-CSF intrinsically controls eosinophil accumulation in the setting of allergic airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.M.; Wardlaw, A.J. Dexamethasone inhibits prolonged survival and autocrine granulocyte-macrophage colony-stimulating factor production by human eosinophils cultured on laminin or tissue fibronectin. J. Allergy Clin. Immunol. 1997, 100, 208–215. [Google Scholar] [CrossRef]
- Broide, D.H.; Paine, M.M.; Firestein, G.S. Eosinophils express interleukin 5 and granulocyte macrophage-colony-stimulating factor mRNA at sites of allergic inflammation in asthmatics. J. Clin. Investig. 1992, 90, 1414–1424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coden, M.E.; Walker, M.T.; Jeong, B.M.; Connelly, A.R.; Nagasaka, R.; Berdnikovs, S. Beyond Il-5: Metabolic Reprogramming and Stromal Support Are Prerequisite for Generation and Survival of Long-Lived Eosinophil. Cells 2021, 10, 815. https://doi.org/10.3390/cells10040815
Coden ME, Walker MT, Jeong BM, Connelly AR, Nagasaka R, Berdnikovs S. Beyond Il-5: Metabolic Reprogramming and Stromal Support Are Prerequisite for Generation and Survival of Long-Lived Eosinophil. Cells. 2021; 10(4):815. https://doi.org/10.3390/cells10040815
Chicago/Turabian StyleCoden, Mackenzie E., Matthew T. Walker, Brian M. Jeong, Andrew R. Connelly, Reina Nagasaka, and Sergejs Berdnikovs. 2021. "Beyond Il-5: Metabolic Reprogramming and Stromal Support Are Prerequisite for Generation and Survival of Long-Lived Eosinophil" Cells 10, no. 4: 815. https://doi.org/10.3390/cells10040815
APA StyleCoden, M. E., Walker, M. T., Jeong, B. M., Connelly, A. R., Nagasaka, R., & Berdnikovs, S. (2021). Beyond Il-5: Metabolic Reprogramming and Stromal Support Are Prerequisite for Generation and Survival of Long-Lived Eosinophil. Cells, 10(4), 815. https://doi.org/10.3390/cells10040815






