Effect of Muscle Cell Preservation on Viability and Differentiation of Hamstring Tendon Graft In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Isolation and Culture of Human TDCs and MDCs
2.3. Co-Culture Assay
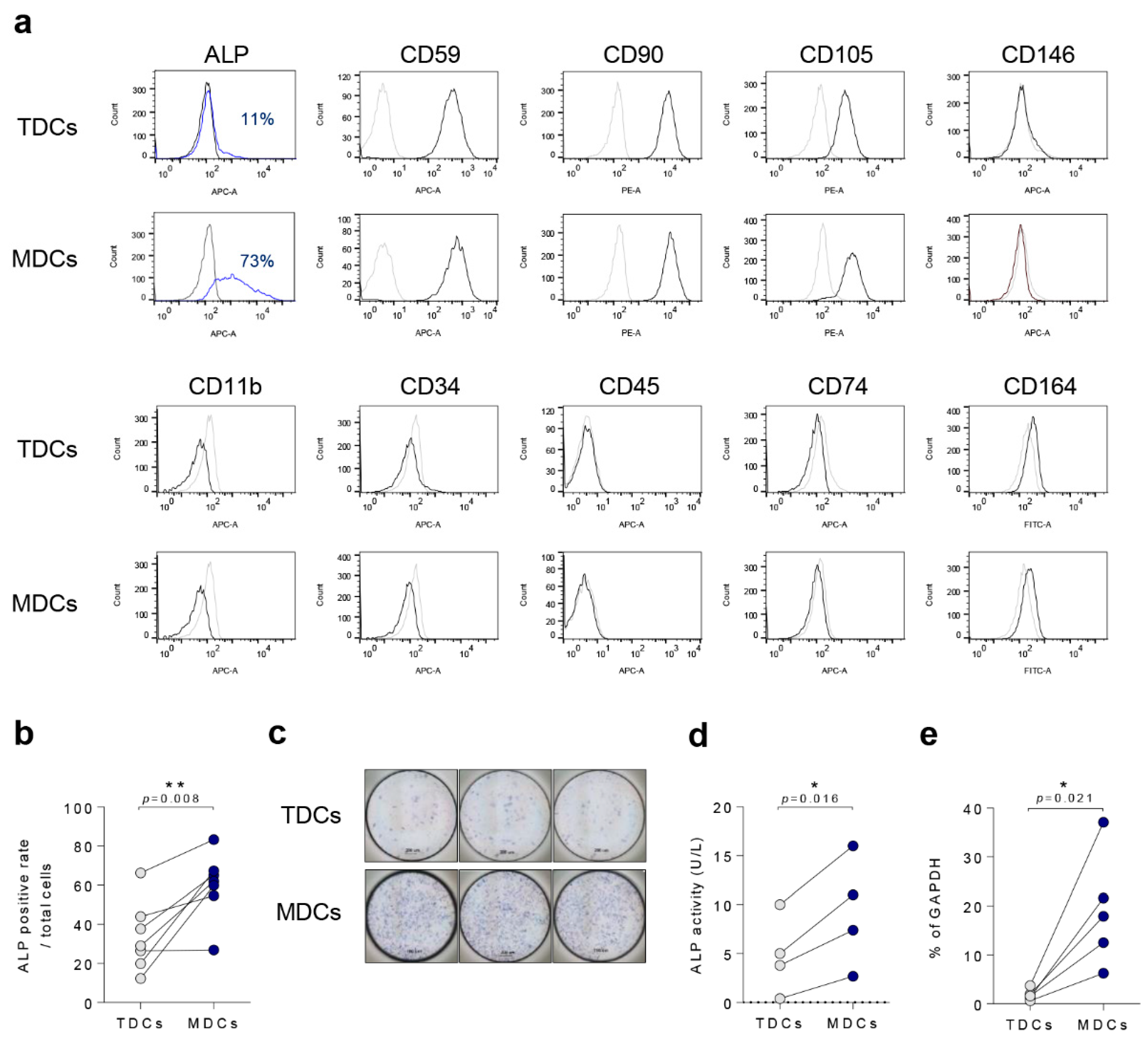
2.4. Flow Cytometry Analysis (FACS)
2.5. ALP Staining and Activity
2.6. Cell Viability Using Water-Soluble Tetrazolium Salts (WST) Assay
2.7. Ligamentous Differentiation and Activities
2.8. Total Collagen Assay
2.9. Osteogenic Differentiation and Activities
2.10. Chondrogenic Differentiation and Activities
2.11. RT-qPCR
2.12. Statistical Analyses
3. Results
3.1. Basal Characteristics of TDCs, MDCs, and Co-Cultured Cells
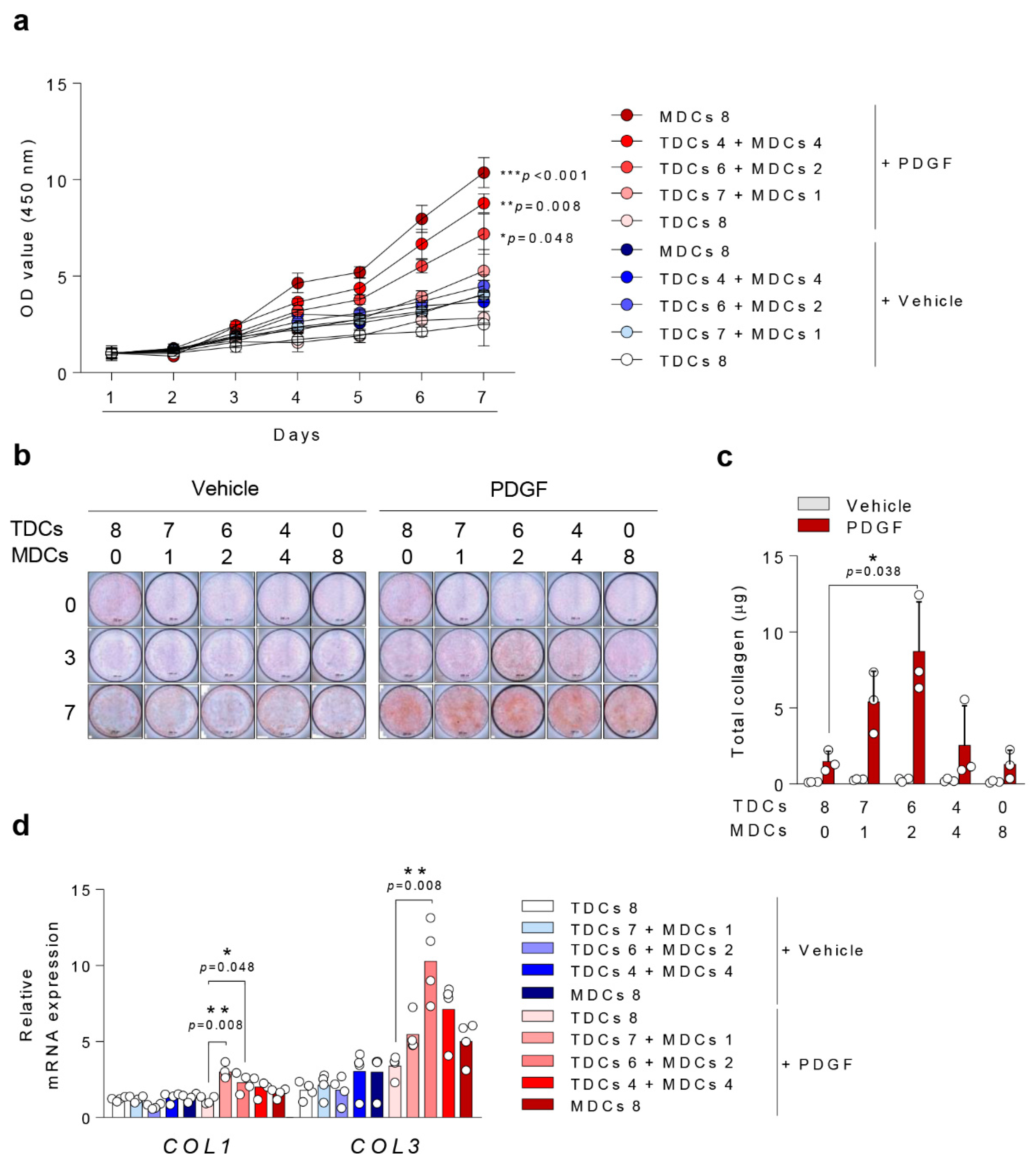
3.2. Cell Viability
3.3. Ligamentous Differentiation and Collagen Synthesis
3.4. Osteogenic Differentiation
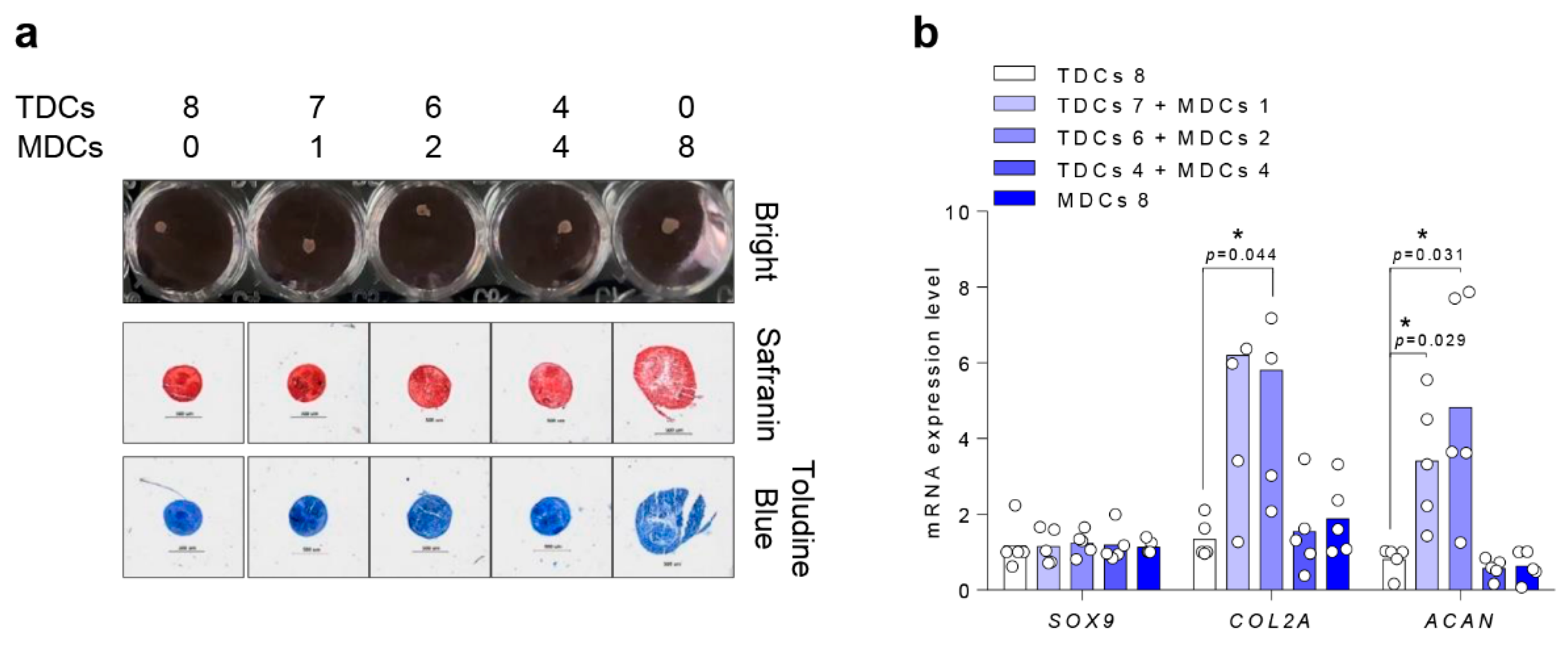
3.5. Chondrogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Duquin, T.R.; Wind, W.M.; Fineberg, M.S.; Smolinski, R.J.; Buyea, C.M. Current trends in anterior cruciate ligament reconstruction. J. Knee Surg. 2009, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. J. Bone Joint Surg. Am. 2000, 82, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Agung, M.; Ochi, M.; Yanada, S.; Adachi, N.; Izuta, Y.; Yamasaki, T.; Toda, K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1307–1314. [Google Scholar] [CrossRef]
- Menetrey, J.; Duthon, V.B.; Laumonier, T.; Fritschy, D. “Biological failure” of the anterior cruciate ligament graft. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Gale, T.; Sundaram, V.; Nagai, K.; Irrgang, J.J.; Anderst, W.; Nakashima, Y.; Tashman, S.; Fu, F.H. The Graft Bending Angle Can Affect Early Graft Healing After Anterior Cruciate Ligament Reconstruction: In Vivo Analysis With 2 Years’ Follow-up. Am. J. Sports Med. 2017, 45, 1829–1836. [Google Scholar] [CrossRef]
- Jansson, K.S.; Costello, K.E.; O’Brien, L.; Wijdicks, C.A.; Laprade, R.F. A historical perspective of PCL bracing. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1064–1070. [Google Scholar] [CrossRef]
- Samuelsson, K.; Andersson, D.; Ahlden, M.; Fu, F.H.; Musahl, V.; Karlsson, J. Trends in surgeon preferences on anterior cruciate ligament reconstructive techniques. Clin. Sports Med. 2013, 32, 111–126. [Google Scholar] [CrossRef]
- Lee, D.W.; Shim, J.C.; Yang, S.J.; Cho, S.I.; Kim, J.G. Functional Effects of Single Semitendinosus Tendon Harvesting in Anatomic Anterior Cruciate Ligament Reconstruction: Comparison of Single versus Dual Hamstring Harvesting. Clin. Orthop. Surg. 2019, 11, 60–72. [Google Scholar] [CrossRef]
- Kyung, H.S. Graft considerations for successful anterior cruciate ligament reconstruction. Knee Surg. Relat. Res. 2019, 31, 1. [Google Scholar] [CrossRef]
- Lu, H.; Chen, C.; Xie, S.; Tang, Y.; Qu, J. Tendon Healing in Bone Tunnel after Human Anterior Cruciate Ligament Reconstruction: A Systematic Review of Histological Results. J. Knee Surg. 2019, 32, 454–462. [Google Scholar] [CrossRef]
- Robert, H.; Es-Sayeh, J.; Heymann, D.; Passuti, N.; Eloit, S.; Vaneenoge, E. Hamstring insertion site healing after anterior cruciate ligament reconstruction in patients with symptomatic hardware or repeat rupture: A histologic study in 12 patients. Arthroscopy 2003, 19, 948–954. [Google Scholar] [CrossRef]
- Eriksson, K.; Kindblom, L.G.; Wredmark, T. Semitendinosus tendon graft ingrowth in tibial tunnel following ACL reconstruction: A histological study of 2 patients with different types of early graft failure. Acta Orthop. Scand. 2000, 71, 275–279. [Google Scholar] [CrossRef]
- Lazarides, A.L.; Eward, W.C.; Green, K.; Cardona, D.M.; Brigman, B.E.; Taylor, D.C. Histological Evaluation of Tendon-Bone Healing of an Anterior Cruciate Ligament Hamstring Graft in a 14-Year-Old Boy. Am. J. Sports Med. 2015, 43, 1935–1940. [Google Scholar] [CrossRef]
- Falconiero, R.P.; DiStefano, V.J.; Cook, T.M. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy 1998, 14, 197–205. [Google Scholar] [CrossRef]
- Sanchez, M.; Anitua, E.; Azofra, J.; Prado, R.; Muruzabal, F.; Andia, I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: Gross morphology and histology. Arthroscopy 2010, 26, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Claes, S.; Verdonk, P.; Forsyth, R.; Bellemans, J. The “ligamentization” process in anterior cruciate ligament reconstruction: What happens to the human graft? A systematic review of the literature. Am. J. Sports Med. 2011, 39, 2476–2483. [Google Scholar] [CrossRef]
- Howell, S.M.; Knox, K.E.; Farley, T.E.; Taylor, M.A. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am. J. Sports Med. 1995, 23, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, M.; Soldati, F.; Szomolanyi, P.; Trattnig, S.; Bartolucci, F.; Fu, F.; Denti, M. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Rougraff, B.T.; Shelbourne, K.D. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 9–14. [Google Scholar] [CrossRef]
- Ahn, G.Y.; Nam, I.H.; Lee, Y.H.; Lee, Y.S.; Choi, Y.D.; Lee, H.H.; Hwang, S.H. Factors Affecting the Extent of Graft Tendon Synovialization after Double-Bundle Anterior Cruciate Ligament Reconstruction: Based on Second-Look Arthroscopic Findings. Clin. Orthop. Surg. 2018, 10, 413–419. [Google Scholar] [CrossRef]
- Kim, S.G.; Jung, J.H.; Song, J.H.; Bae, J.H. Evaluation parameters of graft maturation on second-look arthroscopy following anterior cruciate ligament reconstruction: A systematic review. Knee Surg. Relat. Res. 2019, 31, 2. [Google Scholar] [CrossRef]
- Cuti, T.; Antunovic, M.; Marijanovic, I.; Ivkovic, A.; Vukasovic, A.; Matic, I.; Pecina, M.; Hudetz, D. Capacity of muscle derived stem cells and pericytes to promote tendon graft integration and ligamentization following anterior cruciate ligament reconstruction. Int. Orthop. 2017, 41, 1189–1198. [Google Scholar] [CrossRef]
- Biz, C.; Crimi, A.; Fantoni, I.; Pozzuoli, A.; Ruggieri, P. Muscle stem cells: What’s new in orthopedics? Acta Biomed. 2019, 90, 8–13. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Sung, I.H.; Park, Y.S.; Bae, S.C.; Kim, T.H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 2018, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Park, P.-R.; Jo, S.; Jin, S.-H.; Kim, T.-J. MicroRNA-10b Plays a Role in Bone Formation by Suppressing Interleukin-22 in Ankylosing Spondylitis. J. Rheum. Dis. 2020, 27. [Google Scholar] [CrossRef]
- Lee, J.K.; Jo, S.; Lee, Y.L.; Park, H.; Song, J.S.; Sung, I.H.; Kim, T.H. Anterior cruciate ligament remnant cells have different potentials for cell differentiation based on their location. Sci. Rep. 2020, 10, 3097. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Luetzenberg, R.; Abuagela, N.; Hollenberg, S.; Infanger, M. Spheroid formation and modulation of tenocyte-specific gene expression under simulated microgravity. Muscles Ligaments Tendons J. 2017, 7, 411–417. [Google Scholar] [CrossRef]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Park, H.; Lee, Y.L.; Oh, Y.; Park, J.; Kim, S.Y.; Weon, S.; Choi, S.H.; Yang, J.H.; Jo, S.; et al. TGFbeta1 Suppressed Matrix Mineralization of Osteoblasts Differentiation by Regulating SMURF1-C/EBPbeta-DKK1 Axis. Int. J. Mol. Sci. 2020, 21, 9771. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; van Griensven, M.; Unger, M.; Scheffler, H.; Falldorf, K.; Fentz, A.K.; Seeliger, C.; Schroter, S.; Nussler, A.K.; Balmayor, E.R. Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 994. [Google Scholar] [CrossRef]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W.S. Adult mesenchymal stem cells and cell surface characterization—A systematic review of the literature. Open Orthop. J. 2011, 5, 253–260. [Google Scholar] [CrossRef]
- De Mattei, M.; Fini, M.; Setti, S.; Ongaro, A.; Gemmati, D.; Stabellini, G.; Pellati, A.; Caruso, A. Proteoglycan synthesis in bovine articular cartilage explants exposed to different low-frequency low-energy pulsed electromagnetic fields. Osteoarthr. Cartil. 2007, 15, 163–168. [Google Scholar] [CrossRef][Green Version]
- Ciombor, D.M.; Lester, G.; Aaron, R.K.; Neame, P.; Caterson, B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J. Orthop. Res. 2002, 20, 40–50. [Google Scholar] [CrossRef]
- Diederichs, S.; Gabler, J.; Autenrieth, J.; Kynast, K.L.; Merle, C.; Walles, H.; Utikal, J.; Richter, W. Differential Regulation of SOX9 Protein During Chondrogenesis of Induced Pluripotent Stem Cells Versus Mesenchymal Stromal Cells: A Shortcoming for Cartilage Formation. Stem. Cells Dev. 2016, 25, 598–609. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Jiang, T.; Zheng, L.; Zhao, J.; Zhang, X. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater. Sci. 2018, 6, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Genin, G.M.; Thomopoulos, S. The tendon-to-bone attachment: Unification through disarray. Nat. Mater. 2017, 16, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Kuntz, L.A.; Kunold, E.; Schock, J.; Muller, K.W.; Grabmayr, H.; Stolberg-Stolberg, J.; Pfeiffer, F.; Sieber, S.A.; Burgkart, R.; et al. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017, 16, 664–670. [Google Scholar] [CrossRef]
- Ma, Y.; Murawski, C.D.; Rahnemai-Azar, A.A.; Maldjian, C.; Lynch, A.D.; Fu, F.H. Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: A magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 661–668. [Google Scholar] [CrossRef]
- Sun, L.; Hou, C.; Wu, B.; Tian, M.; Zhou, X. Effect of muscle preserved on tendon graft on intra-articular healing in anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Landry, P.S.; Marino, A.A.; Sadasivan, K.K.; Albright, J.A. Effect of soft-tissue trauma on the early periosteal response of bone to injury. J. Trauma 2000, 48, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Grover, L.M.; Eisenstein, N.; Lewis, M.P.; Liu, Y. Identifying the Cellular Mechanisms Leading to Heterotopic Ossification. Calcif. Tissue Int. 2015, 97, 432–444. [Google Scholar] [CrossRef]
- Mazzocca, A.D.; Chowaniec, D.; McCarthy, M.B.; Beitzel, K.; Cote, M.P.; McKinnon, W.; Arciero, R. In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: Multiple passages result in changes in routine cell markers. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1666–1672. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Dede Eren, A.; Vasilevich, A.; Eren, E.D.; Sudarsanam, P.; Tuvshindorj, U.; de Boer, J.; Foolen, J. Tendon-Derived Biomimetic Surface Topographies Induce Phenotypic Maintenance of Tenocytes In Vitro. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef]

| Gene. | 5′-Forward-3′ | 3′-Reverse-5′ |
|---|---|---|
| Col1 | ACATGCCGTGACTTGAGACTCA | GCCAGACGTGTTTCTTGTCCTT |
| Col2 | GCCGGATCTGTGTCTGTGAC | TGTCCCTTTGGTCCTGGTTG |
| Col3 | CTTCTCTCCAGCCGAGCTTC | CCAGTGTGTTTCGTGCAACC |
| Col6 | CCTGGGCGTCAAAGTCTTCT | AGCACACTTGCTCCACGTTA |
| Col9 | GCCCCAGATTCCCTGTCAAT | CCAACTTGTAAGCCACCTGC |
| Col10 | CTGGACCGGCTGGAATTTCT | CGACCAGGAGCACCATATCC |
| Col11 | ATCACAGGTGATCCCAAGGC | ACGATGTTTGCCTCATCTATCTG |
| ALP | CCAAGGACGCTGGGAAATCT | TATGCATGAGCTGGTAGGCG |
| SOX9 | CTGAACGAGAGCGAGAAGCG | CCCGTTCTTCACCGACTTCC |
| ACAN | CTGGTCAGATGGACACCCCAT | CGTTTGTAGGTGGTGGCTGTG |
| TNC | GGTTGCTGGAGACTGTGGAA | AGGTTTTCCAGAAGGGGCAG |
| OCN | GATGGTCTCAAGCCCTGGTT | CCTCCCGTAGTGCTCCGATA |
| GAPDH | CAAGATCATCAGCAATGCC | CTGTGGTCATGAGTCCTTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Jo, S.; Lee, Y.L.; Weon, S.; Song, J.-S.; Sung, I.-H.; Kim, T.-H. Effect of Muscle Cell Preservation on Viability and Differentiation of Hamstring Tendon Graft In Vitro. Cells 2021, 10, 740. https://doi.org/10.3390/cells10040740
Lee JK, Jo S, Lee YL, Weon S, Song J-S, Sung I-H, Kim T-H. Effect of Muscle Cell Preservation on Viability and Differentiation of Hamstring Tendon Graft In Vitro. Cells. 2021; 10(4):740. https://doi.org/10.3390/cells10040740
Chicago/Turabian StyleLee, Jin Kyu, Sungsin Jo, Young Lim Lee, Subin Weon, Jun-Seob Song, Il-Hoon Sung, and Tae-Hwan Kim. 2021. "Effect of Muscle Cell Preservation on Viability and Differentiation of Hamstring Tendon Graft In Vitro" Cells 10, no. 4: 740. https://doi.org/10.3390/cells10040740
APA StyleLee, J. K., Jo, S., Lee, Y. L., Weon, S., Song, J.-S., Sung, I.-H., & Kim, T.-H. (2021). Effect of Muscle Cell Preservation on Viability and Differentiation of Hamstring Tendon Graft In Vitro. Cells, 10(4), 740. https://doi.org/10.3390/cells10040740






