Regulation of Hypoxic Signaling and Oxidative Stress via the MicroRNA–SIRT2 Axis and Its Relationship with Aging-Related Diseases
Abstract
:1. Introduction
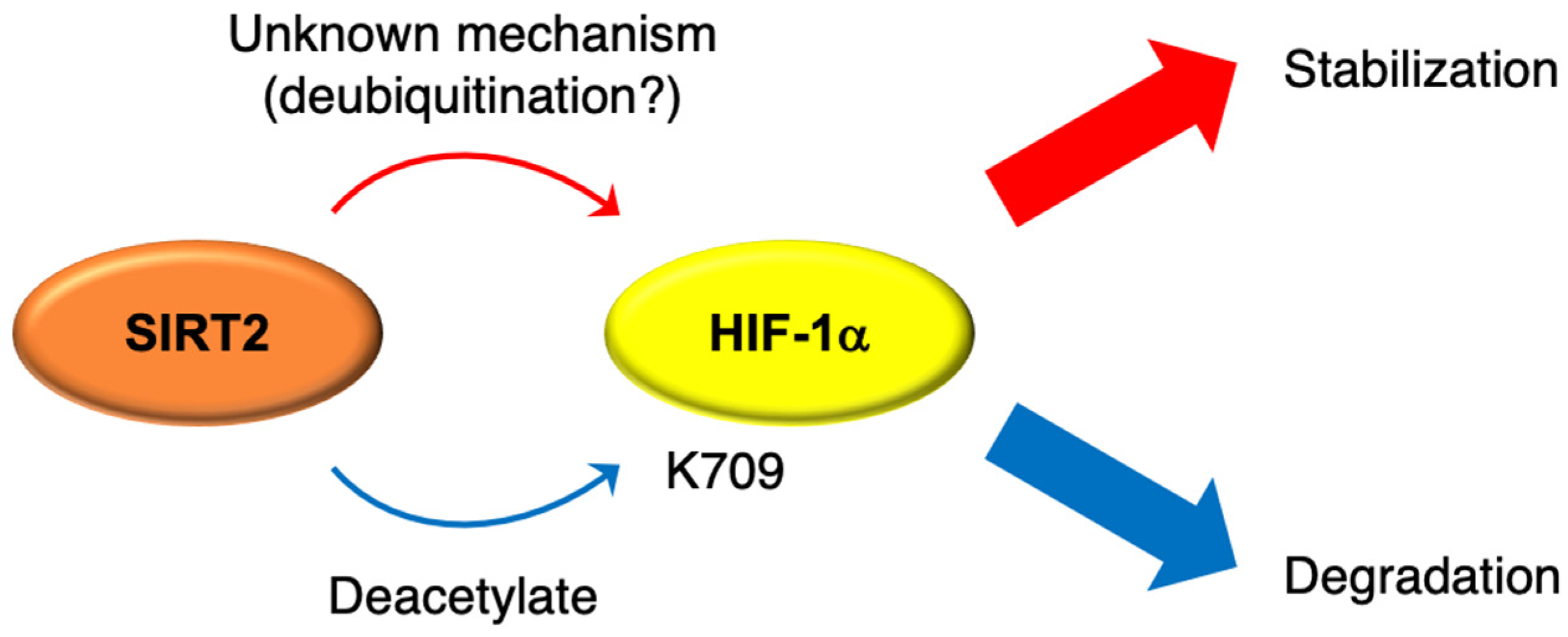
2. Involvement of SIRT2 in Hypoxic Signaling and Oxidative Stress
3. Major miRNAs in Hypoxic Signaling and Oxidative Stress
3.1. miR-130a
3.2. miR-210
3.3. miR-199
3.4. miR-122
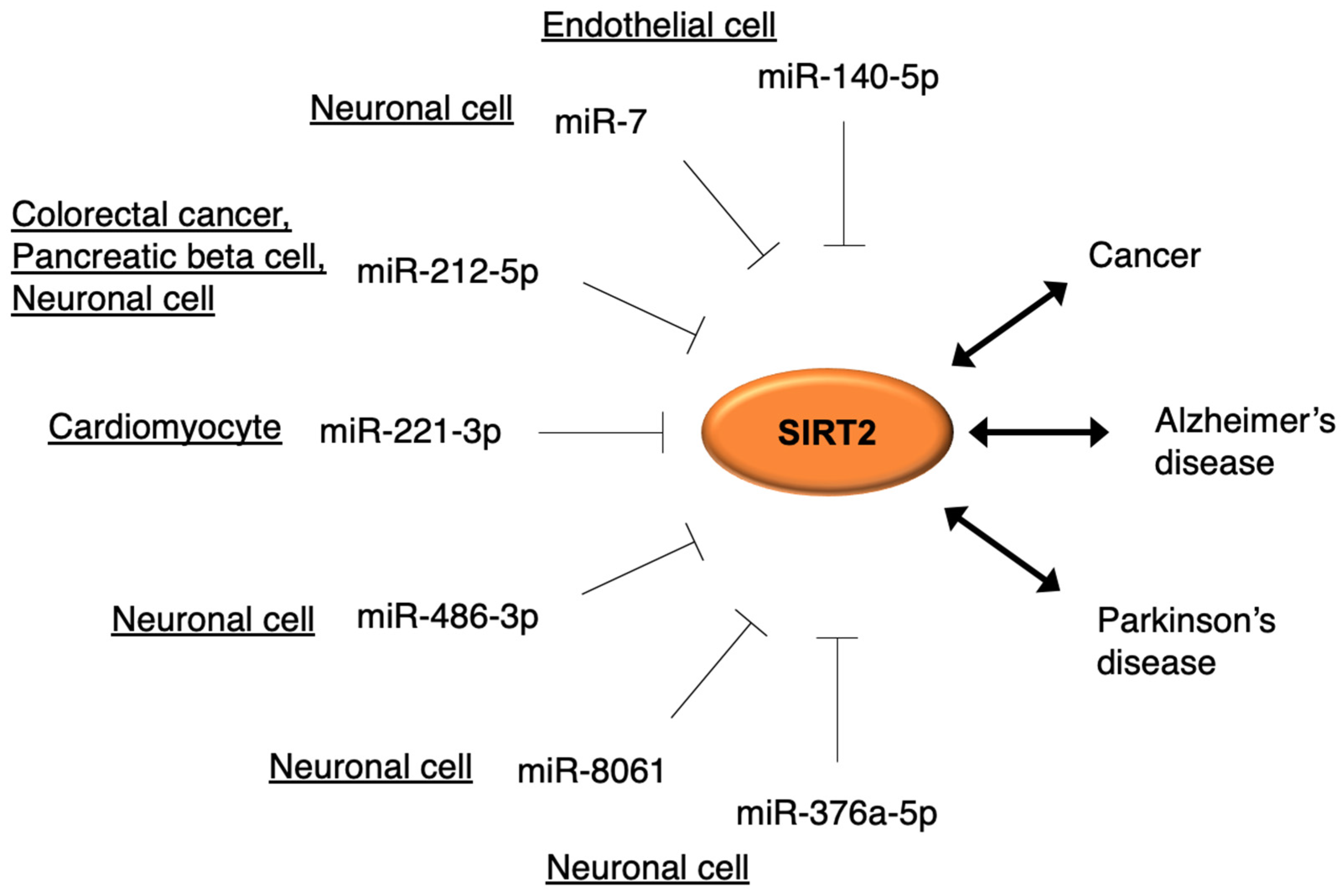
4. Regulation of SIRT2 by miRNA and Its Relation to Cancer and Neurodegenerative Diseases
4.1. miR-212-5p–SIRT2 Axis
4.2. miR-221-3p–SIRT2 Axis
4.3. miR-140-5p–SIRT2 Axis
4.4. miR-7–SIRT2 Axis
4.5. miRNA–SIRT2 in Parkinson’s Disease (miR-486-3p, miR-376a-5p, and miR-8061)
5. SIRT2, miRNAs, Hypoxia, Oxidative Stress, and Neurodegenerative Diseases
6. Splicing Variants of SIRT2 and Another Posttranscriptional Regulation and Their Potential Relation to Neurological Diseases
7. Therapeutic Strategy via miRNA–SIRT2 Inhibition
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haigis, M.C.; Guarente, L.P. Mammalian Sirtuins—Emerging Roles in Physiology, Aging, and Calorie Restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Guarente, L. It Takes Two to Tango: NAD+ and Sirtuins in Aging/Longevity Control. npj Aging Mech. Dis. 2016, 2, 16017. [Google Scholar] [CrossRef] [Green Version]
- Guarente, L. Hypoxic Hookup. Science 2009, 324, 1281–1282. [Google Scholar] [CrossRef]
- Bell, E.L.; Guarente, L. The SirT3 Divining Rod Points to Oxidative Stress. Mol. Cell 2011, 42, 561–568. [Google Scholar] [CrossRef]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging Targets in Neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian MicroRNAs Predominantly Act to Decrease Target MRNA Levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emde, A.; Hornstein, E. Mi RNA s at the Interface of Cellular Stress and Disease. EMBO J 2014, 33, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Macharia, L.W.; Wanjiru, C.M.; Mureithi, M.W.; Pereira, C.M.; Ferrer, V.P.; Moura-Neto, V. MicroRNAs, Hypoxia and the Stem-Like State as Contributors to Cancer Aggressiveness. Front. Genet. 2019, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, J.; Zhou, W.; Cao, B.; Zhou, X.; Zhang, H.; Zhao, Q.; Hong, L.; Fan, D. Reciprocal Regulations between MiRNAs and HIF-1α in Human Cancers. Cell. Mol. Life Sci. 2019, 76, 453–471. [Google Scholar] [CrossRef]
- Joo, H.-Y.; Yun, M.; Jeong, J.; Park, E.-R.; Shin, H.-J.; Woo, S.R.; Jung, J.K.; Kim, Y.-M.; Park, J.-J.; Kim, J.; et al. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochem. Biophys. Res. Commun. 2015, 462, 294–300. [Google Scholar] [CrossRef]
- Laemmle, A.; Lechleiter, A.; Roh, V.; Schwarz, C.; Portmann, S.; Furer, C.; Keogh, A.; Tschan, M.P.; Candinas, D.; Vorburger, S.A.; et al. Inhibition of SIRT1 Impairs the Accumulation and Transcriptional Activity of HIF-1α Protein under Hypoxic Conditions. PLoS ONE 2012, 7, e33433. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-H.; Lee, Y.-M.; Chun, Y.-S.; Chen, J.; Kim, J.-E.; Park, J.-W. Sirtuin 1 Modulates Cellular Responses to Hypoxia by Deacetylating Hypoxia-Inducible Factor 1α. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Bai, M.; Lu, C.; An, L.; Gao, Q.; Xie, W.; Miao, F.; Chen, X.; Pan, Y.; Wang, Q. SIRT1 Relieves Necrotizing Enterocolitis through Inactivation of Hypoxia-Inducible Factor (HIF)-1a. Cell Cycle 2020, 19, 2018–2027. [Google Scholar] [CrossRef]
- Dioum, E.M.; Chen, R.; Alexander, M.S.; Zhang, Q.; Hogg, R.T.; Gerard, R.D.; Garcia, J.A. Regulation of Hypoxia-Inducible Factor 2 Signaling by the Stress-Responsive Deacetylase Sirtuin 1. Science 2009, 324, 1289–1293. [Google Scholar] [CrossRef]
- Bell, E.L.; Emerling, B.M.; Ricoult, S.J.H.; Guarente, L. SirT3 Suppresses Hypoxia Inducible Factor 1α and Tumor Growth by Inhibiting Mitochondrial ROS Production. Oncogene 2011, 30, 2986–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Che, W.; Xue, J.; Zheng, C.; Tang, K.; Zhang, J.; Wen, J.; Xu, Y. SIRT4 Prevents Hypoxia-Induced Apoptosis in H9c2 Cardiomyoblast Cells. Cell. Physiol. Biochem. 2013, 32, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, Y.; Niu, Y.; Wang, L.; Chen, T.; Guo, C.; Liu, Q. Hypoxia-induced MiR-3677-3p Promotes the Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cells by Suppressing SIRT5. J. Cell. Mol. Med. 2020, 24, 8718–8731. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Y.; Zhu, L.; Yang, K.; Liang, K.; Tan, J.; Yu, B. SIRT6 Promotes Angiogenesis and Hemorrhage of Carotid Plaque via Regulating HIF-1α and Reactive Oxygen Species. Cell Death Dis. 2021, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Hubbi, M.E.; Hu, H.; Gilkes, D.M.; Semenza, G.L. Sirtuin-7 Inhibits the Activity of Hypoxia-Inducible Factors. J. Biol. Chem. 2013, 288, 20768–20775. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.D.; Kim, W.; Jeong, J.-W.; Park, J.-W.; Kim, J.-E. AK-1, a SIRT2 Inhibitor, Destabilizes HIF-1α and Diminishes Its Transcriptional Activity during Hypoxia. Cancer Lett. 2016, 373, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-S.; Park, J.-H.; Heo, J.Y.; Jing, K.; Han, J.; Min, K.-N.; Kim, C.; Koh, G.Y.; Lim, K.; Kang, G.-Y.; et al. SIRT2 regulates tumour hypoxia response by promoting HIF-1α hydroxylation. Oncogene 2015, 34, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Liu, Q.; Xue, C.; David, L.L.; Beer, T.M.; Thomas, G.V.; Dai, M.-S.; Qian, D.Z. HIF1α Protein Stability Is Increased by Acetylation at Lysine 709. J. Biol. Chem. 2012, 287, 35496–35505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitsuka, T.; Matsushita, M.; Matsushita, N. SIRT2 Inhibition Activates Hypoxia-Inducible Factor 1α Signaling and Mediates Neuronal Survival. Biochem. Biophys. Res. Commun. 2020, 529, 957–962. [Google Scholar] [CrossRef]
- Hu, A.; Yang, L.; Liang, J.; Lu, D.; Zhang, J.; Cao, F.; Fu, J.; Dai, W.; Zhang, J. SIRT2 Modulates VEGFD-associated Lymphangiogenesis by Deacetylating EPAS1 in Human Head and Neck Cancer. Mol. Carcinog. 2020, 59, 1280–1291. [Google Scholar] [CrossRef]
- Krishnan, J.; Danzer, C.; Simka, T.; Ukropec, J.; Walter, K.M.; Kumpf, S.; Mirtschink, P.; Ukropcova, B.; Gasperikova, D.; Pedrazzini, T.; et al. Dietary obesity-associated Hif1 activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012, 26, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.; Kwak, J.-S.; Yang, S.; Gong, M.-K.; Kim, J.-H.; Rhee, J.; Kim, S.K.; Kim, H.-E.; Ryu, J.-H.; Chun, J.-S. Reciprocal Regulation by Hypoxia-Inducible Factor-2α and the NAMPT-NAD + -SIRT Axis in Articular Chondrocytes Is Involved in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2288–2296. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Xu, T.-Y.; Guan, Y.-F.; Tian, W.-W.; Viollet, B.; Rui, Y.-C.; Zhai, Q.-W.; Su, D.-F.; Miao, C.-Y. Nicotinamide Phosphoribosyltransferase Protects against Ischemic Stroke through SIRT1-Dependent Adenosine Monophosphate-Activated Kinase Pathway. Ann. Neurol. 2011, 69, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Y.; Sun, Y.; Luo, Y.; Shen, Y.; Shao, A. Will Sirtuins Be Promising Therapeutic Targets for TBI and Associated Neurodegenerative Diseases? Front. Neurosci. 2020, 14, 791. [Google Scholar] [CrossRef]
- Saito, K.; Kondo, E.; Matsushita, M. MicroRNA 130 Family Regulates the Hypoxia Response Signal through the P-Body Protein DDX6. Nucleic Acids Res. 2011, 39, 6086–6099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, M.; Haider, T.J.; Vogel, J.; Gassmann, M.; Speich, R.; Trenkmann, M.; Ulrich, S.; Kohler, M.; Huber, L.C. The Hypoxia-Induced MicroRNA-130a Controls Pulmonary Smooth Muscle Cell Proliferation by Directly Targeting CDKN1A. Int. J. Biochem. Cell Biol. 2015, 61, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Zhu, X.; Zhuo, C. H19/MiR-130a-3p/DAPK1 Axis Regulates the Pathophysiology of Neonatal Hypoxic-Ischemia Encephalopathy. Neurosci. Res. 2021, 163, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Fan, C.; Zhao, Y.; Mao, Y.; Li, J.; Zhang, Y.; Teng, J. MicroRNA-130a Regulates Neurological Deficit and Angiogenesis in Rats with Ischaemic Stroke by Targeting XIAP. J. Cell. Mol. Med. 2020, 24, 10987–11000. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, S.; Tong, S.; Sun, X. Exosomal MiR-130a-3p Regulates Osteogenic Differentiation of Human Adipose-Derived Stem Cells through Mediating SIRT7/Wnt/Β-catenin Axis. Cell Prolif. 2020, 53, e12890. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Toledo, E.M.; Gyllborg, D.; Saltó, C.; Carlos Villaescusa, J.; Arenas, E. Niche-Derived Laminin-511 Promotes Midbrain Dopaminergic Neuron Survival and Differentiation through YAP. Sci. Signal. 2017, 10, eaal4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zuo, J. Emerging Roles of MiR-210 and Other Non-Coding RNAs in the Hypoxic Response. Acta Biochim. Biophys. Sin. 2014, 46, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Zhang, Y.-Y.; Hemann, C.; Mahoney, C.E.; Zweier, J.L.; Loscalzo, J. MicroRNA-210 Controls Mitochondrial Metabolism during Hypoxia by Repressing the Iron-Sulfur Cluster Assembly Proteins ISCU1/2. Cell Metab. 2009, 10, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Bertero, T.; Rezzonico, R.; Pottier, N.; Mari, B. Impact of MicroRNAs in the Cellular Response to Hypoxia. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 333, pp. 91–158. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Zeng, J.; Zhao, Z.; Hu, Q. MicroRNA-210-3p Is Transcriptionally Upregulated by Hypoxia Induction and Thus Promoting EMT and Chemoresistance in Glioma Cells. PLoS ONE 2021, 16, e0253522. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Gaetano, C.; Martelli, F. HypoxamiR Regulation and Function in Ischemic Cardiovascular Diseases. Antioxid. Redox Signal. 2014, 21, 1202–1219. [Google Scholar] [CrossRef] [Green Version]
- Zaccagnini, G.; Greco, S.; Longo, M.; Maimone, B.; Voellenkle, C.; Fuschi, P.; Carrara, M.; Creo, P.; Maselli, D.; Tirone, M.; et al. Hypoxia-induced miR-210 modulates the inflammatory response and fibrosis upon acute ischemia. Cell Death Dis. 2021, 12, 435. [Google Scholar] [CrossRef]
- Zaccagnini, G.; Maimone, B.; Di Stefano, V.; Fasanaro, P.; Greco, S.; Perfetti, A.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Hypoxia-Induced MiR-210 Modulates Tissue Response to Acute Peripheral Ischemia. Antioxid. Redox Signal. 2014, 21, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA Expression in the Blood and Brain of Rats Subjected to Transient Focal Ischemia by Middle Cerebral Artery Occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dasgupta, C.; Huang, L.; Meng, X.; Zhang, L. MiRNA-210 Induces Microglial Activation and Regulates Microglia-Mediated Neuroinflammation in Neonatal Hypoxic-Ischemic Encephalopathy. Cell. Mol. Immunol. 2020, 17, 976–991. [Google Scholar] [CrossRef]
- Watts, M.; Williams, S.; Nithianantharajah, J.; Claudianos, C. Hypoxia-Induced MicroRNA-210 Targets Neurodegenerative Pathways. ncRNA 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lei, S.; Long, J.; Liu, X.; Wu, Q. MicroRNA-199a-5p Inhibits Tumor Proliferation in Melanoma by Mediating HIF-1α. Mol. Med. Rep. 2016, 13, 5241–5247. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Huang, R.; Su, Z.; Zhang, M.; Xu, M.; Gong, J.; Chen, N.; Zeng, H.; Chen, X.; Zhou, Q. Downregulation of MiR-199a-5p Promotes Prostate Adeno-Carcinoma Progression through Loss of Its Inhibition of HIF-1α. Oncotarget 2017, 8, 83523–83538. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Hu, H.; Zhang, P.; Cui, W. Downregulation of MicroRNA-199a-5p Attenuates Hypoxia/Reoxygenation-induced Cytotoxicity in Cardiomyocytes by Targeting the HIF-1α-GSK3β-mPTP Axis. Mol. Med. Rep. 2019, 19, 5335–5344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zheng, Y.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. MiR-199a-5p–HIF-1α-STAT3 Positive Feedback Loop Contributes to the Progression of Non-Small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 8, 620615. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, S.T.; Schaal, V.L.; Moore, D.; Guda, R.S.; Koul, S.; Yelamanchili, S.V.; Bevins, R.A.; Pendyala, G. MicroRNA Cluster MiR199a/214 Are Differentially Expressed in Female and Male Rats Following Nicotine Self-Administration. Sci. Rep. 2018, 8, 17464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Wang, H.; Tian, J.; Xu, H. Down-Regulation of ID2-AS1 Alleviates the Neuronal Injury Induced by 1-Methy1-4-Phenylpyridinium in Human Neuroblastoma Cell Line SH-SY5Y Cells Through Regulating MiR-199a-5p/IFNAR1/JAK2/STAT1 Axis. Neurochem. Res. 2021, 46, 2192–2203. [Google Scholar] [CrossRef]
- Csak, T.; Bala, S.; Lippai, D.; Satishchandran, A.; Catalano, D.; Kodys, K.; Szabo, G. MicroRNA-122 Regulates Hypoxia-Inducible Factor-1 and Vimentin in Hepatocytes and Correlates with Fibrosis in Diet-Induced Steatohepatitis. Liver Int. 2015, 35, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Long, J.-K.; Dai, W.; Zheng, Y.-W.; Zhao, S.-P. MiR-122 Promotes Hepatic Lipogenesis via Inhibiting the LKB1/AMPK Pathway by Targeting Sirt1 in Non-Alcoholic Fatty Liver Disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, C.; Wang, M.; Tak, E.; Kim, B.; Emontzpohl, C.; Yang, Y.; Yuan, X.; Kutay, H.; Liang, Y.; Hall, D.R.; et al. Hypoxia-inducible factor–1α–dependent induction of miR122 enhances hepatic ischemia tolerance. J. Clin. Investig. 2021, 131, 140300. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, K.; Zhan, J.; Wu, M. MiR-122/SIRT1 Axis Regulates Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biosci. Rep. 2020, 40, BSR20191908. [Google Scholar] [CrossRef]
- Elhanati, S.; Ben-Hamo, R.; Kanfi, Y.; Varvak, A.; Glazz, R.; Lerrer, B.; Efroni, S.; Cohen, H.Y. Reciprocal Regulation between SIRT6 and MiR-122 Controls Liver Metabolism and Predicts Hepatocarcinoma Prognosis. Cell Rep. 2016, 14, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Gu, R.; Wang, L.; Tang, M.; Li, S.-R.; Liu, R.; Hu, X. LncRNA Rpph1 Protects Amyloid-β Induced Neuronal Injury in SK-N-SH Cells via MiR-122/Wnt1 Axis. Int. J. Neurosci. 2020, 130, 443–453. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. MiRDB: An Online Database for Prediction of Functional MicroRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, X. Prediction of Functional MicroRNA Targets by Integrative Modeling of MicroRNA Binding and Target Expression Data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Li, Z.; Zhang, G.; Shaoyan, S.; Geng, D.; Tao, Z.; Qiu, K.; Liu, S.; Zhou, Y.; Zhang, Y.; et al. SIRT2, a direct target of miR-212-5p, suppresses the proliferation and metastasis of colorectal cancer cells. J. Cell. Mol. Med. 2020, 24, 9985–9998. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Yang, Y.; Tang, N.; Wang, J.; Sun, P.; Yang, N.; Chen, F.; Wu, T.; Sun, T.; Li, Y.; et al. M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice. Diabetol. 2021, 64, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, G.; Davaakhuu, G.; Kaplun, L.; Chung, W.-C.; Rana, A.; Atfi, A.; Miele, L.; Tzivion, G. Sirt2 Deacetylase Is a Novel AKT Binding Partner Critical for AKT Activation by Insulin. J. Biol. Chem. 2014, 289, 6054–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Han, X.; Li, X.; Song, Q.; Lu, M.; Jia, M.; Ding, J.; Hu, G. MicroRNA-212-5p Prevents Dopaminergic Neuron Death by Inhibiting SIRT2 in MPTP-Induced Mouse Model of Parkinson’s Disease. Front. Mol. Neurosci. 2018, 11, 381. [Google Scholar] [CrossRef]
- Bracken, C.P.; Scott, H.S.; Goodall, G.J. A Network-Biology Perspective of MicroRNA Function and Dysfunction in Cancer. Nat. Rev. Genet. 2016, 17, 719–732. [Google Scholar] [CrossRef]
- Lin, J.-F.; Zeng, H.; Zhao, J.-Q. MiR-212-5p Regulates the Proliferation and Apoptosis of AML Cells through Targeting FZD5. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8415–8422. [Google Scholar] [CrossRef]
- Deng, J.-H.; Zheng, G.-Y.; Li, H.-Z.; Ji, Z.-G. MiR-212-5p Inhibits the Malignant Behavior of Clear Cell Renal Cell Carcinoma Cells by Targeting TBX15. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10699–10707. [Google Scholar] [CrossRef]
- Wanet, A.; Tacheny, A.; Arnould, T.; Renard, P. MiR-212/132 Expression and Functions: Within and beyond the Neuronal Compartment. Nucleic Acids Res. 2012, 40, 4742–4753. [Google Scholar] [CrossRef] [Green Version]
- Gillardon, F.; Mack, M.; Rist, W.; Schnack, C.; Lenter, M.; Hildebrandt, T.; Hengerer, B. MicroRNA and Proteome Expression Profiling in Early-Symptomatic α-Synuclein(A30P)-Transgenic Mice. Prot. Clin. Appl. 2008, 2, 697–705. [Google Scholar] [CrossRef]
- Wang, W.-X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of MicroRNA Expression in Normal and Early Alzheimer’s Disease Human Temporal Cortex: White Matter versus Gray Matter. Acta Neuropathol. 2011, 121, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. MiR-212 and MiR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Jiang, Y.; Liang, W.; Wang, Y.; Cao, S.; Yan, H.; Gao, L.; Zhang, L. MiR-212-5p Attenuates Ferroptotic Neuronal Death after Traumatic Brain Injury by Targeting Ptgs2. Mol. Brain 2019, 12, 78. [Google Scholar] [CrossRef]
- Zhuang, L.; Xia, W.; Chen, D.; Ye, Y.; Hu, T.; Li, S.; Hou, M. Exosomal LncRNA–NEAT1 Derived from MIF-Treated Mesenchymal Stem Cells Protected against Doxorubicin-Induced Cardiac Senescence through Sponging MiR-221-3p. J. Nanobiotechnol. 2020, 18, 157. [Google Scholar] [CrossRef]
- Fathi, M.; Ghafouri-Fard, S.; Abak, A.; Taheri, M. Emerging Roles of MiRNAs in the Development of Pancreatic Cancer. Biomed. Pharmacother. 2021, 141, 111914. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, C.; Fan, J.; Hou, Z.; Han, Y. MiR-221-3p Targets Hif-1α to Inhibit Angiogenesis in Heart Failure. Lab. Investig. 2021, 101, 104–115. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, N.; Lu, K.; Liao, Q.; Long, X.; Gou, D.; Bi, F.; Zhou, J. Elevated Plasma MiR-133b and MiR-221-3p as Biomarkers for Early Parkinson’s Disease. Sci. Rep. 2021, 11, 15268. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Peng, J. MicroRNA-140-5p Aggravates Doxorubicin-Induced Cardiotoxicity by Promoting Myocardial Oxidative Stress via Targeting Nrf2 and Sirt2. Redox Biol. 2018, 15, 284–296. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective Effect of Dioscin against Doxorubicin-Induced Cardiotoxicity via Adjusting MicroRNA-140-5p-Mediated Myocardial Oxidative Stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, K.; Liu, S.; Li, W.; Huang, C.; Yang, X. MicroRNA-140-5p Aggravates Hypertension and Oxidative Stress of Atherosclerosis via Targeting Nrf2 and Sirt2. Int. J. Mol. Med. 2019, 43, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Wang, T.; Shi, B.; Wu, Z.; Wang, W.; Yang, Y. Neuroprotective Effects of MicroRNA-140-5p on Ischemic Stroke in Mice via Regulation of the TLR4/NF-ΚB Axis. Brain Res. Bull. 2021, 168, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, X.; Zhai, K.; Xu, R.; Zhang, Y.; Zhao, S.; Qin, X.; Yin, L.; Lou, J. MicroRNA-7 Inhibits Neuronal Apoptosis in a Cellular Parkinson’s Disease Model by Targeting Bax and Sirt2. Am. J. Transl. Res. 2016, 8, 993–1004. [Google Scholar] [PubMed]
- Liu, Y.; Zhang, Y.; Zhu, K.; Chi, S.; Wang, C.; Xie, A. Emerging Role of Sirtuin 2 in Parkinson’s Disease. Front. Aging Neurosci. 2020, 11, 372. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.C.; Chae, Y.-J.; Kabaria, S.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Mouradian, M.M.; Junn, E. MicroRNA-7 Protects against 1-Methyl-4-Phenylpyridinium-Induced Cell Death by Targeting RelA. J. Neurosci. 2014, 34, 12725–12737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junn, E.; Lee, K.-W.; Jeong, B.S.; Chan, T.W.; Im, J.-Y.; Mouradian, M.M. Repression of -Synuclein Expression and Toxicity by MicroRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, K.J.; Murray, T.K.; Bengoa-Vergniory, N.; Llana, O.C.; Cooper, J.; Buckley, A.; Wade-Martins, R.; Uney, J.B.; O’Neill, M.J.; Wong, L.F.; et al. Loss of MicroRNA-7 Regulation Leads to α-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol. Ther. 2017, 25, 2404–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titze-de-Almeida, R.; Titze-de-Almeida, S.S. MiR-7 Replacement Therapy in Parkinson’s Disease. CGT 2018, 18, 143–153. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.; Huang, H.; Chen, X.; Chen, X.; Chen, X.; Mai, H.; Li, X.; Zhao, J.; Yang, J.; et al. miR-486-3p Influences the Neurotoxicity of a-Synuclein by Targeting the SIRT2 Gene and the Polymorphisms at Target Sites Contributing to Parkinson’s Disease. Cell. Physiol. Biochem. 2018, 51, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mai, H.; Chen, X.; Cai, Y.; Cheng, Q.; Chen, X.; Li, X.; Fan, W.; Tang, P.; Ou, M.; et al. Rs2015 Polymorphism in miRNA Target Site of Sirtuin2 Gene Is Associated with the Risk of Parkinson’s Disease in Chinese Han Population. BioMed Res. Int. 2019, 2019, 1498034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Chen, L.; Zhang, S.; Xie, L. Correlation Between SIRT2 3′UTR Gene Polymorphism and the Susceptibility to Alzheimer’s Disease. J. Mol. Neurosci. 2020, 70, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Bulgarelli, J.; Ruberti, S.; Rontauroli, S.; Sacchi, G.; Norfo, R.; Pennucci, V.; Zini, R.; Salati, S.; Prudente, Z.; et al. MYB controls erythroid versus megakaryocyte lineage fate decision through the miR-486-3p-mediated downregulation of MAF. Cell Death Differ. 2015, 22, 1906–1921. [Google Scholar] [CrossRef] [Green Version]
- ElKhouly, A.M.; Youness, R.A.; Gad, M.Z. MicroRNA-486-5p and MicroRNA-486-3p: Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions. Non-Coding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Hong, T.-T.; Sun, Y.; Huang, H.; Chen, F.; Chen, X.; Chen, H.; Dong, S.; Cui, L.; et al. RTN4B-mediated suppression of Sirtuin 2 activity ameliorates β-amyloid pathology and cognitive impairment in Alzheimer’s disease mouse model. Aging Cell 2020, 19, e13194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, L.; Shen, C.; Qi, Q.; Qin, Y.; Xing, J.; Zhou, D.; Qi, Y.; Yan, Z.; Lin, X.; et al. SIRT2-knockdown Rescues GARS-induced Charcot-Marie-Tooth Neuropathy. Aging Cell 2021, 20, e13391. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, H.; Wang, G.; Ren, H. Imbalance of Lysine Acetylation Contributes to the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 7182. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.-C.; McLean, P.J.; et al. Sirtuin 2 Inhibitors Rescue α-Synuclein-Mediated Toxicity in Models of Parkinson’s Disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef]
- Pallos, J.; Bodai, L.; Lukacsovich, T.; Purcell, J.M.; Steffan, J.S.; Thompson, L.M.; Marsh, J.L. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum. Mol. Genet. 2008, 17, 3767–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthi-Carter, R.; Taylor, D.M.; Pallos, J.; Lambert, E.; Amore, A.; Parker, A.; Moffitt, H.; Smith, D.L.; Runne, H.; Gokce, O.; et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 7927–7932. [Google Scholar] [CrossRef] [Green Version]
- Chopra, V.; Quinti, L.; Kim, J.; Vollor, L.; Narayanan, K.L.; Edgerly, C.; Cipicchio, P.M.; Lauver, M.A.; Choi, S.H.; Silverman, R.B.; et al. The Sirtuin 2 Inhibitor AK-7 Is Neuroprotective in Huntington’s Disease Mouse Models. Cell Rep. 2012, 2, 1492–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Oikawa, S.; Kurimoto, S.; Kitamura, Y.; Tada-Oikawa, S.; Kobayashi, H.; Yamashima, T.; Murata, M. Proteomic Analysis of the Monkey Hippocampus for Elucidating Ischemic Resistance. J. Clin. Biochem. Nutr. 2020, 67, 167–173. [Google Scholar] [CrossRef]
- Wu, D.; Lu, W.; Wei, Z.; Xu, M.; Liu, X. Neuroprotective Effect of Sirt2-Specific Inhibitor AK-7 Against Acute Cerebral Ischemia Is P38 Activation-Dependent in Mice. Neuroscience 2018, 374, 61–69. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, Z.-M.; Lu, L.-Y.; Nie, H.; Ding, J.; Ying, W.-H.; Tian, H.-L. SIRT2 Inhibition Exacerbates Neuroinflammation and Blood-Brain Barrier Disruption in Experimental Traumatic Brain Injury by Enhancing NF-ΚB P65 Acetylation and Activation. J. Neurochem. 2016, 136, 581–593. [Google Scholar] [CrossRef] [Green Version]
- Ranadive, N.; Arora, D.; Nampoothiri, M.; Mudgal, J. Sirtuins, a Potential Target in Traumatic Brain Injury and Relevant Experimental Models. Brain Res. Bull. 2021, 171, 135–141. [Google Scholar] [CrossRef]
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 Mediates Oxidative Stress-Induced Apoptosis of Differentiated PC12 Cells. NeuroReport 2014, 25, 838–842. [Google Scholar] [CrossRef]
- Chen, X.; Wales, P.; Quinti, L.; Zuo, F.; Moniot, S.; Hérisson, F.; Rauf, N.A.; Wang, H.; Silverman, R.B.; Ayata, C.; et al. The Sirtuin-2 Inhibitor AK7 Is Neuroprotective in Models of Parkinson’s Disease but Not Amyotrophic Lateral Sclerosis and Cerebral Ischemia. PLoS ONE 2015, 10, e0116919. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guan, Q.; Wang, M.; Yang, L.; Bai, J.; Yan, Z.; Zhang, Y.; Liu, Z. Aging-Related Rotenone-Induced Neurochemical and Behavioral Deficits: Role of SIRT2 and Redox Imbalance, and Neuroprotection by AK-7. DDDT 2015, 9, 2553. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Hanson, P.S.; Morris, C.M. Sirtuin-2 Protects Neural Cells from Oxidative Stress and Is Elevated in Neurodegeneration. Parkinson’s Dis. 2017, 2017, 2643587. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lu, W.; Wu, D. Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front. Neurosci. 2021, 15, 614107. [Google Scholar] [CrossRef]
- Silva, D.F.; Esteves, A.R.; Oliveira, C.R.; Cardoso, S.M. Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer’s-Disease Related Pathology. Mol. Neurobiol. 2017, 54, 4021–4040. [Google Scholar] [CrossRef]
- van Leeuwen, I.M.M.; Higgins, M.; Campbell, J.; McCarthy, A.R.; Sachweh, M.C.C.; Navarro, A.M.; Laín, S. Modulation of P53 C-Terminal Acetylation by Mdm2, P14ARF, and Cytoplasmic SirT2. Mol. Cancer Ther. 2013, 12, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Rack, J.G.M.; VanLinden, M.R.; Lutter, T.; Aasland, R.; Ziegler, M. Constitutive Nuclear Localization of an Alternatively Spliced Sirtuin-2 Isoform. J. Mol. Biol. 2014, 426, 1677–1691. [Google Scholar] [CrossRef] [Green Version]
- Thangaraj, M.P.; Furber, K.L.; Gan, J.K.; Ji, S.; Sobchishin, L.; Doucette, J.R.; Nazarali, A.J. RNA-Binding Protein Quaking Stabilizes Sirt2 MRNA during Oligodendroglial Differentiation. J. Biol. Chem. 2017, 292, 5166–5182. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yin, J.; Cao, D.; Xiao, D.; Zhou, Z.; Liu, Y.; Shou, W. The Emerging Roles of the RNA Binding Protein QKI in Cardiovascular Development and Function. Front. Cell Dev. Biol. 2021, 9, 668659. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Xu, C.; Ba, L.; Liu, Z.; Li, X.; Huang, J.; Simpson, E.; Gao, H.; Cao, D.; et al. QKI is a critical pre-mRNA alternative splicing regulator of cardiac myofibrillogenesis and contractile function. Nat. Commun. 2021, 12, 89. [Google Scholar] [CrossRef]
- Aberg, K.; Saetre, P.; Jareborg, N.; Jazin, E. Human QKI, a Potential Regulator of MRNA Expression of Human Oligodendrocyte-Related Genes Involved in Schizophrenia. Proc. Natl. Acad. Sci. USA 2006, 103, 7482–7487. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, B.; Peuckert, C.; Zimmermann, B.; Jazin, E.; Kettunen, P.; Emilsson, L.S. Gene Expression of Quaking in Sporadic Alzheimer’s Disease Patients Is Both Upregulated and Related to Expression Levels of Genes Involved in Amyloid Plaque and Neurofibrillary Tangle Formation. JAD 2016, 53, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Roshdy, E.; Mustafa, M.; Shaltout, A.E.-R.; Radwan, M.O.; Ibrahim, M.A.A.; Soliman, M.E.; Fujita, M.; Otsuka, M.; Ali, T.F.S. Selective SIRT2 Inhibitors as Promising Anticancer Therapeutics: An Update from 2016 to 2020. Eur. J. Med. Chem. 2021, 224, 113709. [Google Scholar] [CrossRef]
- Di Fruscia, P.; Zacharioudakis, E.; Liu, C.; Moniot, S.; Laohasinnarong, S.; Khongkow, M.; Harrison, I.; Koltsida, K.; Reynolds, C.R.; Schmidtkunz, K.; et al. The Discovery of a Highly Selective 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one SIRT2 Inhibitor that is Neuroprotective in an in vitro Parkinson’s Disease Model. ChemMedChem 2014, 10, 69–82. [Google Scholar] [CrossRef]
- Ai, T.; Wilson, D.J.; More, S.S.; Xie, J.; Chen, L. 5-((3-Amidobenzyl)Oxy)Nicotinamides as Sirtuin 2 Inhibitors. J. Med. Chem. 2016, 59, 2928–2941. [Google Scholar] [CrossRef]
- Radwan, M.O.; Ciftci, H.I.; Ali, T.F.S.; Ellakwa, D.E.; Koga, R.; Tateishi, H.; Nakata, A.; Ito, A.; Yoshida, M.; Okamoto, Y.; et al. Antiproliferative S-Trityl-l-Cysteine -Derived Compounds as SIRT2 Inhibitors: Repurposing and Solubility Enhancement. Molecules 2019, 24, 3295. [Google Scholar] [CrossRef] [Green Version]
- Radwan, M.O.; Ciftci, H.I.; Ali, T.F.S.; Koga, R.; Tateishi, H.; Nakata, A.; Ito, A.; Yoshida, M.; Fujita, M.; Otsuka, M. Structure Activity Study of S-Trityl-Cysteamine Dimethylaminopyridine Derivatives as SIRT2 Inhibitors: Improvement of SIRT2 Binding and Inhibition. Bioorg. Med. Chem. Lett. 2020, 30, 127458. [Google Scholar] [CrossRef]
- Alarcón-Arís, D.; Pavia-Collado, R.; Miquel-Rio, L.; Coppola-Segovia, V.; Ferrés-Coy, A.; Ruiz-Bronchal, E.; Galofré, M.; Paz, V.; Campa, L.; Revilla, R.; et al. Anti-α-synuclein ASO delivered to monoamine neurons prevents α-synuclein accumulation in a Parkinson’s disease-like mouse model and in monkeys. EBioMedicine 2020, 59, 102944. [Google Scholar] [CrossRef]
| Keywords | Hits | Plus Keyword | Hits |
|---|---|---|---|
| SIRT1 | 10,888 | +microRNA | 895 |
| SIRT2 | 1676 | +microRNA | 30 |
| SIRT3 | 1969 | +microRNA | 41 |
| SIRT4 | 283 | +microRNA | 7 |
| SIRT5 | 347 | +microRNA | 7 |
| SIRT6 | 936 | +microRNA | 55 |
| SIRT7 | 371 | +microRNA | 37 |
| SIRT1 × hypoxia | 424 | +microRNA | 57 |
| SIRT2 × hypoxia | 16 | +microRNA | 0 |
| SIRT3 × hypoxia | 103 | +microRNA | 4 |
| SIRT4 × hypoxia | 9 | +microRNA | 1 |
| SIRT5 × hypoxia | 7 | +microRNA | 1 |
| SIRT6 × hypoxia | 39 | +microRNA | 3 |
| SIRT7 × hypoxia | 9 | +microRNA | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaitsuka, T.; Matsushita, M.; Matsushita, N. Regulation of Hypoxic Signaling and Oxidative Stress via the MicroRNA–SIRT2 Axis and Its Relationship with Aging-Related Diseases. Cells 2021, 10, 3316. https://doi.org/10.3390/cells10123316
Kaitsuka T, Matsushita M, Matsushita N. Regulation of Hypoxic Signaling and Oxidative Stress via the MicroRNA–SIRT2 Axis and Its Relationship with Aging-Related Diseases. Cells. 2021; 10(12):3316. https://doi.org/10.3390/cells10123316
Chicago/Turabian StyleKaitsuka, Taku, Masayuki Matsushita, and Nobuko Matsushita. 2021. "Regulation of Hypoxic Signaling and Oxidative Stress via the MicroRNA–SIRT2 Axis and Its Relationship with Aging-Related Diseases" Cells 10, no. 12: 3316. https://doi.org/10.3390/cells10123316
APA StyleKaitsuka, T., Matsushita, M., & Matsushita, N. (2021). Regulation of Hypoxic Signaling and Oxidative Stress via the MicroRNA–SIRT2 Axis and Its Relationship with Aging-Related Diseases. Cells, 10(12), 3316. https://doi.org/10.3390/cells10123316







