Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast
Abstract
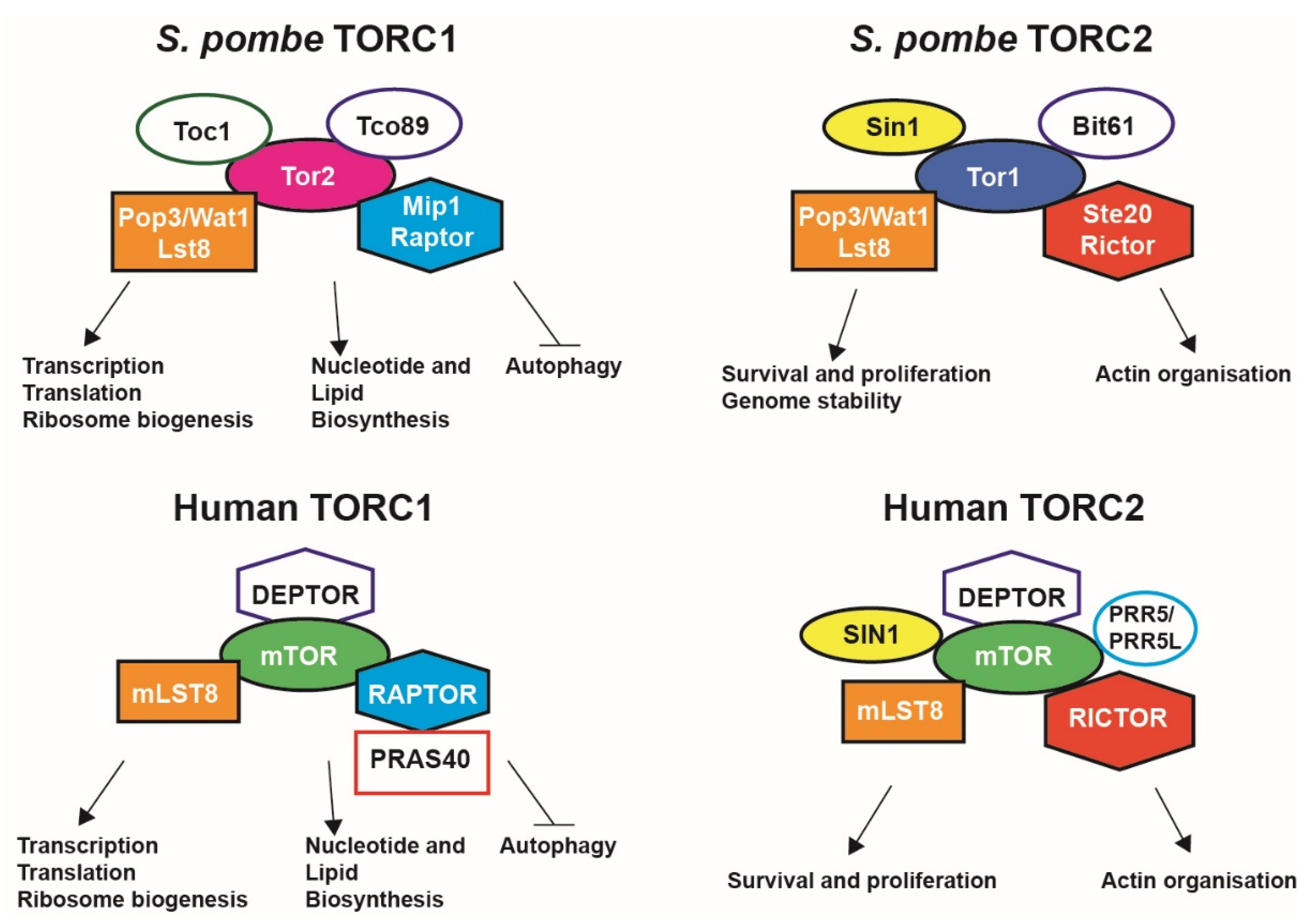
1. The Mechanistic Target of Rapamycin Pathway in Cell Growth and Metabolism
2. DNA Damage Response (DDR) Signaling: Cdc25, Wee1, and DNA Damage CheckPoint Activation
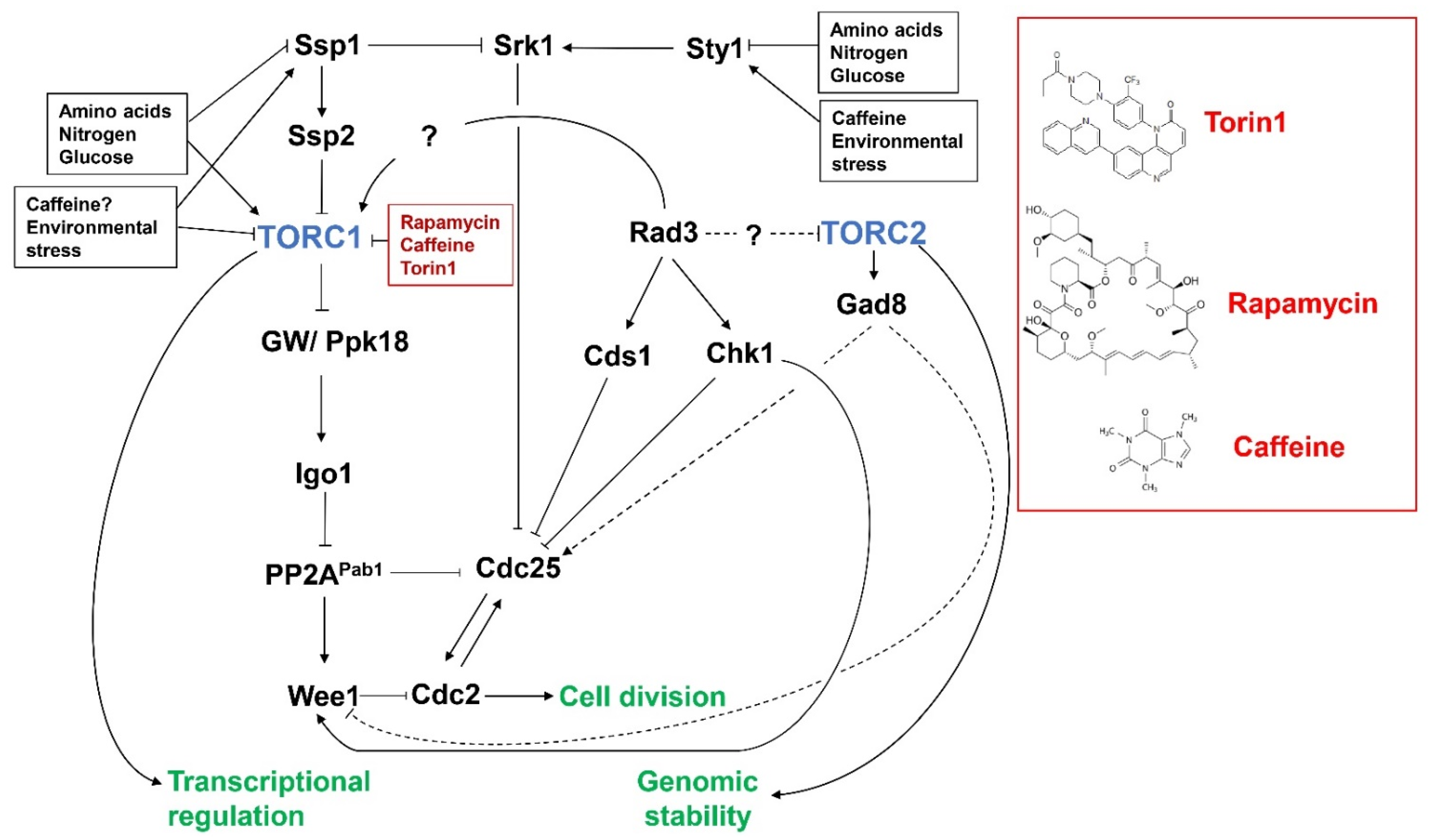
3. TORC1, Caffeine and the DNA Damage Response Pathway
4. Crosstalk between TORC2 and DNA Damage Response Pathways
5. Prospects for mTOR as a Chemo- and Radio-Sensitisation Therapeutic Target
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Kapahi, P.; Chen, D.; Rogers, A.N.; Katewa, S.D.; Li, P.W.-L.; Thomas, E.L.; Kockel, L. With TOR, Less Is More: A Key Role for the Conserved Nutrient-Sensing TOR Pathway in Aging. Cell Metab. 2010, 11, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for Cell Cycle Arrest by the Immunosuppressant Rapamycin in Yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef]
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A Mammalian Protein Targeted by G1-Arresting Rapamycin-Receptor Complex. Nature 1994, 369, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S.; Montagne, J.; Radimerski, T.; Thomas, G.; Hafen, E. Genetic and Biochemical Characterization of DTOR, the Drosophila Homolog of the Target of Rapamycin. Genes Dev. 2000, 14, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Sabers, C.J.; Martin, M.M.; Brunn, G.J.; Williams, J.M.; Dumont, F.J.; Wiederrecht, G.; Abraham, R.T. Isolation of a Protein Target of the FKBP12-Rapamycin Complex in Mammalian Cells. J. Biol. Chem. 1995, 270, 815–822. [Google Scholar] [CrossRef]
- Sabatini, D.M.; Erdjument-Bromage, H.; Lui, M.; Tempst, P.; Snyder, S.H. RAFT1: A Mammalian Protein That Binds to FKBP12 in a Rapamycin-Dependent Fashion and Is Homologous to Yeast TORs. Cell 1994, 78, 35–43. [Google Scholar] [CrossRef]
- Long, X.; Spycher, C.; Han, Z.S.; Rose, A.M.; Müller, F.; Avruch, J. TOR Deficiency in C. Elegans Causes Developmental Arrest and Intestinal Atrophy by Inhibition of MRNA Translation. Curr. Biol. 2002, 12, 1448–1461. [Google Scholar] [CrossRef]
- Long, X.; Lin, Y.; Ortiz-Vega, S.; Yonezawa, K.; Avruch, J. Rheb Binds and Regulates the MTOR Kinase. Curr. Biol. 2005, 15, 702–713. [Google Scholar] [CrossRef]
- Matsumoto, S.; Bandyopadhyay, A.; Kwiatkowski, D.J.; Maitra, U.; Matsumoto, T. Role of the Tsc1-Tsc2 Complex in Signaling and Transport across the Cell Membrane in the Fission Yeast Schizosaccharomyces pombe. Genetics 2002, 161, 1053–1063. [Google Scholar] [PubMed]
- Huang, J.; Manning, B.D. The TSC1-TSC2 Complex: A Molecular Switchboard Controlling Cell Growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, M. Cellular Quiescence: Are Controlling Genes Conserved? Trends Cell Biol. 2009, 19, 705–715. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Nakashima, A.; Ueno, M.; Ushimaru, T.; Aiba, K.; Doi, H.; Uritani, M. Fission Yeast Tor1 Functions in Response to Various Stresses Including Nitrogen Starvation, High Osmolarity, and High Temperature. Curr. Genet. 2001, 39, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, Y.; Nakashima, A.; Yamamoto, M.; Yamashita, A. TORC1-Dependent Phosphorylation Targets in Fission Yeast. Biomolecules 2017, 7, 50. [Google Scholar] [CrossRef]
- Weisman, R.; Choder, M. The Fission Yeast TOR Homolog, tor1+, Is Required for the Response to Starvation and Other Stresses via a Conserved Serine. J. Biol. Chem. 2001, 276, 7027–7032. [Google Scholar] [CrossRef]
- Loewith, R.; Hall, M.N. Target of Rapamycin (TOR) in Nutrient Signaling and Growth Control. Genetics 2011, 189, 1177–1201. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the Role of MTOR in Cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Rallis, C.; Bähler, J. Inhibition of TORC1 Signaling and Increased Lifespan: Gained in Translation? Aging (Albany NY) 2013, 5, 335–336. [Google Scholar] [CrossRef][Green Version]
- Rallis, C.; Codlin, S.; Bähler, J. TORC1 Signaling Inhibition by Rapamycin and Caffeine Affect Lifespan, Global Gene Expression, and Cell Proliferation of Fission Yeast. Aging Cell 2013, 12, 563–573. [Google Scholar] [CrossRef]
- Wanke, V.; Cameroni, E.; Uotila, A.; Piccolis, M.; Urban, J.; Loewith, R.; De Virgilio, C. Caffeine Extends Yeast Lifespan by Targeting TORC1. Mol. Microbiol. 2008, 69, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kuo, C.J.; Crabtree, G.R.; Blenis, J. Rapamycin-FKBP Specifically Blocks Growth-Dependent Activation of and Signaling by the 70 Kd S6 Protein Kinases. Cell 1992, 69, 1227–1236. [Google Scholar] [CrossRef]
- Ma, Y.; Vassetzky, Y.; Dokudovskaya, S. MTORC1 Pathway in DNA Damage Response. Biochim. Biophys. Acta BBA Mol. Cell Res. 2018, 1865, 1293–1311. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular Mechanisms of MTOR-Mediated Translational Control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Nojima, H.; Tokunaga, C.; Eguchi, S.; Oshiro, N.; Hidayat, S.; Yoshino, K.; Hara, K.; Tanaka, N.; Avruch, J.; Yonezawa, K. The Mammalian Target of Rapamycin (MTOR) Partner, Raptor, Binds the MTOR Substrates P70 S6 Kinase and 4E-BP1 through Their TOR Signaling (TOS) Motif. J. Biol. Chem. 2003, 278, 15461–15464. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.-H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a Novel Binding Partner of MTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway That Regulates the Cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Xu, S.; Li, L.; Li, M.; Zhang, M.; Ju, M.; Chen, X.; Gu, H. Impact on Autophagy and Ultraviolet B Induced Responses of Treatment with the MTOR Inhibitors Rapamycin, Everolimus, Torin 1, and Pp242 in Human Keratinocytes. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Gaubitz, C.; Prouteau, M.; Kusmider, B.; Loewith, R. TORC2 Structure and Function. Trends Biochem. Sci. 2016, 41, 532–545. [Google Scholar] [CrossRef]
- Matsuo, T.; Otsubo, Y.; Urano, J.; Tamanoi, F.; Yamamoto, M. Loss of the TOR Kinase Tor2 Mimics Nitrogen Starvation and Activates the Sexual Development Pathway in Fission Yeast. Mol. Cell. Biol. 2007, 27, 3154–3164. [Google Scholar] [CrossRef]
- Du, W.; Halova, L.; Kirkham, S.; Atkin, J.; Petersen, J. TORC2 and the AGC Kinase Gad8 Regulate Phosphorylation of the Ribosomal Protein S6 in Fission Yeast. Biol. Open 2012, 1, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Schonbrun, M.; Laor, D.; López-Maury, L.; Bähler, J.; Kupiec, M.; Weisman, R. TOR Complex 2 Controls Gene Silencing, Telomere Length Maintenance, and Survival under DNA-Damaging Conditions. Mol. Cell. Biol. 2009, 29, 4584–4594. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Rallis, C. The TOR Signaling Pathway in Spatial and Temporal Control of Cell Size and Growth. Front. Cell Dev. Biol. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Kirkham, S.; Halova, L.; Atkin, J.; Franz-Wachtel, M.; Cobley, D.; Krug, K.; Maček, B.; Mulvihill, D.P.; Petersen, J. TOR Complex 2 Localises to the Cytokinetic Actomyosin Ring and Controls the Fidelity of Cytokinesis. J. Cell Sci. 2016, 129, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Ikai, N.; Nakazawa, N.; Hayashi, T.; Yanagida, M. The Reverse, but Coordinated, Roles of Tor2 (TORC1) and Tor1 (TORC2) Kinases for Growth, Cell Cycle and Separase-Mediated Mitosis in Schizosaccharomyces pombe. Open Biol. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Weisman, R. Target of Rapamycin (TOR) Regulates Growth in Response to Nutritional Signals. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Strukenbrock, E.H., James, T.Y., Grow, N.A.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 535–548. [Google Scholar] [CrossRef]
- Schonbrun, M.; Kolesnikov, M.; Kupiec, M.; Weisman, R. TORC2 Is Required to Maintain Genome Stability during S Phase in Fission Yeast. J. Biol. Chem. 2013, 288, 19649–19660. [Google Scholar] [CrossRef]
- Shaltiel, I.A.; Krenning, L.; Bruinsma, W.; Medema, R.H. The Same, Only Different-DNA Damage Checkpoints and Their Reversal throughout the Cell Cycle. J. Cell Sci. 2015, 128, 607–620. [Google Scholar] [CrossRef]
- Jimenez, G.; Yucel, J.; Rowley, R.; Subramani, S. The Rad3+ Gene of Schizosaccharomyces pombe is Involved in Multiple Checkpoint Functions and in DNA Repair. Proc. Natl. Acad. Sci. USA 1992, 89, 4952–4956. [Google Scholar] [CrossRef]
- Bentley, N.J.; Holtzman, D.A.; Flaggs, G.; Keegan, K.S.; DeMaggio, A.; Ford, J.C.; Hoekstra, M.; Carr, A.M. The Schizosaccharomyces pombe Rad3 Checkpoint Gene. EMBO J. 1996, 15, 6641–6651. [Google Scholar] [CrossRef]
- Carr, A.M. DNA Structure Dependent Checkpoints as Regulators of DNA Repair. DNA Repair 2002, 1, 983–994. [Google Scholar] [CrossRef]
- Awasthi, P.; Foiani, M.; Kumar, A. ATM and ATR Signaling at a Glance. J. Cell Sci. 2015, 128, 4255–4262. [Google Scholar] [CrossRef]
- Corcoles-Saez, I.; Dong, K.; Cha, R.S. Versatility of the Mec1ATM/ATR Signaling Network in Mediating Resistance to Replication, Genotoxic, and Proteotoxic Stresses. Curr. Genet. 2019, 65, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Cussiol, J.R.R.; Soares, B.L.; de Oliveira, F.M.B. From Yeast to Humans: Understanding the Biology of DNA Damage Response (DDR) Kinases. Genet. Mol. Biol. 2020, 43, e20190071. [Google Scholar] [CrossRef] [PubMed]
- Crncec, A.; Hochegger, H. Triggering Mitosis. FEBS Lett. 2019, 593, 2868–2888. [Google Scholar] [CrossRef]
- Moseley, J.B. Wee1 and Cdc25: Tools, Pathways, Mechanisms, Questions. Cell Cycle 2017, 16, 599–600. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Walworth, N.C.; Carr, A.M. The G2-Phase DNA-Damage Checkpoint. Trends Cell Biol. 2000, 10, 296–303. [Google Scholar] [CrossRef]
- Karlsson-Rosenthal, C.; Millar, J.B.A. Cdc25: Mechanisms of Checkpoint Inhibition and Recovery. Trends Cell Biol. 2006, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Stracker, T.H.; Roig, I.; Knobel, P.A.; Marjanović, M. The ATM Signaling Network in Development and Disease. Front. Genet. 2013, 4, 37. [Google Scholar] [CrossRef]
- Rhind, N.; Russell, P. Chk1 and Cds1: Linchpins of the DNA Damage and Replication Checkpoint Pathways. J. Cell Sci. 2000, 113 Pt 22, 3889–3896. [Google Scholar]
- Paparatto, D.; Fletcher, D.; Piwowar, K.; Baldino, K.; Morel, C.; Dunaway, S. The Schizosaccharomyces pombe Checkpoint Kinases Chk1 and Cds1 Are Important for Cell Survival in Response to Cisplatin. PLoS ONE 2009, 4, e6181. [Google Scholar] [CrossRef]
- Qu, M.; Yang, B.; Tao, L.; Yates, J.R.; Russell, P.; Dong, M.-Q.; Du, L.-L. Phosphorylation-Dependent Interactions between Crb2 and Chk1 Are Essential for DNA Damage Checkpoint. PLoS Genet. 2012, 8, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Rhind, N.; Russell, P. Mitotic DNA Damage and Replication Checkpoints in Yeast. Curr. Opin. Cell Biol. 1998, 10, 749–758. [Google Scholar] [CrossRef]
- Boddy, M.N.; Furnari, B.; Mondesert, O.; Russell, P. Replication Checkpoint Enforced by Kinases Cds1 and Chk1. Science 1998, 280, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.U.; Bentley, N.J.; Martinho, R.G.; Nielsen, O.; Carr, A.M. Mik1 Levels Accumulate in S Phase and May Mediate an Intrinsic Link between S Phase and Mitosis. Proc. Natl. Acad. Sci. USA 2000, 97, 2579–2584. [Google Scholar] [CrossRef]
- Zeng, Y.; Forbes, K.C.; Wu, Z.; Moreno, S.; Piwnica-Worms, H.; Enoch, T. Replication Checkpoint Requires Phosphorylation of the Phosphatase Cdc25 by Cds1 or Chk1. Nature 1998, 395, 507–510. [Google Scholar] [CrossRef]
- Zeng, Y.; Piwnica-Worms, H. DNA Damage and Replication Checkpoints in Fission Yeast Require Nuclear Exclusion of the Cdc25 Phosphatase via 14-3-3 Binding. Mol. Cell. Biol. 1999, 19, 7410–7419. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Furnari, B.; Mondesert, O.; Russell, P. Nuclear Localization of Cdc25 Is Regulated by DNA Damage and a 14-3-3 Protein. Nature 1999, 397, 172–175. [Google Scholar] [CrossRef]
- Furnari, B.; Blasina, A.; Boddy, M.N.; McGowan, C.H.; Russell, P. Cdc25 Inhibited in vivo and in vitro by Checkpoint Kinases Cds1 and Chk1. Mol. Biol. Cell 1999, 10, 833–845. [Google Scholar] [CrossRef]
- Frazer, C.; Young, P.G. Carboxy-Terminal Phosphorylation Sites in Cdc25 Contribute to Enforcement of the DNA Damage and Replication Checkpoints in Fission Yeast. Curr. Genet. 2012, 58, 217–234. [Google Scholar] [CrossRef]
- Frazer, C.; Young, P.G. Redundant Mechanisms Prevent Mitotic Entry Following Replication Arrest in the Absence of Cdc25 Hyper-Phosphorylation in Fission Yeast. PLoS ONE 2011, 6, e21348. [Google Scholar] [CrossRef][Green Version]
- Alao, J.P.; Sjölander, J.J.; Baar, J.; Özbaki-Yagan, N.; Kakoschky, B.; Sunnerhagen, P. Caffeine Stabilizes Cdc 25 Independently of Rad 3 in Schizosaccharomyces pombe Contributing to Checkpoint Override. Mol. Microbiol. 2014, 92, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Kovelman, R.; Russell, P. Stockpiling of Cdc25 during a DNA Replication Checkpoint Arrest in Schizosaccharomyces pombe. Mol. Cell. Biol. 1996, 16, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, J.M.; O’Connell, M.J. The G(2) DNA Damage Checkpoint Targets Both Wee1 and Cdc25. J. Cell Sci. 2000, 113 Pt 10, 1727–1736. [Google Scholar]
- López-Avilés, S.; Lambea, E.; Moldón, A.; Grande, M.; Fajardo, A.; Rodríguez-Gabriel, M.A.; Hidalgo, E.; Aligue, R. Activation of Srk1 by the Mitogen-Activated Protein Kinase Sty1/Spc1 Precedes Its Dissociation from the Kinase and Signals Its Degradation. Mol. Biol. Cell 2008, 19, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Toone, W.M.; Chen, D.; Bähler, J.; Jones, N.; Morgan, B.A.; Quinn, J. The Srk1 Protein Kinase Is a Target for the Sty1 Stress-Activated MAPK in Fission Yeast. J. Biol. Chem. 2002, 277, 33411–33421. [Google Scholar] [CrossRef] [PubMed]
- Atkin, J.; Halova, L.; Ferguson, J.; Hitchin, J.R.; Lichawska-Cieslar, A.; Jordan, A.M.; Pines, J.; Wellbrock, C.; Petersen, J. Torin1-Mediated TOR Kinase Inhibition Reduces Wee1 Levels and Advances Mitotic Commitment in Fission Yeast and HeLa Cells. J. Cell Sci. 2014, 127, 1346–1356. [Google Scholar] [CrossRef]
- Shiozaki, K.; Russell, P. Cell-Cycle Control Linked to Extracellular Environment by MAP Kinase Pathway in Fission Yeast. Nature 1995, 378, 739–743. [Google Scholar] [CrossRef]
- Degols, G.; Russell, P. Discrete Roles of the Spc1 Kinase and the Atf1 Transcription Factor in the UV Response of Schizosaccharomyces pombe. Mol. Cell. Biol. 1997, 17, 3356–3363. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. P38MAPK: Stress Responses from Molecular Mechanisms to Therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Rupeš, I.; Jia, Z.; Young, P.G. Ssp1 Promotes Actin Depolymerization and Is Involved in Stress Response and New End Take-Off Control in Fission Yeast. Mol. Biol. Cell 1999, 10, 1495–1510. [Google Scholar] [CrossRef]
- Yanagida, M.; Ikai, N.; Shimanuki, M.; Sajiki, K. Nutrient Limitations Alter Cell Division Control and Chromosome Segregation through Growth-Related Kinases and Phosphatases. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Freitag, S.I.; Wong, J.; Young, P.G. Genetic and Physical Interaction of Ssp1 CaMKK and Rad24 14-3-3 during Low PH and Osmotic Stress in Fission Yeast. Open Biol. 2014, 4, 130127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez-Hierro, A.; Lambea, E.; Giménez-Zaragoza, D.; López-Avilés, S.; Yance-Chávez, T.; Montserrat, M.; Pujol, M.J.; Bachs, O.; Aligue, R. Ssp1 CaMKK: A Sensor of Actin Polarization That Controls Mitotic Commitment through Srk1 in Schizosaccharomyces pombe. PLoS ONE 2015, 10, e0143037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davie, E.; Forte, G.M.A.; Petersen, J. Nitrogen Regulates AMPK to Control TORC1 Signaling. Curr. Biol. 2015, 25, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hartmuth, S.; Petersen, J. Fission Yeast Tor1 Functions as Part of TORC1 to Control Mitotic Entry through the Stress MAPK Pathway Following Nutrient Stress. J. Cell Sci. 2009, 122, 1737–1746. [Google Scholar] [CrossRef]
- Alao, J.P.; Huis in ’t Veld, P.J.; Buhse, F.; Sunnerhagen, P. Hyperosmosis Enhances Radiation and Hydroxyurea Resistance of Schizosaccharomyces pombe Checkpoint Mutants through the Spindle Checkpoint and Delayed Cytokinesis. Mol. Microbiol. 2010, 77, 143–157. [Google Scholar] [CrossRef]
- Alao, J.P.; Sunnerhagen, P. Caffeine as a Tool for Investigating the Integration of Cdc25 Phosphorylation, Activity and Ubiquitin-Dependent Degradation in Schizosaccharomyces pombe. Cell Div. 2020, 15. [Google Scholar] [CrossRef]
- Weisman, R.; Cohen, A.; Gasser, S.M. TORC 2—A New Player in Genome Stability. EMBO Mol. Med. 2014, 6, 995–1002. [Google Scholar] [CrossRef]
- Fletcher, J.; Griffiths, L.; Caspari, T. Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe. Cells 2018, 7, 15. [Google Scholar] [CrossRef]
- Castro, A.; Lorca, T. Greatwall Kinase at a Glance. J. Cell Sci. 2018, 131, jcs222364. [Google Scholar] [CrossRef]
- Pérez-Hidalgo, L.; Moreno, S. Coupling TOR to the Cell Cycle by the Greatwall–Endosulfine–PP2A-B55 Pathway. Biomolecules 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Lopez-Aviles, S. Express Yourself: How PP2A-B55Pab1 Helps TORC1 Talk to TORC2. Curr. Genet. 2018, 64, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Alcaide-Gavilán, M.; Anastasia, S.D.; Kellogg, D.R. Wee1 and Cdc25 Are Controlled by Conserved PP2A-Dependent Mechanisms in Fission Yeast. Cell Cycle 2017, 16, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Nemoto, T.; Nabeshima, K.; Kondoh, H.; Niwa, H.; Yanagida, M. The Regulatory Subunits of Fission Yeast Protein Phosphatase 2A (PP2A) Affect Cell Morphogenesis, Cell Wall Synthesis and Cytokinesis. Genes Cells 1996, 1, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The Enigmatic Effects of Caffeine in Cell Cycle and Cancer. Cancer Lett. 2007, 247, 26–39. [Google Scholar] [CrossRef]
- Osman, F.; McCready, S. Differential Effects of Caffeine on DNA Damage and Replication Cell Cycle Checkpoints in the Fission Yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1998, 260, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Busby, E.C.; Tibbetts, R.S.; Roos, P.; Taya, Y.; Karnitz, L.M.; Abraham, R.T. Inhibition of ATM and ATR Kinase Activities by the Radiosensitizing Agent, Caffeine. Cancer Res. 1999, 59, 4375–4382. [Google Scholar]
- Blasina, A.; Price, B.D.; Turenne, G.A.; McGowan, C.H. Caffeine Inhibits the Checkpoint Kinase ATM. Curr. Biol. 1999, 9, 1135–1138. [Google Scholar] [CrossRef]
- Moser, B.A.; Brondello, J.M.; Baber-Furnari, B.; Russell, P. Mechanism of Caffeine-Induced Checkpoint Override in Fission Yeast. Mol. Cell. Biol. 2000, 20, 4288–4294. [Google Scholar] [CrossRef][Green Version]
- Cortez, D. Caffeine Inhibits Checkpoint Responses without Inhibiting the Ataxia-Telangiectasia-Mutated (ATM) and ATM- and Rad3-Related (ATR) Protein Kinases. J. Biol. Chem. 2003, 278, 37139–37145. [Google Scholar] [CrossRef]
- Calvo, I.A.; Gabrielli, N.; Iglesias-Baena, I.; García-Santamarina, S.; Hoe, K.-L.; Kim, D.U.; Sansó, M.; Zuin, A.; Pérez, P.; Ayté, J.; et al. Genome-Wide Screen of Genes Required for Caffeine Tolerance in Fission Yeast. PLoS ONE 2009, 4, e6619. [Google Scholar] [CrossRef]
- Campos, A.; Clemente-Blanco, A. Cell Cycle and DNA Repair Regulation in the Damage Response: Protein Phosphatases Take Over the Reins. Int. J. Mol. Sci. 2020, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Norbury, C.; Harris, A.L.; Toda, T. Caffeine Can Override the S-M Checkpoint in Fission Yeast. J. Cell Sci. 1999, 112 Pt 6, 927–937. [Google Scholar]
- Reinke, A.; Chen, J.C.-Y.; Aronova, S.; Powers, T. Caffeine Targets TOR Complex I and Provides Evidence for a Regulatory Link between the FRB and Kinase Domains of Tor1p. J. Biol. Chem. 2006, 281, 31616–31626. [Google Scholar] [CrossRef]
- Alao, J.P.; Johansson-Sjölander, J.; Rallis, C.; Sunnerhagen, P. Caffeine Stabilises Fission Yeast Wee1 in a Rad24-Dependent Manner but Attenuates Its Expression in Response to DNA Damage. Microorganisms 2020, 8, 1512. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.; Gonzalez, S.; Hillson, O.; Tunnacliffe, E.; Codlin, S.; Tallada, V.A.; Bähler, J.; Rallis, C. The GATA Transcription Factor Gaf1 Represses TRNAs, Inhibits Growth, and Extends Chronological Lifespan Downstream of Fission Yeast TORC1. Cell Rep. 2020, 30, 3240–3249.e4. [Google Scholar] [CrossRef] [PubMed]
- Pataki, E.; Simhaev, L.; Engel, H.; Cohen, A.; Kupiec, M.; Weisman, R. TOR Complex 2- Independent Mutations in the Regulatory PIF Pocket of Gad8AKT1/SGK1 Define Separate Branches of the Stress Response Mechanisms in Fission Yeast. PLoS Genet. 2020, 16, e1009196. [Google Scholar] [CrossRef] [PubMed]
- Ochotorena, I.L.; Hirata, D.; Kominami, K.; Potashkin, J.; Sahin, F.; Wentz-Hunter, K.; Gould, K.L.; Sato, K.; Yoshida, Y.; Vardy, L.; et al. Conserved Wat1/Pop3 WD-Repeat Protein of Fission Yeast Secures Genome Stability through Microtubule Integrity and May Be Involved in MRNA Maturation. J. Cell Sci. 2001, 114, 2911–2920. [Google Scholar]
- Verma, S.K.; Ranjan, R.; Kumar, V.; Siddiqi, M.I.; Ahmed, S. Wat1/Pop3, a Conserved WD Repeat Containing Protein Acts Synergistically with Checkpoint Kinase Chk1 to Maintain Genome Ploidy in Fission Yeast S. pombe. PLoS ONE 2014, 9, e89587. [Google Scholar] [CrossRef][Green Version]
- Tenzer, A.; Pruschy, M. Potentiation of DNA-Damage-Induced Cytotoxicity by G2 Checkpoint Abrogators. Curr. Med. Chem. Anti Cancer Agents 2003, 3, 35–46. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Iliakis, G.; Wang, Y. Caffeine-Induced Radiosensitization Is Independent of Nonhomologous End Joining of DNA Double-Strand Breaks. Radiat. Res. 2003, 159, 426–432. [Google Scholar] [CrossRef]
- Sinn, B.; Tallen, G.; Schroeder, G.; Grassl, B.; Schulze, J.; Budach, V.; Tinhofer, I. Caffeine Confers Radiosensitization of PTEN-Deficient Malignant Glioma Cells by Enhancing Ionizing Radiation-Induced G1 Arrest and Negatively Regulating Akt Phosphorylation. Mol. Cancer Ther. 2010, 9, 480–488. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for Cancer Prevention and Treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Nakajima, A. AMPK: Therapeutic Target for Diabetes and Cancer Prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The Strange Case of AMPK and Cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alao, J.-P.; Legon, L.; Rallis, C. Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast. Cells 2021, 10, 305. https://doi.org/10.3390/cells10020305
Alao J-P, Legon L, Rallis C. Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast. Cells. 2021; 10(2):305. https://doi.org/10.3390/cells10020305
Chicago/Turabian StyleAlao, John-Patrick, Luc Legon, and Charalampos Rallis. 2021. "Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast" Cells 10, no. 2: 305. https://doi.org/10.3390/cells10020305
APA StyleAlao, J.-P., Legon, L., & Rallis, C. (2021). Crosstalk between the mTOR and DNA Damage Response Pathways in Fission Yeast. Cells, 10(2), 305. https://doi.org/10.3390/cells10020305







