Structural and (Pseudo-)Enzymatic Properties of Neuroglobin: Its Possible Role in Neuroprotection
Abstract
1. Introduction
2. The Ngb Structure
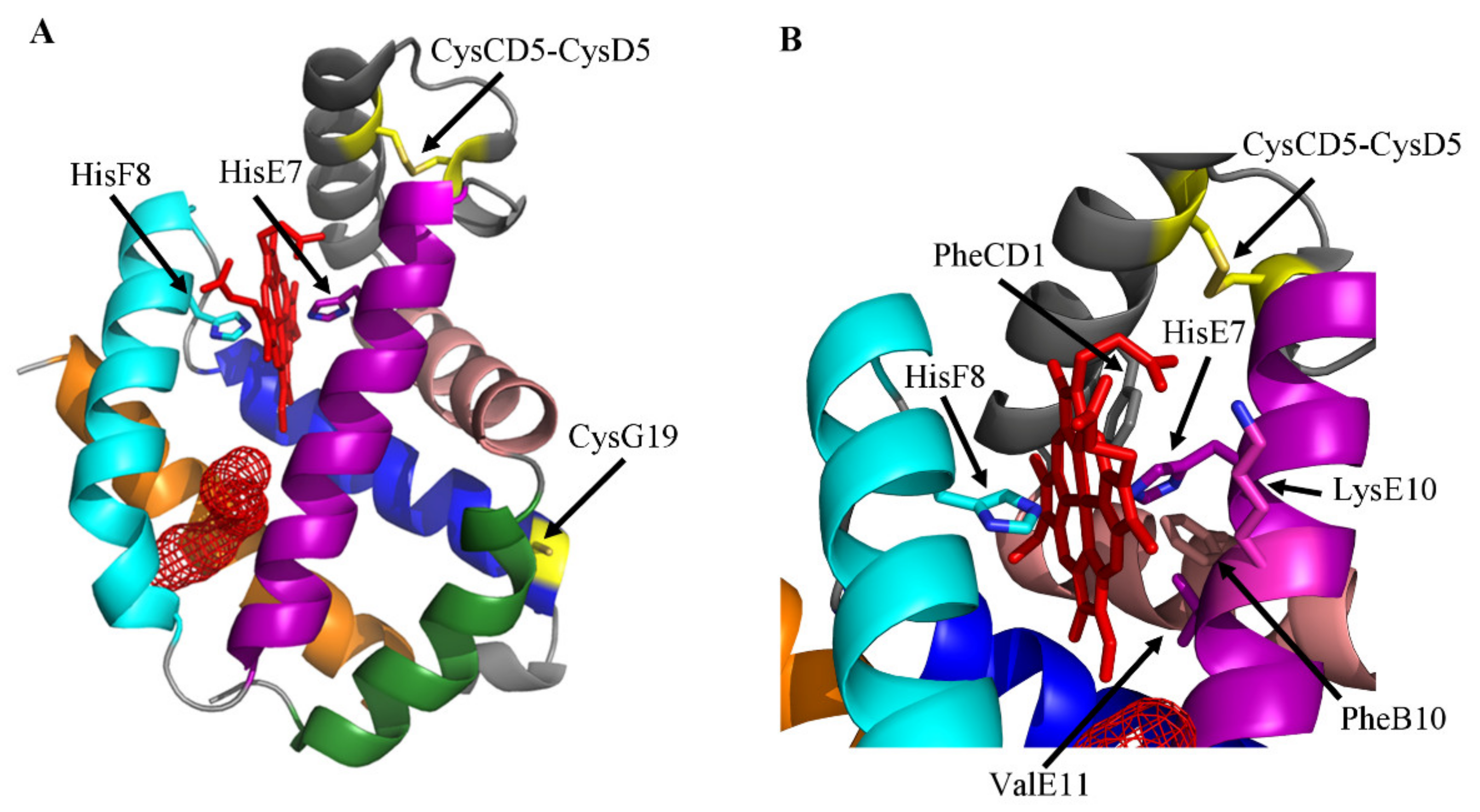
3. Ngb (Pseudo-)Enzymatic Properties
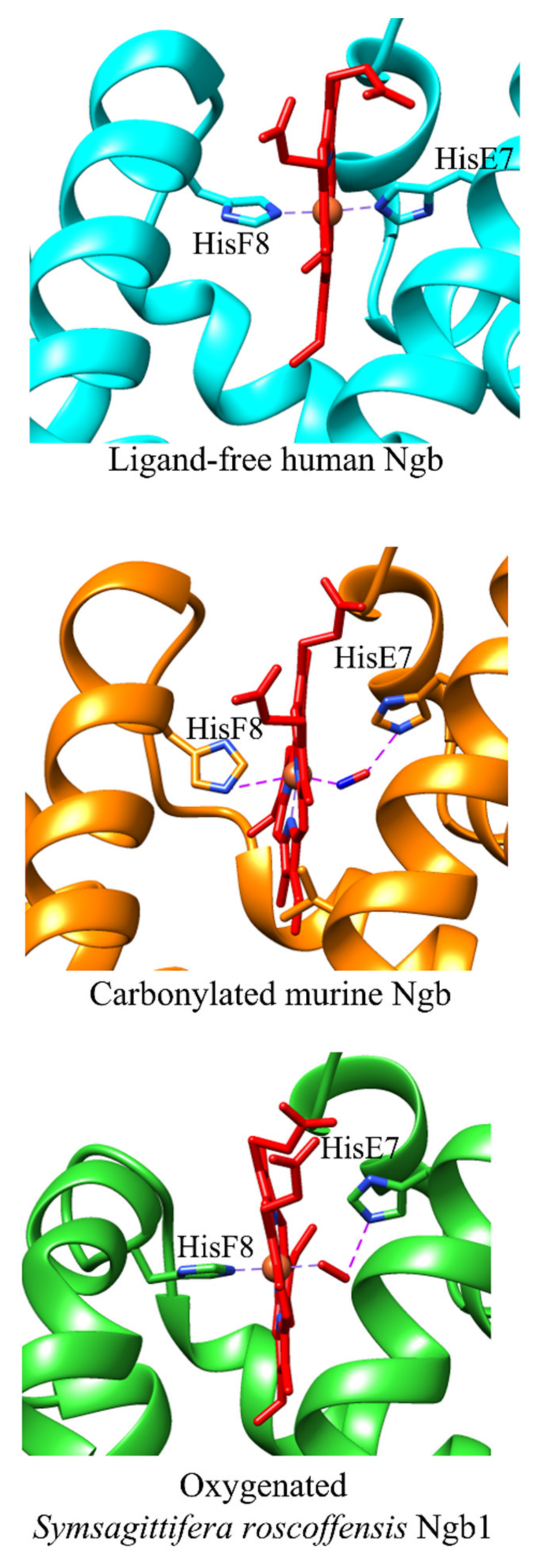
4. The Cleavage of the HisE7-Heme-Fe Bond Modulates the Ngb Reactivity
5. Ngb(II) Oxygenation
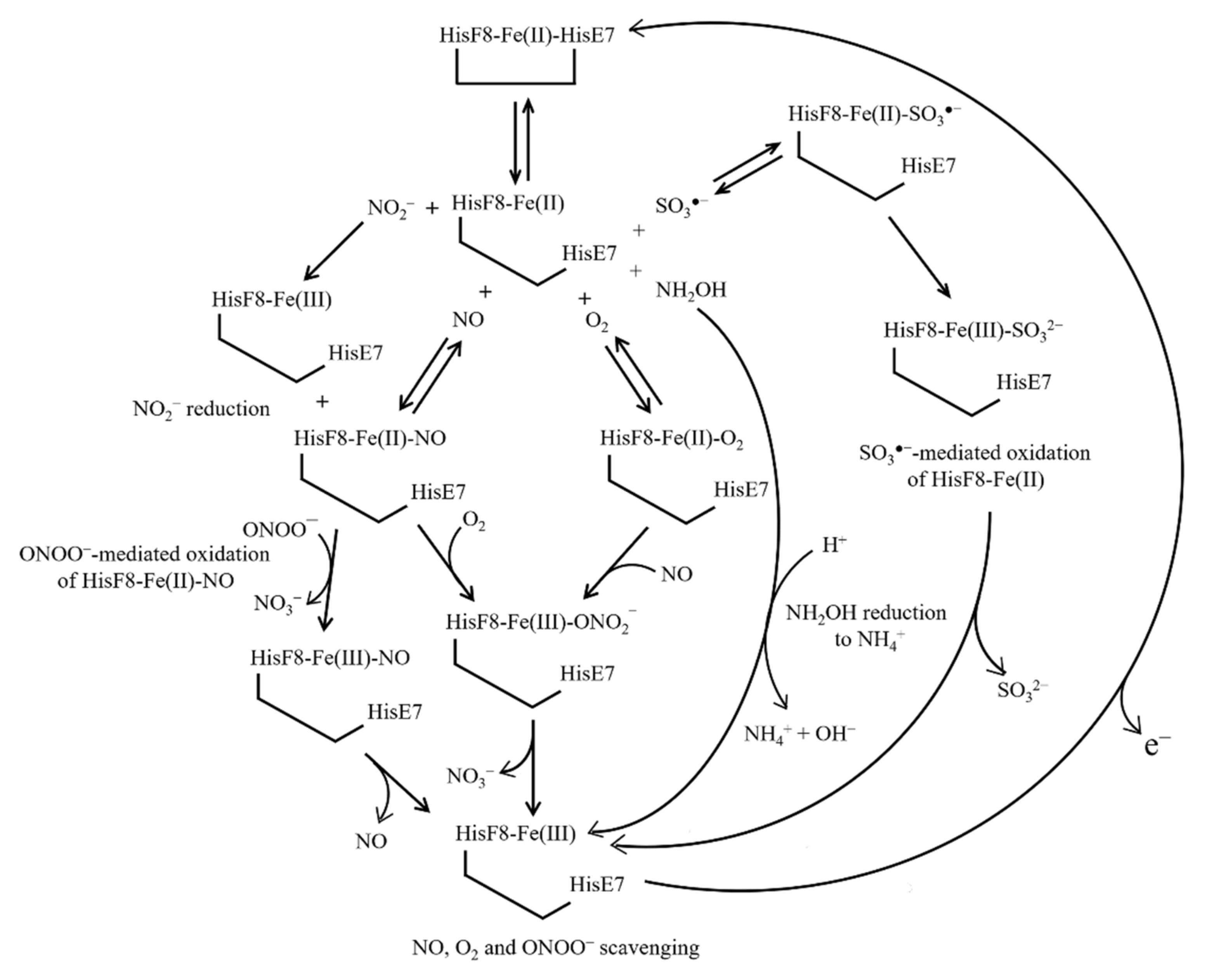
6. Ngb(II) Nitrosylation
7. NO and O2 Scavenging from Ngb(II)–O2 and Ngb(II)–NO
8. NO2− Reduction by Ngb(II)
9. NH2OH Reduction by Ngb(II)
10. Ngb(II) Oxidation by ONOO− and SO3●−
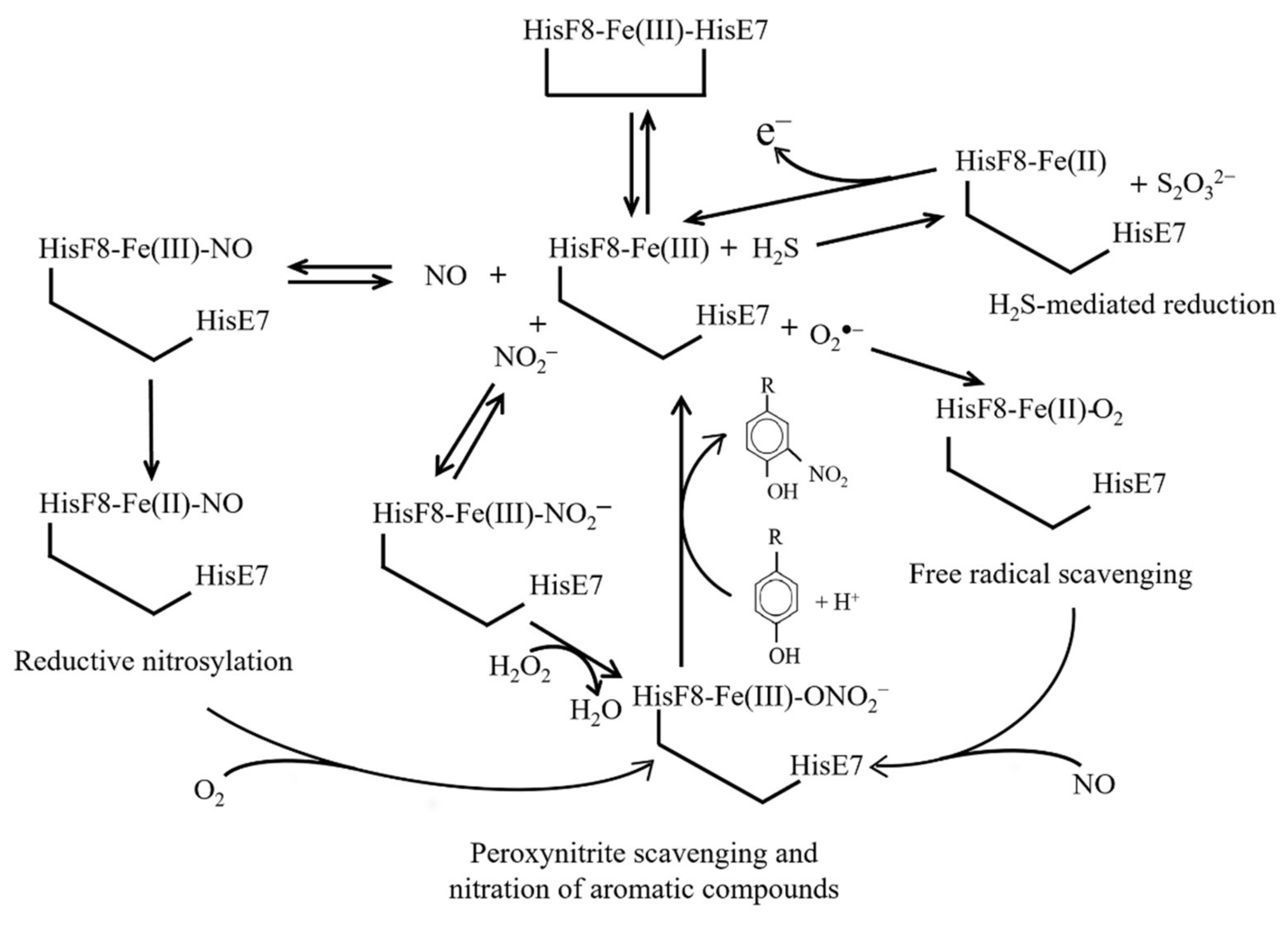
11. Reductive Nitrosylation of Ngb(III)
12. H2S-Mediated Reduction of Ngb(III)
13. Free Radical Scavenging from Ngb(III)
14. Nitration of Aromatic Compounds by Ngb(III)-NO2−
15. Possible Patho-Physiological Roles of Neuroglobin
16. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adenovirus associated vector |
| Adgb | androglobin |
| Cygb | cytoglobin |
| Hb | hemoglobin |
| Mb | myoglobin |
| Ngb(II) | ferrous Ngb |
| Ngb(III) | ferric Ngb |
| GbE | globin E |
| GbX | globin X |
| GbY | globin Y |
| Mb | myoglobin |
| Ngb | neuroglobin |
| mice Ngb-Tg | transgenic mice overexpressing Ngb |
| RGC | retinal ganglion cell |
References
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland Publishing, Co.: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Perutz, M.F. Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Annu. Rev. Biochem. 1979, 48, 327–386. [Google Scholar] [CrossRef]
- Bunn, H.F.; Forget, B.G. Hemoglobin: Molecular, Genetic and Clinical Aspects; W.B. Saunders Co.: Philadelphia, PA, USA, 1986. [Google Scholar]
- Bolognesi, M.; Bordo, D.; Rizzi, M.; Tarricone, C.; Ascenzi, P. Nonvertebrate hemoglobins: Structural bases for reactivity. Progr. Biophys. Mol. Biol. 1997, 68, 29–68. [Google Scholar] [CrossRef]
- Gilles-Gonzalez, M.A.; Gonzalez, G. Heme-based sensors: Defining characteristics, recent developments, and regulatory hypotheses. J. Inorg. Biochem. 2005, 99, 1–22. [Google Scholar] [CrossRef]
- Pesce, A.; Bolognesi, M.; Nardini, M. The diversity of 2/2 (truncated) globins. Adv. Microb. Physiol. 2013, 63, 49–78. [Google Scholar] [CrossRef]
- Pesce, A.; Bolognesi, M.; Nardini, M. Protoglobin: Structure and ligand-binding properties. Adv. Microb. Physiol. 2013, 63, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Brunori, M. A molecule for all seasons: The heme. J. Porphyr. Phthalocyanines 2016, 29, 1–16. [Google Scholar] [CrossRef]
- Vinogradov, S.N.; Hoogewijs, D.; Bailly, X.; Arredondo-Peter, R.; Gough, J.; Dewilde, S.; Moens, L.; Vanfleteren, J.R. A phylogenomic profile of globins. BMC Evol. Biol. 2006, 6, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.N.; Moens, L. Diversity of globin function: Enzymatic, transport, storage, and sensing. J. Biol. Chem. 2008, 283, 8773–8777. [Google Scholar] [CrossRef]
- Wajcman, H.; Kiger, L.; Marden, M.C. Structure and function evolution in the superfamily of globins. Crit. Rev. Biol. 2009, 332, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Burmester, T.; Hankeln, T. Function and evolution of vertebrate globins. Acta Physiol. 2014, 211, 501–514. [Google Scholar] [CrossRef]
- Keppner, A.; Maric, D.; Correia, M.; Koay, T.W.; Orlando, I.M.C.; Vinogradov, S.N.; Hoogewijs, D. Lessons from the post-genomic era: Globin diversity beyond oxygen binding and transport. Redox Biol. 2020, 37, 101687–101705. [Google Scholar] [CrossRef] [PubMed]
- Burmester, T.; Weich, B.; Reinhardt, S.; Hankeln, T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523. [Google Scholar] [CrossRef]
- Burmester, T.; Ebner, B.; Weich, B.; Hankeln, T. Cytoglobin: A novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 2002, 19, 416–421. [Google Scholar] [CrossRef]
- Trent, J.T., III; Hargrove, M.S. A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 2002, 277, 19538–19545. [Google Scholar] [CrossRef]
- Fuchs, C.; Burmester, T.; Hankeln, T. The amphibian globin gene repertoire as revealed by the Xenopus genome. Genome Res. 2006, 112, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kugelstadt, D.; Haberkamp, M.; Hankeln, T.; Burmester, T. Neuroglobin, cytoglobin, and a novel, eye-specific globin from chicken. Biochem. Biophys. Res. Commun. 2004, 325, 719–725. [Google Scholar] [CrossRef]
- Roesner, A.; Fuchs, C.; Hankeln, T.; Burmester, T. A globin gene of ancient evolutionary origin in lower vertebrates: Evidence for two distinct globin families in animals. Mol. Biol. Evol. 2005, 22, 12–20. [Google Scholar] [CrossRef]
- Hoogewijs, D.; Ebner, B.; Germani, F.; Hoffmann, F.G.; Fabrizius, A.; Moens, L.; Burmester, T.; Dewilde, S.; Storz, J.F.; Vinogradov, S.N.; et al. Androglobin: A chimeric globin in metazoans that is preferentially expressed in mammalian testes. Mol. Biol. Evol. 2012, 29, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Pesce, A.; Couture, M.; Dewilde, S.; Guertin, M.; Yamauchi, K.; Ascenzi, P.; Moens, L.; Bolognesi, M. A novel two-over-two alpha-helical sandwich fold is characteristic of the truncated hemoglobin family. EMBO J. 2000, 19, 2424–2434. [Google Scholar] [CrossRef]
- Montfort, W.R.; Weichsel, A.; Andersen, J.F. Nitrophorins and related antihemostatic lipocalins from Rhodnius prolixus and other blood-sucking arthropods. Biochim. Biophys. Acta 2000, 1482, 110–118. [Google Scholar] [CrossRef]
- Andersen, J.F. Structure and mechanism in salivary proteins from blood-feeding arthropods. Toxicon 2010, 56, 1120–1129. [Google Scholar] [CrossRef]
- Champagne, D.E. Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol. Haemos. Thromb. 2005, 34, 221–227. [Google Scholar] [CrossRef]
- De Simone, G.; Ascenzi, P.; Polticelli, F. Nitrobindin: An ubiquitous family of all β-barrel heme-proteins. IUBMB Life 2016, 68, 423–428. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Ascenzi, P.; di Masi, A.; Polticelli, F. Nitrophorins and nitrobindins: Structure and function. Biomol. Concepts 2017, 8, 105–118. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Vita, G.M.; Polticelli, F.; Pesce, A.; Nardini, M.; Bolognesi, M.; Ciaccio, C.; Coletta, M.; Turilli, E.S.; et al. Mycobacterial and human nitrobindins: Structure and function. Antioxid. Redox Signal. 2020, 33, 229–246. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ciaccio, C.; Coletta, M.; Ascenzi, P. NO Scavenging through reductive nitrosylation of ferric Mycobacterium tuberculosis and Homo sapiens nitrobindins. Int. J. Mol. Sci. 2021, 21, 9395. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Pesce, A.; Bolognesi, M.; Ciaccio, C.; Tognaccini, L.; Smulevich, G.; Abbruzzetti, S.; Viappiani, C.; Bruno, S.; et al. Mycobacterial and human ferrous nitrobindins: Spectroscopic and reactivity properties. Int. J. Mol. Sci. 2021, 22, 1674. [Google Scholar] [CrossRef] [PubMed]
- Tilton, R.F.; Kuntz, I.D.; Petsko, G.A. Cavities in proteins: Structure of a metmyoglobin xenon complex solved at 1.9 Å. Biochemistry 1984, 23, 2849–2857. [Google Scholar] [CrossRef]
- Milani, M.; Pesce, A.; Ouellet, Y.; Ascenzi, P.; Guertin, M.; Bolognesi, M. Mycobacterium tuberculosis hemoglobin N displays a protein tunnel suited for O2 diffusion to the heme. EMBO J. 2001, 20, 3902–3909. [Google Scholar] [CrossRef]
- Pesce, A.; Dewilde, S.; Nardini, M.; Moens, L.; Ascenzi, P.; Hankeln, T.; Burmester, T.; Bolognesi, M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure 2003, 11, 1087–1095. [Google Scholar] [CrossRef]
- De Sanctis, D.; Dewilde, S.; Pesce, A.; Moens, L.; Ascenzi, P.; Hankeln, T.; Burmester, T.; Bolognesi, M. Mapping protein matrix cavities in human cytoglobin through Xe atom binding. Biochem. Biophys. Res. Commun. 2004, 316, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Vallone, B.; Nienhaus, K.; Brunori, M.; Nienhaus, G.U. The structure of murine neuroglobin: Novel pathways for ligand migration and binding. Proteins 2004, 56, 85–92. [Google Scholar] [CrossRef]
- Vallone, B.; Nienhaus, K.; Matthes, A.; Brunori, M.; Nienhaus, G.U. The structure of carbonmonoxy neuroglobin reveals a heme-sliding mechanism for control of ligand affinity. Proc. Natl. Acad. Sci. USA 2004, 101, 17351–17356. [Google Scholar] [CrossRef]
- Nardini, M.; Pesce, A.; Thijs, L.; Saito, J.A.; Dewilde, S.; Alam, M.; Ascenzi, P.; Coletta, M.; Ciaccio, C.; Moens, L.; et al. Archaeal protoglobin structure indicates new ligand diffusion paths and modulation of haem-reactivity. EMBO Rep. 2008, 9, 157–163. [Google Scholar] [CrossRef]
- Arroyo-Mañez, P.; Bikiel, D.E.; Boechi, L.; Capece, L.; Di Lella, S.; Estrin, D.A.; Martí, M.A.; Moreno, D.M.; Nadra, A.D.; Petruk, A.A. Protein dynamics and ligand migration interplay as studied by computer simulation. Biochim. Biophys. Acta 2011, 1814, 1054–1064. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Fattibene, P.; Ciaccio, C.; Platas-Iglesias, C.; Coletta, M.; Pesce, A.; Ascenzi, P. Oxygen-mediated oxidation of ferrous nitrosylated Nitrobindins. J. Inorg. Biochem. 2021, 224, 111579–111588. [Google Scholar] [CrossRef]
- Karplus, M.; Petsko, G.A. Molecular dynamics simulations in biology. Nature 1990, 347, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Karplus, M. Allostery and cooperativity revisited. Protein Sci. 2008, 17, 1295–1307. [Google Scholar] [CrossRef]
- Elber, R. Ligand diffusion in globins: Simulations versus experiment. Curr. Opin. Struct. Biol. 2010, 20, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, Z.; Cho, K.-S.; Chen, H.; Malik, M.T.A.; Chen, X.; Lo, E.H.; Wang, X.; Chen, D.F. Neuroglobin is an endogenous neuroprotectant for retinal ganglion cells against glaucomatous damage. Am. J. Pathol. 2011, 179, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Augustin, S.; Cwerman-Thibault, H.; Bouaita, A.; Forster, V.; Célier, C.; Rustin, P.; Marden, M.C.; Sahel, J.-A.; Corral-Debrinski, M. Neuroglobin involvement in respiratory chain function and retinal ganglion cell integrity. Biochim. Biophys. Acta 2012, 1823, 2261–2273. [Google Scholar] [CrossRef]
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From structure to function in health and disease. Mol. Aspects Med. 2016, 52, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Cipolletti, M.; Marino, M. Compensatory role of neuroglobin in nervous and non-nervous cancer cells in response to the nutrient deprivation. PLoS ONE 2017, 12, e0189179. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M. Neuroglobin: A novel player in the oxidative stress response of cancer cells. Oxid. Med. Cell. Longev. 2019, 2019, 6315034. [Google Scholar] [CrossRef]
- Van Acker, Z.P.; Van Raemdonck, G.A.; Logie, E.; Van Acker, S.I.; Baggerman, G.; Van den Berghe, W.; Ponsaerts, P.; Dewilde, S. Connecting the dots in the neuroglobin-protein interaction network of an unstressed and ferroptotic cell death neuroblastoma model. Cells 2019, 8, 873. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Cracco, P.; Montalesi, E.; Solar Fernandez, V.; Stuart, J.A.; Marino, M. Neuroglobin and mitochondria: The impact on neurodegenerative diseases. Arch. Biochem. Biophys. 2021, 701, 10882–108831. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Aslani, S.; Barreto, G.E.; Báez-Jurado, E.; Kiaie, N.; Jamialahmadi, T.; Sahebkar, A. The potential of mitochondrial modulation by neuroglobin in treatment of neurological disorders. Free Radic. Biol. Med. 2021, 162, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Jager, M.; Laguerre, L.; Kiger, L.; Correc, G.; Leroux, C.; Vinogradov, S.; Czjzek, M.; Marden, M.C.; Bailly, X. Neuroglobins, pivotal proteins associated with emerging neural systems and precursors of metazoan globin diversity. J. Biol. Chem. 2013, 288, 6957–6967. [Google Scholar] [CrossRef]
- Bashford, D.; Chothia, C.; Lesk, A.M. Determinants of a protein fold. Unique features of the globin amino acid sequences. J. Mol. Biol. 1987, 196, 199–216. [Google Scholar] [CrossRef]
- Du, W.; Syvitski, R.; Dewilde, S.; Moens, L.; La Mar, G.N. Solution 1H NMR characterization of equilibrium heme orientational disorder with functional consequences in mouse neuroglobin. J. Am. Chem. Soc. 2003, 125, 8080–8081. [Google Scholar] [CrossRef]
- Guimarães, B.G.; Hamdane, D.; Lechauve, C.; Marden, M.C.; Golinelli-Pimpaneau, B. The crystal structure of wild-type human brain neuroglobin reveals flexibility of the disulfide bond that regulates oxygen affinity. Acta Crystallogr. D. Biol. Crystallogr. 2014, 70, 1005–1014. [Google Scholar] [CrossRef]
- Walker, F.A. The heme environment of mouse Neuroglobin: Histidine imidazole plane orientations obtained from solution NMR and EPR spectroscopy as compared with X-ray crystallography. J. Biol. Inorg. Chem. 2006, 11, 391–397. [Google Scholar] [CrossRef]
- Perutz, M.F. Myoglobin and haemoglobin: Role of distal residues in reactions with haem ligands. Trends Biochem. Sci. 1989, 14, 42–44. [Google Scholar] [CrossRef]
- Brancaccio, A.; Cutruzzola, F.; Allocatelli, C.T.; Brunori, M.; Smerdon, S.J.; Wilkinson, A.J.; Dou, Y.; Keenan, D.; Ikeda-Saito, M.; Brantley, R.E., Jr.; et al. Structural factors governing azide and cyanide binding to mammalian metmyoglobins. J. Biol. Chem. 1994, 269, 13843–13853. [Google Scholar] [CrossRef]
- Draghi, F.; Miele, A.E.; Travaglini-Allocatelli, C.; Vallone, B.; Brunori, M.; Gibson, Q.H.; Olson, J.S. Controlling ligand binding in myoglobin by mutagenesis. J. Biol. Chem. 2002, 277, 7509–7519. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, T.; Mueller, U.; Schulze, J.; Brunori, M.; Vallone, B. The structure of neuroglobin at high Xe and Kr pressure reveals partial conservation of globin internal cavities. Biophys. J. 2009, 97, 1700–1708. [Google Scholar] [CrossRef]
- Abraini, J.H.; Marassio, G.; David, H.N.; Vallone, B.; Prangé, T.; Colloc’h, N. Crystallographic studies with xenon and nitrous oxide provide evidence for protein-dependent processes in the mechanisms of general anesthesia. Anesthesiology 2014, 121, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Avella, G.; Ardiccioni, C.; Scaglione, A.; Moschetti, T.; Rondinelli, C.; Montemiglio, L.C.; Savino, C.; Giuffrè, A.; Brunori, M.; Vallone, B. Engineering the internal cavity of Neuroglobin demonstrates the role of haem-sliding mechanism. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 1640–1648. [Google Scholar] [CrossRef]
- Anselmi, M.; Brunori, M.; Vallone, B.; Di Nola, A. Molecular dynamics simulation of deoxy and carboxy murine Neuroglobin in water. Biophys. J. 2007, 93, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, M.; Brunori, M.; Vallone, B.; Di Nola, A. Molecular dynamics simulation of the neuroglobin crystal: Comparison with the simulation in solution. Biophys. J. 2008, 95, 4157–4162. [Google Scholar] [CrossRef][Green Version]
- Lutz, S.; Nienhaus, K.; Nienhaus, G.U.; Meuwly, M. Ligand migration between internal docking sites in photodissociated carbonmonoxy Neuroglobin. J. Phys. Chem. B 2009, 113, 15334–15343. [Google Scholar] [CrossRef]
- Nienhaus, K.; Lutz, S.; Meuwly, M.; Nienhaus, G.U. Structural identification of spectroscopic substates in neuroglobin. Chem. Phys. Chem. 2010, 11, 119–129. [Google Scholar] [CrossRef]
- Anselmi, M.; Di Nola, A.; Amadei, A. Kinetics of carbon monoxide migration and binding in solvated Neuroglobin as revealed by molecular dynamics simulations and quantum mechanical calculations. J. Phys. Chem. B 2011, 115, 2436–2446. [Google Scholar] [CrossRef]
- Nienhaus, K.; Lutz, S.; Meuwly, M.; Nienhaus, G.U. Reaction-pathway selection in the structural dynamics of a heme protein. Chemistry 2013, 19, 3558–3562. [Google Scholar] [CrossRef]
- Lutz, S.; Meuwly, M. Structural characterization of spectroscopic substates in carbonmonoxy neuroglobin. Faraday Discuss. 2011, 150, 375–390. [Google Scholar] [CrossRef]
- Stourac, J.; Vavra, O.; Kokkonen, P.; Filipovic, J.; Pinto, G.; Brezovsky, J.; Damborsky, J.; Bednar, D. Caver Web 1.0: Identification of tunnels and channels in proteins and analysis of ligand transport. Nucleic Acids Res. 2019, 47, W414–W422. [Google Scholar] [CrossRef] [PubMed]
- Lilkova, E.; Petkov, P.; Ilieva, N.; Litov, L. The PyMOL molecular graphics system version 2.0, Software. Schrödinger LLC.: New York, NY, USA, 2015. [Google Scholar]
- Brunori, M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem. Sci. 2001, 26, 21–23. [Google Scholar] [CrossRef]
- Schmidt, M.; Giessl, A.; Laufs, T.; Hankeln, T.; Wolfrum, U.; Burmester, T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J. Biol. Chem. 2003, 278, 1932–1935. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M.; Vallone, B. A globin for the brain. FASEB J. 2006, 20, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Gustincich, S.; Marino, M. Mammalian nerve globins in search of functions. IUBMB Life 2014, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, D.; Kiger, L.; Dewilde, S.; Green, B.N.; Pesce, A.; Uzan, J.; Burmester, T.; Hankeln, T.; Bolognesi, M.; Moens, L.; et al. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003, 278, 51713–51721. [Google Scholar] [CrossRef]
- Vinck, E.; Van Doorslaer, S.; Dewilde, S.; Moens, L. Structural change of the heme pocket due to disulfide bridge formation is significantly larger for neuroglobin than for cytoglobin. J. Am. Chem. Soc. 2004, 126, 4516–4517. [Google Scholar] [CrossRef]
- Hamdane, D.; Kiger, L.; Hoa, G.H.B.; Dewilde, S.; Uzan, J.; Burmester, T.; Hankeln, T.; Moens, L.; Marden, M.C. High pressure enhances hexacoordination in neuroglobin and other globins. J. Biol. Chem. 2005, 280, 36809–36814. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, T.; Tejero, J.; Chen, B.B.; Blood, A.B.; Frizzell, S.; Shapiro, C.; Tiso, M.; Hood, B.L.; Wang, X.; Zhao, X.; et al. 14-3-3 binding and phosphorylation of neuroglobin during hypoxia modulate six-to-five heme pocket coordination and rate of nitrite reduction to nitric oxide. J. Biol. Chem. 2011, 286, 42679–42689. [Google Scholar] [CrossRef] [PubMed]
- Kiger, L.; Uzan, J.; Dewilde, S.; Burmester, T.; Hankeln, T.; Moens, L.; Hamdane, D.; Baudin-Creuza, V.; Marden, M.C. Neuroglobin ligand binding kinetics. IUBMB Life 2004, 56, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M.; Giuffrè, A.; Nienhaus, K.; Nienhaus, G.U.; Scandurra, F.M.; Vallone, B. Neuroglobin, nitric oxide, and oxygen: Functional pathways and conformational changes. Proc. Natl. Acad. Sci. USA 2005, 102, 8483–8488. [Google Scholar] [CrossRef]
- Giuffrè, A.; Moschetti, T.; Vallone, B.; Brunori, M. Neuroglobin: Enzymatic reduction and oxygen affinity. Biochem. Biophys. Res. Commun. 2008, 367, 893–898. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, D.; Pesce, A.; Nardini, M.; Bolognesi, M.; Bocedi, A.; Ascenzi, P. Structure-function relationships in the growing hexa-coordinate hemoglobin sub-family. IUBMB Life 2004, 56, 643–651. [Google Scholar] [CrossRef]
- Pesce, A.; De Sanctis, D.; Nardini, M.; Dewilde, S.; Moens, L.; Hankeln, T.; Burmester, T.; Ascenzi, P.; Bolognesi, M. Reversible hexa- to penta-coordination of the heme Fe atom modulates ligand binding properties of neuroglobin and cytoglobin. IUBMB Life 2004, 56, 657–664. [Google Scholar] [CrossRef]
- Kakar, S.; Hoffman, F.G.; Storz, J.F.; Fabian, M.; Hargrove, M.S. Structure and reactivity of hexacoordinate hemoglobins. Biophys. Chem. 2010, 152, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Dewilde, S.; Kiger, L.; Burmester, T.; Hankeln, T.; Baudin-Creuza, V.; Aerts, T.; Marden, M.C.; Caubergs, R.; Moens, L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 2001, 276, 38949–38955. [Google Scholar] [CrossRef] [PubMed]
- Trent, J.T., III; Watts, R.A.; Hargrove, M.S. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J. Biol. Chem. 2001, 276, 30106–30110. [Google Scholar] [CrossRef]
- Fago, A.; Mathews, A.J.; Dewilde, S.; Moens, L.; Brittain, T. The reactions of Neuroglobin with CO: Evidence for two forms of the ferrous protein. J. Inorg. Biochem. 2006, 100, 1339–1343. [Google Scholar] [CrossRef]
- Smagghe, B.J.; Sarath, G.; Ross, E.; Hilbert, J.; Hargrove, M.S. Slow ligand binding kinetics dominate ferrous hexacoordinate hemoglobin reactivities and reveal differences between plants and other species. Biochemistry 2006, 45, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.H.; Battistoni, A.; Lechauve, C.; Marden, M.C.; Kiger, L.; Desbois, A.; Pilet, E.; de Rosny, E.; Liebl, U. Ultrafast heme-residue bond formation in six-coordinate heme proteins: Implications for functional ligand exchange. Biochemistry 2008, 47, 5718–5723. [Google Scholar] [CrossRef]
- Abbruzzetti, S.; Faggiano, S.; Bruno, S.; Spyrakis, F.; Mozzarelli, A.; Dewilde, S.; Moens, L.; Viappiani, C. Ligand migration through the internal hydrophobic cavities in human neuroglobin. Proc. Natl. Acad. Sci. USA 2009, 106, 18984–18989. [Google Scholar] [CrossRef] [PubMed]
- Skommer, J.; Helbo, S.; Henty, K.; Brittain, T. Ligand binding, reactivity and biological activity of a distal pocket mutant of neuroglobin. Int. J. Biol. Macromol. 2012, 51, 284–290. [Google Scholar] [CrossRef]
- Herold, S.; Fago, A.; Weber, R.E.; Dewilde, S.; Moens, L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J. Biol. Chem. 2004, 279, 22841–22847. [Google Scholar] [CrossRef] [PubMed]
- Bocahut, A.; Derrien, V.; Bernad, S.; Sebban, P.; Sacquin-Mora, S.; Guittet, E.; Lescop, E. Heme orientation modulates histidine dissociation and ligand binding kinetics in the hexacoordinated human neuroglobin. J. Biol. Inorg. Chem. 2013, 18, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, J.; Meuwly, M. Atomistic simulation of adiabatic reactive processes based on multi-state potential energy surfaces. J. Chem. Theory Comput. 2008, 4, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Trashin, S.; de Jong, M.; Luyckx, E.; Dewilde, S.; De Wael, K. Electrochemical evidence for neuroglobin activity on NO at physiological concentrations. J. Biol. Chem. 2016, 291, 18959–18966. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Giacometti, G.M.; Antonini, E.; Rotilio, G.; Brunori, M. Equilibrium and kinetic evidence for a transition between six- and five-coordinate ferrous heme in the nitric oxide derivative of Aplysia myoglobin. J. Biol. Chem. 1981, 256, 5383–5386. [Google Scholar] [CrossRef]
- Giacometti, G.M.; Ascenzi, P.; Bolognesi, M.; Brunori, M. Reactivity of ferric Aplysia myoglobin towards anionic ligands in the acidic region. Proposal for a structural model. J. Mol. Biol. 1981, 146, 363–374. [Google Scholar] [CrossRef]
- Orlowski, S.; Nowak, W. Topology and thermodynamics of gaseous ligands diffusion paths in human neuroglobin. Biosystems 2008, 94, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Bocahut, A.; Bernad, S.; Sebban, P.; Sacquin-Mora, S. Relating the diffusion of small ligands in human Neuroglobin to its structural and mechanical properties. J. Phys. Chem. B 2009, 113, 16257–16267. [Google Scholar] [CrossRef] [PubMed]
- Capece, L.; Marti, M.A.; Bidon-Chanal, A.; Nadra, A.; Luque, F.J.; Estrin, D.A. High pressure reveals structural determinants for globin hexacoordination: Neuroglobin and myoglobin cases. Proteins 2009, 75, 885–894. [Google Scholar] [CrossRef]
- Astudillo, L.; Bernad, S.; Derrien, V.; Sebban, P.; Miksovska, J. Probing the role of the internal disulfide bond in regulating conformational dynamics in neuroglobin. Biophys. J. 2010, 99, L16–L18. [Google Scholar] [CrossRef] [PubMed]
- Bocahut, A.; Bernad, S.; Sebban, P.; Sacquin-Mora, S. Frontier residues lining globin internal cavities present specific mechanical properties. J. Am. Chem. Soc. 2011, 133, 8753–8761. [Google Scholar] [CrossRef]
- Yang, Y.; Allemand, F.; Guca, E.; Vallone, B.; Delbecq, S.; Roumestand, C. 1H, 15N and 13C backbone resonance assignments of murine met-neuroglobin, free and in complex with cyanide. Biomol. NMR Assign. 2015, 9, 153–156. [Google Scholar] [CrossRef]
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef]
- McBryde, F.D.; Malpas, S.C.; Paton, J.F. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol. 2017, 219, 274–287. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain glucose metabolism: Integration of energetics with function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Tang, B.L. Glucose, glycolysis, and neurodegenerative diseases. J. Cell. Physiol. 2020, 235, 7653–7662. [Google Scholar] [CrossRef]
- Fago, A.; Hundahl, C.; Malte, H.; Weber, R.E. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life 2001, 56, 689–696, Erratum in IUBMB Life 2005, 57, 461. [Google Scholar] [CrossRef]
- Fago, A.; Hundahl, C.; Dewilde, S.; Gilany, K.; Moens, L.; Weber, R.E. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin: Molecular mechanisms and physiological significance. J. Biol. Chem. 2004, 279, 44417–44426. [Google Scholar] [CrossRef]
- Uzan, J.; Dewilde, S.; Burmester, T.; Hankeln, T.; Moens, L.; Hamdane, D.; Marden, M.C.; Kiger, L. Neuroglobin and other hexacoordinated hemoglobins show a weak temperature dependence of oxygen binding. Biophys. J. 2004, 87, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, C.; Fago, A.; Dewilde, S.; Moens, L.; Hankeln, T.; Burmester Weber, R.E. Oxygen binding properties of non-mammalian nerve globins. FEBS J. 2006, 273, 1323–1329. [Google Scholar] [CrossRef]
- Hundahl, C.; Kelsen, J.; Kjaer, K.; Rønn, L.C.B.; Weber, R.E.; Geuens, E.; Hay-Schmidt, A.; Nyengaard, A.J.R. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCA in spontaneously hypertensive rats. Brain Res. 2006, 1085, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, S.; Dewilde, S.; Kiger, L.; Nistor, S.V.; Goovaerts, E.; Marden, M.C.; Moens, L. Nitric oxide binding properties of neuroglobin: A characterization by EPR and flash photolysis. J. Biol. Chem. 2003, 278, 4919–4925. [Google Scholar] [CrossRef]
- Nienhaus, K.; Kriegl, J.M.; Nienhaus, G.U. Structural dynamics in the active site of murine neuroglobin and its effects on ligand binding. J. Biol. Chem. 2004, 279, 22944–22952. [Google Scholar] [CrossRef] [PubMed]
- Džoljić, E.; Grbatinić, I.; Kostić, V. Why is nitric oxide important for our brain? Funct. Neurol. 2015, 30, 159–163. [Google Scholar] [CrossRef]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.O.; Xie, L.; Khan, A.A.; Greenberg, D.A. Neuroglobin protects against nitric oxide toxicity. Neurosci. Lett. 2008, 430, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Smagghe, B.J.; Trent, J.T., 3rd; Hargrove, M.S. NO dioxygenase activity in hemoglobins is ubiquitous in vitro but limited by reduction in vivo. PLoS ONE 2008, 30, e2039. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Tejero, J.; Basu, S.; Azarov, I.; Wang, X.; Simplaceanu, V.; Frizzell, S.; Jayaraman, T.; Geary, L.; Shapiro, C.; et al. Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 2011, 286, 18277–18289. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Sparacino-Watkins, C.E.; Ragireddy, V.; Frizzell, S.; Gladwin, M.T. Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Biochemistry 2015, 54, 722–733, Erratum in Biochemistry 2015, 54, 3716. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.G.; Dewilde, S.; Fago, A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J. Inorg. Biochem. 2008, 102, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Sturms, R.; DiSpirito, A.A.; Fulton, D.B.; Hargrove, M.S. Hydroxylamine reduction to ammonium by plant and cyanobacterial hemoglobins. Biochemistry 2011, 50, 10829–10835. [Google Scholar] [CrossRef]
- Athwal, N.S.; Alagurajan, J.; Andreotti, A.H.; Hargrove, M.S. Role of reversible histidine coordination in hydroxylamine reduction by plant hemoglobins (Phytoglobins). Biochemistry 2016, 55, 5809–5817. [Google Scholar] [CrossRef] [PubMed]
- Alagurajan, J.; Singh Athwal, N.; Hargrove, M.S. Steady-state kinetics of phytoglobin-catalyzed reduction of hydroxylamine to ammonium. Biochemistry 2018, 57, 4824–4832. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.R.; Gardner, D.P.; Gardner, A.P. Globins scavenge sulfur trioxide anion radical. J. Biol. Chem. 2015, 290, 27204–27214. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef]
- Rizzi, M.; Wittenberg, J.B.; Coda, A.; Fasano, M.; Ascenzi, P.; Bolognesi, M. Structure of the sulfide-reactive hemoglobin from the clam Lucina pectinata. Crystallographic analysis at 1.5 Å resolution. J. Mol. Biol. 1994, 244, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Wittenberg, J.B.; Coda, A.; Ascenzi, P.; Bolognesi, M. Structural bases for sulfide recognition in Lucina pectinata hemoglobin I. J. Mol. Biol. 1996, 258, 1–5. [Google Scholar] [CrossRef]
- Brittain, T.; Yosaatmadja, Y.; Henty, K. The interaction of human neuroglobin with hydrogen sulphide. IUBMB Life 2008, 60, 135–138. [Google Scholar] [CrossRef]
- Ruetz, M.; Kumutima, J.; Lewis, B.E.; Filipovic, M.R.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. A distal ligand mutes the interaction of hydrogen sulfide with human neuroglobin. J. Biol. Chem. 2017, 292, 6512–6528. [Google Scholar] [CrossRef]
- Yamashita, T.; Hafsi, L.; Masuda, E.; Tsujino, H.; Uno, T. Ferric human neuroglobin scavenges superoxide to form oxy adduct. Chem. Pharm. Bull. 2014, 62, 613–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ezhevskaya, M.; Trandafir, F.; Moens, L.; Dewilde, S.; Van Doorslaer, S. EPR investigation of the role of B10 phenylalanine in neuroglobin: Evidence that B10Phe mediates structural changes in the heme region upon disulfide-bridge formation. J. Inorg. Biochem. 2011, 105, 1131–1137. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Ren, C.; Lu, Y.; Gao, Y.; Zheng, X.; Zhang, C. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins 2011, 79, 115–125. [Google Scholar] [CrossRef]
- Sacksteder, C.A.; Qian, W.-J.; Knyushko, T.V.; Wang, H.; Chin, M.H.; Lacan, G.; Melega, W.P.; Camp, D.G., II; Smith, R.D.; Smith, D.J.; et al. Endogenously nitrated proteins in mouse brain: Links to neurodegenerative diseases. Biochemistry 2006, 45, 8009–8022. [Google Scholar] [CrossRef]
- Nicolis, S.; Monzani, E.; Ciaccio, C.; Ascenzi, P.; Moens, L.; Casella, L. Reactivity and endogenous modification by nitrite and hydrogen peroxide: Does human neuroglobin act only as a scavenger? Biochem. J. 2007, 407, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Fordel, E.; Thijs, L.; Martinet, W.; Lenjou, M.; Laufs, T.; Van Bockstaele, D.; Moens, L.; Dewilde, S. Neuroglobin and Cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci. Lett. 2006, 410, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Y.; Sun, J.; Xiang, Y.; Yang, J.; Dai, S.; Zhang, X. Neuroglobin attenuates beta amyloid-induced apoptosis through inhibiting caspases activity by activating PI3K/Akt signaling pathway. J. Mol. Neurosci. 2016, 58, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945–956. [Google Scholar] [CrossRef]
- De Vidania, S.; Palomares-Perz, I.; Frank-Garcia, A.; Saito, T.; Saido, T.C.; Draffin, J.; Szaruga, M.; Chavez-Gutierrez, L.; Calero, M.; Medina, M.; et al. Prodromal Alzheimer’s disease: Constitutive upregulation of Neuroglobin prevents the initiation of Alzheimer’s pathology. Front. Neurosci. 2020, 14, 562581–562598. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Augustin, S.; Roussel, D.; Sahel, J.-A.; Corral-Debrinski, M. Neuroglobin involvement in visual pathways through the optic nerve. Bochim. Biophys. Acta 2013, 1834, 1772–1778. [Google Scholar] [CrossRef]
- Moschetti, T.; Giuffrè, A.; Ardiccioni, C.; Vallone, B.; Modjtahedi, N.; Kroemer, G.; Brunori, M. Failure of apoptosis-inducing factor to act as neuroglobin reductase. Biochem. Biophys. Res. Commun. 2009, 390, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cwerman-Thibault, H.; Lechauve, C.; Augustin, S.; Roussel, D.; Reboussin, E.; Mohammad, A.; Degardin-Chicaud, J.; Simonutti, M.; Liang, H.; Brignole-Baudouin, F.; et al. Neuroglobin can prevent or reverse glaucomatous progression in DBA/2J mice. Mol. Ther. Meth. Clin. Dev. 2017, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.A.; Gaffney, E.A.; Luthert, P.J.; Foss, A.J.E.; Byrne, H.M. Retinal oxygen distribution and the role of neuroglobin. J. Math. Biol. 2016, 73, 1179–1204. [Google Scholar] [CrossRef]
- Shi, S.-Y.; Feng, X.-M.; Li, Y.; Li, X.; Chen, X.-L. Expression of neuroglobin in ocular hypertension induced acute hypoxic-ischemic retinal injury in rats. Int. J. Ophthalmol. 2011, 4, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, K.; Mao, X.O.; Zhu, Y.; Greenberg, D.A. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. USA 2001, 98, 15306–15311. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Peel, A.; Mao, X.O.; Xie, L.; Greenberg, D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Wang, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Miles, E.; Graboski, J.; Chen, S.; Ellerby, L.M.; Jin, K.; et al. Neuroglobin-overexpressing transgenic mice aare resistant to cerebral and myocardial ischemia. Proc. Natl. Acad. Sci. USA 2006, 103, 17944–17948. [Google Scholar] [CrossRef] [PubMed]
- Raida, Z.; Hundahl, C.A.; Nyengaard, J.R.; Hay-Schmidt, A. Neuroglobin over expressing mice: Expression pattern and effect on brain ischemic infarct size. PLoS ONE 2013, 8, e76565. [Google Scholar] [CrossRef]
- Tun, S.B.B.; Barathi, V.A.; Luu, C.D.; Lynn, M.N.; Chan, A.S.Y. Effects of exogenous neuroglobin (Ngb) on retinal inflammatory chemokines and microglia in a rat model of transient hypoxia. Sci. Rep. 2019, 9, 18799–18885. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Tang, Y.; Yang, M.; Long, S.; Shu, P.; Li, J.; Xiao, Y.; Tang, F.; Wei, C.; et al. Neuroglobin regulates Wnt/β-catenin and NFkB signaling pathway through Dvl1. Int. J. Mol. Sci. 2018, 19, 2133. [Google Scholar] [CrossRef]
- Chan, A.S.Y.; Saraswathy, S.; Rehak, M.; Ueki, M.; Rao, N.A. Neuroglobin protection in retinal ischemia. Invest. Ophtalmol. Visual. Sci. 2012, 53, 704–711. [Google Scholar] [CrossRef]
- Singh, S.; Zhuo, M.; Gorgun, F.M.; Englander, E.W. Overexpressed neuroglobin raises threshold for nitric oxide-induced impairment of mitochondrial respiratory activities and stress signaling in primary cortical neurons. Nitric Oxide 2013, 32, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, N.; Li, Y.; Xu, J.; Wang, X. Neuroglobin overexpression inhibits oxygen-glucose deprivation-induced mitochondrial permeability transition pore opening in primary cultured mouse cortical neurons. Neurobiol. Dis. 2013, 56, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, B.; Hempel, C.; Kurtzhals, J.A.; Penkowa, M. In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia 2010, 58, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.C.; Cossa, A.C.; Perosa, S.R.; de Oliveira, E.M.; da Silva, J.A., Jr.; da Silva Fernandes, M.J.; da Silva, I.R.; Higa, E.M.; da Graça Naffah-Mazzacoratti, M.; Cavalheiro, E.A.; et al. Neuroglobin is up-regulated in the cerebellum of pups exposed to maternal epileptic seizures. Int. J. Dev. Neurosci. 2011, 29, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Qiu, S.; Chen, X.-C.; Dai, Z.-H.; Huang, Y.-C.; Li, Y.-N.; Cai, R.-H.; Lei, H.-T.; Gu, H.-Y. Neuroglobin-A potential biological marker of retinal damage induced by led light. Neuroscience 2014, 270, 158–167. [Google Scholar] [CrossRef]
- Sugitani, K.; Koriyama, Y.; Sera, M.; Arai, K.; Ogai, K.; Wakasugi, K. A novel function of neuroglobin for neurodegeneration in mice after optic nerve injury. Biochem. Biophys. Res. Comm. 2017, 493, 1254–1259. [Google Scholar] [CrossRef]
- Kamioka, Y.; Fujikawa, C.; Ogai, K.; Sugitani, K.; Watanabe, S.; Kato, S.; Wakasugi, K. Functional characterization of fish neuroglobin: Zebrafish neuroglobin is highly expressed in amacrine cells after optic nerve injury and can translocate into ZF4 cells. Biochim. Biophys. Acta 2013, 1834, 1779–1788. [Google Scholar] [CrossRef]
- Bønding, S.H.; Henty, K.; Dingley, A.J.; Brittain, T. The binding of cytochrome c to neuroglobin: A docking and surface plasmon resonance study. Int. J. Biol. Macromol. 2008, 43, 295–299. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, E.; Fiocchetti, M.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin upregulation induced by 17β-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013, 4, e508. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Skommer, J.; Henty, K.; Birch, N.; Brittain, T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis 2010, 15, 401–411. [Google Scholar] [CrossRef]
- Tejero, J. Negative surface charges in neuroglobin modulate the interaction with cytochrome c. Biochem. Biophys. Res. Comm. 2020, 523, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kastner, R.; Haberkamp, M.; Schmitz, C.; Hankeln, T.; Burmester, T. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 2006, 1103, 173–180. [Google Scholar] [CrossRef]
- Kelsen, J.; Hundahl, C.A.; Hay-Schmidt, A. Neuroglobin: Endogenous neuroprotectant or maintenance of homeostasis? Stroke 2008, 39, e177–e178. [Google Scholar] [CrossRef]
- Raida, Z.; Hundahl, C.A.; Kelsen, J.; Nyengaard, J.R.; Hay-Schmidt, A. Reduced infarct size in neuroglobin-null mice after experimental stroke in vivo. Exp. Transl. Stroke Med. 2012, 4, 15–16. [Google Scholar] [CrossRef]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; D’Urso, S.; Longo, S.; Vagnozzi, R.; Signoretti, S.; Clementi, E.; Giardina, B.; et al. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic. Biol. Med. 2014, 69, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Fabrizius, A.; Andre, D.; Laufs, T.; Bicker, A.; Reuss, S.; Porto, E.; Burmester, T.; Hankeln, T. Critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience 2016, 337, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, C.A.; Fahrenkrug, J.; Luuk, H.; Hay-Schmidt, A.; Hannibal, J. Restricted expression of neuroglobin in the mouse retina and co-localization with melanopsin and tyrosine hydroxylase. Biochem. Biophys. Res. Commun. 2012, 425, 100–106. [Google Scholar] [CrossRef]
- Cai, B.; Lin, Y.; Xue, X.H.; Fang, L.; Wang, N.; Wu, Z.Y. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp. Neurol. 2011, 227, 224–231. [Google Scholar] [CrossRef]
- Khan, A.A.; Mao, X.O.; Banwait, S.; DerMardirossian, C.M.; Bokoch, G.M.; Jin, K.; Greenberg, D.A. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008, 22, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Solar Fernandez, V.; Fiocchetti, M.; Cipolletti, M.; Segatto, M.; Cercola, P.; Massari, A.; Ghinassi, S.; Cavaliere, F.; Marino, M. Neuroglobin: A new possible marker of estrogen-responsive breast cancer. Cells 2021, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Sun, Y.; Jin, K.; Mao, X.O.; Chen, S.; Ellerby, L.M.; Greenberg, D.A. A neuroglobin-overexpressing transgenic mouse. Gene 2007, 398, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Augustin, S.; Cwerman-Thibault, H.; Reboussin, E.; Roussel, D.; Lai-Kuen, R.; Saubamea, B.; Sahel, J.-A.; Debeir, T.; Corral-Debrinski, M. Neuroglobin gene therapy prevents optic atrophy and preserves durably visual function in Harlequin mice. Mol. Ther. 2014, 22, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simone, G.; Sbardella, D.; Oddone, F.; Pesce, A.; Coletta, M.; Ascenzi, P. Structural and (Pseudo-)Enzymatic Properties of Neuroglobin: Its Possible Role in Neuroprotection. Cells 2021, 10, 3366. https://doi.org/10.3390/cells10123366
De Simone G, Sbardella D, Oddone F, Pesce A, Coletta M, Ascenzi P. Structural and (Pseudo-)Enzymatic Properties of Neuroglobin: Its Possible Role in Neuroprotection. Cells. 2021; 10(12):3366. https://doi.org/10.3390/cells10123366
Chicago/Turabian StyleDe Simone, Giovanna, Diego Sbardella, Francesco Oddone, Alessandra Pesce, Massimo Coletta, and Paolo Ascenzi. 2021. "Structural and (Pseudo-)Enzymatic Properties of Neuroglobin: Its Possible Role in Neuroprotection" Cells 10, no. 12: 3366. https://doi.org/10.3390/cells10123366
APA StyleDe Simone, G., Sbardella, D., Oddone, F., Pesce, A., Coletta, M., & Ascenzi, P. (2021). Structural and (Pseudo-)Enzymatic Properties of Neuroglobin: Its Possible Role in Neuroprotection. Cells, 10(12), 3366. https://doi.org/10.3390/cells10123366








