Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin
Abstract
1. Introduction
2. Materials and Methods
2.1. Gametes Collection, Fertilization Procedure, and Embryos Observation
2.2. Visualization of the Egg-Incorporated Sperm
2.3. Scanning Electron Microscopy (SEM)
2.4. Transmission Electron Microscopy (TEM)
2.5. Chemicals and Reagents
2.6. Microinjection, Ca2+ Imaging, and Confocal Microscopy
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
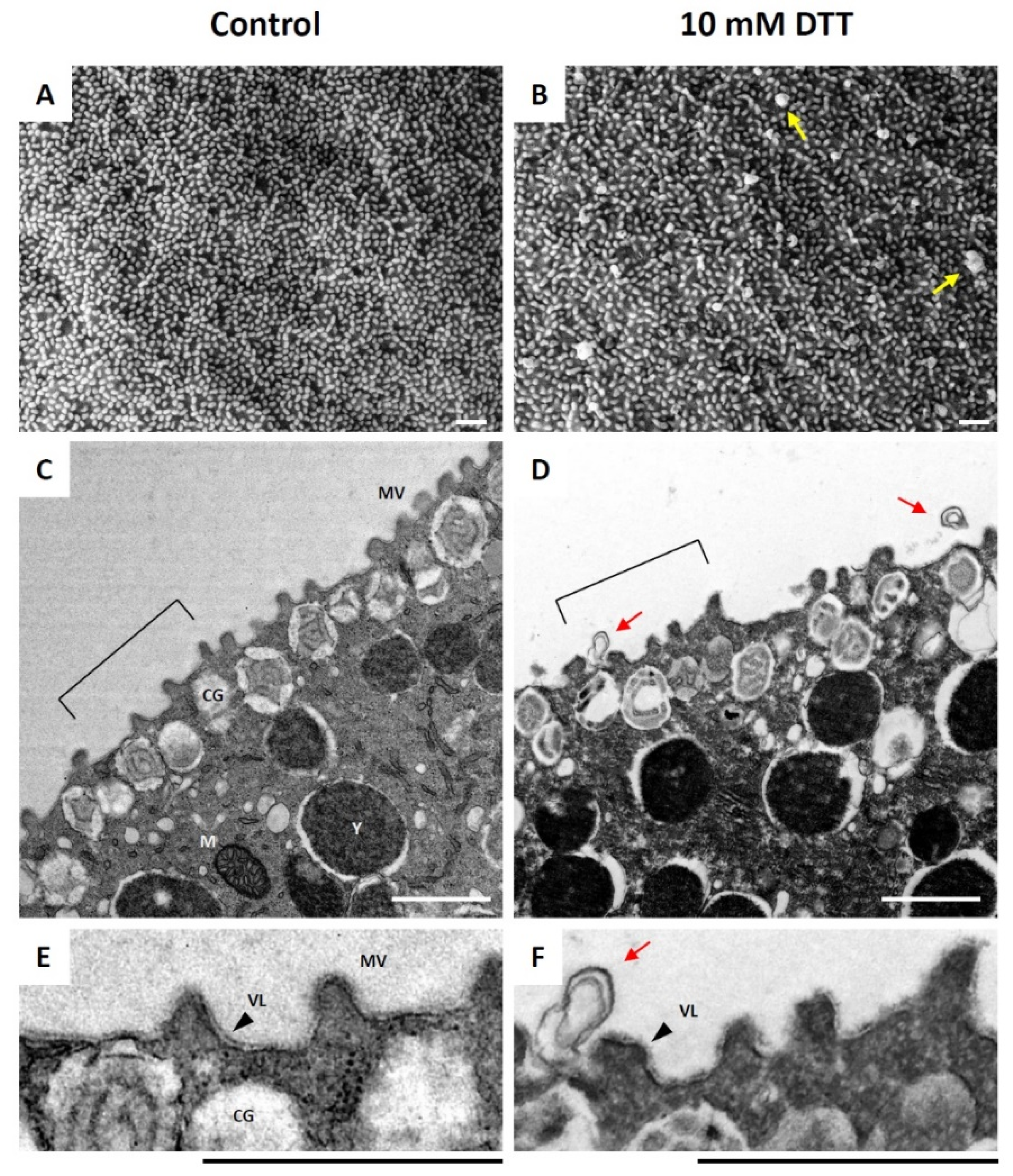
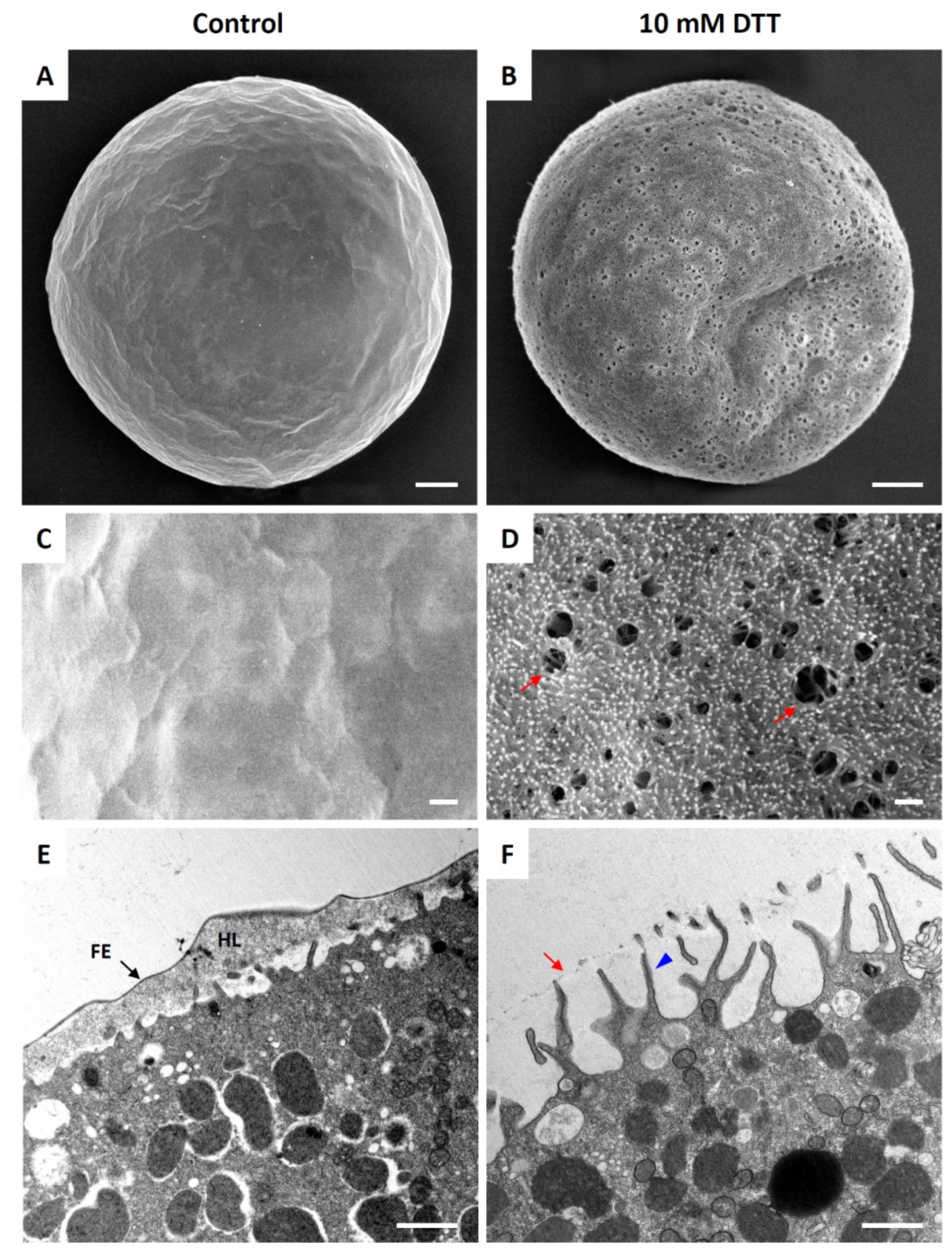
3.1. DTT Induces Ultrastructural Changes on the Surface of Unfertilized Sea Urchin Eggs
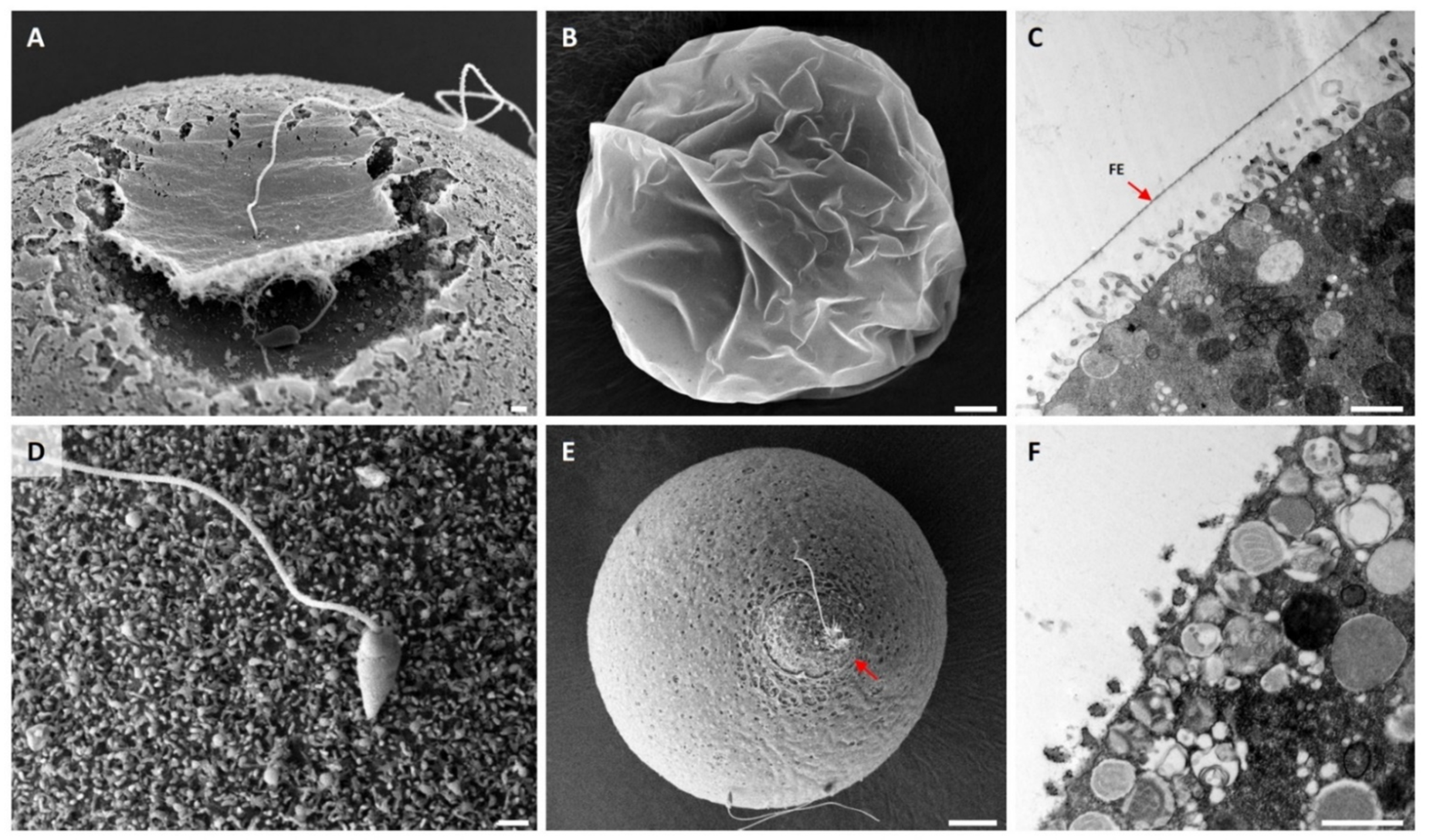
3.2. Effect of DTT Pretreatment of the Eggs on the Fertilization Process
3.3. Effect of DTT Pretreatment of the Eggs on the Later Stage of Fertilization
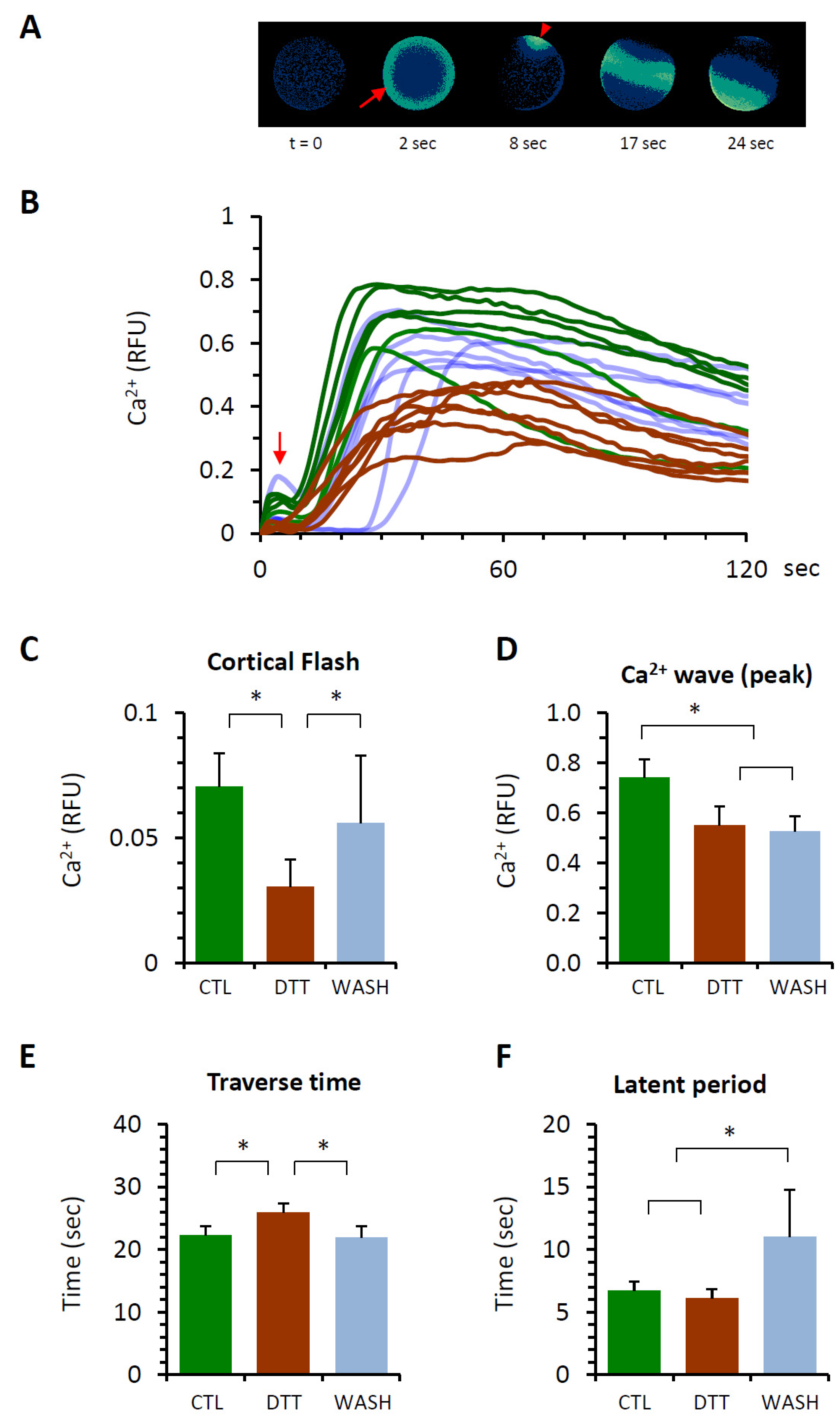
3.4. DTT Pretreatment of the Eggs Alters the Ca2+ Response at Fertilization
3.5. Effect of DTT Pretreatment of the Eggs on Monospermic Fertilization
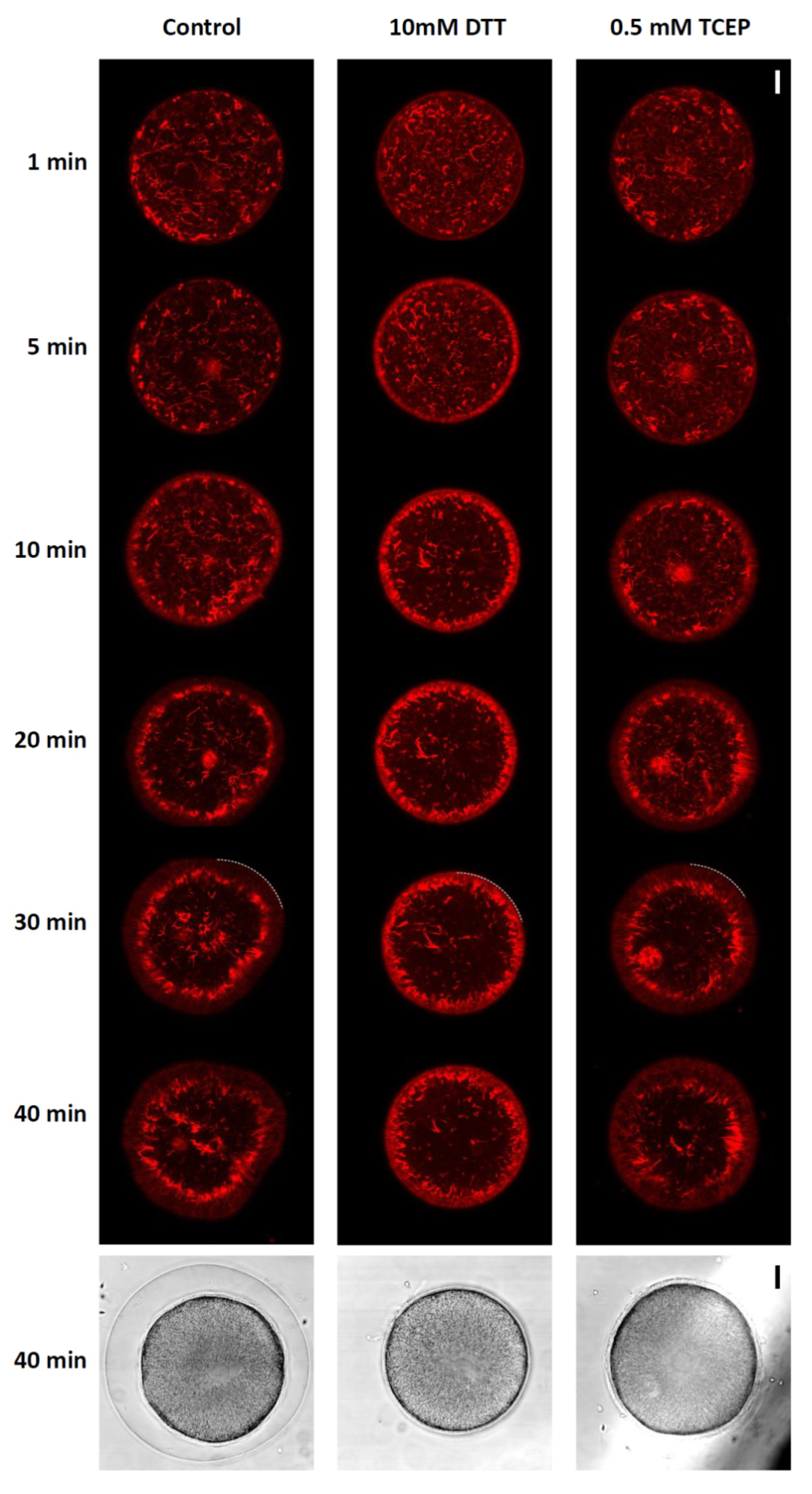
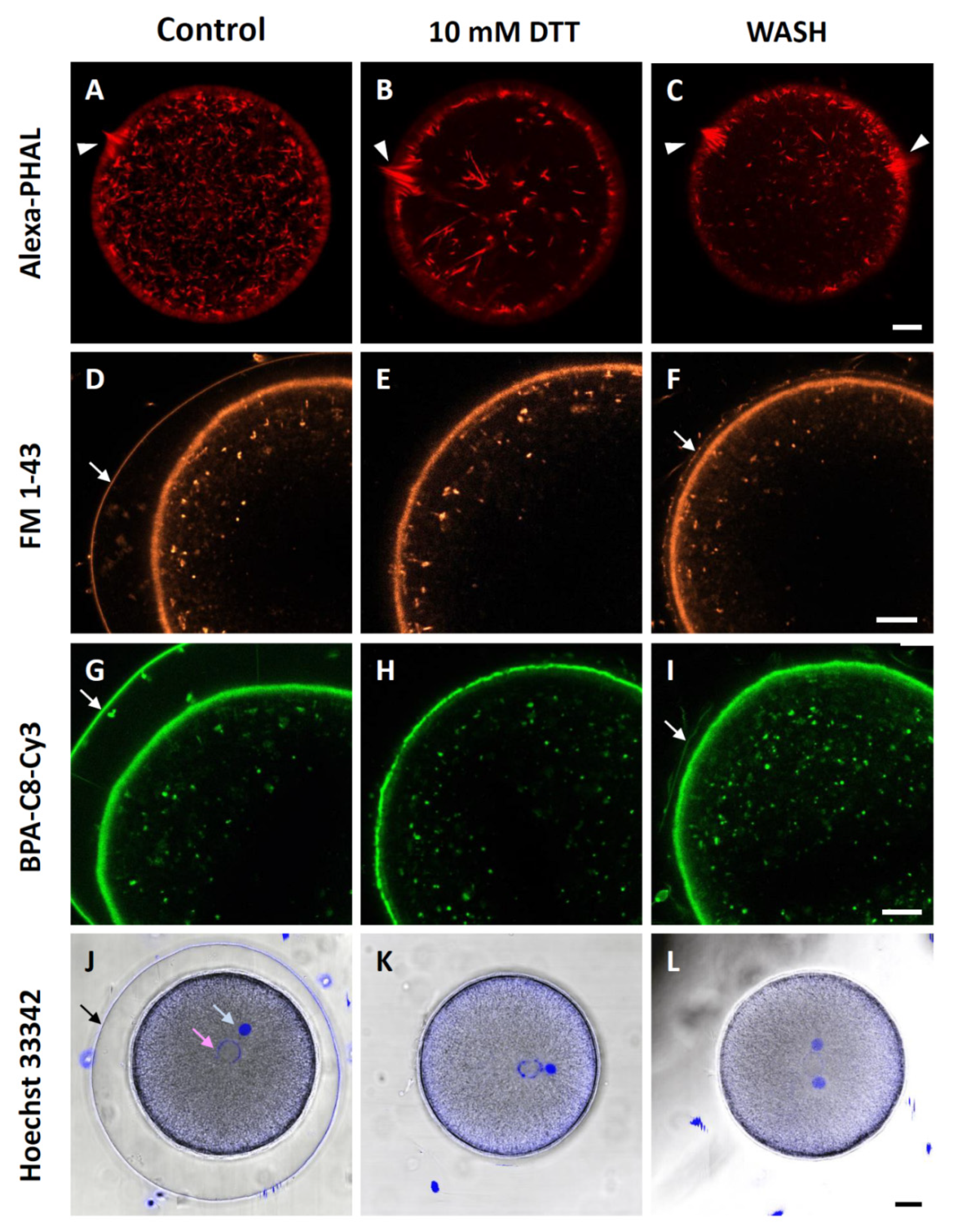
3.6. F-Actin Mobilization during Egg Activation Is Inhibited by DTT
3.7. Effect of DTT Pretreatment on the Late Events of Fertilization
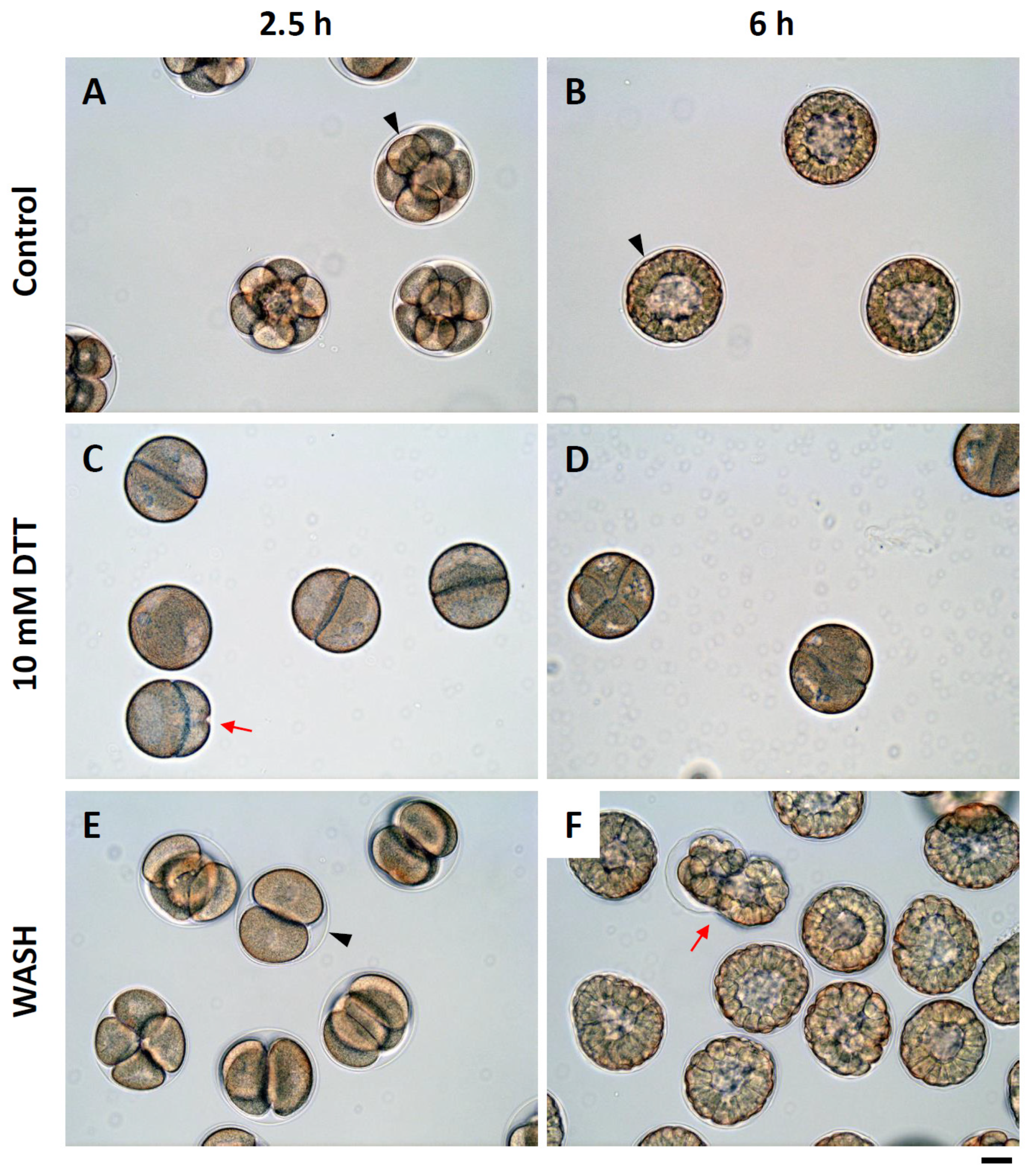
3.8. Effect of DTT Pretreatment of the Eggs on Early Embryonic Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, G.E.; Brokaw, C.J.; Garbers, D.L.; Vacquier, V.D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 1985, 101, 2324–2329. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Solzin, J.; Hildebrand, E.; Brown, J.E.; Helbig, A.; Hagen, V.; Beyermann, M.; Pampaloni, F.; Weyand, I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat. Cell Biol. 2003, 5, 109–117. [Google Scholar] [CrossRef]
- Ramírez-Gómez, H.V.; Jimenez Sabinina, V.; Velázquez Pérez, M.; Beltran, C.; Carneiro, J.; Wood, C.D.; Tuval, I.; Darszon, A.; Guerrero, A. Sperm chemotaxis is driven by the slope of the chemoattractant concentration field. eLife 2020, 9, e50532. [Google Scholar] [CrossRef]
- Aketa, K. On the sperm-egg bonding as the initial step of fertilization in the sea urchin. Embryologia (Nagoya) 1967, 9, 238–245. [Google Scholar] [CrossRef]
- Aketa, K.; Onitake, K.; Tsuzuki, H. Tryptic disruption of sperm-binding site of sea urchin egg surface. Exp. Cell Res. 1972, 71, 27–32. [Google Scholar] [CrossRef]
- Glabe, C.G.; Vacquier, V.D. Isolation and characterization of the vitelline layer of sea urchin eggs. J. Cell Biol. 1977, 75, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Epel, D.; Douglas, L.A. Sea urchin eggs release protease activity at fertilization. Nature 1972, 237, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Moy, G.W. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 1977, 74, 2456–2460. [Google Scholar] [CrossRef]
- Chandler, D.E.; Heuser, J. The vitelline layer of the sea urchin egg and its modification during fertilization. A freeze-fracture study using quick-freezing and deep-etching. J. Cell Biol. 1980, 84, 618–632. [Google Scholar] [CrossRef]
- Glabe, C.G.; Lennarz, W.J. Species-specific sperm adhesion in sea urchins. A quantitative investigation of bindin-mediated egg agglutination. J. Cell Biol. 1979, 83, 595–604. [Google Scholar] [CrossRef]
- Vacquier, V.D. Activation of sea urchin spermatozoa during fertilization. Trends Biochem. Sci. 1986, 11, 77–81. [Google Scholar] [CrossRef]
- Kamei, N.; Glabe, C.G. The species-specific egg receptor for sea urchin sperm adhesion is EBR1, a novel ADAMTS protein. Genes Dev. 2003, 17, 2502–2507. [Google Scholar] [CrossRef]
- Vacquier, V.D. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012, 425, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Wessel, G.M.; Wada, Y.; Yajima, M.; Kiyomoto, M. Bindin is essential for fertilization in the sea urchin. Proc. Natl. Acad. Sci. USA 2021, 118, e2109636118. [Google Scholar] [CrossRef]
- Epel, D.; Weaver, A.M.; Mazia, D. Methods for removal of the vitelline membrane of sea urchin eggs: I. Use of dithiothreitol (Cleland Reagent). Exp. Cell Res. 1970, 61, 64–68. [Google Scholar] [CrossRef]
- Schatten, G. Motility during fertilization. Int. Rev. Cytol. 1982, 79, 59–163. [Google Scholar] [CrossRef]
- Ohlendieck, K.; Lennarz, W.J. Role of the sea urchin egg receptor for sperm in gamete interactions. Trends Biochem. Sci. 1995, 20, 29–33. [Google Scholar] [CrossRef]
- Santella, L.; Vasilev, F.; Chun, J.T. Fertilization in echinoderms. Biochem. Biophys. Res. Commun. 2012, 425, 588–594. [Google Scholar] [CrossRef]
- Gilkey, J.C.; Jaffe, L.F.; Ridgway, E.B.; Reynolds, G.T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J. Cell Biol. 1978, 76, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Lim, D.; Moccia, F. Calcium and fertilization: The beginning of life. Trends Biochem. Sci. 2004, 29, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S. Thirty years of calcium signals at fertilization. Semin. Cell Dev. Biol. 2006, 17, 233–243. [Google Scholar] [CrossRef]
- Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef]
- Stein, P.; Savy, V.; Williams, A.M.; Williams, C.J. Modulators of calcium signalling at fertilization. Open Biol. 2020, 10, 200118. [Google Scholar] [CrossRef]
- Parrington, J.; Davis, L.C.; Galione, A.; Wessel, G. Flipping the switch: How a sperm activates the egg at fertilization. Dev. Dyn. 2007, 236, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M. Actin filament translocations in sea urchin eggs. Cell Motil. Cytoskelet. 1996, 34, 48–56. [Google Scholar] [CrossRef]
- Tilney, L.G.; Jaffe, L.A. Actin, microvilli, and the fertilization cone of sea urchin eggs. J. Cell Biol. 1980, 87, 771–782. [Google Scholar] [CrossRef]
- Chun, J.T.; Puppo, A.; Vasilev, F.; Gragnaniello, G.; Garante, E.; Santella, L. The biphasic increase of PIP2 in the fertilized eggs of starfish: New roles in actin polymerization and Ca2+ signaling. PLoS ONE 2010, 5, e14100. [Google Scholar] [CrossRef][Green Version]
- Vasilev, F.; Chun, J.T.; Gragnaniello, G.; Garante, E.; Santella, L. Effects of ionomycin on egg activation and early development in starfish. PLoS ONE 2012, 7, e39231. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Santella, L.; Chun, J.T. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells 2020, 9, 63. [Google Scholar] [CrossRef]
- Santella, L.; Chun, J.T. Actin, more than just a housekeeping protein at the scene of fertilization. Sci. China Life Sci. 2011, 54, 733–743. [Google Scholar] [CrossRef][Green Version]
- Santella, L.; Limatola, N.; Chun, J.T. Calcium and actin in the saga of awakening oocytes. Biochem. Biophys. Res. Commun. 2015, 460, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.T.; Vasilev, F.; Limatola, N.; Santella, L. Fertilization in Starfish and Sea Urchin: Roles of Actin. Results Probl. Cell Differ. 2018, 65, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Limatola, N.; Chun, J.T. Cellular and molecular aspects of oocyte maturation and fertilization: A perspective from the actin cytoskeleton. Zool. Lett. 2020, 6, 5. [Google Scholar] [CrossRef]
- Vasilev, F.; Ezhova, Y.; Chun, J.T. Signaling Enzymes and Ion Channels Being Modulated by the Actin Cytoskeleton at the Plasma Membrane. Int. J. Mol. Sci. 2021, 22, 10366. [Google Scholar] [CrossRef]
- Foltz, K.R.; Partin, J.S.; Lennarz, W.J. Sea urchin egg receptor for sperm: Sequence similarity of binding domain and hsp70. Science 1993, 259, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Partin, J.S.; Lennarz, W.J. The biologically active form of the sea urchin egg receptor for sperm is a disulfide-bonded homo-multimer. J. Cell Biol. 1994, 125, 817–824. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Chun, J.T.; Limatola, N.; Vasilev, F.; Santella, L. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem. Biophys. Res. Commun. 2014, 450, 1166–1174. [Google Scholar] [CrossRef]
- Eddy, E.M.; Shapiro, B.M. Changes in the topography of the sea urchin egg after fertilization. J. Cell Biol. 1976, 71, 35–48. [Google Scholar] [CrossRef]
- Green, J.D.; Summers, R.G. Formation of the cortical concavity at fertilization in the sea urchin egg. Dev. Growth Differ. 1980, 22, 821–829. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019, 27, 241–249. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Mabuchi, I. Effects of phalloidin microinjection and localization of fluorescein-labeled phalloidin in living sand dollar eggs. Cell Motil. 1982, 2, 103–113. [Google Scholar] [CrossRef]
- Wessel, G.M.; Wong, J.L. Cell surface changes in the egg at fertilization. Mol. Reprod. Dev. 2009, 76, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Buck, W.R. Sources of calcium in sea urchin eggs during the fertilization response. Dev. Biol. 1993, 157, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Lange, K. Microvillar ion channels: Cytoskeletal modulation of ion fluxes. J. Theor. Biol. 2000, 206, 561–584. [Google Scholar] [CrossRef]
- Vasilev, F.; Limatola, N.; Chun, J.T.; Santella, L. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 2019, 15, 757–775. [Google Scholar] [CrossRef]
- Gillot, I.; Ciapa, B.; Payan, P.; Sardet, C. The calcium content of cortical granules and the loss of calcium from sea urchin eggs at fertilization. Dev. Biol. 1991, 146, 396–405. [Google Scholar] [CrossRef]
- Dale, B.; De Belice, L.J.; Taglietti, V. Membrane noise and conductance increase during single spermatozoon-egg interactions. Nature 1978, 275, 217–219. [Google Scholar] [CrossRef]
- Cline, D.J.; Redding, S.E.; Brohawn, S.G.; Psathas, J.N.; Schneider, J.P.; Thorpe, C. New water-soluble phosphines as reductants of peptide and protein disulfide bonds: Reactivity and membrane permeability. Biochemistry 2004, 43, 15195–15203. [Google Scholar] [CrossRef]
- Han, J.C.; Han, G.Y. A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal. Biochem. 1994, 220, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Getz, E.B.; Xiao, M.; Chakrabarty, T.; Cooke, R.; Selvin, P.R. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal. Biochem. 1999, 273, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Byrd, W.; Belisle, B.W. Microvillar elongation following parthenogenetic activation of sea urchin eggs. Exp. Cell Res. 1985, 159, 211–223. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Chun, J.T. Unpublished Data, Department of Research Infrastructures for Marine Biological Resources, Stazione Zoologica Anton Dohrn: Napoli, Italy, 2021; manuscript in preparation.
- Nusco, G.A.; Chun, J.T.; Ercolano, E.; Lim, D.; Gragnaniello, G.; Kyozuka, K.; Santella, L. Modulation of calcium signalling by the actin-binding protein cofilin. Biochem. Biophys. Res. Commun. 2006, 348, 109–114. [Google Scholar] [CrossRef]
- Puppo, A.; Chun, J.T.; Gragnaniello, G.; Garante, E.; Santella, L. Alteration of the cortical actin cytoskeleton deregulates Ca2+ signaling, monospermic fertilization, and sperm entry. PLoS ONE 2008, 3, e3588. [Google Scholar] [CrossRef]
- Chun, J.T.; Vasilev, F.; Santella, L. Antibody against the actin-binding protein depactin attenuates Ca2+ signaling in starfish eggs. Biochem. Biophys. Res. Commun. 2013, 441, 301–307. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Altered actin cytoskeleton in ageing eggs of starfish affects fertilization process. Exp. Cell Res. 2019, 381, 179–190. [Google Scholar] [CrossRef]
- Fisher, G.W.; Rebhun, L.I. Sea urchin egg cortical granule exocytosis is followed by a burst of membrane retrieval via uptake into coated vesicles. Dev. Biol. 1983, 99, 456–472. [Google Scholar] [CrossRef]
- Smith, R.M.; Baibakov, B.; Ikebuchi, Y.; White, B.H.; Lambert, N.A.; Kaczmarek, L.K.; Vogel, S.S. Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J. Cell Biol. 2000, 148, 755–767. [Google Scholar] [CrossRef]
- Terasaki, M. Visualization of exocytosis during sea urchin egg fertilization using confocal microscopy. J. Cell Sci. 1995, 108, 2293–2300. [Google Scholar] [CrossRef]
- Emans, N.; Zimmermann, S.; Fischer, R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 2002, 14, 71–86. [Google Scholar] [CrossRef]
- Nedeva, I.; Koripelly, G.; Caballero, D.; Chièze, L.; Guichard, B.; Romain, B.; Pencreach, E.; Lehn, J.M.; Carlier, M.F.; Riveline, D. Synthetic polyamines promote rapid lamellipodial growth by regulating actin dynamics. Nat. Commun. 2013, 4, 2165. [Google Scholar] [CrossRef] [PubMed]
- Oriol-Audit, C. Polyamine-induced actin polymerization. Eur. J. Biochem. 1978, 87, 371–376. [Google Scholar] [CrossRef]
- Wallace, H.M. Polyamines: Specific metabolic regulators or multifunctional polycations? Biochem. Soc. Trans. 1998, 26, 569–571. [Google Scholar] [CrossRef]
- Hamaguchi, M.S.; Hiramoto, Y. Fertilization process in the heart-urchin, clypeaster japonicus observed with a differential interference microscope. Dev. Growth Differ. 1980, 22, 517–530. [Google Scholar] [CrossRef]
- Holy, J.; Schatten, G. Spindle pole centrosomes of sea urchin embryos are partially composed of material recruited from maternal stores. Dev. Biol. 1991, 147, 343–353. [Google Scholar] [CrossRef]
- Zarski, D.; Krejszeff, S.; Palińska, K.; Targońska, K.; Kupren, K.; Fontaine, P.; Kestemont, P.; Kucharczyk, D. Cortical reaction as an egg quality indicator in artificial reproduction of pikeperch, Sander lucioperca. Reprod. Fertil. Dev. 2012, 24, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.; Lucero, A.J.; Shuster, C.B. Calcium-responsive contractility during fertilization in sea urchin eggs. Dev. Dyn. 2006, 235, 1042–1052. [Google Scholar] [CrossRef]
- Santella, L. Polyspermy-preventing mechanisms in sea urchin eggs: New developments for an old problem. Biochem. Biophys. Res. Commun. 2019, 520, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Cline, C.A.; Schatten, G. Microfilaments during sea urchin fertilization: Fluorescence detection with rhodaminyl phalloidin. Gamete Res. 1986, 14, 277–291. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Effects of salinity and pH of seawater on the reproduction of the sea urchin Paracentrotus lividus. Biol. Bull. 2020, 239, 13–23. [Google Scholar] [CrossRef]
- Limatola, N.; Bertocci, I.; Chun, J.T.; Musco, L.; Munari, M.; Caramiello, D.; Danovaro, R.; Santella, L. Oxygen supersaturation mitigates the impact of the regime of contaminated sediment reworking on sea urchin fertilization process. Mar. Environ. Res. 2020, 158, 104951. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Fertilization and development of Arbacia lixula eggs are affected by osmolality conditions. Biosystems 2021, 206, 104448. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374. [Google Scholar] [CrossRef]
- Huber, P.J.; Brunner, U.T.; Schaub, M.C. Disulfide formation within the regulatory light chain of skeletal muscle myosin. Biochemistry 1989, 28, 9116–9123. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, S. Freezing of actin. Reversible oxidation of a sulfhydryl group and structural change. J. Biochem. 1976, 80, 595–609. [Google Scholar] [CrossRef]
- Tang, J.X.; Janmey, P.A.; Stossel, T.P.; Ito, T. Thiol oxidation of actin produces dimers that enhance the elasticity of the F-actin network. Biophys. J. 1999, 76, 2208–2215. [Google Scholar] [CrossRef]
- Grintsevich, E.E.; Phillips, M.; Pavlov, D.; Phan, M.; Reisler, E.; Muhlrad, A. Antiparallel dimer and actin assembly. Biochemistry 2010, 49, 3919–3927. [Google Scholar] [CrossRef][Green Version]
- Schatten, G.; Mazia, D. The surface events of fertilization: The movements of the spermatozoon through the sea urchin egg surface and the roles of the surface layers. J. Supramol. Struct. 1976, 5, 343–369. [Google Scholar] [CrossRef]
- Schatten, G.; Mazia, D. The penetration of the spermatozoon through the sea urchin egg surface at fertilization. Observations from the outside on whole eggs and from the inside on isolated surfaces. Exp. Cell Res. 1976, 98, 325–337. [Google Scholar] [CrossRef]
- Just, E.E. The Biology of the Cell Surface; P. Blakiston’s Son & Co.: Philadelphia, PA, USA, 1939. [Google Scholar]
| Frequency (%) | NSW | 10mM DTT | DTT/WASH | 0.5mM TCEP | TCEP/WASH |
|---|---|---|---|---|---|
| Mean | 4 | 11 | 60 *,# | 0 | 0 |
| SD | 4.18 | 21.9 | 35.9 | 0 | 0 |
| N | 5 | 5 | 5 | 1 | 1 |
| Sperm Per Egg | NSW | 10mM DTT | DTT/WASH | 0.5mM TCEP | TCEP/WASH |
|---|---|---|---|---|---|
| Mean | 1.04 | 1.13 | 2.38 * | 1 | 1 |
| SD | 0.2 | 0.42 | 1.62 | 0 | 0 |
| n | 100 | 100 | 100 | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limatola, N.; Chun, J.T.; Cherraben, S.; Schmitt, J.-L.; Lehn, J.-M.; Santella, L. Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin. Cells 2021, 10, 3573. https://doi.org/10.3390/cells10123573
Limatola N, Chun JT, Cherraben S, Schmitt J-L, Lehn J-M, Santella L. Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin. Cells. 2021; 10(12):3573. https://doi.org/10.3390/cells10123573
Chicago/Turabian StyleLimatola, Nunzia, Jong Tai Chun, Sawsen Cherraben, Jean-Louis Schmitt, Jean-Marie Lehn, and Luigia Santella. 2021. "Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin" Cells 10, no. 12: 3573. https://doi.org/10.3390/cells10123573
APA StyleLimatola, N., Chun, J. T., Cherraben, S., Schmitt, J.-L., Lehn, J.-M., & Santella, L. (2021). Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin. Cells, 10(12), 3573. https://doi.org/10.3390/cells10123573








