Interleukin-1α Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects and Cell Preparation
2.2. Cell Cultures
2.3. ELISA
2.4. Reverse Transcription Quantitative Real-Time PCR
2.5. Western Blot
2.6. Statistical Analyses
3. Results
3.1. IL6 and IL8 Secretion from HBF Treated with Eosinophil-Derived Supernatants Is Dependent on the IL1 Receptor
3.2. IL1β Is Dispensable for the Induction of IL6/IL8 in HBF Treated with Eosinophil-Derived Supernatants
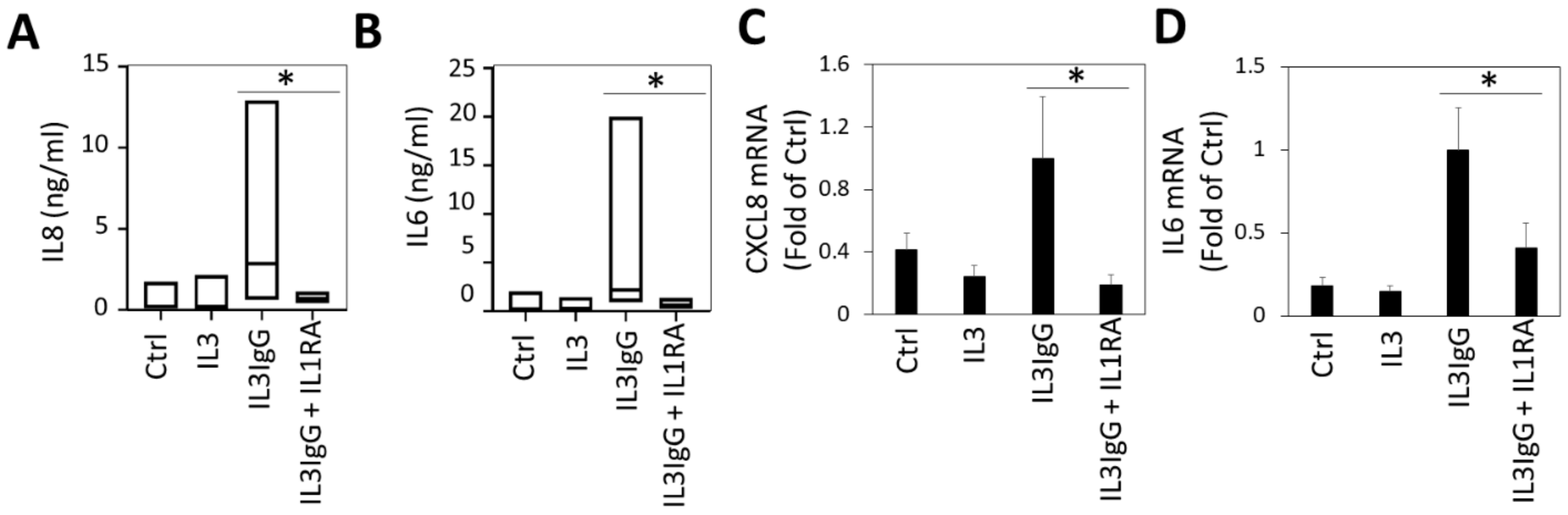
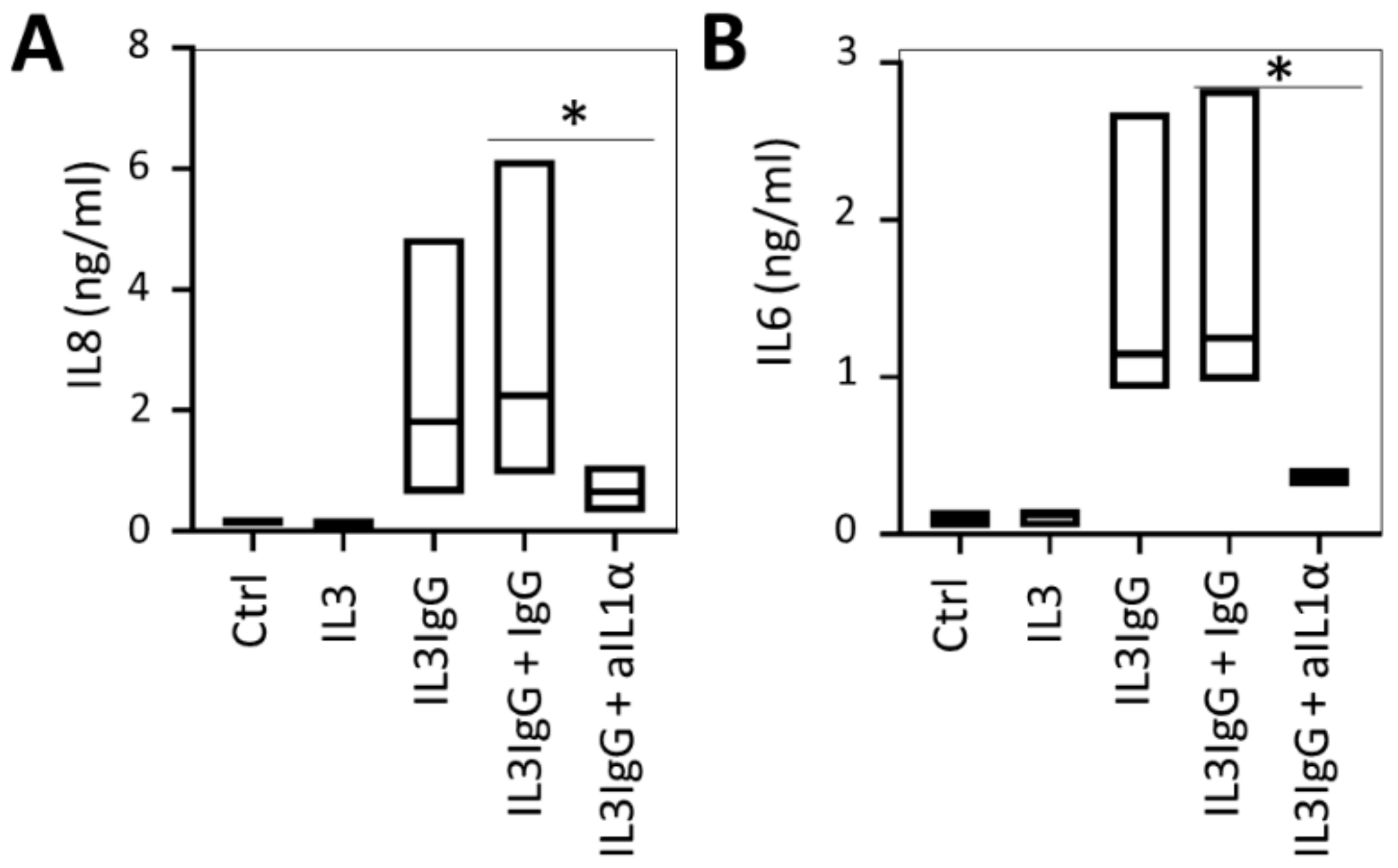
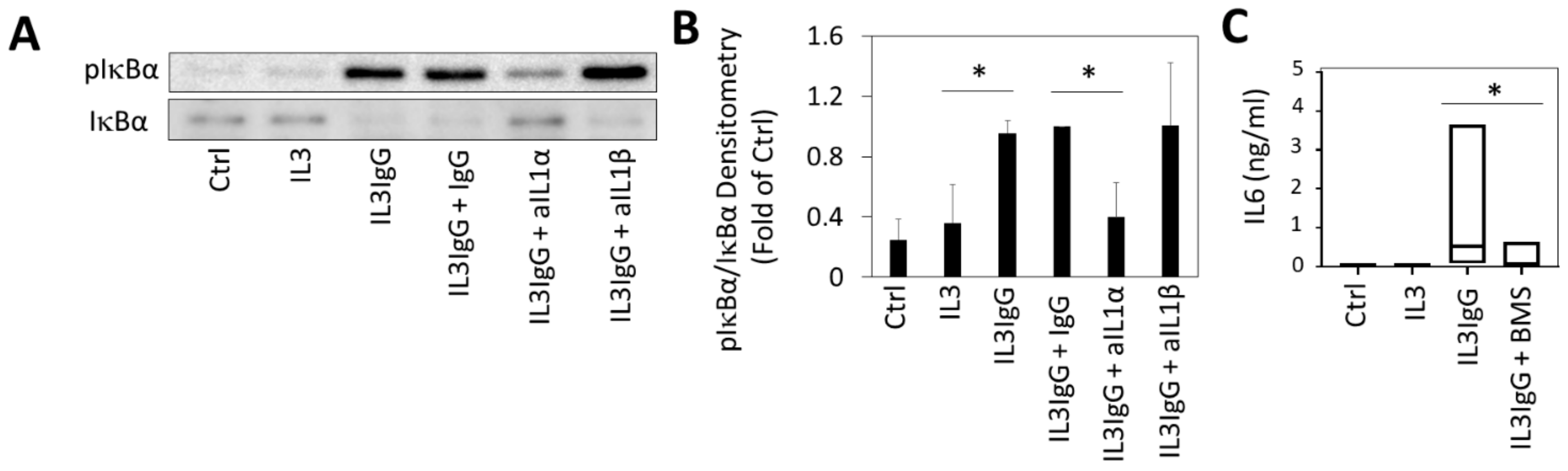
3.3. IL1α Is Essential for the Induction of IL6/IL8 in HBF Treated with Eosinophil-Derived Supernatants
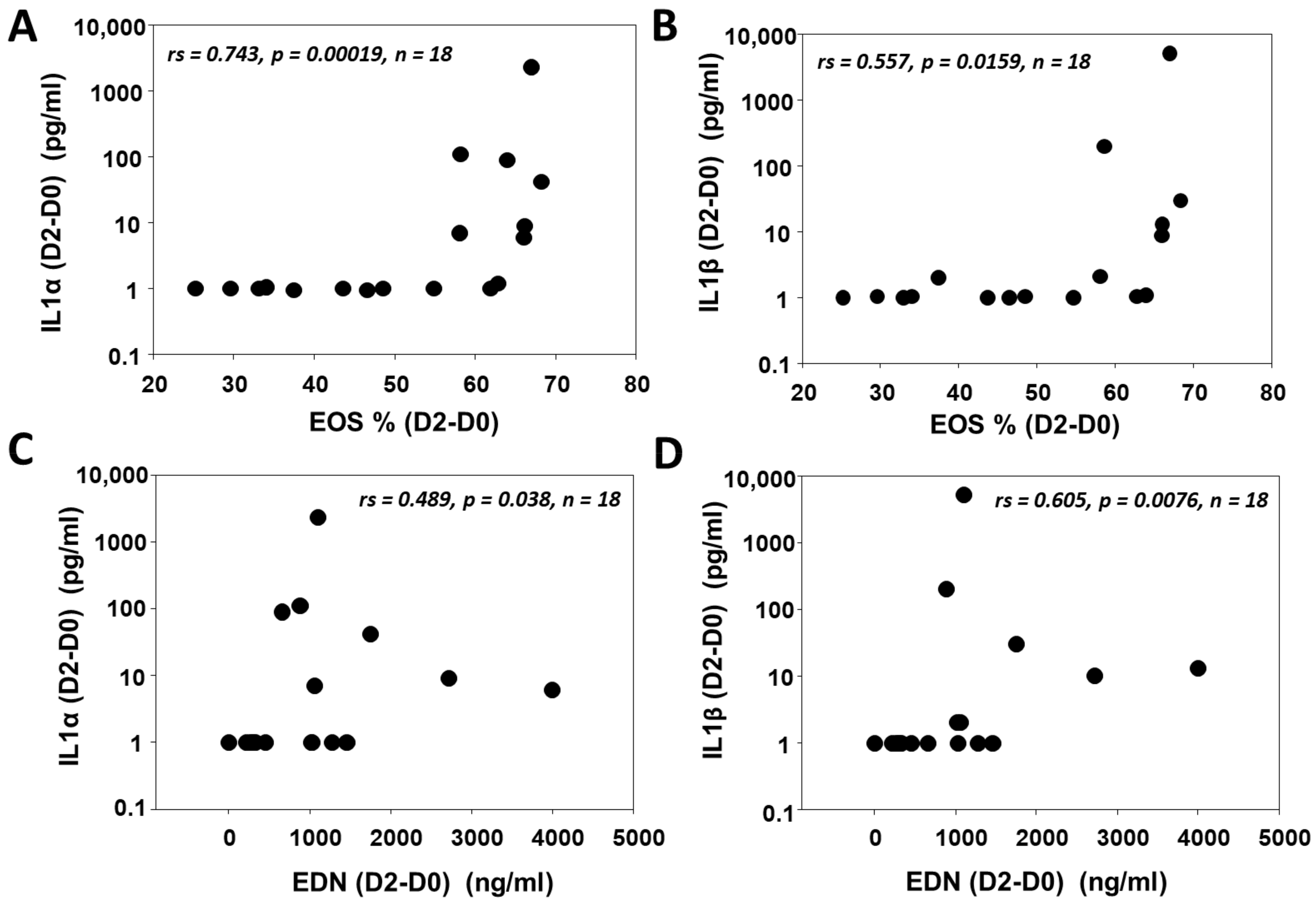
3.4. IL1 (α/β) Is Associated with Eosinophilic Inflammation after SBP-Ag
3.5. Release of IL6 in HBF Stimulated with Eosinophil-Derived Supernatants Is Dependent on NFkB Signaling
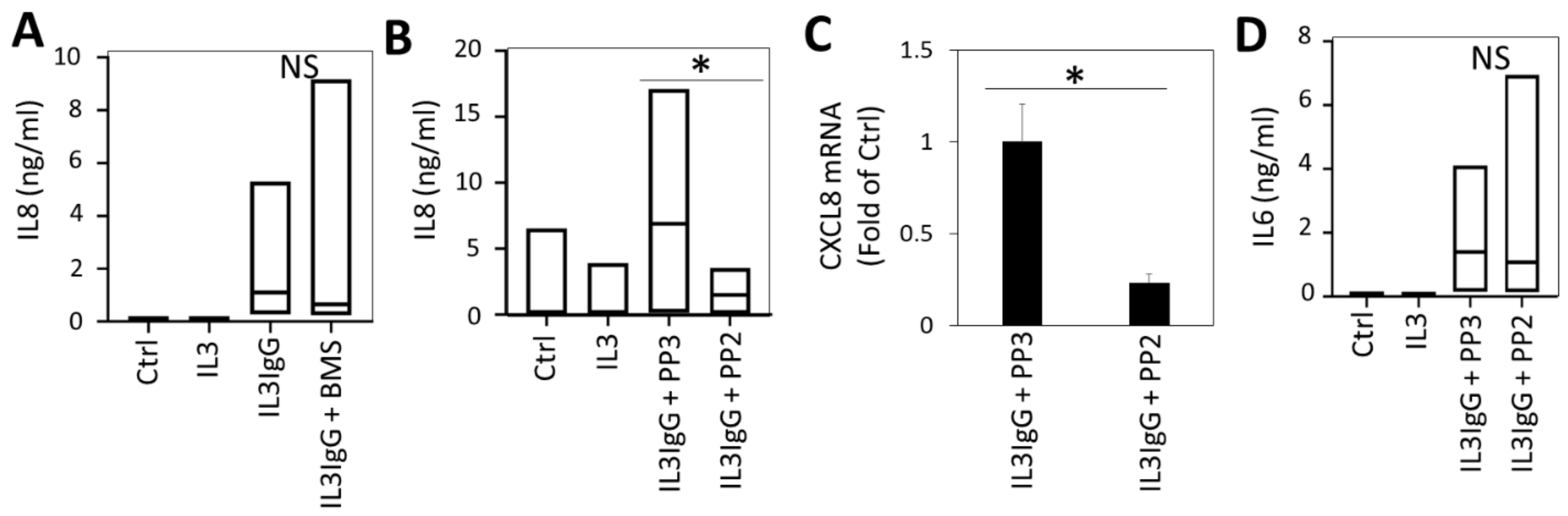
3.6. IL6 Expression by HBF Requires Janus Kinase (JAK)/Signal Transducer and Activator of Transcription Protein (STAT) Signaling
3.7. Src-Dependent Signaling Is Required for IL8 Expression by HBF in Response to Eosinophil-Derived Supernatants, While NFκB Is Dispensable
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
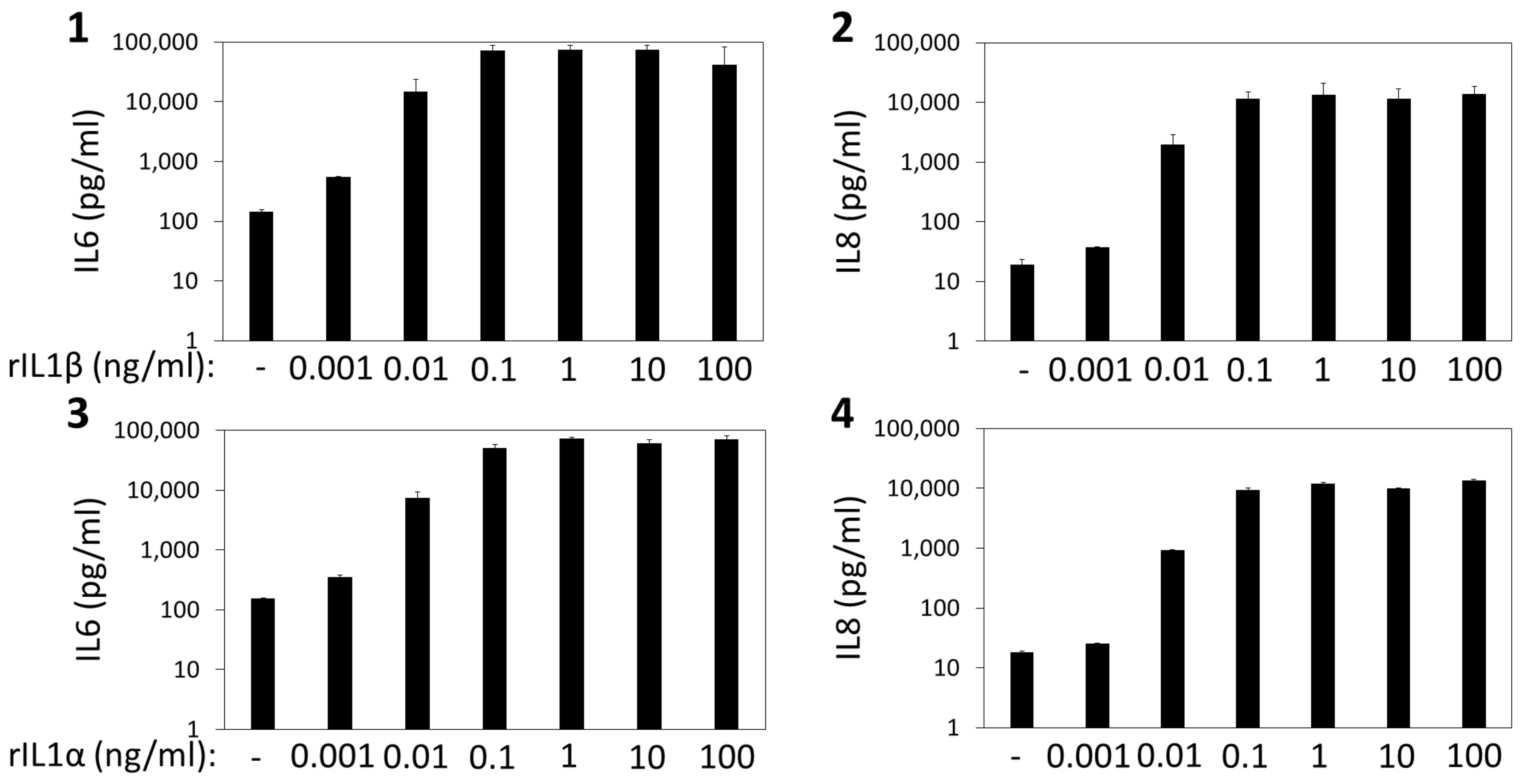
Appendix B
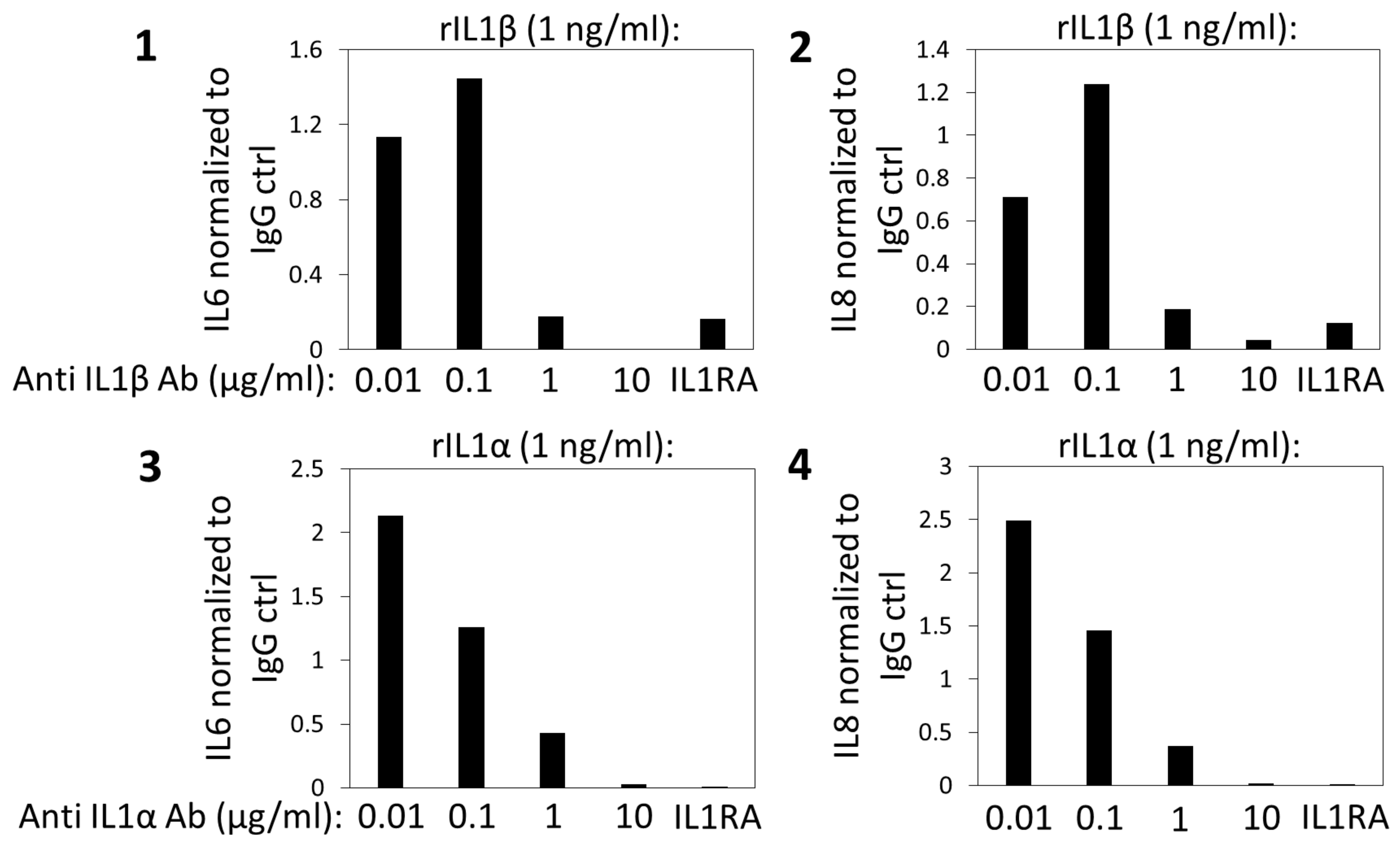
Appendix C

Appendix D
| Number | 18 |
|---|---|
| Sex | 9 F, 9 M |
| Age (yrs) | 22 ± 1 * |
| Baseline FEV1 (% Predicted) | 96.9 ± 2.1 * |
| Antigens Used for Challenge | 8 Ragweed, 8 Dust mite, 2 Cat dander |
Appendix E
| Cell Type | Before Challenge | After Challenge |
|---|---|---|
| Eosinophil | 2.2 ± 3.9 * | 53.6 ± 15.8 * |
| Macrophage/monocyte | 87.8 ± 8.1 * | 34.1 ± 12.9 * |
| Neutrophil | 1.5 ± 1.4 * | 4.3 ± 3.7 * |
| Lymphocytes | 8.4 ± 6.4 * | 7.9 ± 4.1 * |
References
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barneon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Malinovschi, A.; Fonseca, J.A.; Jacinto, T.; Alving, K.; Janson, C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J. Allergy Clin. Immunol. 2013, 132, 821–827. [Google Scholar] [CrossRef]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.; Weller, P.F. Eosinophil secretion of granule-derived cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef]
- Esnault, S.; Leet, J.P.; Johansson, M.W.; Barretto, K.T.; Fichtinger, P.S.; Fogerty, F.J.; Bernau, K.; Mathur, S.K.; Mosher, D.F.; Sandbo, N.; et al. Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling. Clin. Exp. Allergy 2020, 50, 198–212. [Google Scholar] [CrossRef]
- Bernau, K.; Leet, J.P.; Esnault, S.; Noll, A.L.; Evans, M.D.; Jarjour, N.N.; Sandbo, N. Eosinophil-degranulation products drive a proinflammatory fibroblast phenotype. J. Allergy Clin. Immunol. 2018, 142, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Bernau, K.; Torr, E.E.; Bochkov, Y.A.; Jarjour, N.N.; Sandbo, N. RNA-sequencing analysis of lung primary fibroblast response to eosinophil-degranulation products predicts downstream effects on inflammation, tissue remodeling and lipid metabolism. Respir. Res. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhsen, S.; Johnson, J.R.; Hamid, Q. Remodeling in asthma. J. Allergy Clin. Immunol. 2011, 128, 451–462. [Google Scholar] [CrossRef]
- Humbles, A.A.; Lloyd, C.M.; McMillan, S.J.; Friend, D.S.; Xanthou, G.; McKenna, E.E.; Ghiran, S.; Gerard, N.P.; Yu, C.; Orkin, S.H.; et al. A critical role for eosinophils in allergic airways remodeling. Science 2004, 305, 1776–1779. [Google Scholar] [CrossRef]
- Mostaco-Guidolin, L.B.; Osei, E.T.; Ullah, J.; Hajimohammadi, S.; Fouadi, M.; Li, X.; Li, V.; Shaheen, F.; Yang, C.X.; Chu, F.; et al. Defective fibrillar collagen organization by fibroblasts contributes to airway remodeling in asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 431–443. [Google Scholar] [CrossRef]
- Fang, C.L.; Yin, L.J.; Sharma, S.; Kierstein, S.; Wu, H.F.; Eid, G.; Haczku, A.; Corrigan, C.J.; Ying, S. Resistin-like molecule-beta (RELM-beta) targets airways fibroblasts to effect remodelling in asthma: From mouse to man. Clin. Exp. Allergy 2015, 45, 940–952. [Google Scholar] [CrossRef]
- Trivedi, S.G.; Lloyd, C.M. Eosinophils in the pathogenesis of allergic airways disease. Cell. Mol. Life Sci. 2007, 64, 1269–1289. [Google Scholar] [CrossRef]
- Ayars, G.H.; Altman, L.C.; Gleich, G.J.; Loegering, D.A.; Baker, C.B. Eosinophil- and eosinophil granule-mediated pneumocyte injury. J. Allergy Clin. Immunol. 1985, 76, 595–604. [Google Scholar] [CrossRef]
- Ohno, I.; Nitta, Y.; Yamauchi, K.; Hoshi, H.; Honma, M.; Woolley, K.; O’Byrne, P.; Tamura, G.; Jordana, M.; Shirato, K.; et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am. J. Respir. Cell Mol. Biol. 1996, 15, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Mathur, S.K.; Espenshade, B.M.; Mori, Y.; Varga, J.; Ackerman, S.J. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: Implications in fibrogenesis. J. Allergy Clin. Immunol. 2005, 116, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Dolgachev, V.; Berlin, A.A.; Lukacs, N.W. Eosinophil activation of fibroblasts from chronic allergen-induced disease utilizes stem cell factor for phenotypic changes. Am. J. Pathol. 2008, 172, 68–76. [Google Scholar] [CrossRef]
- Esnault, S.; Johansson, M.W.; Kelly, E.A.; Koenderman, L.; Mosher, D.F.; Jarjour, N.N. IL-3 up-regulates and activates human eosinophil CD32 and alphaMbeta2 integrin causing degranulation. Clin. Exp. Allergy 2017, 47, 488–498. [Google Scholar] [CrossRef]
- Terada, M.; Kelly, E.A.; Jarjour, N.N. Increased thrombin activity after allergen challenge: A potential link to airway remodeling? Am. J. Respir. Crit. Care Med. 2004, 169, 373–377. [Google Scholar] [CrossRef]
- Kelly, E.A.; Esnault, S.; Liu, L.Y.; Evans, M.D.; Johansson, M.W.; Mathur, S.; Mosher, D.F.; Denlinger, L.C.; Jarjour, N.N. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Kelly, E.A.; Sorkness, R.L.; Evans, M.D.; Busse, W.W.; Jarjour, N.N. Airway factor XIII associates with type 2 inflammation and airway obstruction in asthmatic patients. J. Allergy Clin. Immunol. 2016, 137, 767–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esnault, S.; Khosravi, M.; Kelly, E.A.; Liu, L.Y.; Bochkov, Y.A.; Tattersall, M.C.; Jarjour, N.N. Increased IL-6 and Potential IL-6 trans-signalling in the airways after an allergen challenge. Clin. Exp. Allergy 2021. [Google Scholar] [CrossRef]
- Sandbo, N.; Ngam, C.; Torr, E.; Kregel, S.; Kach, J.; Dulin, N. Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of Mdiaj. Biol. Chem. 2013, 288, 15466–15473. [Google Scholar] [CrossRef] [PubMed]
- Bernau, K.; Ngam, C.; Torr, E.E.; Acton, B.; Kach, J.; Dulin, N.O.; Sandbo, N. Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis. Respir. Res. 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Johnson, S.H.; DeLain, L.P.; Haedt, M.J.; Noll, A.L.; Sandbo, N.; Jarjour, N.N. Matrix metalloproteinase-9-dependent release of IL-1beta by human eosinophils. Mediat. Inflamm. 2019, 2019, 7479107. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Nettenstrom, L.M.; Cook, E.B.; Seroogy, C.M.; Jarjour, N.N. Human eosinophils release IL-1ss and increase expression of IL-17A in activated CD4 + T lymphocytes. Clin. Exp. Allergy 2012, 42, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Kelly, E.A.; Schwantes, E.A.; Liu, L.Y.; DeLain, L.P.; Hauer, J.A.; Bochkov, Y.A.; Denlinger, L.C.; Malter, J.S.; Mathur, S.K.; et al. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PLoS ONE 2013, 8, e67560. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, N.; Kimber, I.; Rothwell, N.J.; Pinteaux, E. Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. Br. J. Pharm. 2008, 153, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Weller, P.F.; Rand, T.H.; Barrett, T.; Elovic, A.; Wong, D.T.; Finberg, R.W. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J. Immunol. 1993, 150, 2554–2562. [Google Scholar]
- Chen, C.J.; Kono, H.; Golenbock, D.; Reed, G.; Akira, S.; Rock, K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007, 13, 851–856. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.J.; Filer, A.; Buckley, C.D. The role of stromal cells in the persistence of chronic inflammation. Clin. Exp. Immunol. 2013, 171, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrod, T.; Park, J.H.; Harder, J.; Iwakura, Y.; Nunez, G. Cutting edge: Critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J. Immunol. 2008, 181, 8194–8198. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Kelly, E.A.; Dodge, A.M.; McCartney, J.G.; Meyer, K.C.; Cornwell, R.D.; Jackson, M.J.; Evans, M.D.; Jarjour, N.N. Safety of and cellular response to segmental bronchoprovocation in allergic asthma. PLoS ONE 2013, 8, e51963. [Google Scholar] [CrossRef]
- Kimura, H.; Inukai, Y.; Takii, T.; Furutani, Y.; Shibata, Y.; Hayashi, H.; Sakurada, S.; Okamoto, T.; Inoue, J.; Oomoto, Y.; et al. Molecular analysis of constitutive IL-1alpha gene expression in human melanoma cells: Autocrine stimulation through NF-kappaB activation by endogenous IL-1alpha. Cytokine 1998, 10, 872–879. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; McCarthy, S.A.; Watkins, S.C.; Wright, T.M. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J. Rheumatol. 2004, 31, 1946–1954. [Google Scholar]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernau, K.; Leet, J.P.; Floerke, H.; Bruhn, E.M.; Noll, A.L.; McDermott, I.S.; Esnault, S.; Jarjour, N.N.; Sandbo, N. Interleukin-1α Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation. Cells 2021, 10, 528. https://doi.org/10.3390/cells10030528
Bernau K, Leet JP, Floerke H, Bruhn EM, Noll AL, McDermott IS, Esnault S, Jarjour NN, Sandbo N. Interleukin-1α Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation. Cells. 2021; 10(3):528. https://doi.org/10.3390/cells10030528
Chicago/Turabian StyleBernau, Ksenija, Jonathan P. Leet, Heather Floerke, Ellen M. Bruhn, Andrea L. Noll, Ivy S. McDermott, Stephane Esnault, Nizar N. Jarjour, and Nathan Sandbo. 2021. "Interleukin-1α Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation" Cells 10, no. 3: 528. https://doi.org/10.3390/cells10030528
APA StyleBernau, K., Leet, J. P., Floerke, H., Bruhn, E. M., Noll, A. L., McDermott, I. S., Esnault, S., Jarjour, N. N., & Sandbo, N. (2021). Interleukin-1α Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation. Cells, 10(3), 528. https://doi.org/10.3390/cells10030528







