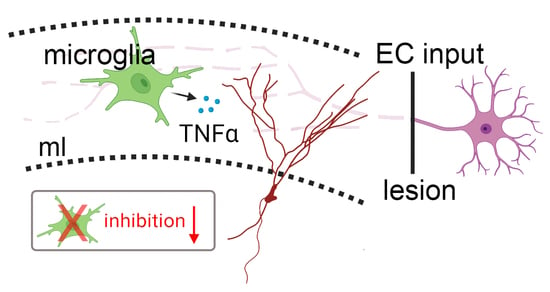
Scavenging Tumor Necrosis Factor α Does Not Affect Inhibition of Dentate Granule Cells Following In Vitro Entorhinal Cortex Lesion
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Preparation of Tissue Cultures
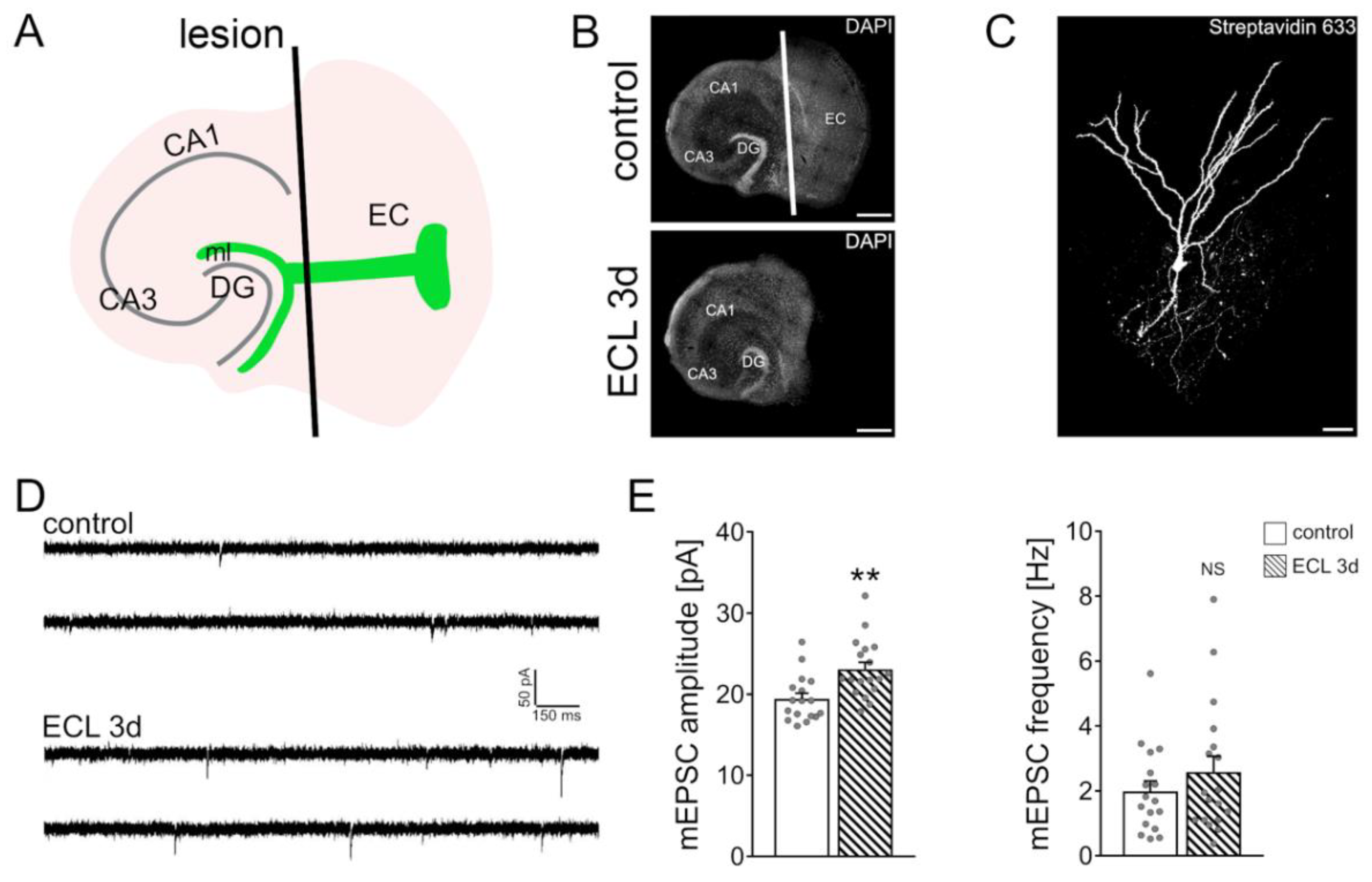
2.4. Entorhinal Cortex Lesion
2.5. Pharmacology
2.6. Whole-Cell Patch-Clamp Recordings
2.7. Immunohistochemistry and Imaging
2.8. Post-Hoc Identification of Recorded Neurons
2.9. Live-Cell Microscopy
2.10. Quantification and Statistics
2.11. Digital Illustrations
3. Results
3.1. Partial Denervation of Dentate Granule Cells Induces a Compensatory Adjustment of Excitatory Synaptic Strength
3.2. TNFα Does Not Affect Inhibitory Neurotransmission onto Denervated Dentate Granule Cells
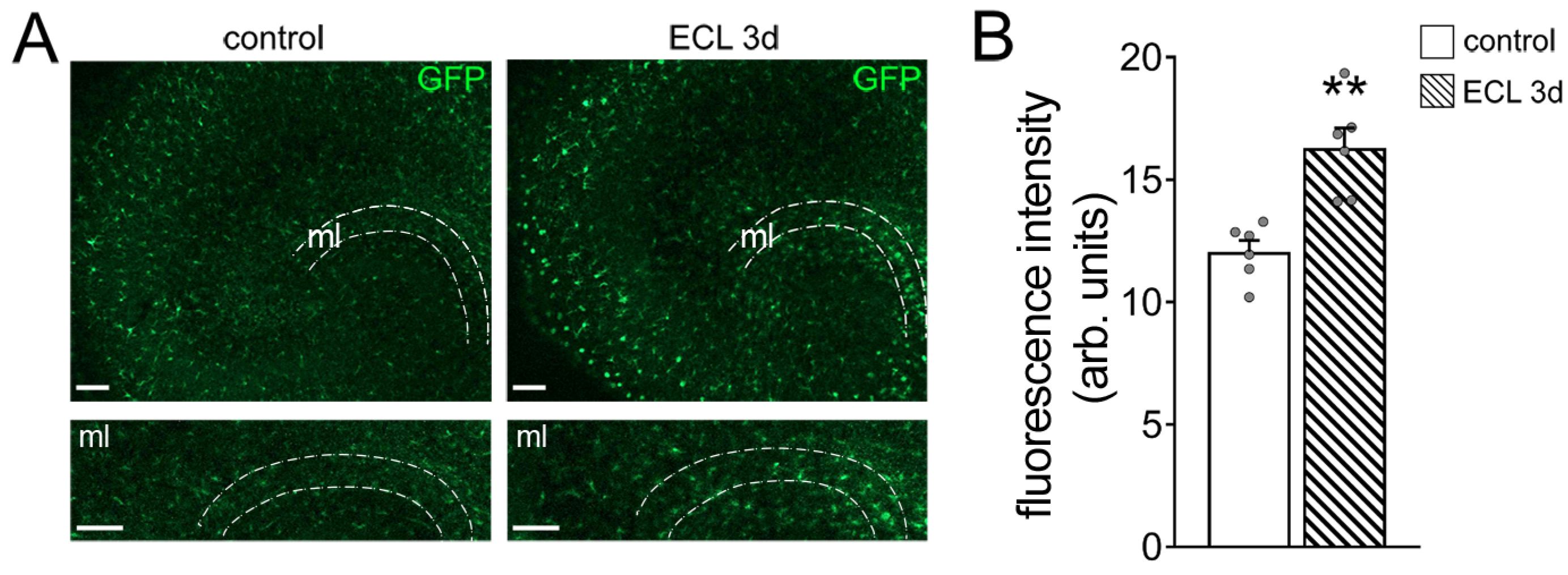
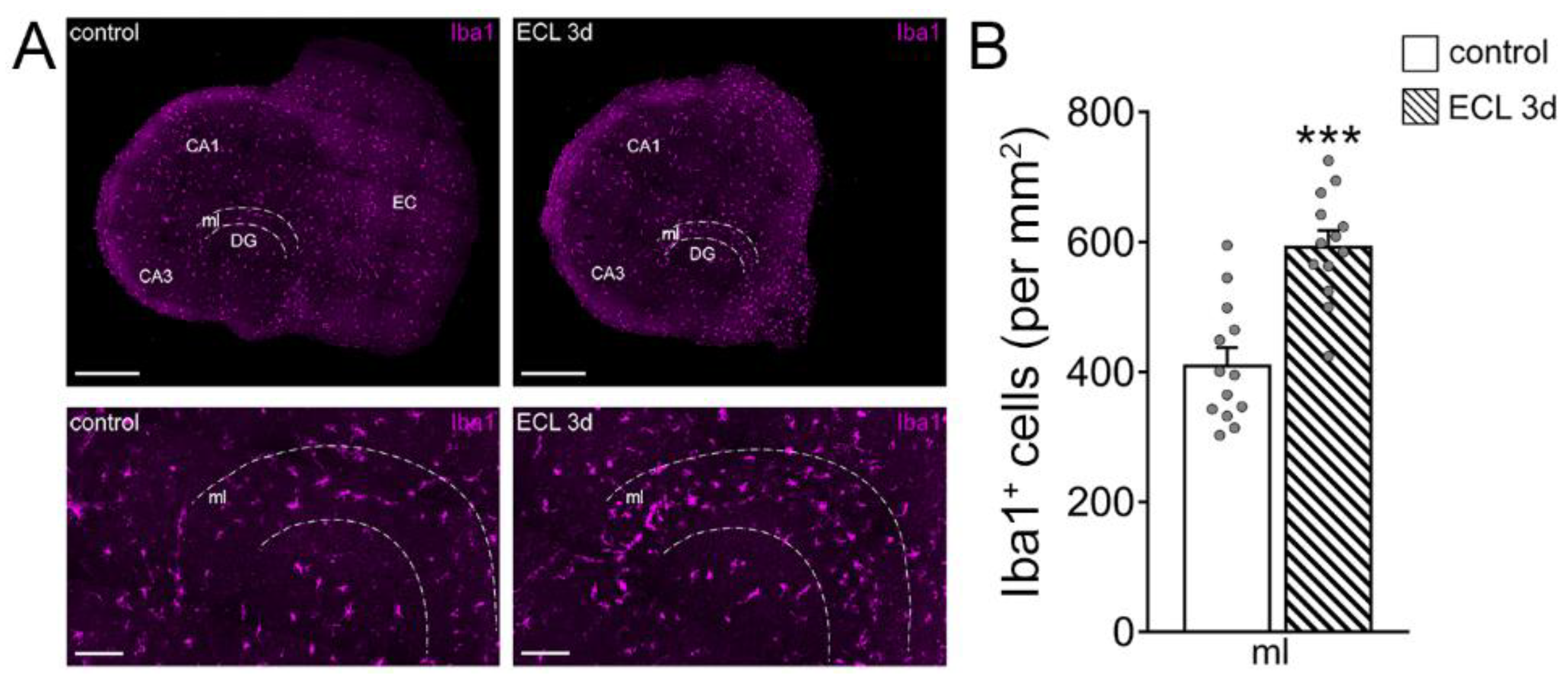
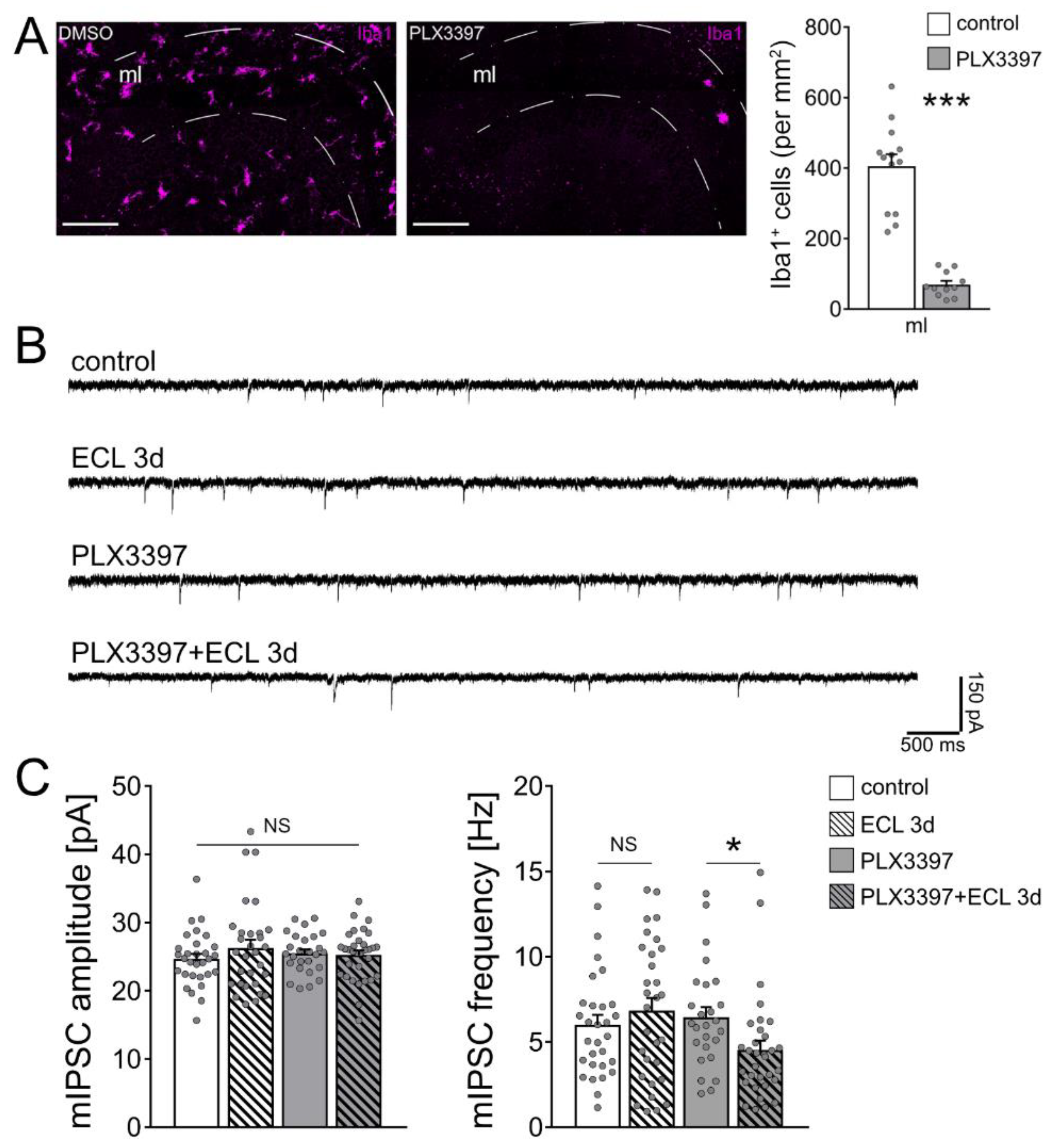
3.3. Microglia Maintain Inhibition of Partially Denervated Dentate Granule Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehghani, N.; Peyrache, A.; Telenczuk, B.; Le Van Quyen, M.; Halgren, E.; Cash, S.S.; Hatsopoulos, N.G.; Destexhe, A. Dynamic Balance of Excitation and Inhibition in Human and Monkey Neocortex. Sci. Rep. 2016, 6, 23176. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.; Abbott, L.F.; Sompolinsky, H. Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity. Proc. Natl. Acad. Sci. USA 2017, 114, E9366–E9375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yu, Y. Synaptic E-I Balance Underlies Efficient Neural Coding. Front. Neurosci. 2018, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Sprekeler, H. Functional consequences of inhibitory plasticity: Homeostasis, the excitation-inhibition balance and beyond. Curr. Opin. Neurobiol. 2017, 43, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.; Hübener, M.; Bonhoeffer, T. Interactions between synaptic homeostatic mechanisms: An attempt to reconcile BCM theory, synaptic scaling, and changing excitation/inhibition balance. Curr. Opin. Neurobiol. 2017, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pozo, K.; Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef]
- Fernandes, D.; Carvalho, A.L. Carvalho. Mechanisms of homeostatic plasticity in the excitatory synapse. J. Neurochem. 2016, 139, 973–996. [Google Scholar] [CrossRef]
- Keck, T.; Toyoizumi, T.; Chen, L.; Doiron, B.; Feldman, D.E.; Fox, K.; Gerstner, W.; Haydon, P.G.; Hübener, M.; Lee, H.-K.; et al. Integrating Hebbian and homeostatic plasticity: The current state of the field and future research directions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160158. [Google Scholar] [CrossRef]
- Yu, X.; Chung, S.; Chen, D.-Y.; Wang, S.; Dodd, S.J.; Walters, J.R.; Isaac, J.T.; Koretsky, A.P. Thalamocortical inputs show post-critical-period plasticity. Neuron 2012, 74, 731–742. [Google Scholar] [CrossRef]
- Vlachos, A.; Becker, D.; Jedlicka, P.; Winkels, R.; Roeper, J.; Deller, T. Entorhinal denervation induces homeostatic synaptic scaling of excitatory postsynapses of dentate granule cells in mouse organotypic slice cultures. PLoS ONE 2012, 7, e32883. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, A.; Ikenberg, B.; Lenz, M.; Becker, D.; Reifenberg, K.; Orth, C.B.; Deller, T. Synaptopodin regulates denervation-induced homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Ikenberg, B.; Schiener, S.; Maggio, N.; Vlachos, A. NMDA-receptor inhibition restores Protease-Activated Receptor 1 (PAR1) mediated alterations in homeostatic synaptic plasticity of denervated mouse dentate granule cells. Neuropharmacology 2014, 86, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.; Keller, G.B.; Jacobsen, R.I.; Eysel, U.; Bonhoeffer, T.; Hübener, M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 2013, 80, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Franzoni, E.; Jacobsen, R.I.; Erdelyi, F.; Szabo, G.; Clopath, C.; Keller, G.B.; Keck, T. Deprivation-Induced Homeostatic Spine Scaling In Vivo Is Localized to Dendritic Branches that Have Undergone Recent Spine Loss. Neuron 2017, 96, 871–882.e5. [Google Scholar] [CrossRef]
- Deller, T.; Frotscher, M.; Nitsch, R. Morphological evidence for the sprouting of inhibitory commissural fibers in response to the lesion of the excitatory entorhinal input to the rat dentate gyrus. J. Neurosci. 1995, 15, 6868–6878. [Google Scholar] [CrossRef]
- Simburger, E.; Plaschke, M.; Kirsch, J.; Nitsch, R. Distribution of the receptor-anchoring protein gephyrin in the rat dentate gyrus and changes following entorhinal cortex lesion. Cereb. Cortex 2000, 10, 422–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinocourt, C.; Aungst, S.; Yang, K.; Thompson, S.M. Homeostatic increase in excitability in area CA1 after Schaffer collateral transection in vivo. Epilepsia 2011, 52, 1656–1665. [Google Scholar] [CrossRef]
- Lenz, M.; Galanis, C.; Kleidonas, D.; Fellenz, M.; Deller, T.; Vlachos, A. Denervated mouse dentate granule cells adjust their excitatory but not inhibitory synapses following in vitro entorhinal cortex lesion. Exp. Neurol. 2019, 312, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef]
- Pribiag, H.; Stellwagen, D. TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J. Neurosci. 2013, 33, 15879–15893. [Google Scholar] [CrossRef]
- Becker, D.; Zahn, N.; Deller, T.; Vlachos, A. Tumor necrosis factor alpha maintains denervation-induced homeostatic synaptic plasticity of mouse dentate granule cells. Front. Cell. Neurosci. 2013, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Deller, T.; Vlachos, A. Tumor necrosis factor (TNF)-receptor 1 and 2 mediate homeostatic synaptic plasticity of denervated mouse dentate granule cells. Sci. Rep. 2015, 5, 12726. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Müller, P.-F.; Wolf, Y.; Varol, D.; Yona, S.; Brendecke, S.M.; Kierdorf, K.; Staszewski, O.; Datta, M.; et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 2013, 16, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Welser-Alves, J.V.; Milner, R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 2013, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Lenz, M.; Eichler, A.; Kruse, P.; Strehl, A.; Rodriguez-Rozada, S.; Goren, I.; Yogev, N.; Frank, S.; Waisman, A.; Deller, T.; et al. Interleukin 10 Restores Lipopolysaccharide-Induced Alterations in Synaptic Plasticity Probed by Repetitive Magnetic Stimulation. Front. Immunol. 2020, 11, 614509. [Google Scholar] [CrossRef]
- Del Turco, D.; Deller, T. Organotypic entorhino-hippocampal slice cultures—A tool to study the molecular and cellular regulation of axonal regeneration and collateral sprouting in vitro. Methods Mol. Biol. 2007, 399, 55–66. [Google Scholar] [CrossRef]
- Rappert, A.; Bechmann, I.; Pivneva, T.; Mahlo, J.; Biber, K.; Nolte, C.; Kovac, A.D.; Gerard, C.; Boddeke, H.W.G.M.; Nitsch, R.; et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J. Neurosci. 2004, 24, 8500–8509. [Google Scholar] [CrossRef]
- Han, J.; Harris, R.A.; Zhang, X.-M. An updated assessment of microglia depletion: Current concepts and future directions. Mol. Brain 2017, 10, 25. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflam. 2020, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.; Kleidonas, D.; Turi, Z.; Kirsch, M.; Pfeifer, D.; Masuda, T.; Prinz, M.; Lenz, M.; Vlachos, A. Microglia mediate synaptic plasticity induced by 10 Hz repetitive magnetic stimulation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Leslie, K.R.; Desai, N.S.; Rutherford, L.C.; Nelson, S. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998, 391, 892–896. [Google Scholar] [CrossRef]
- Thiagarajan, T.C.; Lindskog, M.; Tsien, R.W. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 2005, 47, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Swanwick, C.C.; Murthy, N.R.; Kapur, J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol. Cell. Neurosci. 2006, 31, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kilman, V.; van Rossum, M.C.; Turrigiano, G.G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. 2002, 22, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Strehl, A.; Galanis, C.; Radic, T.; Schwarzacher, S.W.; Deller, T.; Vlachos, A. Dopamine Modulates Homeostatic Excitatory Synaptic Plasticity of Immature Dentate Granule Cells in Entorhino-Hippocampal Slice Cultures. Front. Mol. Neurosci. 2018, 11, 303. [Google Scholar] [CrossRef]
- Vlachos, A.; Helias, M.; Becker, D.; Diesmann, M.; Deller, T. NMDA-receptor inhibition increases spine stability of denervated mouse dentate granule cells and accelerates spine density recovery following entorhinal denervation in vitro. Neurobiol. Dis. 2013, 59, 267–276. [Google Scholar] [CrossRef]
- Joseph, A.; Turrigiano, G.G. All for One But Not One for All: Excitatory Synaptic Scaling and Intrinsic Excitability Are Coregulated by CaMKIV, Whereas Inhibitory Synaptic Scaling Is Under Independent Control. J. Neurosci. 2017, 37, 6778–6785. [Google Scholar] [CrossRef]
- Ge, Y.; Kang, Y.; Cassidy, R.; Moon, K.-M.; Lewis, R.; Wong, R.O.; Foster, L.J.; Craig, A.M. Clptm1 Limits Forward Trafficking of GABAA Receptors to Scale Inhibitory Synaptic Strength. Neuron 2018, 97, 596–610.e8. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Malenka. Control of synaptic strength by glial TNFalpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Vlachos, A. Tumor necrosis factor (TNF) modulates synaptic plasticity in a concentration-dependent manner through intracellular calcium stores. J. Mol. Med. 2018, 96, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Bourgognon, J.M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Volterra, A. TNFalpha in synaptic function: Switching gears. Trends Neurosci. 2012, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Jiang, J.; Wang, L.; Misrani, A.; Huo, Q.; Han, Y.; Long, C.; Yang, L. Microglial activation results in neuron-type-specific increase in mPFC GABAergic transmission and abnormal behavior in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lau, L.T.; Yu, A.C. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma 2001, 18, 351–359. [Google Scholar] [CrossRef]
- Rodgers, K.; Lin, Y.; Langan, T.J.; Iwakura, Y.; Chou, R.C. Innate Immune Functions of Astrocytes are Dependent Upon Tumor Necrosis Factor-Alpha. Sci. Rep. 2020, 10, 7047. [Google Scholar] [CrossRef]
- Park, K.M.; Bowers, W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleidonas, D.; Vlachos, A. Scavenging Tumor Necrosis Factor α Does Not Affect Inhibition of Dentate Granule Cells Following In Vitro Entorhinal Cortex Lesion. Cells 2021, 10, 3232. https://doi.org/10.3390/cells10113232
Kleidonas D, Vlachos A. Scavenging Tumor Necrosis Factor α Does Not Affect Inhibition of Dentate Granule Cells Following In Vitro Entorhinal Cortex Lesion. Cells. 2021; 10(11):3232. https://doi.org/10.3390/cells10113232
Chicago/Turabian StyleKleidonas, Dimitrios, and Andreas Vlachos. 2021. "Scavenging Tumor Necrosis Factor α Does Not Affect Inhibition of Dentate Granule Cells Following In Vitro Entorhinal Cortex Lesion" Cells 10, no. 11: 3232. https://doi.org/10.3390/cells10113232
APA StyleKleidonas, D., & Vlachos, A. (2021). Scavenging Tumor Necrosis Factor α Does Not Affect Inhibition of Dentate Granule Cells Following In Vitro Entorhinal Cortex Lesion. Cells, 10(11), 3232. https://doi.org/10.3390/cells10113232