Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review
Abstract
1. Introduction
2. Search Methods for the Review
3. Functional Neuroimaging Markers of ADHD That Could Provide Targets for Neurotherapeutics
3.1. Electrophysiological Biomarkers
3.2. fMRI Biomarkers
4. Neurotherapeutics in ADHD
4.1. Neurofeedback
4.1.1. EEG-NF
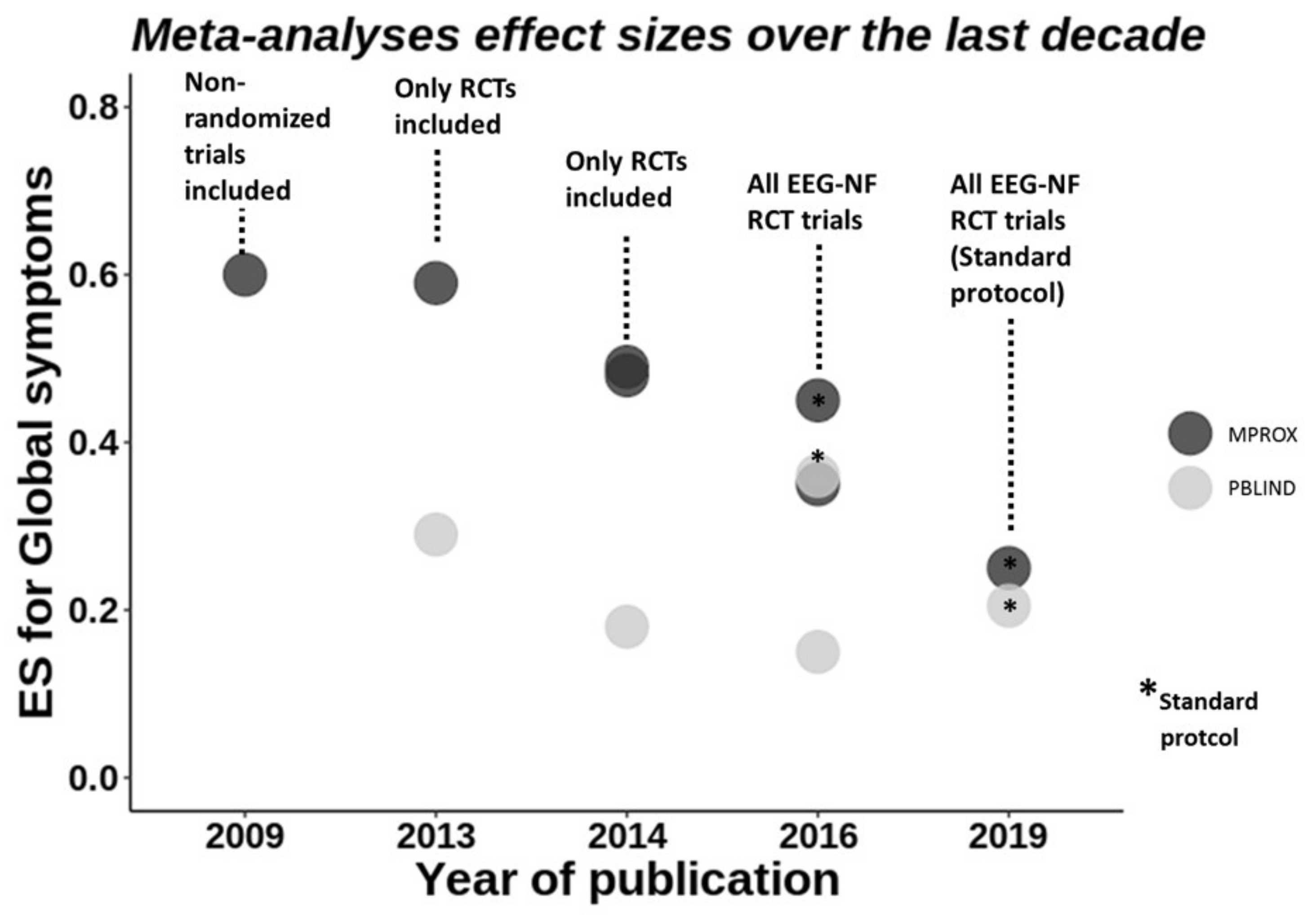
Meta-Analyses of EEG-NF
Other Aspects of EEG-NF
4.1.2. fMRI-Neurofeedback
4.1.3. NIRS Neurofeedback
4.1.4. Conclusions from Neurofeedback Studies
4.2. Brain Stimulation
4.2.1. Repetitive Transcranial Magnetic Stimulation (rTMS)
4.2.2. Transcranial Direct Current Stimulation (tDCS)
| Stimulation Protocol | Outcome Measures (Bold/Underlined = Improvement; Cursive = Impairment) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Design | n | Mean Age | Anode/Cathode | mA | Sessions | Timing a | Duration (mins) | Clinical | Cognitive |
| Children | ||||||||||
| † Bandeira et al., 2016 [168] | Open label | 9 | 11 | L DLPFC/R SOA | 2 | 5 | Online | 28 | Patient Global Impression of Improvement | Visual Attention Test (OM); NEPSY-II-inhibition (Switch errors); Digit Span; Corsi Cubes |
| Breitling et al., 2016 [169] | Single-blind, sham-controlled, randomised, crossover | 21 | 14 | R IFC/L Cheek | 1 | 1 | Online | 20 | n/t | Flanker (Incongruent trials: COM c,d & RTV c) e |
| L Cheek/R IFC | 1 | 1 | Online | 20 | n/t | Flanker | ||||
| Munz et al., 2015 [167] | Double-blind, sham-controlled, randomised, crossover | 14 | 12 | L DLPFC/R Cheek; R DLPFC/L Cheek | 0.25 | 1 | Offline | 25 (5 on, 1 off) | n/t | Go/No-Go (Go RT & RTV); Motor memory; Alertness |
| Nejati et al., 2020, Exp 1 [171] | Double-blind, sham-controlled, randomised, crossover | 15 | 10 | L DLPFC/R DLPFC | 1 | 1 | Offline | 15 | n/t | Go/No-Go; N-back (Acc, RT); Stroop (Incongruent trials: COM & RT); WCST (Completion time) |
| Nejati et al., 2020, Exp 2 [171] | Double-blind, sham-controlled, randomised, crossover | 10 | 9 | L DLPFC/R SOA | 1 | 1 | Offline | 15 | n/t | Go/No-Go; N-back (Acc c, RT) d; WCST (Total categories completed, total & pers errors) d |
| R SOA/L DLPFC | 1 | 1 | Offline | 15 | n/t | Go/No-Go (No--Go acc) d; N-back; WCST (Total categories completed, total & pers errors c) d | ||||
| Prehn-Kristensen et al., 2014 [166] | Double-blind, sham-controlled, randomised, parallel | 12 | 12 | L DLPFC/R Cheek; R DLPFC/L Cheek | 0.25 | 1 | Offline | 25 (5 on, 1 off) | n/t | Declarative Memory (Acc); Alertness; Digit Span |
| Soff et al., 2017 [164] | Double-blind, sham-controlled, randomised, crossover | 15 | 14 | L DLPFC/Vertex | 1 | 5 | Online | 20 | FBB-ADHD(Inattention f) g,h | QbTest (Inattention f; hyperactivity i) g,h |
| Soltaninejad et al., 2019 [161] | Single-blind, sham-controlled, randomised, crossover | 20 | 16 | L DLPFC/R SOA | 1.5 | 1 | Online | 15 | n/t | Go/No-Go (Go Acc) c,d; Stroop |
| R SOA/L DLPFC | 1.5 | 1 | Online | 15 | n/t | Go/No-Go (NoGo Acc) c,j; Stroop | ||||
| ‡ Soltaninejad et al., 2015 [161] | Single-blind, sham-controlled, randomised, crossover | 20 | 16 | rIFC/L SOA | 1 | 1 | Online | 15 | n/t | Go/No-Go (Go Acc); Stroop |
| Sotnikova et al., 2017 [165] | Double-blind, sham-controlled, randomised, crossover | 13 | 14 | L DLPFC/Vertex | 1 | 1 | Online | 20 | n/t | QbTest (RT, RTV k, OMs, Acc) l |
| Breitling et al., 2020 [169] | Double-blind, sham- and HD-tDCS controlled, randomised, crossover | ADHD: 15HC: 15 | 13 (10–16) | R IFC/L SOA | 1 | 3 with CT | Online | 20 | n/t | WM task; ERPs N200; P300 |
| Salehinejad et al., 2020 [170] | Single-blind, sham-controlled, randomised, cross-over | 19 | 9 (8–12) | 1 | 2 | Online | 23 | n/t | ANT (orienting); GNG; SAT; Stroop | |
| † Westwood et al., 2021 [162] | Double-blind, sham-controlled, randomised, parallel | 50 | 14 | R IFC/L SOA | 1 | 15 | Online | 20 | ADHD-RS; Conners 3P | GNG; Stop; Simon; WCST; CPT; MCT; time estimation; NIH WM; Verbal Fluency |
| Nejati et al., 2020 [171] | Double-blind, sham-controlled, randomised, cross-over | 20 | 9 | L DLPFC/R vmPFC R DLPFC/L vmPFC Sham | 1 | 1 | Online | 20 | n/t | BART; CDDT (k20, k10) |
| † Berger et al., 2021 [174] | Double-blind, active controlled, randomised, cross-over | 19 | 7–12 | L DLPFC (tDCS)/R SOA L DLPFC/R IFC (tRNS) | 0.75 | 5 | Online | 5 | n/t | ADHD-RS; Working & short-term memory, Moxo-CPT (all improved with tRNS vs. tDCS) |
| Adults | ||||||||||
| † Allenby et al., 2018 [177] | Double-blind, sham-controlled, randomised, crossover | 37 | 32 | L DLPFC/R SOA | 2 | 3 | Online | 20 | n/t | Conners CPT (COM m); Stop Task |
| Cachoeira et al., 2017 [178] | Double-blind, sham-controlled, randomised, parallel | A: 9 S: 8 | A: 31 S: 34 | R DLPFC/L DLPFC | 2 | 5 | Offline | 20 | ADHD Checklist (Inattention, Total) n; SDS (after tDCS); ADHD total score 2 weeks | None |
| Cosmo et al., 2015 [175] | Double-blind, sham-controlled, randomised, parallel | A: 30 S: 30 | A: 32 S: 33 | LDLPFC/R DLPFC | 1 | 1 | Offline | 20 | n/t | Go/No-Go |
| Jacoby et al., 2018 [176] | Single-blind, sham-controlled, randomised, crossover | 20 | 23 | L&R DLPFC/Cerebellum | 1.8 | 1 | Offline | 20 | n/t | CPT (multi-button presses) |
| Dubreuil-Vall et al., 2020 [179] | Double-blind, sham-controlled, randomised, crossover | 37 | 18–67 | L DLPFC/R SOA R DLPFC/R SOA | 2 | 1 | Offline | 30 | n/t | Flanker (incongruent RT) n = 18; L P300; L N200. Stop (go RTs); L P200. n = 19 Flanker; Stop |
4.2.3. Other Stimulation Methods
5. Overall Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. Functional brain imaging across development: A review. Eur. Child Adolesc. Psychiatry 2013, 22, 719–731. [Google Scholar] [CrossRef]
- Rubia, K. “Cool” inferior fronto-striatal dysfunction in Attention Deficit Hyperactivity Disorder (ADHD) versus “hot” ventromedial orbitofronto-limbic dysfunction in conduct disorder: A review. Biol. Psychiatry 2011, 69, e69–e87. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Sonuga-Barke, E.J.S.; Nigg, J.T.; Sergeant, G.A. Recent Developments in Neuropsychological Models of Childhood Psychiatric Disorders. In Biological Child Psychiatry; Banaschewski, T., Rohde, L.A., Eds.; Karger: Basel, Switzerland, 2008; Volume 24, pp. 195–226. [Google Scholar]
- Pievsky, M.A.; McGrath, R.E. The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses. Arch. Clin. Neuropsychol. 2018, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Halari, R.; Christakou, A.; Taylor, E. Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1919–1931. [Google Scholar] [CrossRef]
- Noreika, V.; Falter, C.M.; Rubia, K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia 2013, 51, 235–266. [Google Scholar] [CrossRef] [PubMed]
- Plichta, M.M.; Scheres, A. Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014, 38, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Groen, Y.; Gaastra, G.F.; Lewis-Evans, B.; Tucha, O. Risky Behavior in Gambling Tasks in Individuals with ADHD—A Systematic Literature Review. PLoS ONE 2013, 8, e74909. [Google Scholar] [CrossRef]
- Nigg, J.T.; Stavro, G.; Ettenhofer, M.; Hambrick, D.Z.; Miller, T.; Henderson, J.M. Executive functions and adhd in adults: Evidence for selective effects on ADHD symptom domains. J. Abnorm. Psychol. 2005, 114, 706–717. [Google Scholar] [CrossRef]
- Roberts, B.A.; Martel, M.M.; Nigg, J.T. Are There Executive Dysfunction Subtypes Within ADHD? J. Atten. Disord. 2017, 21, 284–293. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Rubia, K.; Alzamora, A.; Cubillo, A.; Smith, A.B.; Radua, J.; Brammer, M.J. Effects of stimulants on brain function in ADHD: A systematic review and meta-analysis. Biol. Psychiatry 2014, 76, 616–628. [Google Scholar] [CrossRef]
- Coghill, D.R.; Seth, S.; Pedroso, S.; Usala, T.; Currie, J.; Gagliano, A. Effects of Methylphenidate on Cognitive Functions in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: Evidence from a Systematic Review and a Meta-Analysis. Biol. Psychiatry 2014, 76, 603–615. [Google Scholar] [CrossRef]
- Pievsky, M.A.; McGrath, R.E. Neurocognitive effects of methylphenidate in adults with attention-deficit/hyperactivity disorder: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 447–455. [Google Scholar] [CrossRef]
- Swanson, J.M. Debate: Are Stimulant Medications for Attention-Deficit/Hyperactivity Disorder Effective in the Long Term? (Against). J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Coghill, D. Debate: Are Stimulant Medications for Attention-Deficit/Hyperactivity Disorder Effective in the Long Term? (For). J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 938–939. [Google Scholar] [CrossRef]
- Connolly, J.J.; Glessner, J.T.; Elia, J.; Hakonarson, H. ADHD & Pharmacotherapy: Past, Present and Future: A Review of the Changing Landscape of Drug Therapy for Attention Deficit Hyperactivity Disorder. Ther. Innov. Regul. Sci. 2015, 49, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Lubar, J.F.; Shouse, M.N. EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR). Appl. Psychophysiol. Biofeedback 1976, 1, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, J.H. EEG issues in children with minimal brain dysfunction. Semin. Psychiatry 1973, 5, 35–46. [Google Scholar] [PubMed]
- Satterfield, J.H.; Lesser, L.I.; Saul, R.E.; Cantwell, D.P. EEG aspects in the diagnosis and treatment of minimal brain dysfunction. Ann. N. Y. Acad. Sci. 1973, 205, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Makeig, S. Clinical Utility of EEG in Attention-Deficit/Hyperactivity Disorder: A Research Update. Neurotherapeutics 2012, 9, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Rugino, T.A.; Hornig, M.; Stein, M.A. Integration of an EEG biomarker with a clinician’s ADHD evaluation. Brain Behav. 2015, 5, e00330. [Google Scholar] [CrossRef]
- Liechti, M.; Valko, L.; Müller, U.C.; Döhnert, M.; Drechsler, R.; Steinhausen, H.-C.; Brandeis, D. Diagnostic Value of Resting Electroencephalogram in Attention-Deficit/Hyperactivity Disorder Across the Lifespan. Brain Topogr. 2013, 26, 135–151. [Google Scholar] [CrossRef]
- Buyck, I.; Wiersema, J.R. State-related electroencephalographic deviances in attention deficit hyperactivity disorder. Res. Dev. Disabil. 2014, 35, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Buyck, I.; Wiersema, J.R. Resting electroencephalogram in attention deficit hyperactivity disorder: Developmental course and diagnostic value. Psychiatry Res. 2014, 216, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Buyck, I.; Wiersema, J.R. Electroencephalographic Activity Before and After Cognitive Effort in Children with Attention Deficit/Hyperactivity Disorder. Clin. EEG Neurosci. 2015, 46, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; Karamacoska, D.; Johnstone, S.J. The EEG Theta/Beta Ratio: A marker of Arousal or Cognitive Processing Capacity? Appl. Psychophysiol. Biofeedback 2019, 44, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Conners, C.K.; Kraemer, H.C. A decade of EEG Theta/Beta Ratio Research in ADHD: A meta-analysis. J. Atten. Disord. 2012, 17, 374–383. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.; Dupuy, F.E.; Heckel, L.D.; McCarthy, R.; Selikowitz, M.; Johnstone, S. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2011, 122, 1333–1341. [Google Scholar] [CrossRef]
- Bussalb, A.; Collin, S.; Barthélemy, Q.; Ojeda, D.; Bioulac, S.; Blasco-Fontecilla, H.; Brandeis, D.; Ouakil, D.P.; Ros, T.; Mayaud, L. Is there a cluster of high theta-beta ratio patients in attention deficit hyperactivity disorder? Clin. Neurophysiol. 2019, 130, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Johnstone, S.J.; Li, H.; Barry, R.J.; Clarke, A.R.; Zhao, Q.; Song, Y.; Liu, L.; Qian, Q.; Wang, Y.; et al. Time Effects on Resting EEG in Children With/Without AD/HD. Brain Topogr. 2019, 32, 286–294. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Johnstone, S. Resting state EEG power research in Attention-Deficit/Hyperactivity Disorder: A review update. Clin. Neurophysiol. 2020, 131, 1463–1479. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Arns, M.; Barterian, J.; Bergman, R.; Black, S.; Conners, C.K.; Connor, S.; Dasgupta, S.; Debeus, R.; Higgins, T.; et al. Double-Blind Placebo-Controlled Randomized Clinical Trial of Neurofeedback for Attention-Deficit/Hyperactivity Disorder With 13-Month Follow-up. J. Am. Acad. Child Adolesc. Psychiatry 2020, 60, 841–855. [Google Scholar] [CrossRef]
- Bioulac, S.; Purper-Ouakil, D.; Ros, T.; Blasco-Fontecilla, H.; Prats, M.; Mayaud, L.; Brandeis, D. Personalized at-home neurofeedback compared with long-acting methylphenidate in an european non-inferiority randomized trial in children with ADHD. BMC Psychiatry 2019, 19, 237. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003, 114, 171–183. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Barry, R.; Clarke, A.R. Ten years on: A follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2013, 124, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Aggensteiner, P.-M.; Baumeister, S.; Holz, N.E.; Banaschewski, T.; Brandeis, D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Gamma, A.; Kara, O. Event-Related Potentials for Diagnosing Children and Adults with ADHD. J. Atten. Disord. 2020, 24, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; E van Hulzen, K.J.; Medland, S.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef]
- Nakao, T.; Radua, C.; Rubia, K.; Mataix-Cols, D. Gray matter volume abnormalities in ADHD and the effects of stimulant medication: Voxel-based meta-analysis. Am. J. Psychiatry 2011, 168, 1154–1163. [Google Scholar] [CrossRef]
- Norman, L.; Carlisi, C.O.; Lukito, S.; Hart, H.; Mataix-Cols, D.; Radua, J.; Rubia, K. Comparative meta-analysis of functional and structural deficits in ADHD and OCD. JAMA Psychiatry 2016, 73, 815–825. [Google Scholar] [CrossRef]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef]
- Hoogman, M.; Muetzel, R.; Guimaraes, J.P.; Shumskaya, E.; Mennes, M.; Zwiers, M.P.; Jahanshad, N.; Sudre, G.; Wolfers, T.; Earl, E.A.; et al. Brain Imaging of the Cortex in ADHD: A Coordinated Analysis of Large-Scale Clinical and Population-Based Samples. Am. J. Psychiatry 2019, 176, 531–542. [Google Scholar] [CrossRef]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Malek, M.; Watson, B.; Sharp, W.; Evans, A.; Greenstein, D. Development of Cortical Surface Area and Gyrification in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2012, 72, 191–197. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Ouyang, L.; He, N.; Liao, Y.; Liu, Q.; Zhou, M.; Wu, M.; Huang, X.; Gong, Q. A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 2016, 68, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Cortese, S.; Castellanos, F. Research Review: Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: Meta-analyses and reflections on head motion. J. Child Psychol. Psychiatry 2017, 59, 193–202. [Google Scholar] [CrossRef]
- Rubia, K. Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front. Hum. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Hart, H.; Radua, J.; Mataix, D.; Rubia, K. Meta-analysis of fMRI studies of inhibition and attention in ADHD: Exploring task-specific, stimulant medication and age effects. JAMA Psychiatry 2013, 70, 185–198. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.; Skokauskas, N.; Frodl, T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: A meta-analysis. Psychol. Med. 2014, 44, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Du, M.; Wu, M.; Chen, T.; Huang, X.; Du, X.; Bi, F.; Kemp, G.; Gong, Q. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology 2015, 29, 874–881. [Google Scholar] [CrossRef]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies. Am. J. Psychiatry 2012, 169, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Hart, H.; Radua, J.; Mataix, D.; Rubia, K. Meta-analysis of fMRI studies of timing functions in ADHD. Neurosci. Biobehav. Rev. 2012, 36, 2248–2256. [Google Scholar] [CrossRef]
- Wiener, M.; Turkeltaub, P.; Coslett, H. The image of time: A voxel-wise meta-analysis. NeuroImage 2010, 49, 1728–1740. [Google Scholar] [CrossRef]
- Van Ewijk, H.; Weeda, W.D.; Heslenfeld, D.J.; Luman, M.; Hartman, C.A.; Hoekstra, P.J.; Faraone, S.; Franke, B.; Buitelaar, J.K.; Oosterlaan, J. Neural correlates of visuospatial working memory in attention-deficit/hyperactivity disorder and healthy controls. Psychiatry Res. Neuroimaging 2015, 233, 233–242. [Google Scholar] [CrossRef]
- Sripada, C.; Kessler, D.; Fang, Y.; Welsh, R.C.; Kumar, K.P.; Angstadt, M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014, 35, 4693–4705. [Google Scholar] [CrossRef] [PubMed]
- Sripada, C.S.; Kessler, D.; Angstadt, M. Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc. Natl. Acad. Sci. USA 2014, 111, 14259–14264. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Christakou, A.; MRC AIMS Consortium; Murphy, C.M.; Chantiluke, K.; Cubillo, A.; Smith, A.B.; Giampietro, V.; Daly, E.; Ecker, C.; Robertson, D.; et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with Autism. Mol. Psychiatry 2013, 18, 236–244. [Google Scholar] [CrossRef]
- Salavert, J.; Ramos-Quiroga, J.A.; Moreno-Alcázar, A.; Caseras, X.; Palomar, G.; Radua, J.; Bosch, R.; Salvador, R.; McKenna, P.J.; Casas, M.; et al. Functional Imaging Changes in the Medial Prefrontal Cortex in Adult ADHD. J. Atten. Disord. 2018, 22, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Bozhilova, N.S.; Michelini, G.; Kuntsi, J.; Asherson, P. Mind wandering perspective on attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 2018, 92, 464–476. [Google Scholar] [CrossRef]
- Rubia, K.; Criaud, M.; Wulff, M.; Alegria, A.; Brinson, H.; Barker, G.; Stahl, D.; Giampietro, V. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. NeuroImage 2019, 188, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L. The plastic human brain. Restor. Neurol. Neurosci. 2009, 27, 521–538. [Google Scholar] [CrossRef]
- Rapoport, J.L.; Gogtay, N. Brain Neuroplasticity in Healthy, Hyperactive and Psychotic Children: Insights from Neuroimaging. Neuropsychopharmacology 2008, 33, 181–197. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Neuroplasticity: Changes in grey matter induced by training—Newly honed juggling skills show up as a transient feature on a brain-imaging scan. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef]
- Draganski, B.; May, A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008, 192, 137–142. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Kempermann, G.; Kuhn, H.-G.; Winkler, J.; Büchel, C.; May, A. Temporal and Spatial Dynamics of Brain Structure Changes during Extensive Learning. J. Neurosci. 2006, 26, 6314–6317. [Google Scholar] [CrossRef] [PubMed]
- Dodich, A.; Zollo, M.; Crespi, C.; Cappa, S.; Martinez, D.L.; Falini, A.; Canessa, N. Short-term Sahaja Yoga meditation training modulates brain structure and spontaneous activity in the executive control network. Brain Behav. 2019, 9, e01159. [Google Scholar] [CrossRef] [PubMed]
- Ashkan, K.; Shotbolt, P.; David, A.; Samuel, M. Deep brain stimulation: A return journey from psychiatry to neurology. Postgrad. Med. J. 2013, 89, 323–328. [Google Scholar] [CrossRef][Green Version]
- Anderson, V.; Spencer-Smith, M.; Wood, A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 2011, 134, 2197–2221. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.; Valero-Cabré, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Schachar, R.J.; Tannock, R.; Logan, G. Inhibitory Control, Impulsiveness, and Attention-Deficit Hyperactivity Disorder. Clin. Psychol. Rev. 1993, 13, 721–739. [Google Scholar] [CrossRef]
- Arns, M.; Heinrich, H.; Strehl, U. Evaluation of neurofeedback in ADHD: The long and winding road. Biol. Psychol. 2014, 95, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Enriquez-Geppert, S.; Zotev, V.; Young, K.D.; Wood, G.; Whitfield-Gabrieli, S.; Wan, F.; Vuilleumier, P.; Vialatte, F.; Van De Ville, D.; et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain 2020, 143, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; de Ridder, S.; Strehl, U.; Breteler, M.; Coenen, A. Efficacy of Neurofeedback Treatment in ADHD: The Effects on Inattention, Impulsivity and Hyperactivity: A Meta-Analysis. Clin. EEG Neurosci. 2009, 40, 180–189. [Google Scholar] [CrossRef]
- Arns, M.; Clark, C.R.; Trullinger, M.; Debeus, R.; Mack, M.; Aniftos, M. Neurofeedback and Attention-Deficit/Hyperactivity-Disorder (ADHD) in Children: Rating the Evidence and Proposed Guidelines. Appl. Psychophysiol. Biofeedback 2020, 45, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Holtmann, M.; Aggensteiner, P.; Daley, D.; Santosh, P.; Simonoff, E.; Stevenson, J.; Stringaris, A.; et al. Neurofeedback for Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Clinical and Neuropsychological Outcomes from Randomized Controlled Trials. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 444–455. [Google Scholar] [CrossRef]
- Van Doren, J.; Arns, M.; Heinrich, H.; Vollebregt, M.A.; Strehl, U.; Loo, S.K. Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2018, 28, 293–305. [Google Scholar] [CrossRef]
- Micoulaud-Franchi, J.-A.; Geoffroy, P.A.; Fond, G.; Lopez, R.; Bioulac, S.; Philip, P. EEG neurofeedback treatments in children with ADHD: An updated meta-analysis of randomized controlled trials. Front. Hum. Neurosci. 2014, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Matías, P.; Yela-Bernabé, J.R.; Crego, A.; Sánchez-Zaballos, E. What Do Meta-Analyses Have to Say About the Efficacy of Neurofeedback Applied to Children With ADHD? Review of Previous Meta-Analyses and a New Meta-Analysis. J. Atten. Disord. 2021, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.; Brandeis, D.; Cortese, S.; Daley, D.; Danckaerts, M.; Dopfner, M.; Ferrin, M.; Holtmann, M.; Van der Oord, S. Evidence for Efficacy of Neurofeedback in ADHD? Response. Am. J. Psychiatry 2013, 170, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, S.; Yuan, Y.; Zhang, J. Effects of neurofeedback versus methylphenidate for the treatment of ADHD: Systematic review and meta-analysis of head-to-head trials. Evidence-Based Ment. Health 2019, 22, 111–117. [Google Scholar] [CrossRef]
- Lambez, B.; Harwood-Gross, A.; Golumbic, E.Z.; Rassovsky, Y. Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 120, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bussalb, A.; Congedo, M.; Barthélemy, Q.; Ojeda, D.; Acquaviva, E.; Delorme, R.; Mayaud, L. Clinical and Experimental Factors Influencing the Efficacy of Neurofeedback in ADHD: A Meta-Analysis. Front. Psychiatry 2019, 10, 35. [Google Scholar] [CrossRef]
- Hodgson, K.; Hutchinson, A.; Denson, L. Nonpharmacological treatments for ADHD: A meta-analytic review. J. Atten. Disord. 2014, 18, 275–282. [Google Scholar] [CrossRef]
- Norris, S.L. Challenges in Using Nonrandomized Studies in Systematic Reviews of Treatment Interventions. Ann. Intern. Med. 2005, 142, 1112–1119. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Brandeis, D.; Cortese, S.; Daley, D.; Ferrin, M.; Holtmann, M.; Stevenson, J.; Danckaerts, M.; Van Der Oord, S.; Döpfner, M.; et al. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. Am. J. Psychiatry 2013, 170, 275–289. [Google Scholar] [CrossRef]
- Strehl, U.; Aggensteiner, P.; Wachtlin, D.; Brandeis, D.; Albrecht, B.; Arana, M.; Bach, C.; Banaschewski, T.; Bogen, T.; Flaig-Röhr, A.; et al. Neurofeedback of Slow Cortical Potentials in Children with Attention-Deficit/Hyperactivity Disorder: A Multicenter Randomized Trial Controlling for Unspecific Effects. Front. Hum. Neurosci. 2017, 11, 135. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef]
- Geladé, K.; Janssen, T.W.P.; Bink, M.; Van Mourik, R.; Maras, A.; Oosterlaan, J. Behavioral Effects of Neurofeedback Compared to Stimulants and Physical Activity in Attention-Deficit/Hyperactivity Disorder: A Randomized Controlled Trial. J. Clin. Psychiatry 2016, 77, e1270–e1277. [Google Scholar] [CrossRef] [PubMed]
- Aggensteiner, P.-M.; Brandeis, D.; Millenet, S.; Hohmann, S.; Ruckes, C.; Beuth, S.; Albrecht, B.; Schmitt, G.; Schermuly, S.; Wörz, S.; et al. Slow cortical potentials neurofeedback in children with ADHD: Comorbidity, self-regulation and clinical outcomes 6 months after treatment in a multicenter randomized controlled trial. Eur. Child Adolesc. Psychiatry 2019, 28, 1087–1095. [Google Scholar] [CrossRef]
- Wood, G.; Kober, S.E. EEG Neurofeedback Is Under Strong Control of Psychosocial Factors. Appl. Psychophysiol. Biofeedback 2018, 43, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.T.; Lifshitz, M.; Raz, A. The self-regulating brain and neurofeedback: Experimental science and clinical promise. Cortex 2016, 74, 247–261. [Google Scholar] [CrossRef]
- Thibault, R.; Lifshitz, M.; Raz, A. Neurofeedback or neuroplacebo? Brain 2017, 140, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Lifshitz, M.; Raz, A. The climate of neurofeedback: Scientific rigour and the perils of ideology. Brain 2018, 141, e11. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Raz, A. Neurofeedback: The power of psychosocial therapeutics. Lancet Psychiatry 2016, 3, e18. [Google Scholar] [CrossRef]
- Schönenberg, M.; Weingärtner, A.-L.; Weimer, K.; Scheeff, J. Believing is achieving—On the role of treatment expectation in neurofeedback applications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110129. [Google Scholar] [CrossRef]
- Zuberer, A.; Brandeis, D.; Drechsler, R. Are treatment effects of neurofeedback training in children with ADHD related to the successful regulation of brain activity? A review on the learning of regulation of brain activity and a contribution to the discussion on specificity. Front. Hum. Neurosci. 2015, 9, 135. [Google Scholar] [CrossRef]
- Gevensleben, H.; Albrecht, B.; Lã¼Tcke, H.; Auer, T.; Dewiputri, W.I.; Schweizer, R.; Moll, G.; Heinrich, H.; Rothenberger, A. Neurofeedback of slow cortical potentials: Neural mechanisms and feasibility of a placebo-controlled design in healthy adults. Front. Hum. Neurosci. 2014, 8, 990. [Google Scholar] [CrossRef]
- Drechsler, R.; Straub, M.; Doehnert, M.; Heinrich, H.; Steinhausen, H.-C.; Brandeis, D. 1Controlled evaluation of a neurofeedback training of slow cortical potentials in children with Attention Deficit/Hyperactivity Disorder (ADHD). Behav. Brain Funct. 2007, 3, 35. [Google Scholar] [CrossRef]
- Strehl, U.; Leins, U.; Goth, G.; Klinger, C.; Hinterberger, T.; Birbaumer, N. Self-regulation of Slow Cortical Potentials: A New Treatment for Children With Attention-Deficit/Hyperactivity Disorder. Pediatrics 2006, 118, e1530–e1540. [Google Scholar] [CrossRef]
- Janssen, T.W.P.; Bink, M.; Geladé, K.; Van Mourik, R.; Maras, A.; Oosterlaan, J. A randomized controlled trial into the effects of neurofeedback, methylphenidate, and physical activity on EEG power spectra in children with ADHD. J. Child Psychol. Psychiatry 2016, 57, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Wangler, S.; Gevensleben, H.; Albrecht, B.; Studer, P.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Neurofeedback in children with ADHD: Specific event-related potential findings of a randomized controlled trial. Clin. Neurophysiol. 2011, 122, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Gevensleben, H.; Kleemeyer, M.; Rothenberger, L.G.; Studer, P.; Flaig-Röhr, A.; Moll, G.H.; Rothenberger, A.; Heinrich, H. Neurofeedback in ADHD: Further Pieces of the Puzzle. Brain Topogr. 2014, 27, 20–32. [Google Scholar] [CrossRef]
- Gevensleben, H.; Moll, G.H.; Rothenberger, A.; Heinrich, H. Neurofeedback in attention-deficit/hyperactivity disorder—Different models, different ways of application. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.T.; MacPherson, A.; Lifshitz, M.; Roth, R.R.; Raz, A. Neurofeedback with fMRI: A critical systematic review. NeuroImage 2018, 172, 786–807. [Google Scholar] [CrossRef]
- Zilverstand, A.; Sorger, B.; Slaats-Willemse, D.; Kan, C.C.; Goebel, R.; Buitelaar, J.K. fMRI Neurofeedback Training for Increasing Anterior Cingulate Cortex Activation in Adult Attention Deficit Hyperactivity Disorder. An Exploratory Randomized, Single-Blinded Study. PLoS ONE 2017, 12, e0170795. [Google Scholar] [CrossRef]
- Alegria, A.A.; Wulff, M.; Brinson, H.; Barker, G.; Norman, L.J.; Brandeis, D.; Stahl, D.; David, A.; Taylor, E.A.; Giampietro, V.; et al. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum. Brain Mapp. 2017, 38, 3190–3209. [Google Scholar] [CrossRef]
- Criaud, M.; Wulff, M.; Alegria, A.; Barker, G.; Giampietro, V.; Rubia, K. Increased left inferior fronto-striatal activation during error monitoring after fMRI neurofeedback of right inferior frontal cortex in adolescents with attention deficit hyperactivity disorder. NeuroImage Clin. 2020, 27, 102311. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, A.; Smith, A.B.; Barrett, N.; Giampietro, V.; Brammer, M.J.; Simmons, A.; Rubia, K. Shared and Drug-Specific Effects of Atomoxetine and Methylphenidate on Inhibitory Brain Dysfunction in Medication-Naive ADHD Boys. Cereb. Cortex 2014, 24, 174–185. [Google Scholar] [CrossRef]
- Rubia, K.; Halari, R.; Taylor, E.; Brammer, M. Methylphenidate normalises fronto-cingulate underactivation during error processing in children with Attention-Deficit Hyperactivity Disorder. Biol. Psychiatry 2011, 70, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zuberer, A.; Minder, F.; Brandeis, D.; Drechsler, R. Mixed-Effects Modeling of Neurofeedback Self-Regulation Performance: Moderators for Learning in Children with ADHD. Neural Plast. 2018, 2018, 2464310. [Google Scholar] [CrossRef]
- Lam, S.-L.; Criaud, M.; Alegria, A.; Barker, G.J.; Giampietro, V.; Rubia, K. Neurofunctional and behavioural measures associated with fMRI-neurofeedback learning in adolescents with Attention-Deficit/Hyperactivity Disorder. NeuroImage Clin. 2020, 27, 102291. [Google Scholar] [CrossRef]
- Marx, A.-M.; Ehlis, A.-C.; Furdea, A.; Holtmann, M.; Banaschewski, T.; Brandeis, D.; Rothenberger, A.; Gevensleben, H.; Freitag, C.M.; Fuchsenberger, Y.; et al. Near-infrared spectroscopy (NIRS) neurofeedback as a treatment for children with attention deficit hyperactivity disorder (ADHD)—A pilot study. Front. Hum. Neurosci. 2015, 8. [Google Scholar] [CrossRef]
- Aase, H.; Sagvolden, T. Infrequent, but not frequent, reinforcers produce more variable responding and deficient sustained attention in young children with attention-deficit/hyperactivity disorder (ADHD). J. Child Psychol. Psychiatry 2005, 47, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Strehl, U. Evidence for Efficacy of Neurofeedback in ADHD? Am. J. Psychiatry 2013, 170, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.S.; Hinshaw, S.P.; Swanson, J.M.; Arnold, L.E.; Vitiello, B.; Jensen, P.; Epstein, J.N.; Hoza, B.; Hechtman, L.; Abikoff, H.B.; et al. The MTA at 8 Years: Prospective Follow-up of Children Treated for Combined-Type ADHD in a Multisite Study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 484–500. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Rubia, K.; Rossi, G.; Sartori, G.; Balottin, U. Striatal Dopamine Transporter Alterations in ADHD: Pathophysiology or Adaptation to Psychostimulants? A Meta-Analysis. Am. J. Psychiatry 2012, 169, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, R.S.M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Demirtas-Tatlidede, A.; Vahabzadeh-Hagh, A.M.; Pascual-Leone, A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 2013, 64, 566–578. [Google Scholar] [CrossRef]
- Ruf, S.P.; Fallgatter, A.J.; Plewnia, C. Augmentation of working memory training by transcranial direct current stimulation (tDCS). Sci. Rep. 2017, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Au, J.; Buschkuehl, M.; Abagis, T.; Zabel, C.; Jaeggi, S.M.; Jonides, J. Individual Differences and Long-term Consequences of tDCS-augmented Cognitive Training. J. Cogn. Neurosci. 2017, 29, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.-F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Mann, C.; Götz, M.; Gerlicher, A.; Saase, V.; Yuen, K.S.; Aedo-Jury, F.; Gonzalez-Escamilla, G.; Stroh, A.; Kalisch, R. Increased Neural Activity in Mesostriatal Regions after Prefrontal Transcranial Direct Current Stimulation and l-DOPA Administration. J. Neurosci. 2019, 39, 5326–5335. [Google Scholar] [CrossRef] [PubMed]
- Borwick, C.; Lal, R.; Lim, L.W.; Stagg, C.J.; Aquili, L. Dopamine depletion effects on cognitive flexibility as modulated by tDCS of the dlPFC. Brain Stimul. 2020, 13, 105–108. [Google Scholar] [CrossRef]
- Fukai, M.; Bunai, T.; Hirosawa, T.; Kikuchi, M.; Ito, S.; Minabe, Y.; Ouchi, Y. Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: A study with positron emission tomography. Transl. Psychiatry 2019, 9, 115. [Google Scholar] [CrossRef]
- Adelhöfer, N.; Mückschel, M.; Teufert, B.; Ziemssen, T.; Beste, C. Anodal tDCS affects neuromodulatory effects of the norepinephrine system on superior frontal theta activity during response inhibition. Brain Struct. Funct. 2019, 224, 1291–1300. [Google Scholar] [CrossRef]
- Mishima, T.; Nagai, T.; Yahagi, K.; Akther, S.; Oe, Y.; Monai, H.; Kohsaka, S.; Hirase, H. Transcranial Direct Current Stimulation (tDCS) Induces Adrenergic Receptor-Dependent Microglial Morphological Changes in Mice. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Moretti, J.; Poh, E.Z.; Rodger, J. rTMS-Induced Changes in Glutamatergic and Dopaminergic Systems: Relevance to Cocaine and Methamphetamine Use Disorders. Front. Neurosci. 2020, 14, 137. [Google Scholar] [CrossRef]
- Poh, E.Z.; Hahne, D.; Moretti, J.; Harvey, A.R.; Clarke, M.W.; Rodger, J. Simultaneous quantification of dopamine, serotonin, their metabolites and amino acids by LC-MS/MS in mouse brain following repetitive transcranial magnetic stimulation. Neurochem. Int. 2019, 131, 104546. [Google Scholar] [CrossRef]
- Kuo, M.-F.; Nitsche, M.A. Effects of Transcranial Electrical Stimulation on Cognition. Clin. EEG Neurosci. 2012, 43, 192–199. [Google Scholar] [CrossRef]
- Ziemann, U.; Siebner, H.R. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008, 1, 60–66. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.; Cantello, R.M.; Cincotta, M.; De Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Janicak, P.G.; Dokucu, M.E. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatry Dis. Treat. 2015, 11, 1549–1560. [Google Scholar] [CrossRef]
- Mehta, U.M.; Naik, S.S.; Thanki, M.V.; Thirthalli, J. Investigational and Therapeutic Applications of Transcranial Magnetic Stimulation in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 89. [Google Scholar] [CrossRef]
- Parkin, B.L.; Ekhtiari, H.; Walsh, V.F. Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron 2015, 87, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Bloch, Y. Transcranial magnetic stimulation (TMS) as a treatment in ADHD. Isr. J. Psychiatry Relat. Sci. 2012, 49, 18. [Google Scholar]
- Weaver, L.; Rostain, A.L.; Mace, W.; Akhtar, U.; Moss, E.; O’Reardon, J.P. Transcranial Magnetic Stimulation (TMS) in the Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents and Young Adults. J. ECT 2012, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y.; Friedwald, K.; Levkovitz, Y.; Zangen, A.; Alyagon, U.; Nitzan, U.; Segev, A.; Maoz, H.; Koubi, M.; Bloch, Y. Randomised sham-controlled study of high-frequency bilateral deep transcranial magnetic stimulation (dTMS) to treat adult attention hyperactive disorder (ADHD): Negative results. World J. Biol. Psychiatry 2017, 19, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Alyagon, U.; Shahar, H.; Hadar, A.; Barnea-Ygael, N.; Lazarovits, A.; Shalev, H.; Zangen, A. Alleviation of ADHD symptoms by non-invasive right prefrontal stimulation is correlated with EEG activity. NeuroImage Clin. 2020, 26, 102206. [Google Scholar] [CrossRef]
- Gómez, L.; Vidal, B.; Morales, L.; Báez, M.; Maragoto, C.; Galvizu, R.; Vera, H.; Cabrera, I.; Zaldívar, M.; Sánchez, A. Low Frequency Repetitive Transcranial Magnetic Stimulation in Children With Attention Deficit/Hyperactivity Disorder. Preliminary Results. Brain Stimul. 2014, 7, 760–762. [Google Scholar] [CrossRef]
- Cao, P.; Xing, J.; Cao, Y.; Cheng, Q.; Sun, X.; Kang, Q.; Dai, L.; Zhou, X.; Song, Z. Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatry Dis. Treat. 2018, 14, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Santos, L.; Peterson, M.; Ehinger, M. Safety of Noninvasive Brain Stimulation in Children and Adolescents. Brain Stimul. 2015, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, E.; Ciechanski, P.; Kuo, H.; Giuffre, A.; Kahl, C.; King, R.; Cole, L.; Godfrey, H.; Seeger, T.; Swansburg, R.; et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: Prospective single center evidence from 3.5 million stimulations. Brain Stimul. 2020, 13, 565–575. [Google Scholar] [CrossRef]
- Boggio, P.S.; Ferrucci, R.; Mameli, F.; Martins, D.; Martins, O.; Vergari, M.; Tadini, L.; Scarpini, E.; Fregni, F.; Priori, A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012, 5, 223–230. [Google Scholar] [CrossRef]
- Kuo, M.-F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage 2014, 85, 948–960. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011, 32, 1236–1249. [Google Scholar] [CrossRef]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Mulert, C.; Grossheinrich, N.; Rupprecht, R.; Möller, H.-J.; Hegerl, U.; et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as d-amphetamine. Psychiatry Res. Neuroimaging 2007, 156, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.-F.; Chen, P.-S.; Nitsche, M.A. The application of tDCS for the treatment of psychiatric diseases. Int. Rev. Psychiatry 2017, 29, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A.; Najian, A.; Javadi, A.-H. Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. J. Atten. Disord. 2020, 24, 1928–1943. [Google Scholar] [CrossRef]
- Rubia, K.; Lim, L.; Ecker, C.; Halari, R.; Giampietro, V.; Simmons, A.; Brammer, M.; Smith, A.B. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. NeuroImage 2013, 83, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Taylor, E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage 2003, 20, 351–358. [Google Scholar] [CrossRef]
- Rubia, K.; Smith, A.B.; Taylor, E.; Brammer, M. Linear age-correlated functional development of right inferior fron-to-striato-cerebellar networks during response inhibition and anterior Cingulate during error-related processes. Hum. Brain Mapp. 2007, 28, 1163–1177. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Bonath, B.; Tegelbeckers, J.; Flechtner, H.-H.; Krauel, K. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS). Front. Cell. Neurosci. 2016, 10, 72. [Google Scholar] [CrossRef]
- Soltaninejad, Z.; Nejati, V.; Ekhtiari, H. Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. J. Atten. Disord. 2019, 23, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, Z.; Nejati, V.; Ekhtiari, H. Effect of transcranial Direct Current Stimulation on Remediation of Inhibitory Con-trol on right Inferior Frontal Gyrus in Attention Deficit and Hyperactivity Symptoms. Rehabil. Med. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Westwood, S.J.; Criaud, M.; Lam, S.-L.; Lukito, S.; Wallace-Hanlon, S.; Kowalczyk, O.S.; Kostara, A.; Mathew, J.; Agbedjro, D.; Wexler, B.E.; et al. Transcranial direct current stimulation (tDCS) combined with cognitive training in adolescent boys with ADHD: A double-blind, randomised, sham-controlled trial. Psychol. Med. 2021, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Westwood, S.J.; Bozhilova, N.; Criaud, M.; Lam, S.L.; Lukito, S.; Wallace-Hanlon, S.; Kowalczyk, O.S.; Kostara, A.; Mathew, J.; Agbedjro, D.; et al. The effect of transcranial direct current stimulation (tDCS) combined with cognitive training on EEG spectral power in adolescent boys with ADHD: A double-blind, randomised, sham-controlled trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Soff, C.; Sotnikova, A.; Christiansen, H.; Becker, K.; Siniatchkin, M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J. Neural Transm. 2017, 124, 133–144. [Google Scholar] [CrossRef]
- Sotnikova, A.; Soff, C.; Tagliazucchi, E.; Becker, K.; Siniatchkin, M. Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topogr. 2017, 30, 656–672. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Munz, M.; Göder, R.; Wilhelm, I.; Korr, K.; Vahl, W.; Wiesner, C.; Baving, L. Transcranial Oscillatory Direct Current Stimulation during Sleep Improves Declarative Memory Consolidation in Children With Attention-deficit/hyperactivity Disorder to a Level Comparable to Healthy Controls. Brain Stimul. 2014, 7, 793–799. [Google Scholar] [CrossRef]
- Munz, M.T.; Prehn-Kristensen, A.; Thielking, F.; Mölle, M.; Göder, R.; Baving, L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front. Cell. Neurosci. 2015, 9, 307. [Google Scholar] [CrossRef]
- Bandeira, I.D.; Guimarães, R.S.Q.; Jagersbacher, J.G.; Barretto, T.L.; De Jesus-Silva, J.R.; Santos, S.N.; Argollo, N.; Lucena, R. Transcranial Direct Current Stimulation in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder (ADHD): A Pilot Study. J. Child Neurol. 2016, 31, 918–924. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Tegelbeckers, J.; Flechtner, H.-H.; Krauel, K. Comparison between conventional and HD-tDCS of the right inferior frontal gyrus in children and adolescents with ADHD. Clin. Neurophysiol. 2020, 131, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Ghayerin, E.; Nejati, V.; Yavari, F.; Nitsche, M.A. Domain-specific Involvement of the Right Posterior Parietal Cortex in Attention Network and Attentional Control of ADHD: A Randomized, Cross-over, Sham-controlled tDCS Study. Neuroscience 2020, 444, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Khorrami, A.S.; Nitsche, M.A. Transcranial Direct Current Stimulation Improves Reward Processing in Children With ADHD. J. Atten. Disord. 2020, 25, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and Do Not Know about Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Ward, L.M. The benefits of noise in neural systems: Bridging theory and experiment. Nat. Rev. Neurosci. 2011, 12, 415–425. [Google Scholar] [CrossRef]
- Berger, I.; Dakwar-Kawar, O.; Grossman, E.S.; Nahum, M.; Kadosh, R.C. Scaffolding the attention-deficit/hyperactivity disorder brain using transcranial direct current and random noise stimulation: A randomized controlled trial. Clin. Neurophysiol. 2021, 132, 699–707. [Google Scholar] [CrossRef]
- Cosmo, C.; Baptista, A.F.; de Araújo, A.; Rosário, R.S.D.; Miranda, J.G.V.; Montoya, P.; De Sena, E. A Randomized, Double-Blind, Sham-Controlled Trial of Transcranial Direct Current Stimulation in Attention-Deficit/Hyperactivity Disorder. PLoS ONE 2015, 10, e0135371. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, N.; Lavidor, M.; Jacoby, N.; Lavidor, M. Null tDCS Effects in a Sustained Attention Task: The Modulating Role of Learning. Front. Psychol. 2018, 9, 476. [Google Scholar] [CrossRef]
- Allenby, C.; Falcone, M.; Bernardo, L.; Wileyto, E.P.; Rostain, A.; Ramsay, J.; Lerman, C.; Loughead, J. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018, 11, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Cachoeira, C.T.; Leffa, D.T.; Mittelstadt, S.D.; Mendes, L.S.T.; Brunoni, A.R.; Pinto, J.V.; Blazius, V.; Machado, V.; Bau, C.; Rohde, L.A.; et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder A pilot randomized controlled study. Psychiatry Res. 2017, 247, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil-Vall, L.; Gomez-Bernal, F.; Villegas, A.C.; Cirillo, P.; Surman, C.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. Transcranial Direct Current Stimulation to the Left Dorsolateral Prefrontal Cortex Improves Cognitive Control in Patients With Attention-Deficit/Hyperactivity Disorder: A Randomized Behavioral and Neurophysiological Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 6, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Wischnewski, M.; Nejati, V.; Vicario, C.M.; Nitsche, M.A. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits. PLoS ONE 2019, 14, e0215095. [Google Scholar] [CrossRef]
- Westwood, S.J.; Radua, J.; Rubia, K. Non-Invasive Brain Stimulation in Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Psychol. Med. 2021, 6, 1–16, in press. [Google Scholar] [CrossRef]
- Moliadze, V.; Schmanke, T.; Andreas, S.; Lyzhko, E.; Freitag, C.M.; Siniatchkin, M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin. Neurophysiol. 2015, 126, 1392–1399. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Knudsen, E.I. Sensitive Periods in the Development of the Brain and Behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Kekic, M.; Boysen, E.; Campbell, I.C.; Schmidt, U. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J. Psychiatr. Res. 2016, 74, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.H.; Brunoni, A.R.; Nikolin, S.; Loo, C.K. Transcranial Direct Current Stimulation in Psychiatric Disorders: A Comprehensive Review. Psychiatr. Clin. N. Am. 2018, 41, 447–463. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Kim, S.; Stephenson, M.; Morris, P.G.; Jackson, S.R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7T magnetic resonance spectroscopy study. NeuroImage 2014, 99, 237–243. [Google Scholar] [CrossRef]
- Silvanto, J.; Muggleton, N.; Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008, 12, 447–454. [Google Scholar] [CrossRef]
- Krause, B.; Márquez-Ruiz, J.; Kadosh, R.C. The effect of transcranial direct current stimulation: A role for cortical excitation/inhibition balance? Front. Hum. Neurosci. 2013, 7, 602. [Google Scholar] [CrossRef]
- Kadosh, R.C.; Levy, N.; O’Shea, J.; Shea, N.; Savulescu, J. The neuroethics of non-invasive brain stimulation. Curr. Biol. 2012, 22, R108–R111. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Dowker, A.; Kadosh, R.C. Cognitive Enhancement or Cognitive Cost: Trait-Specific Outcomes of Brain Stimulation in the Case of Mathematics Anxiety. J. Neurosci. 2014, 34, 16605–16610. [Google Scholar] [CrossRef]
- Iuculano, T.; Kadosh, R.C. The Mental Cost of Cognitive Enhancement. J. Neurosci. 2013, 33, 4482–4486. [Google Scholar] [CrossRef]
- Shiozawa, P.; Da Silva, M.E.; De Carvalho, T.C.; Cordeiro, Q.; Brunoni, A.R.; Fregni, F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: A systematic review. Arq. Neuro-Psiquiatria 2014, 72, 542–547. [Google Scholar] [CrossRef]
- McGough, J.J.; Loo, S.K.; Sturm, A.; Cowen, J.; Leuchter, A.F.; Cook, I.A. An Eight-week, Open-trial, Pilot Feasibility Study of Trigeminal Nerve Stimulation in Youth with Attention-deficit/Hyperactivity Disorder. Brain Stimul. 2015, 8, 299–304. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005, 493, 99–110. [Google Scholar] [CrossRef]
- Cook, I.A.; Espinoza, R.; Leuchter, A.F. Neuromodulation for depression: Invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg. Clin. N. Am. 2014, 25, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.; Kadosh, R.C. Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev. Cogn. Neurosci. 2013, 6, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Zaghi, S.; Heine, N.; Fregni, F. Brain stimulation for the treatment of pain: A review of costs, clinical effects, and mecha-nisms of treatment for three different central neuromodulatory approaches. J. Pain Manag. 2009, 2, 339–352. [Google Scholar] [PubMed]
- Garcia Pimenta, M.; Brown, T.; Arns, M.; Enriquez-Geppert, S. Treatment Efficacy and Clinical Effectiveness of EEG Neu-rofeedback as a Personalized and Multimodal Treatment in ADHD: A Critical Review. Neuropsychiatr. Dis. Treat. 2021, 17, 637–648. [Google Scholar] [CrossRef]
| Stimulation Protocol | Outcome Measures (Bold/Underlined = Improvement) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Design | N | Age | Target | Sessions | Frequency | Duration | Clinical | Cognitive |
| Children | |||||||||
| Cao et al., 2020 [147] | Single-blind, randomised, parallel (2 active controls: ATX, ATX-rTMS; no sham) | 64 (~20 each) | 6–13 | R DLPFC a | 20 | 18 Hz (100% MT) | 2000 pulses (4 s on, 26 s off) | SNAP-IV | CPT; WISC; IGT |
| Gomez et al., 2014 [146] | Open label | 10 | 7–12 | L DLPFC | 5 | 1 Hz (90% MT) | 1500 pulses (on, off n/r) | DSM-IV ADHD symptom checklist (hyperactivity/imp., inattention) | n/t |
| Adults | |||||||||
| Bloch et al., 2010 [142] | Single-blind, sham-controlled, randomised, crossover | 13 | NR (adults) | R DLPFC a | 1 | 20 Hz (100% MT) | 1680 pulses (2 s on, 30 s off) | PANAS (inattention, total score; mood, anxiety, hyperactivity); VAS (inattention, mood) b | n/t |
| Paz et al., 2018 [144] | Double-blind, sham-controlled, randomised, parallel | A: 13 S: 9 | A: 32 S: 30 | L DLPFC c | 20 | 18 Hz (120% MT) | 1980 pulses (2 s on, 20 s off) | CAARS | TOVA |
| Weaver et al., 2012 [143] | Single-blind, sham-controlled, randomised, crossover | 9 | 18 | R DLPFC a | 10 | 10 Hz (100% MT) | 2000 pulses (4 s on, 26 s off) | CGI-I scale; ADHD-IV scale | WAIS/WISC-IV; Connors CPT; DKEFS; Buschke Selective Reminding Test; Symbol Digit Coding test; Finger Oscillation tasks |
| Alyagon et al., 2020 [145] | Double-semi-blind, randomised, active and sham-controlled | 52 (15, 14, 14) | 21–46 | R IFC & DLPFC | 15 | 18 Hz (120% MT) | 1440 pulses (2 s on, 20 s off) | CAARS (global ADHD symptoms; hyperactivity/impulsiveness) (BAARS-IV (hyperactivity/impulsiveness), BRIEF-A, BDI) | STROOP; STOP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubia, K.; Westwood, S.; Aggensteiner, P.-M.; Brandeis, D. Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review. Cells 2021, 10, 2156. https://doi.org/10.3390/cells10082156
Rubia K, Westwood S, Aggensteiner P-M, Brandeis D. Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review. Cells. 2021; 10(8):2156. https://doi.org/10.3390/cells10082156
Chicago/Turabian StyleRubia, Katya, Samuel Westwood, Pascal-M. Aggensteiner, and Daniel Brandeis. 2021. "Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review" Cells 10, no. 8: 2156. https://doi.org/10.3390/cells10082156
APA StyleRubia, K., Westwood, S., Aggensteiner, P.-M., & Brandeis, D. (2021). Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review. Cells, 10(8), 2156. https://doi.org/10.3390/cells10082156





