Regulation of Eosinophilia in Asthma—New Therapeutic Approaches for Asthma Treatment
Abstract
1. Introduction
2. Glucocorticosteroids
3. Targeting T2 Cytokines
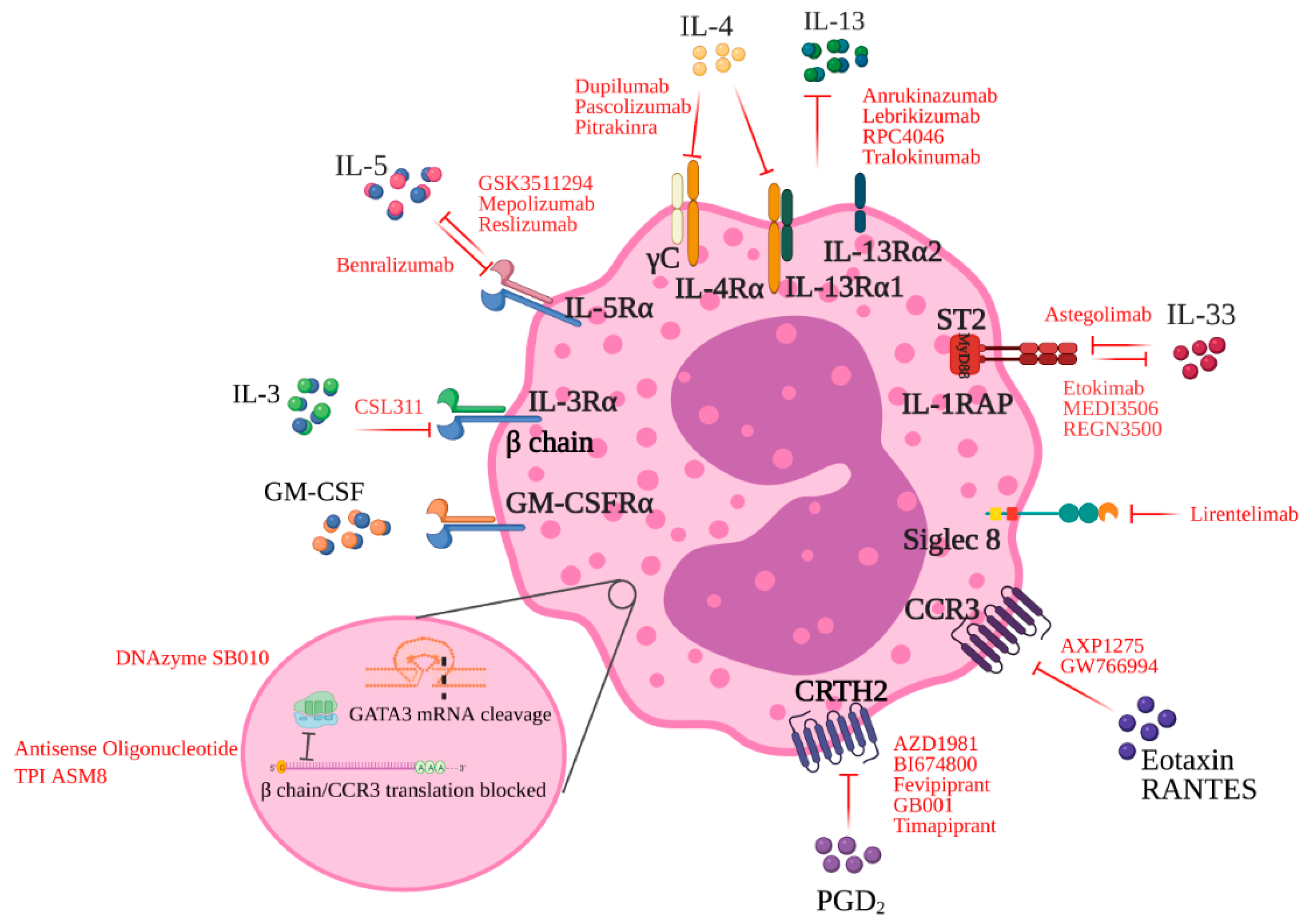
3.1. The IL-3/5/GM-CSF Axis
3.2. IL-4 and IL-13 Blockade
4. Targeting Specific Receptors on Eosinophils
4.1. CCR3 Blockade
4.2. CCR3 and Common β-Chain Blockade
4.3. CRTH2 Antagonism
4.4. Regulation of Eosinophil Apoptosis
5. Targeting Upstream Pathways of T2 Cytokines
5.1. GATA-3 DNAzyme
5.2. Anti-TSLP
5.3. Anti-IL-33
5.4. Targeting ILC2s
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Schuijs, M.J.; Willart, M.A.; Hammad, H.; Lambrecht, B.N. Cytokine targets in airway inflammation. Curr. Opin. Pharmacol. 2013, 13, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Blumenreich, M.S. The White Blood Cell and Differential Count. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Gauvreau, G.M.; Watson, R.M.; O’Byrne, P.M. Kinetics of allergen-induced airway eosinophilic cytokine production and airway inflammation. Am. J. Respir. Crit. Care Med. 1999, 160, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Chabra, R.; Gupta, M. Allergic and Environmental Induced Asthma; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.; Fabbri, L.M.; Pavord, I.D.; Papi, A.; Petruzzelli, S.; Lange, P. Asthma progression and mortality: The role of inhaled corticosteroids. Eur. Respir. J. 2019, 54, 1900491. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Ritondo, B.L.; Puxeddu, E.; Pane, G.; Cazzola, M.; Calzetta, L. Experimental Glucocorticoid Receptor Agonists for the Treatment of Asthma: A Systematic Review. J. Exp. Pharmacol. 2020, 12, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, D.K.; Mandlik, S.K. New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol. Immunotoxicol. 2020, 42, 59–73. [Google Scholar] [CrossRef]
- Giembycz, M.A.; Lindsay, M.A. Pharmacology of the eosinophil. Pharmacol. Rev. 1999, 51, 213–340. [Google Scholar]
- Shalit, M.; Sekhsaria, S.; Malech, H.L. Modulation of growth and differentiation of eosinophils from human peripheral blood CD34+ cells by IL5 and other growth factors. Cell Immunol. 1995, 160, 50–57. [Google Scholar] [CrossRef]
- Guida, L.; O’Hehir, R.E.; Hawrylowicz, C.M. Synergy between dexamethasone and interleukin-5 for the induction of major histocompatibility complex class II expression by human peripheral blood eosinophils. Blood 1994, 84, 2733–2740. [Google Scholar] [CrossRef]
- Tomioka, K.; MacGlashan, D.W., Jr.; Lichtenstein, L.M.; Bochner, B.S.; Schleimer, R.P. GM-CSF regulates human eosinophil responses to F-Met peptide and platelet activating factor. J. Immunol. 1993, 151, 4989–4997. [Google Scholar]
- Lamas, A.M.; Marcotte, G.V.; Schleimer, R.P. Human endothelial cells prolong eosinophil survival. Regulation by cytokines and glucocorticoids. J. Immunol. 1989, 142, 3978–3984. [Google Scholar]
- Lamas, A.M.; Leon, O.G.; Schleimer, R.P. Glucocorticoids inhibit eosinophil responses to granulocyte-macrophage colony-stimulating factor. J. Immunol. 1991, 147, 254–259. [Google Scholar] [PubMed]
- Cox, G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J. Immunol. 1995, 154, 4719–4725. [Google Scholar] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.E.; Barnes, P.J. Defective phagocytosis in airways disease. Chest 2012, 141, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ishmael, F.T.; Fang, X.; Galdiero, M.R.; Atasoy, U.; Rigby, W.F.C.; Gorospe, M.; Cheadle, C.; Stellato, C. Role of the RNA-binding protein tristetraprolin in glucocorticoid-mediated gene regulation. J. Immunol. 2008, 180, 8342–8353. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. Lung dendritic cells in respiratory viral infection and asthma: From protection to immunopathology. Annu. Rev. Immunol. 2012, 30, 243–270. [Google Scholar] [CrossRef]
- Ito, K.; Yamamura, S.; Essilfie-Quaye, S.; Cosio, B.; Ito, M.; Barnes, P.J.; Adcock, I.M. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J. Exp. Med. 2006, 203, 7–13. [Google Scholar] [CrossRef]
- Umland, S.P.; Schleimer, R.P.; Johnston, S.L. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm. Pharmacol. Ther. 2002, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.M.; Leitch, A.E.; Riley, N.A.; Duffin, R.; Haslett, C.; Rossi, A.G. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol. Sci. 2008, 29, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc. Am. Thorac. Soc. 2004, 1, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Truong-Tran, Q.A.; Tancowny, B.; Harris, K.E.; Schleimer, R.P. Glucocorticoids enhance or spare innate immunity: Effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J. Immunol. 2007, 179, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Szefler, S.J.; Leung, D.Y.; Sloan, S.I.; Rex, M.D.; Martin, R.J. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am. J. Respir. Crit. Care Med. 1997, 156 Pt 1, 737–743. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Kupczyk, M.; Haque, S.; Middelveld, R.J.; Dahlén, B.; Dahlén, S.E. Phenotypic predictors of response to oral glucocorticosteroids in severe asthma. Respir. Med. 2013, 107, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Nanzer, A.M.; Pfeffer, P.E.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: Potential benefits of calcitriol. J. Allergy Clin. Immunol. 2015, 136, 628–637.e4. [Google Scholar] [CrossRef] [PubMed]
- Nabe, T. Steroid-Resistant Asthma and Neutrophils. Biol. Pharm. Bull. 2020, 43, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Boulet, L.-P.; Leigh, R.; Cockcroft, D.W.; Killian, K.J.; Davis, B.E.; Deschesnes, F.; Watson, R.M.; Swystun, V.; Mårdh, C.K.; et al. A nonsteroidal glucocorticoid receptor agonist inhibits allergen-induced late asthmatic responses. Am. J. Respir. Crit. Care Med. 2015, 191, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.F.; Williamson, D.J.; Gamble, J.R.; Begley, C.G.; Harlan, J.M.; Klebanoff, S.J.; Waltersdorph, A.; Wong, G.; Clark, S.C.; Vadas, M.A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J. Clin. Investig. 1986, 78, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.F.; Sanderson, C.J.; Gamble, J.R.; Campbell, H.D.; Young, I.G.; Vadas, M.A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J. Exp. Med. 1988, 167, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Owen, W.F.; Jr Silberstein, D.S.; Woods, J.; Soberman, R.J.; Austen, K.F.; Stevens, R.L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J. Clin. Investig. 1988, 81, 1986–1992. [Google Scholar] [CrossRef]
- Mori, Y.; Iwasaki, H.; Kohno, K.; Yoshimoto, G.; Kikushige, Y.; Okeda, A.; Uike, N.; Niiro, H.; Takenaka, K.; Nagafuji, K.; et al. Identification of the human eosinophil lineage-committed progenitor: Revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 2009, 206, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S.; Damia, R.; Zeibecoglou, K.; Molet, S.; North, J.; Yamada, T.; Barry Kay, A.; Hamid, Q. CD34(+)/interleukin-5Ralpha messenger RNA+ cells in the bronchial mucosa in asthma: Potential airway eosinophil progenitors. Am. J. Respir. Cell Mol. Biol. 1999, 20, 9–13. [Google Scholar] [CrossRef]
- Sehmi, R.; Baatjes, A.J.; Denburg, J.A. Hemopoietic progenitor cells and hemopoietic factors: Potential targets for treatment of allergic inflammatory diseases. Curr. Drug Targets Inflamm. Allergy 2003, 2, 271–278. [Google Scholar] [CrossRef]
- Hui, C.C.; McNagny, K.M.; Denburg, J.A.; Siracusa, M.C. In situ hematopoiesis: A regulator of TH2 cytokine-mediated immunity and inflammation at mucosal surfaces. Mucosal. Immunol. 2015, 8, 701–711. [Google Scholar] [CrossRef]
- Sehmi, R.; Howie, K.; Sutherland, D.R.; Schragge, W.; O’Byrne, P.M.; Denburg, J.A. Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. Am. J. Respir. Cell Mol. Biol. 1996, 15, 645–655. [Google Scholar] [CrossRef]
- Shen, Z.J.; Malter, J.S. Determinants of eosinophil survival and apoptotic cell death. Apoptosis 2015, 20, 224–234. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Shen, Z.J.; Johansson, M.W.; Malter, J.S.; Jarjour, N.N. IL-3 Maintains Activation of the p90S6K/RPS6 Pathway and Increases Translation in Human Eosinophils. J. Immunol. 2015, 195, 2529–2539. [Google Scholar] [CrossRef]
- Tai, P.C.; Sun, L.; Spry, C.J. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin. Exp. Immunol. 1991, 85, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Abu-Ghazaleh, R.; Kita, H.; Sanderson, C.J.; Gleich, G.J. Regulatory effect of cytokines on eosinophil degranulation. J. Immunol. 1990, 144, 642–646. [Google Scholar] [PubMed]
- Horie, S.; Gleich, G.J.; Kita, H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J. Allergy Clin. Immunol. 1996, 98, 371–381. [Google Scholar] [CrossRef]
- Reimert, C.M.; Skov, P.S.; Poulsen, L.K. A microtiter assay for activation of eosinophils. Simultaneous monitoring of eosinophil adhesion and degranulation. Allergy 1998, 53, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Johansson, M.W.; Kelly, E.A.; Koenderman, L.; Mosher, D.F.; Jarjour, N.N. IL-3 up-regulates and activates human eosinophil CD32 and αMβ2 integrin causing degranulation. Clin. Exp. Allergy 2017, 47, 488–498. [Google Scholar] [CrossRef]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.; Weller, P.F. Eosinophil secretion of granule-derived cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Martinez-Moczygemba, M.; Huston, D.P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J. Allergy Clin. Immunol. 2003, 112, 653–665, quiz 66. [Google Scholar]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Puggioni, F.; Passalacqua, G.; Canonica, G.W. Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma. Front. Med. 2017, 4, 135. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Virchow, J.C.; Murphy, K.; Maspero, J.F.; Jacobs, J.; Adir, Y.; Humbert, M.; Castro, M.; Marsteller, D.A.; McElhattan, J.; et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: Results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2020, 8, 461–474. [Google Scholar] [PubMed]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar] [CrossRef]
- Mukherjee, M.; Lim, H.F.; Thomas, S.; Miller, D.; Kjarsgaard, M.; Tan, B.; Sehmi, R.; Khalidi, N.; Nair, P. Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy. Allergy Asthma Clin. Immunol. 2017, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, A.; Trikha, A.; Calhoun, W.J. Benralizumab--a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity—A novel approach for the treatment of asthma. Expert Opin. Biol. Ther. 2012, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Nair, P.; Barker, P.; Goldman, M. Glucocorticoid Sparing of Benralizumab in Asthma. N. Engl. J. Med. 2017, 377, 1205. [Google Scholar] [PubMed]
- Ferguson, G.T.; FitzGerald, J.M.; Bleecker, E.R.; Laviolette, M.; Bernstein, D.; LaForce, C.; Mansfield, L.; Barker, P.; Wu, Y.; Jison, M.; et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2017, 5, 568–576. [Google Scholar] [CrossRef]
- Busse, W.; Chupp, G.; Nagase, H.; Albers, F.C.; Doyle, S.; Shen, Q.; Bratton, D.J.; Gunsoy, N.B. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J. Allergy Clin. Immunol. 2019, 143, 190–200.e20. [Google Scholar] [CrossRef]
- Bourdin, A.; Husereau, D.; Molinari, N.; Golam, S.; Siddiqui, M.K.; Lindner, L.; Xu, X. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: A systematic review. Eur. Respir. J. 2018, 52, 1801393. [Google Scholar] [CrossRef] [PubMed]
- Asquith, K.L.; Ramshaw, H.S.; Hansbro, P.M.; Beagley, K.W.; Lopez, A.F.; Foster, P.S. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J. Immunol. 2008, 180, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Panousis, C.; Dhagat, U.; Edwards, K.M.; Rayzman, V.; Hardy, M.P.; Braley, H.; Gauvreau, G.M.; Hercus, T.R.; Smith, S.; Sehmi, R.; et al. CSL311, a novel, potent, therapeutic monoclonal antibody for the treatment of diseases mediated by the common β chain of the IL-3, GM-CSF and IL-5 receptors. MAbs 2016, 8, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Wilson, N.J.; Pant, H.; Brown, C.L.; Busfield, S.; Ng, M.; Alhamdoosh, M.; Woodman, N.; Schembri, M.; Tumes, D.J.; et al. Anti-β(c) mAb CSL311 inhibits human nasal polyp pathophysiology in a humanized mouse xenograft model. Allergy 2020, 75, 475–478. [Google Scholar] [CrossRef]
- Junttila, I.S.; Mizukami, K.; Dickensheets, H.; Meier-Schellersheim, M.; Yamane, H.; Donnelly, R.P.; Paul, W.E. Tuning sensitivity to IL-4 and IL-13: Differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J. Exp. Med. 2008, 205, 2595–2608. [Google Scholar] [CrossRef]
- Minty, A.; Chalon, P.; Derocq, J.M.; Dumont, X.; Guillemot, J.C.; Kaghad, M.; Labit, C.; Leplatois, P.; Liauzun, P.; Miloux, B.; et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 1993, 362, 248–250. [Google Scholar] [CrossRef]
- Zurawski, G.; de Vries, J.E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 1994, 15, 19–26. [Google Scholar] [CrossRef]
- Corry, D.B. IL-13 in allergy: Home at last. Curr. Opin. Immunol. 1999, 11, 610–614. [Google Scholar] [CrossRef]
- Burd, P.R.; Thompson, W.C.; Max, E.E.; Mills, F.C. Activated mast cells produce interleukin 13. J. Exp. Med. 1995, 181, 1373–1380. [Google Scholar] [CrossRef]
- Li, H.; Sim, T.C.; Alam, R. IL-13 released by and localized in human basophils. J. Immunol. 1996, 156, 4833–4838. [Google Scholar]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef]
- Luttmann, W.; Knoechel, B.; Foerster, M.; Matthys, H.; Virchow, J.C.; Jr Kroegel, C. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J. Immunol. 1996, 157, 1678–1683. [Google Scholar]
- Kalayci, O.; Sonna, L.A.; Woodruff, P.G.; Camargo, C.A.; Luster, A.D., Jr.; Lilly, C.M. Monocyte chemotactic protein-4 (MCP-4; CCL-13): A biomarker of asthma. J. Asthma 2004, 41, 27–33. [Google Scholar] [CrossRef]
- Leung, T.F.; Wong, C.K.; Chan, I.H.; Ip, W.K.; Lam, C.W.; Wong, G.W. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J. Allergy Clin. Immunol. 2002, 110, 404–409. [Google Scholar] [CrossRef]
- Schmid-Grendelmeier, P.; Altznauer, F.; Fischer, B.; Bizer, C.; Straumann, A.; Menz, G.; Blaser, K.; Wüthrich, B.; Simon, H.U. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J. Immunol. 2002, 169, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Boulet, L.-P.; Cockcroft, D.W.; Fitzgerald, J.M.; Carlsten, C.; Davis, B.E.; Deschesnes, F.; Duong, M.; Durn, B.L.; Howie, K.J. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am. J. Respir. Crit. Care Med. 2011, 183, 1007–1014. [Google Scholar] [CrossRef]
- Wenzel, S.; Wilbraham, D.; Fuller, R.; Getz, E.B.; Longphre, M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: Results of two phase 2a studies. Lancet 2007, 370, 1422–1431. [Google Scholar] [CrossRef]
- Scheerens, H.; Arron, J.R.; Zheng, Y.; Putnam, W.S.; Erickson, R.W.; Choy, D.F.; Harris, J.M.; Lee, J.; Jarjour, N.N.; Matthews, J.G. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin. Exp. Allergy 2014, 44, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.D.; Gonzalez Edick, M.; Ferrando, R.E.; Solon, M.; Baca, M.; Mesh, K.; Bradding, P.; Gauvreau, G.M.; Sumino, K.; FitzGerald, J.M. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin. Exp. Allergy 2020, 50, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Brusselle, G.G.; Korn, S.; Kuna, P.; Magnan, A.; Cohen, D.; Bowen, K.; Piechowiak, T.; Wang, M.M.; Colice, G. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur. Respir. J. 2019, 53, 1800948. [Google Scholar] [CrossRef]
- Russell, R.J.; Chachi, L.; FitzGerald, J.M.; Backer, V.; Olivenstein, R.; Titlestad, I.L.; Ulrik, C.S.; Harrison, T.; Singh, D.; Chaudhuri, R. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): A multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir. Med. 2018, 6, 499–510. [Google Scholar] [CrossRef]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Pavord, I.D.; Siddiqui, S.; Papi, A.; Corren, J.; Sher, L.D.; Bardin, P.; Langton, D.; Park, H.S.; Rice, M.S.; Deniz, Y. Dupilumab Efficacy in Patients Stratified by Baseline Treatment Intensity and Lung Function. J. Asthma Allergy 2020, 13, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, A.; Papi, A.A.; Corren, J.; Virchow, J.C.; Rice, M.S.; Deniz, Y.; Djandji, M.; Rowe, P.; Pavord, I.D. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy 2021, 76, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Ponath, P.D.; Qin, S.; Ringler, D.J.; Clark-Lewis, I.; Wang, J.; Kassam, N.; Smith, H.; Shi, X.; Gonzalo, J.A.; Newman, W. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J. Clin. Investig. 1996, 97, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Humbles, A.A.; Lu, B.; Friend, D.S.; Okinaga, S.; Lora, J.; Al-Garawi, A.; Martin, T.R.; Gerard, N.P.; Gerard, C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc. Natl. Acad. Sci. USA 2002, 99, 1479–1484. [Google Scholar] [CrossRef]
- Garcia-Zepeda, E.A.; Rothenberg, M.E.; Ownbey, R.T.; Celestin, J.; Leder, P.; Luster, A.D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 1996, 2, 449–456. [Google Scholar] [CrossRef]
- Fulkerson, P.C.; Fischetti, C.A.; McBride, M.L.; Hassman, L.M.; Hogan, S.P.; Rothenberg, M.E. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl. Acad. Sci. USA 2006, 103, 16418–16423. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Hassanshahi, G.; Khorramdelazad, H.; Zainodini, N.; Koochakzadeh, L. An Overlook to the Characteristics and Roles Played by Eotaxin Network in the Pathophysiology of Food Allergies: Allergic Asthma and Atopic Dermatitis. Inflammation 2016, 39, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; El-Gammal, A.I.; O’Byrne, P.M. Allergen-induced airway responses. Eur. Respir. J. 2015, 46, 819–831. [Google Scholar] [CrossRef]
- Boulet, L.P.; Gauvreau, G.; Boulay, M.E.; O’Byrne, P.; Cockcroft, D.W.; Clinical Investigative Collaboration. The allergen bronchoprovocation model: An important tool for the investigation of new asthma anti-inflammatory therapies. Allergy 2007, 62, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; FitzGerald, J.M.; Boulet, L.P.; Watson, R.M.; Hui, L.; Villineuve, H.; Scime, T.X.; Schlatman, A.R.; Obminski, C.; Kum, J. The effects of a CCR3 inhibitor, AXP1275, on allergen-induced airway responses in adults with mild-to-moderate atopic asthma. Clin. Exp. Allergy 2018, 48, 445–451. [Google Scholar] [CrossRef]
- Neighbour, H.; Boulet, L.P.; Lemiere, C.; Sehmi, R.; Leigh, R.; Sousa, A.R.; Martin, J.; Dallow, N.; Gilbert, J.; Allen, A. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 2014, 44, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Boulet, L.P.; Cockcroft, D.W.; Baatjes, A.; Cote, J.; Deschesnes, F.; Davis, B.; Strinich, T.; Howie, K.; Duong, M. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am. J. Respir. Crit. Care Med. 2008, 177, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Pageau, R.; Séguin, R.; Carballo, D.; Gauthier, J.; D’Anjou, H.; Campbell, H.; Watson, R.; Mistry, M.; Parry-Billings, M. Dose-response effects of TPI ASM8 in asthmatics after allergen. Allergy 2011, 66, 1242–1248. [Google Scholar] [CrossRef]
- Song, D.J.; Shim, M.H.; Lee, N.; Yoo, Y.; Choung, J.T. CCR3 Monoclonal Antibody Inhibits Eosinophilic Inflammation and Mucosal. Injury in a Mouse Model of Eosinophilic Gastroenteritis. Allergy Asthma Immunol. Res. 2017, 9, 360–367. [Google Scholar] [CrossRef]
- Shen, H.H.; Xu, F.; Zhang, G.S.; Wang, S.B.; Xu, W.H. CCR3 monoclonal antibody inhibits airway eosinophilic inflammation and mucus overproduction in a mouse model of asthma. Acta Pharmacol. Sin. 2006, 27, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Tanaka, K.; Yoshie, O.; Ogawa, K.; Kenmotsu, K.; Takamori, Y.; Ichimasa, M.; Sugamura, K.; Nakamura, M.; Takano, S. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001, 193, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Barrow, A.; Pettipher, R. Interaction between prostaglandin D and chemoattractant receptor-homologous molecule expressed on Th2 cells mediates cytokine production by Th2 lymphocytes in response to activated mast cells. Clin. Exp. Immunol. 2009, 156, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, N.W.; Berlin, A.A.; Franz-Bacon, K.; Sásik, R.; Sprague, L.J.; Ly, T.W.; Hardiman, G.; Boehme, S.A.; Bacon, K.B. CRTH2 antagonism significantly ameliorates airway hyperreactivity and downregulates inflammation-induced genes in a mouse model of airway inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L767–L779. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Pavord, I.; Chuchalin, A.; Bell, J.; Hunter, M.; Lewis, T.; Parker, D.; Payton, M.; Collins, L.P.; Pettipher, R. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin. Exp. Allergy 2012, 42, 38–48. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Hopkins, R.; Saunders, M.; Chantry, D.; Anderson, L.; Aitchison, R.; Eberhardt, C.; Bell, S.; Cole, J.; Wolfe, J.; et al. Safety and efficacy of ARRY-502, a potent, selective, Oral CRTh2 antagonist, in patients with mild to moderate Th2-driven asthma. J. Allergy Clin. Immunol. 2014, 133, AB4. [Google Scholar] [CrossRef]
- Gonem, S.; Berair, R.; Singapuri, A.; Hartley, R.; Laurencin, M.F.M.; Bacher, G.; Holzhauer, B.; Bourne, M.; Mistry, V.; Pavord, I.D. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: A single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 699–707. [Google Scholar] [CrossRef]
- Brightling, C.E.; Gaga, M.; Inoue, H.; Li, J.; Maspero, J.; Wenzel, S.; Maitra, S.; Lawrence, D.; Brockhaus, F.; Lehmann, T. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): Two phase 3 randomised controlled trials. Lancet Respir. Med. 2021, 9, 43–56. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Chang, A.T.; Youngblood, B.A.; Bochner, B.S. Eosinophil and mast cell Siglecs: From biology to drug target. J. Leukoc Biol. 2020, 108, 73–81. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Brock, E.C.; Leung, J.; Falahati, R.; Bryce, P.J.; Bright, J.; Williams, J.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int. Arch. Allergy Immunol. 2019, 180, 91–102. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Leung, J.; Falahati, R.; Williams, J.; Schanin, J.; Brock, E.C.; Singh, B.; Chang, A.T.; O’Sullivan, J.A.; Schleimer, R.P. Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Gonzalez, J.R.; Schanin, J.; Peters, M.C.; Lambrecht, B.N.; Brock, E.C.; Charbit, A.; Ansel, K.M.; Youngblood, B.A.; Fahy, J.V. An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin. Exp. Allergy 2020, 50, 904–914. [Google Scholar] [CrossRef]
- Altrichter, S.; Staubach, P.; Pasha, M.; Rasmussen, H.; Singh, B.; Chang, A.; Bernstein, J.; Siebenhaar, F.; Maurer, M. Efficacy and safety data of AK002, an anti-Siglec-8 monoclonal antibody, in patients with multiple forms of uncontrolled chronic urticaria (CU): Results from an open-label phase 2a study. Allergy 2019, 74, 117–129. [Google Scholar]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Sel, S.; Wegmann, M.; Dicke, T.; Henke, W.; Yildirim, A.O.; Renz, H.; Garn, H. Effective prevention and therapy of experimental allergic asthma using a GATA-3-specific DNAzyme. J. Allergy Clin. Immunol. 2008, 121, 910–916.e5. [Google Scholar] [CrossRef]
- Ghonim, M.A.; Pyakurel, K.; Ju, J.; Rodriguez, P.C.; Lammi, M.R.; Davis, C.; Abughazleh, M.Q.; Mansy, M.S.; Naura, A.S.; Boulares, A.H. DNA-dependent protein kinase inhibition blocks asthma in mice and human endothelial and CD4+ T-cell function without causing severe combined immunodeficiency. J. Allergy Clin. Immunol. 2015, 135, 425–440. [Google Scholar] [CrossRef]
- Klein-Hessling, S.; Jha, M.K.; Santner-Nanan, B.; Berberich-Siebelt, F.; Baumruker, T.; Schimpl, A.; Serfling, E. Protein kinase A regulates GATA-3-dependent activation of IL-5 gene expression in Th2 cells. J. Immunol. 2003, 170, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ghaffar, O.; Olivenstein, R.; Taha, R.A.; Soussi-Gounni, A.; Zhang, D.H.; Ray, A.; Hamid, Q. Gene expression of the GATA-3 transcription factor is increased in atopic asthma. J. Allergy Clin. Immunol. 1999, 103 Pt 1, 215–222. [Google Scholar] [CrossRef]
- Erpenbeck, V.J.; Hohlfeld, J.M.; Discher, M.; Krentel, H.; Hagenberg, A.; Braun, A.; Krug, N. Increased messenger RNA expression of c-maf and GATA-3 after segmental allergen challenge in allergic asthmatics. Chest 2003, 123 (Suppl. S3), 370s–371s. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.; Hamid, Q.; Cameron, L.; Olivenstein, R. T helper type 2 cytokine receptors and associated transcription factors GATA-3, c-MAF, and signal transducer and activator of transcription factor-6 in induced sputum of atopic asthmatic patients. Chest 2003, 123, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Erpenbeck, V.J.; Hagenberg, A.; Krentel, H.; Discher, M.; Braun, A.; Hohlfeld, J.M.; Krug, N. Regulation of GATA-3, c-maf and T-bet mRNA expression in bronchoalveolar lavage cells and bronchial biopsies after segmental allergen challenge. Int. Arch Allergy Immunol. 2006, 139, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Dicke, T.; Pali-Schöll, I.; Kaufmann, A.; Bauer, S.; Renz, H.; Garn, H. Absence of unspecific innate immune cell activation by GATA-3-specific DNAzymes. Nucleic Acid Ther. 2012, 22, 117–126. [Google Scholar] [CrossRef]
- Fuhst, R.; Runge, F.; Buschmann, J.; Ernst, H.; Praechter, C.; Hansen, T.; von Erichsen, J.; Turowska, A.; Hoymann, H.G.; Müller, M. Toxicity profile of the GATA-3-specific DNAzyme hgd40 after inhalation exposure. Pulm. Pharmacol. Ther. 2013, 26, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.; Lim, J.C.; Soo, H.L.; Stanslas, J. Molecularly targeted therapies for asthma: Current development, challenges and potential clinical translation. Pulm. Pharmacol. Ther. 2016, 40, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Renz, H. GATA-3-specific DNAzyme—A novel approach for stratified asthma therapy. Eur. J. Immunol. 2017, 47, 22–30. [Google Scholar] [CrossRef]
- Turowska, A.; Librizzi, D.; Baumgartl, N.; Kuhlmann, J.; Dicke, T.; Merkel, O.; Homburg, U.; Höffken, H.; Renz, H.; Garn, H. Biodistribution of the GATA-3-specific DNAzyme hgd40 after inhalative exposure in mice, rats and dogs. Toxicol. Appl. Pharmacol. 2013, 272, 365–372. [Google Scholar] [CrossRef]
- Krug, N.; Hohlfeld, J.M.; Kirsten, A.M.; Kornmann, O.; Beeh, K.M.; Kappeler, D.; Korn, S.; Ignatenko, S.; Timmer, W.; Rogon, C. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N. Engl. J. Med. 2015, 372, 1987–1995. [Google Scholar] [CrossRef]
- Greulich, T.; Hohlfeld, J.M.; Neuser, P.; Lueer, K.; Klemmer, A.; Schade-Brittinger, C.; Harnisch, S.; Garn, H.; Renz, H.; Homburg, U. A GATA3-specific DNAzyme attenuates sputum eosinophilia in eosinophilic COPD patients: A feasibility randomized clinical trial. Respir. Res. 2018, 19, 55. [Google Scholar] [CrossRef]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Wong, C.K.; Hu, S.; Cheung, P.F.; Lam, C.W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef]
- Shikotra, A.; Choy, D.F.; Ohri, C.M.; Doran, E.; Butler, C.; Hargadon, B.; Shelley, M.; Abbas, A.R.; Austin, C.D.; Jackman, J. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012, 129, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Hohlfeld, J.M.; Grant, S.; Jain, M.; Cabanski, M.; Pertel, P.; Boulet, L.P.; Cockcroft, D.W.; Davis, B.; Fitzgerald, J.M.; et al. Efficacy and Safety of an Inhaled Anti-TSLP Antibody Fragment in Adults with Mild Atopic Asthma. Am. J. Respir. Crit. Care Med. 2020, 201, A4207. [Google Scholar]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017, 8, 14937. [Google Scholar] [CrossRef]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Griffiths, J.; Hellqvist, Å.; Bowen, K.; Kaur, P.; Almqvist, G. Efficacy and Safety of Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma: Results from the Phase 3 NAVIGATOR Study. J. Allergy Clin. Immunol. 2021, 147, AB249. [Google Scholar]
- Bartemes, K.R.; Iijima, K.; Kobayashi, T.; Kephart, G.M.; McKenzie, A.N.; Kita, H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012, 188, 1503–1513. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, J.; Zhao, J.; Liu, F.; Chen, Y.; Bi, L.; Liu, S.; Dong, L. IL-33 promotes airway remodeling and is a marker of asthma disease severity. J. Asthma. 2014, 51, 863–869. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hur, J.; Kang, H.S.; Choi, J.Y.; Rhee, C.K.; Kang, J.Y.; Kim, Y.K.; Lee, S.Y. Blockade of thymic stromal lymphopoietin and CRTH2 attenuates airway inflammation in a murine model of allergic asthma. Korean J. Intern Med. 2020, 35, 619–629. [Google Scholar] [CrossRef]
- Wechsler, M.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; Nivens, C.; Amin, N.; et al. SAR440340, An Anti-IL-33 Monoclonal Antibody, Demonstrated a Significant Reduction of LOAC Events and Improved Pre-BD FEV1 in Patients with Moderate to Severe Asthma: Results from the Phase 2 Proof of Concept Study. Am. J. Respir. Cell Mol. Biol. 2020, 201, A4269. [Google Scholar]
- Londei, M.; Marquette-Hamoudi, A.; Phenis, K.; Pinkstaff, J.; Sacco, N.; Pavord, I.D. Single-dose Phase 2a trial of etokimab (anti-IL-33) in severe eosinophilic asthma. Allergy 2019, 74, 128. [Google Scholar]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kephart, G.M.; Fox, S.J.; Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 2014, 134, 671–678.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Fan, X.L.; Yu, Q.N.; Qin, Z.L.; Chen, D.; Xu, R.; Chen, D.H.; Lin, Z.B.; Wen, W.; Fu, Q.L. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin. Immunol. 2017, 183, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Mehta, H.; Bissonnette, R.; Sarfati, M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J. Dermatol. Sci. 2017, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Fakhri, S.; Citardi, M.J.; Porter, P.C.; Corry, D.B.; Kheradmand, F.; Liu, Y.J.; Luong, A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2013, 188, 432–439. [Google Scholar] [CrossRef]
- Kwon, B.I.; Hong, S.; Shin, K.; Choi, E.H.; Hwang, J.J.; Lee, S.H. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. Am. J. Respir. Crit. Care Med. 2013, 188, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.-P.; Lemiere, C.; Martin, J. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Smith, S.G.; Salter, B.; El-Gammal, A.; Oliveria, J.P.; Obminski, C.; Watson, R.; O’Byrne, P.M.; Gauvreau, G.M.; Sehmi, R. Allergen-induced Increases in Sputum Levels of Group 2 Innate Lymphoid Cells in Subjects with Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef]
- Wilhelm, C.; Hirota, K.; Stieglitz, B.; Van Snick, J.; Tolaini, M.; Lahl, K.; Sparwasser, T.; Helmby, H.; Stockinger, B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 2011, 12, 1071–1077. [Google Scholar] [CrossRef]
- Bal, S.M.; Bernink, J.H.; Nagasawa, M.; Groot, J.; Shikhagaie, M.M.; Golebski, K.; Van Drunen, C.M.; Lutter, R.; Jonkers, R.E.; Hombrink, P. IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 2016, 17, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.M.; Fallon, G.P.; Matthews, J.D.; Smith, P.; Jolin, E.H.; McKenzie, N.A. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 2000, 13, 573–583. [Google Scholar] [CrossRef]
| Mechanism of Action | Name | Dosing and Route | Adverse Events | Asthma Approval | Current Clinical Trial Phase | Findings | Other Populations Investigated | Ongoing Clinical Trials |
|---|---|---|---|---|---|---|---|---|
| Anti-IL-5: Binds to IL-5, preventing IL-5 from binding to the receptor on eosinophils | GSK3511294 | Long-acting SC injection | Unknown | N/A | 3 | 3 Phase 3 trials currently recruiting | Mild asthma—results pending | NCT04719832 NCT04718103 NCT04718389 |
| Mepolizumab | Fixed dose—100 mg SC every 4 weeks | Rarely causes hypersensitivity reactions Risk of herpes zoster infection | Uncontrolled severe eosinophilic asthma aged ≥6 years | - | ↓ Exacerbations ~50% ↓ OCS use Facilitates OCS weaning ↑ FEV1 | COPD EoE | NCT04075331 NCT03656380 | |
| Reslizumab | Weight adjusted dose—3 mg/kg IV infusion every 4 weeks | Black box warning: 0.3% of patients reported anaphylaxis | Uncontrolled severe eosinophilic asthma aged ≥18 years | - | ↓ Exacerbations ~50–60% ↑ FEV1 | EGPA | NCT02947945 | |
| Anti-IL-5R: Binds to the α subunit of the IL-5 receptor on eosinophils and basophils resulting in apoptosis | Benralizumab | Fixed dose—30 mg SC every 4 weeks for 12 weeks then every 8 weeks | Rarely causes hypersensitivity reactions | Uncontrolled severe eosinophilic asthma aged ≥12 years | - | ↓ Exacerbations ~ 50–70% ↓ OCS use Facilitates OCS weaning ↑ FEV1 | Atopic Dermatitis EoE | NCT03563066 NCT04543409 |
| Anti-IL-13: Binds to IL-13 cytokine at the binding site of the IL-13Rα receptors, preventing binding to IL-13Rα1 and α2. Lebrikizumab also blocks binding to IL-4R α. | Anrukinzumab | IV infusion every 2 weeks | No safety concerns in Phase 2 studies | N/A | - | N/A | Mild AAs—↓allergen induced FEV1 at Day 14 but not Day 35. Ulcerative Colitis | NCT01284062 |
| Lebrikizumab | Fixed dose—250 mg SC every 4 weeks | No safety concerns in a Phase 3 study | N/A | - | Inconsistent effect on AER across 2 phase 3 clinical trials ↑ FEV1 ↓ Feno | Atopic Dermatitis | NCT04250350 NCT04392154 | |
| RPC4046 | SC injection | Unknown in asthma population | N/A | 1 | N/A | EoE | NCT02098473 NCT04753697 | |
| Tralokinumab | Fixed dose—300 mg SC every 2 weeks | Increased risk of hyper-eosinophilia | N/A | 3 | Inconsistent effect on annualised AER across 2 phase 3 clinical trials | Atopic Dermatitis | NCT04556461 | |
| Anti-IL4/IL-13: Blocks the IL-4Rα where it binds to IL-4 and IL-13, blocking IL-4 and IL-13 signalling | Dupilumab | Age and weight based—200 or 300 mg SC every 2 weeks | Rarely causes hypersensitivity reactions Increased risk of injection site reactions | Uncontrolled severe eosinophilic asthma aged ≥12 years | - | ↓ Exacerbations ~50–60% ↓ OCS use Facilitates OCS weaning ↑ FEV1 | Peanut Allergy CRSsNP EoE Aspirin Intolerance | NCT03793608 NCT04362501 NCT03633617 NCT04442256 |
| Pascolizumab | Monthly IV infusion | Unknown | N/A | - | A Phase 2 pilot study in symptomatic steroid naïve asthma failed to show efficacy. | Further development terminated | NCT00024544 | |
| Pitrakinra | Inhalation or SC | No safety concerns in a Phase 2 study | N/A | - | A Phase 2b study found no significant difference in AER over placebo at any dose. | Further development terminated | NCT00801853 | |
| Anti-IL-33: Monoclonal IgG MAb that potently and specifically bind IL-33 | MEDI3506 | SC or IV | Unknown | N/A | 2 | Trial currently recruiting | COPD Atopic Dermatitis Diabetic Kidney Disease | NCT04570657 NCT04631016 NCT04212169 NCT04170543 |
| REGN3500 | SC every 2 weeks | No safety concerns in a Phase 2 study | N/A | 2 | ↓ LOAC compared to placebo, however dupilumab had a greater effect | COPD | NCT04701983 NCT04751487 | |
| Anti-ST2: MAb binds to ST2, the subunit of IL-33 receptor | Astegolimab | SC every 4 weeks | Unknown | N/A | 2b | Results awaited | Atopic Dermatitis COPD Covid-19 | NCT03747575 NCT03615040 NCT04386616 NCT02918019 |
| Anti-TSLP: Monoclonal antibody binds to TSLP preventing its interaction with its receptor | CSJ117 | Inhaled TSLP antibody fragment | Unknown | N/A | 2 | Trial currently recruiting | Mild AAs—↓ allergen induced responses | NCT04410523 |
| Tezepelumab | Fixed dose—210 mg every 4 weeks | Similar safety finding between tezepelumab and placebo * | N/A | 3 | ↓ Exacerbations * ↑ FEV1 * | COPD Severe Steroid Dependent Asthma | NCT04039113 NCT03406078 | |
| CRTh2: DP2 Antagonist | AZD1981 | Once or twice daily tablet | No safety concerns in Phase 2 studies | N/A | - | No change in FEV1 or asthma control | COPD | NCT00690482 |
| BI674800 | Inhaled twice daily | No safety concerns in Phase 2 studies | N/A | - | Inconsistent effect on FEV1 and ACQ across 2 phase 2 clinical trials | Further development terminated | NCT01090024 NCT01092143 | |
| Fevipiprant | Once daily tablet | No safety concerns in Phase 3 studies | N/A | 3 | AER—22% reduction in overall asthma population, 23% in eosinophil-high population | COPD—terminated | NCT03810183 | |
| GB001 | Once daily tablet | Unknown | N/A | 2b | Asthma worsening or AER—no benefit | CRSsNP CRSwNP | NCT03683576 NCT03956862 | |
| Timapiprant | Once daily tablet | No safety concerns in Phase 2 studies | 2 | No significant difference in sputum eosinophils or FEV1 | Moderate asthma—↓ sputum eosinophils, ↑ FEV1 Atopic dermatitis | NCT02660489 | ||
| GATA-3 DNAzyme: Specifically, and selectively targets GATA3 | SB010 | Inhalation once daily | No safety concerns in a small Phase 2a study | N/A | 2a | N/A | Mild AAs—↓allergen induced responses COPD | |
| βc receptor: blocking binding/synthesis of common βc receptor –of IL-3, GM-CSGF and IL-5 | CSL311 | Dose ascending study ongoing | Unknown | N/A | 1 | N/A | Mild Asthma | NCT04082754 |
| TPI ASM8 | Once daily inhalation | No safety concerns in a small Phase 2 studies | N/A | 2b | Attenuation of allergen-induced late asthmatic response | Further development terminated | NCT01158898 NCT00550797 NCT00822861 NCT00402948 | |
| Anti-Siglec 8: MAb binds to Siglec-8 inducing apoptosis of eosinophils | Lirentelimab | Monthly IV infusion | Unknown | N/A | - | - | EoE Chronic Urticaria | NCT04620811 NCT04322708 |
| Decreases eosinophil maturation | Dexpramipex-ole | Once daily tablet | Increased risk of neutropenia | N/A | 2 | Trial currently recruiting | CRSwNP Amyotrophic lateral sclerosis | NCT04046939 |
| JAK inhibitor | AZD0449 | Inhaled therapy | Unknown | N/A | 1 | N/A | Mild allergic asthma | NCT03766399 |
| Anti-CD4 MAb that induces Treg activation | Tregalizumab | SC injection | Unknown | N/A | 2 | N/A | Mild allergic asthma Rheumatoid arthritis | NCT04673591 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusack, R.P.; Whetstone, C.E.; Xie, Y.; Ranjbar, M.; Gauvreau, G.M. Regulation of Eosinophilia in Asthma—New Therapeutic Approaches for Asthma Treatment. Cells 2021, 10, 817. https://doi.org/10.3390/cells10040817
Cusack RP, Whetstone CE, Xie Y, Ranjbar M, Gauvreau GM. Regulation of Eosinophilia in Asthma—New Therapeutic Approaches for Asthma Treatment. Cells. 2021; 10(4):817. https://doi.org/10.3390/cells10040817
Chicago/Turabian StyleCusack, Ruth P., Christiane E. Whetstone, Yanqing Xie, Maral Ranjbar, and Gail M. Gauvreau. 2021. "Regulation of Eosinophilia in Asthma—New Therapeutic Approaches for Asthma Treatment" Cells 10, no. 4: 817. https://doi.org/10.3390/cells10040817
APA StyleCusack, R. P., Whetstone, C. E., Xie, Y., Ranjbar, M., & Gauvreau, G. M. (2021). Regulation of Eosinophilia in Asthma—New Therapeutic Approaches for Asthma Treatment. Cells, 10(4), 817. https://doi.org/10.3390/cells10040817






