Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant’s Cultivation and Al3+ Tolerance Test
2.3. RNA Extraction and Semi-Quantitative Reverse Transcription-PCR (sqRT-PCR)
2.4. Root Extract Preparation for Determination of Protein Content and Azocaseinolytic and Gelatinolytic Activities
2.5. Protein Assay
2.6. Azocaseinolytic Activity Assay
2.7. Zymography of Gelatinolytic Activity
2.8. Root Structural Studies
2.8.1. Light Microscopy
2.8.2. Transmission Electron Microscopy (TEM)
2.9. Statistical Analysis
3. Results
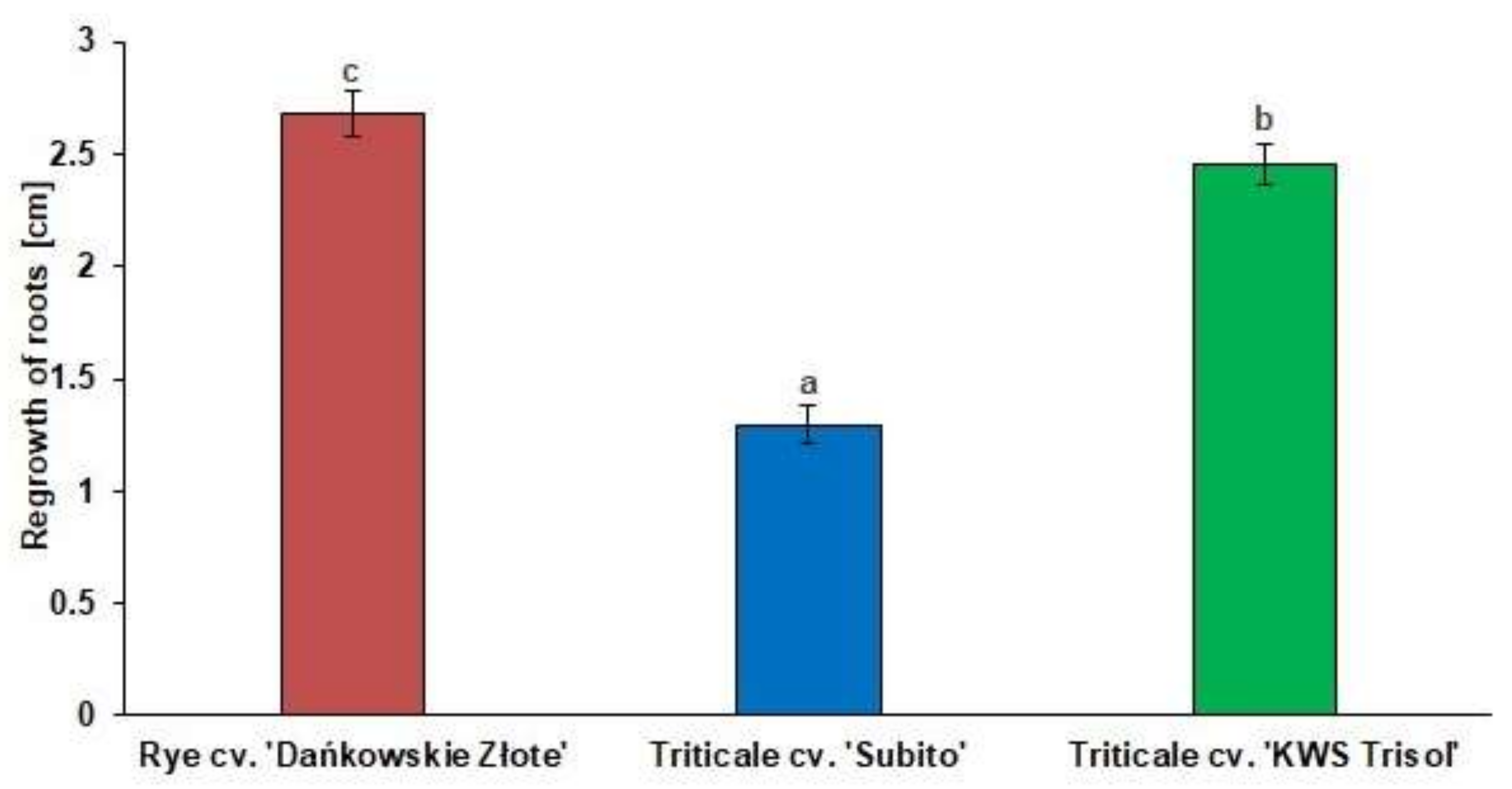
3.1. Al3+ Tolerance Test
3.2. Gene Expression of PhyCys (TrcC-8 and TrcC-9) in the Response of Cereal Roots to Al3+
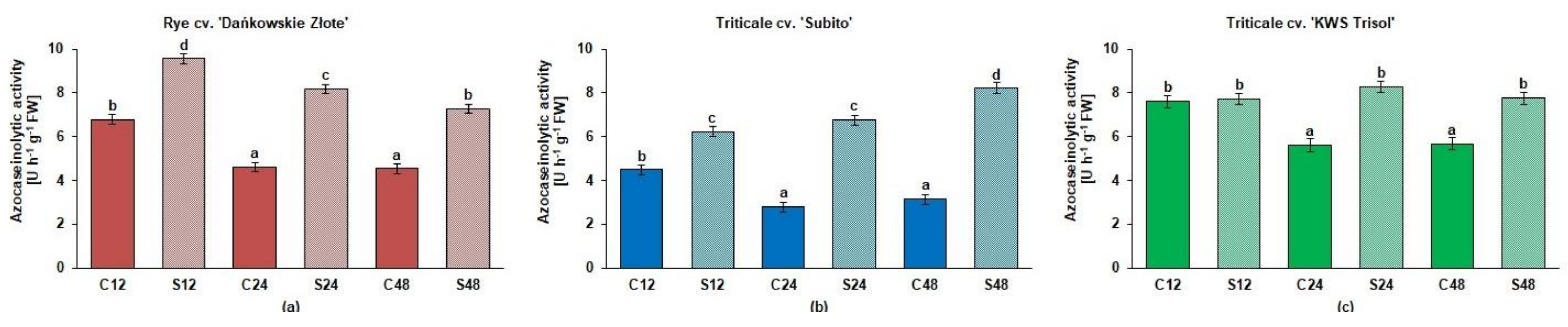
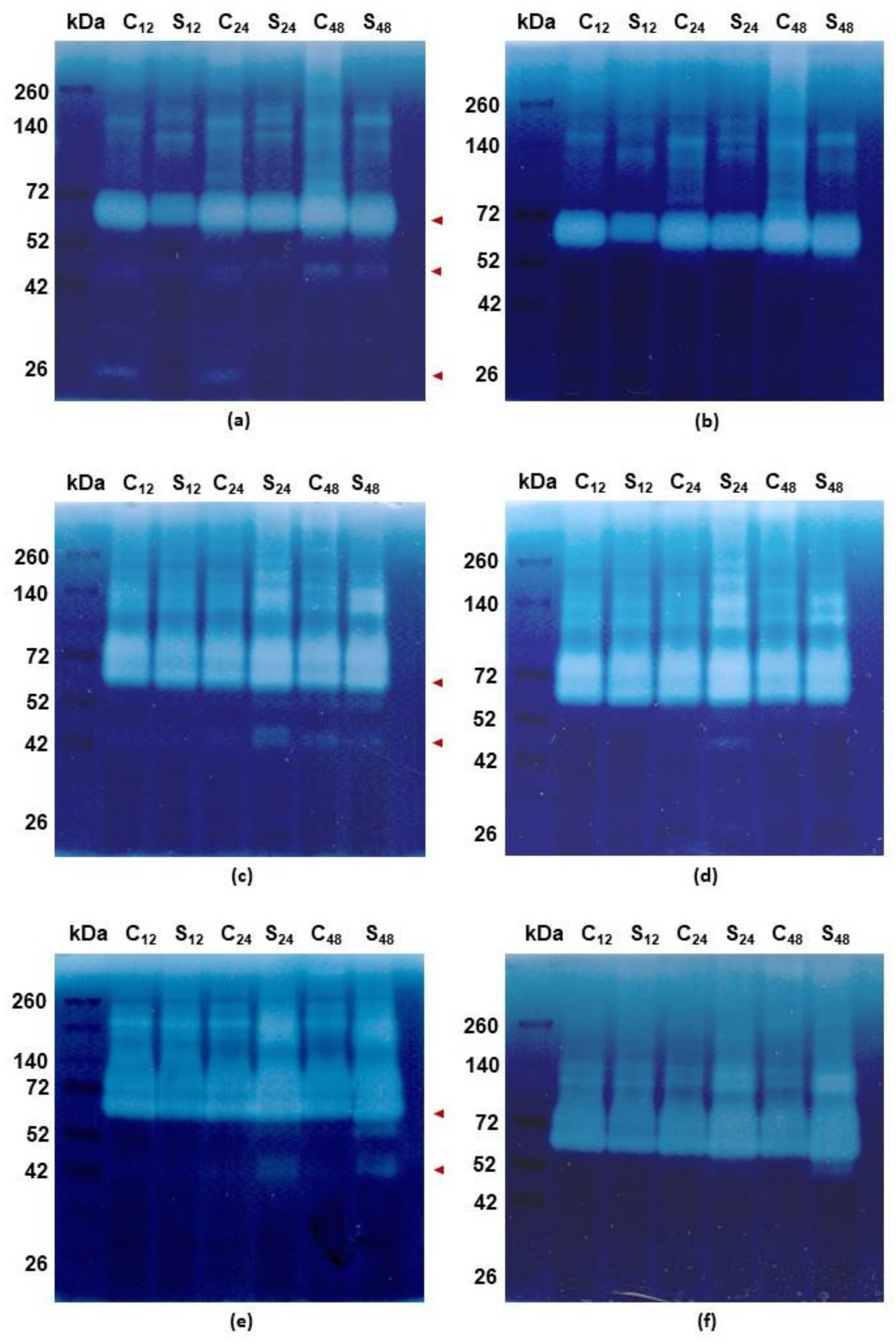
3.3. Spectrophotometric and Zymographic Profiling of Proteolytic Activity in Roots of Rye and Triticales under Al3+
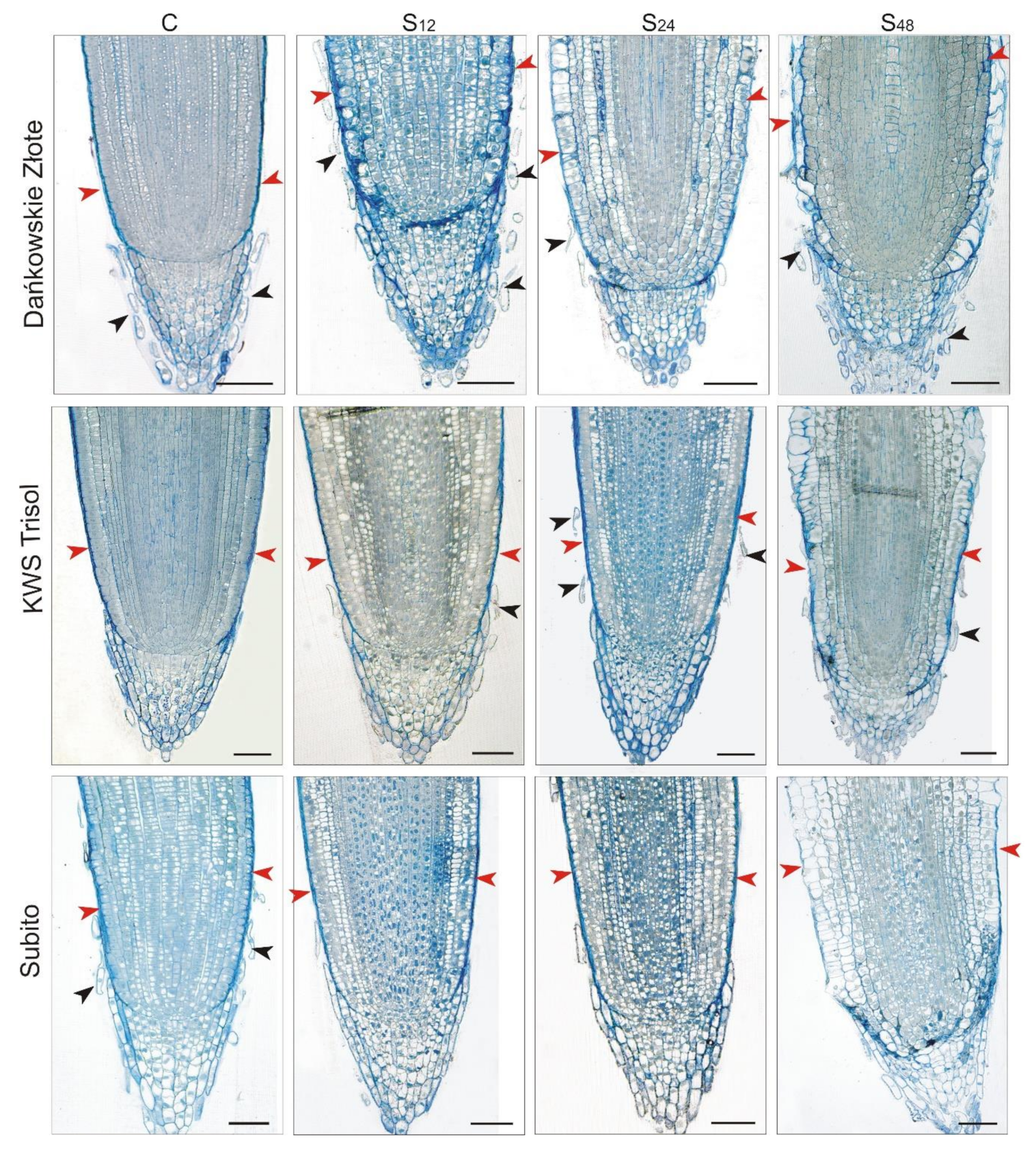
3.4. Structural Changes Observed under Light Microscope in Roots of Rye and Triticales during Al3+ Stress
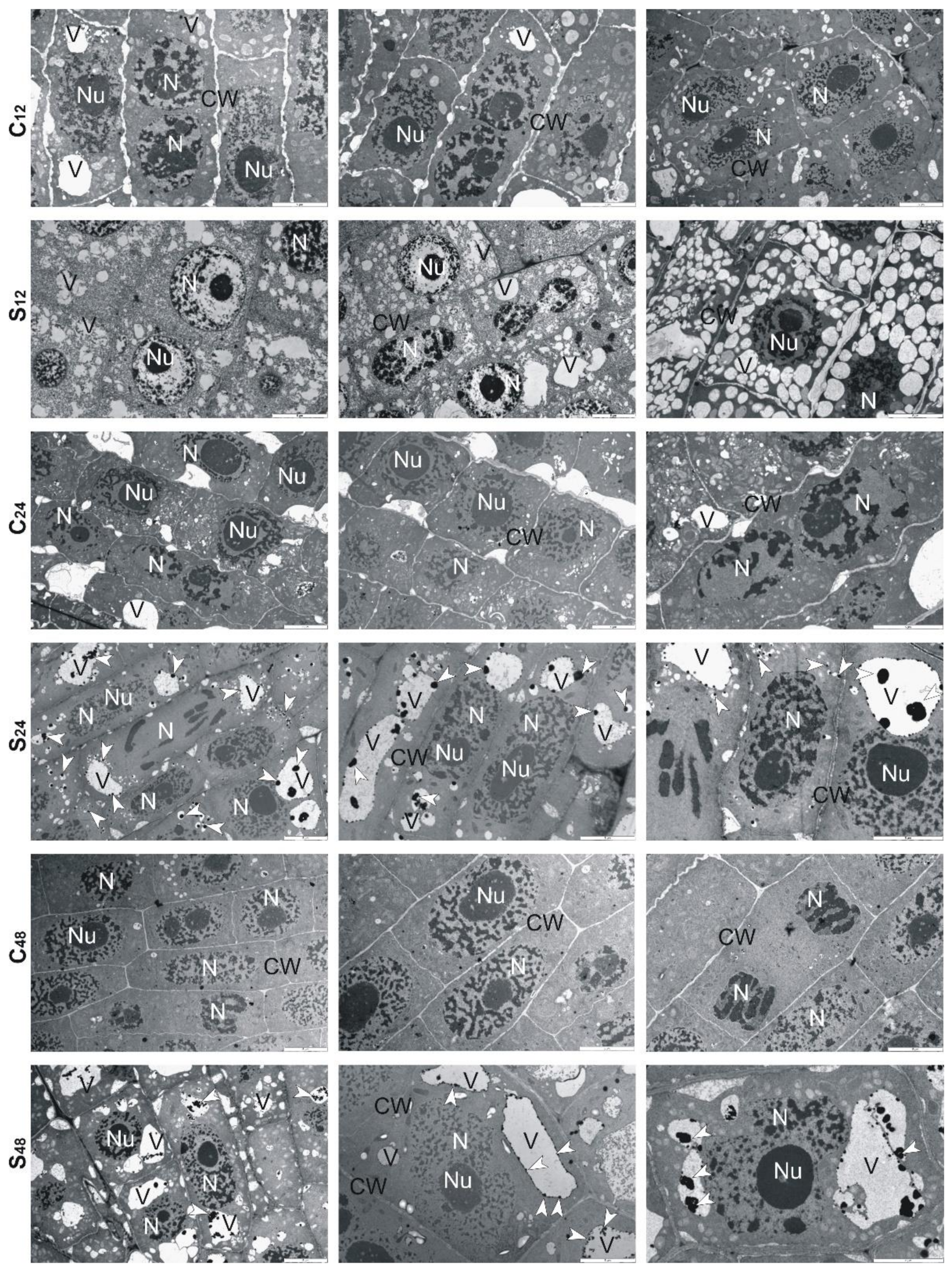
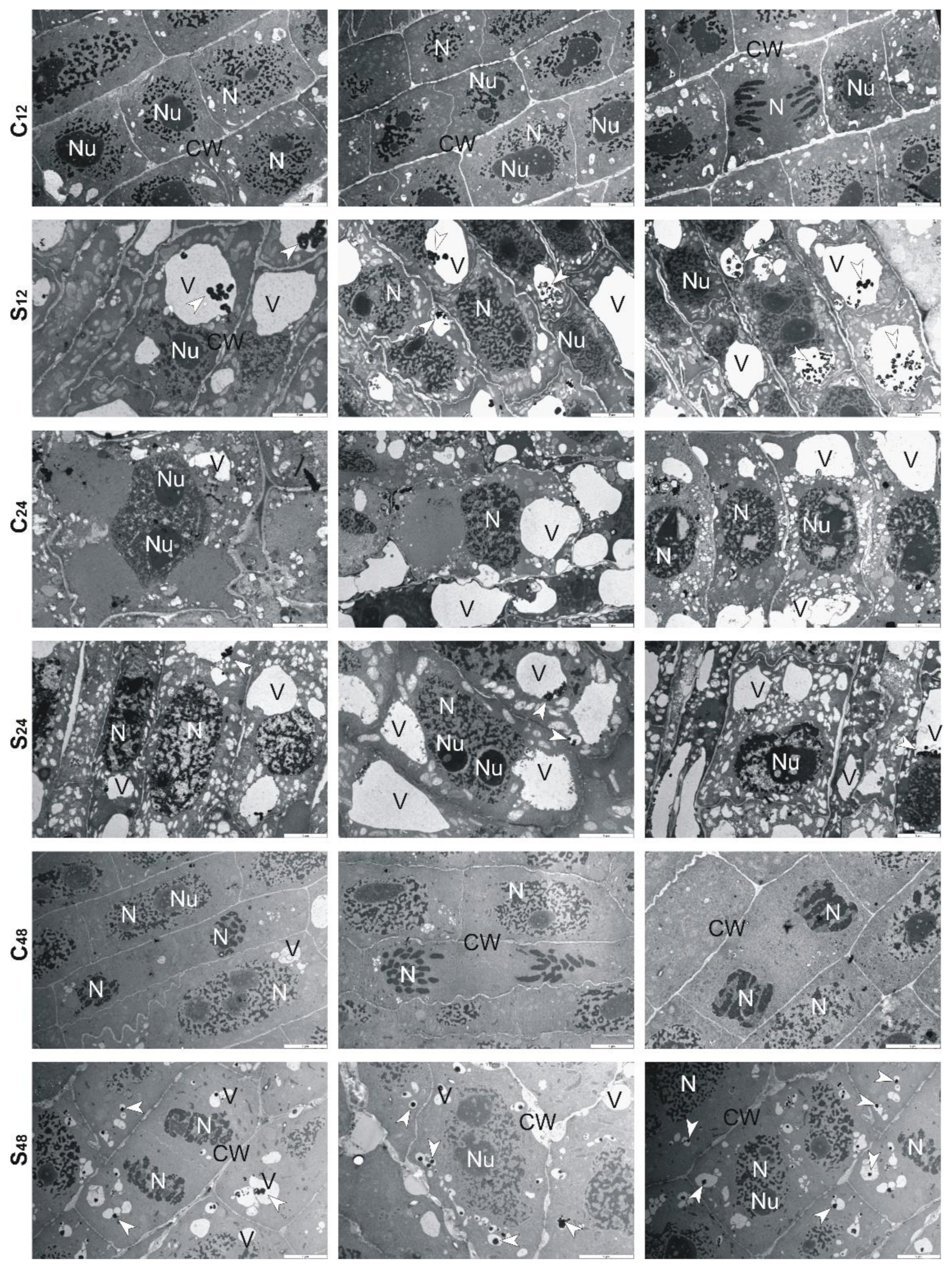
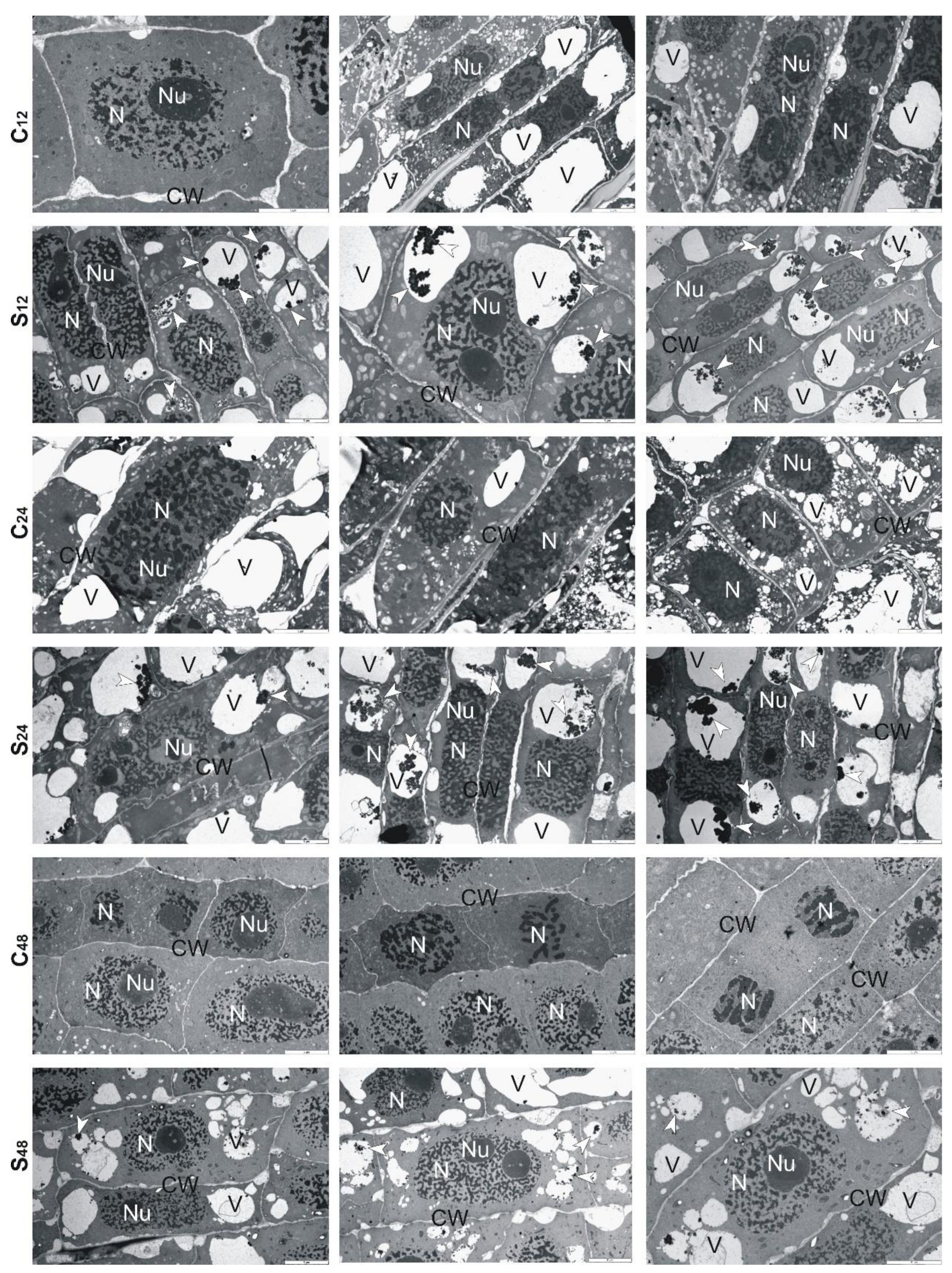
3.5. Structural Changes Observed under Transmission Electron Microscope in Roots of Rye and Triticales during Al3+ Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sojka, D.; Šnebergerová, P.; Robbertse, L. Protease Inhibition—An Established Strategy to Combat Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 5762. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoorn, R.A.L.; Klemenčič, M. Plant proteases: From molecular mechanisms to functions in development and immunity. J. Exp. Bot. 2021, 72, 3337–3339. [Google Scholar] [CrossRef]
- Szewińska, J.; Simińska, J.; Bielawski, W. The roles of cysteine proteases and phytocystatins in development and germination of cereal seeds. J. Plant Physiol. 2016, 207, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, J.A.A. Indispensable Role of Proteases in Plant Innate Immunity. Int. J. Mol. Sci. 2018, 19, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labudda, M.; Różańska, E.; Prabucka, B.; Muszyńska, E.; Marecka, D.; Kozak, M.; Dababat, A.A.; Sobczak, M. Activity profiling of barley vacuolar processing enzymes provides new insights into the plant and cyst nematode interaction. Mol. Plant Pathol. 2019, 21, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Labudda, M.; Różańska, E.; Szewińska, J.; Sobczak, M.; Dzik, J.M. Protease activity and phytocystatin expression in Arabidopsis thaliana upon Heterodera schachtii infection. Plant Physiol. Biochem. 2016, 109, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in proteolytic activity and protein carbonylation in shoots of Alyssum montanum ecotypes under multi-metal stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M. Effects of lead, cadmium and zinc on protein changes in Silene vulgaris shoots cultured in vitro. Ecotoxicol. Environ. Saf. 2020, 204, 111086. [Google Scholar] [CrossRef] [PubMed]
- Gietler, M.; Nykiel, M.; Zagdanska, B. Changes in the reduction state of ascorbate and glutathione, protein oxidation and hydrolysis leading to the development of dehydration intolerance in Triticum aestivum L. seedlings. Plant Growth Regul. 2015, 79, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Stoychev, V.; Simova-Stoilova, L.; Vaseva, I.; Kostadinova, A.; Nenkova, R.; Feller, U.; Demirevska, K. Protein changes and proteolytic degradation in red and white clover plants subjected to waterlogging. Acta Physiol. Plant. 2013, 35, 1925–1932. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B.; Mohanty, P. Salt-stress Induced Alterations in Protein Profile and Protease Activity in the Mangrove Bruguiera parviflora. Z. Nat. C 2004, 59, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439. [Google Scholar] [CrossRef] [Green Version]
- Eberl, F.; Fabisch, T.; Luck, K.; Köllner, T.G.; Vogel, H.; Gershenzon, J.; Unsicker, S.B. Poplar protease inhibitor expression differs in an herbivore specific manner. BMC Plant Biol. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hibbetts, K.; Hines, B.; Williams, D. An Overview of Proteinase Inhibitors. J. Veter. Intern. Med. 1999, 13, 302–308. [Google Scholar] [CrossRef]
- Kidrič, M.; Kos, J.; Sabotič, J. Proteases and Their Endogenous Inhibitors in the Plant Response to Abiotic Stress. Bot. Serbica 2014, 38, 139–158. [Google Scholar]
- Martinez, M.; Santamaria, M.E.; Diaz-Mendoza, M.; Arnaiz, A.; Carrillo, L.; Ortego, F.; Diaz, I. Phytocystatins: Defense Proteins against Phytophagous Insects and Acari. Int. J. Mol. Sci. 2016, 17, 1747. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, M.; Szewińska, J.; Mielecki, M.; Nykiel, M.; Imai, R.; Bielawski, W.; Orzechowski, S. A triticale water-deficit-inducible phytocystatin inhibits endogenous cysteine proteinases in vitro. J. Plant Physiol. 2015, 174, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mosharrof, M.; Uddin, K.; Jusop, S.; Sulaiman, M.; Shamsuzzaman, S.; Haque, A. Changes in Acidic Soil Chemical Properties and Carbon Dioxide Emission Due to Biochar and Lime Treatments. Agriculture 2021, 11, 219. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total. Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [Green Version]
- Klug, B.; Horst, W.J. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum). New Phytol. 2010, 187, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Ryan, P.R. Aluminum toxicity. In Encyclopedia of Applied Plant Sciences; 2 nd ed., Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 211–218. [Google Scholar]
- Kichigina, N.E.; Puhalsky, J.V.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Loskutov, S.I.; Safronova, V.I.; Tikhonovich, I.A.; Vishnyakova, M.A.; Semenova, E.V.; et al. Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants 2017, 23, 851–863. [Google Scholar] [CrossRef]
- Niedziela, A.; Bednarek, P.T.; Labudda, M.; Mańkowski, D.R.; Anioł, A. Genetic mapping of a 7R Al tolerance QTL in triticale (x Triticosecale Wittmack). J. Appl. Genet. 2014, 55, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Herrera, L.A.; Garkava-Gustavsson, L.; Åhman, I. A systematic review of rye (Secale cereale L.) as a source of resistance to pathogens and pests in wheat (Triticum aestivum L.). Hereditas 2017, 154, 1–9. [Google Scholar] [CrossRef]
- Collins, N.C.; Shirley, N.; Saeed, M.; Pallotta, M.; Gustafson, J.P. An ALMT1 Gene Cluster Controlling Aluminum Tolerance at the Alt4 Locus of Rye (Secale cereale L.). Genetics 2008, 179, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Moneim, D.A.; Contreras, R.; Silva-Navas, J.; Gallego, F.J.; Figueiras, A.M.; Benito, C. Pectin methylesterase gene and aluminum tolerance in Secale cereale. Environ. Exp. Bot. 2014, 107, 125–133. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Han, A.; Teixeira, J.; Matos, M.; Fidalgo, F. Oxidative Metabolism of Rye (Secale cereale L.) after Short Term Exposure to Aluminum: Uncovering the Glutathione–Ascorbate Redox Network. Front. Plant Sci. 2016, 7, 685. [Google Scholar] [CrossRef]
- Aniol, A.; Gustafson, J.P. Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Can. J. Genet. Cytol. 1984, 26, 701–705. [Google Scholar] [CrossRef]
- Niedziela, A.; Mańkowski, D.; Bednarek, P.T. Diversity Arrays Technology-based PCR markers for marker assisted selection of aluminum tolerance in triticale (x Triticosecale Wittmack). Mol. Breed. 2015, 35, 209. [Google Scholar] [CrossRef] [Green Version]
- Hamel, F.; Breton, C.; Houde, M. Isolation and characterization of wheat aluminum-regulated genes: Possible involvement of aluminum as a pathogenesis response elicitor. Planta 1998, 205, 531–538. [Google Scholar] [CrossRef]
- Richards, K.D.; Snowden, K.C.; Gardner, R.C. Wali6 and wali7 (Genes Induced by Aluminum in Wheat (Triticum aestivum L.) Roots). Plant Physiol. 1994, 105, 1455–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milla, M.A.R.; Butler, E.; Huete, A.R.; Wilson, C.F.; Anderson, O.; Gustafson, J.P. Expressed Sequence Tag-Based Gene Expression Analysis under Aluminum Stress in Rye. Plant Physiol. 2002, 130, 1706–1716. [Google Scholar] [CrossRef] [Green Version]
- Aniol, A. Induction of Aluminum Tolerance in Wheat Seedlings by Low Doses of Aluminum in the Nutrient Solution. Plant Physiol. 1984, 76, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Bielawski, W.; Prabucka, B. Endopeptidases of Triticale Seeds. Biol. Plant. 2001, 44, 283–288. [Google Scholar] [CrossRef]
- Delisle, G.; Champoux, M.; Houde, M. Characterization of Oxalate Oxidase and Cell Death in Al-Sensitive and Tolerant Wheat Roots. Plant Cell Physiol. 2001, 42, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Zhang, S.; Xing, C.; Wang, F.; Ning, W.; Lei, Z. Developmental characteristics and aluminum resistance of root border cells in rice seedlings. Plant Sci. 2011, 180, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qu, M.; Fang, J.; Shen, R.F.; Feng, Y.M.; Liu, J.Y.; Bian, J.F.; Wu, L.S.; He, Y.M.; Yu, M. Alkali-Soluble Pectin Is the Primary Target of Aluminum Immobilization in Root Border Cells of Pea (Pisum sativum). Front. Plant Sci. 2016, 7, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagayama, T.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Changes in the Distribution of Pectin in Root Border Cells Under Aluminum Stress. Front. Plant Sci. 2019, 10, 1216. [Google Scholar] [CrossRef]
- Shah, K.; Nongkynrih, J.M. Metal hyperaccumulation and bioremediation. Biol. Plant. 2007, 51, 618–634. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell Wall Polysaccharides Are Specifically Involved in the Exclusion of Aluminum from the Rice Root Apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants: Vacuolar Functions in HM Detoxification. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Reese, R.N.; McCall, R.D.; Roberts, L.W. Cadmium-induced ultrastructural changes in suspension-cultured tobacco cells (Nicotiana tabacum L. var. Xanthi). Environ. Exp. Bot. 1986, 26, 169–173. [Google Scholar] [CrossRef]
- Vitória, A.; Da Cunha, M.; Azevedo, R. Ultrastructural changes of radish leaf exposed to cadmium. Environ. Exp. Bot. 2006, 58, 47–52. [Google Scholar] [CrossRef]
- Hasan, K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudkowska, M.; Zagdańska, B. Multifunctional role of plant cysteine proteinases. Acta Biochim. Pol. 2004, 51, 609–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piszczek, E.; Gutman, W. Caspase-like proteases and their role in programmed cell death in plants. Acta Physiol. Plant. 2007, 29, 391–398. [Google Scholar] [CrossRef]
- Szewińska, J.; Prabucka, B.; Krawczyk, M.; Mielecki, M.; Bielawski, W. The participation of phytocystatin TrcC-4 in the activity regulation of EP8, the main prolamin degrading cysteine endopeptidase in triticale seeds. Plant Growth Regul. 2012, 69, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Khan, N.A.; Bano, B. In-sights into the effect of heavy metal stress on the endogenous mustard cystatin. Int. J. Biol. Macromol. 2017, 105, 1138–1147. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewińska, J.; Różańska, E.; Papierowska, E.; Labudda, M. Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress. Cells 2021, 10, 3046. https://doi.org/10.3390/cells10113046
Szewińska J, Różańska E, Papierowska E, Labudda M. Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress. Cells. 2021; 10(11):3046. https://doi.org/10.3390/cells10113046
Chicago/Turabian StyleSzewińska, Joanna, Elżbieta Różańska, Ewa Papierowska, and Mateusz Labudda. 2021. "Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress" Cells 10, no. 11: 3046. https://doi.org/10.3390/cells10113046
APA StyleSzewińska, J., Różańska, E., Papierowska, E., & Labudda, M. (2021). Proteolytic and Structural Changes in Rye and Triticale Roots under Aluminum Stress. Cells, 10(11), 3046. https://doi.org/10.3390/cells10113046






