Moving the Research Forward: The Best of British Biology Using the Tractable Model System Dictyostelium discoideum
Abstract
1. Introduction
- A simple system
- –
- A compact genome with reduced functional redundancy compared with mammalian cells, allowing clear analysis of mutant phenotypes;
- Developmental biology
- –
- Distinct lifecycles of single and multicellular phases, allowing the analysis of decisions specifically affecting cell proliferation and differentiation;
- Biochemistry
- –
- The rapid and inexpensive production of multiple grams of isogenic cells, for example, to analyse enzyme activity, metabolic changes, organelle function or development;
- Genetics and cell biology
- Microscopy
- –
- Advanced microscopy techniques to visualize, monitor and analyse protein and RNA dynamics;
- Pharmacology
- –
- Pharmacogenetic approaches, where screening of mutant-cell libraries can identify the genetic basis of cell or developmental behaviour or how drugs or related compounds act in cells (i.e., mechanism of action studies);
- –
- The rapid analysis of compound effects on normal cell function, including acute and chronic exposure and structure/activity studies.
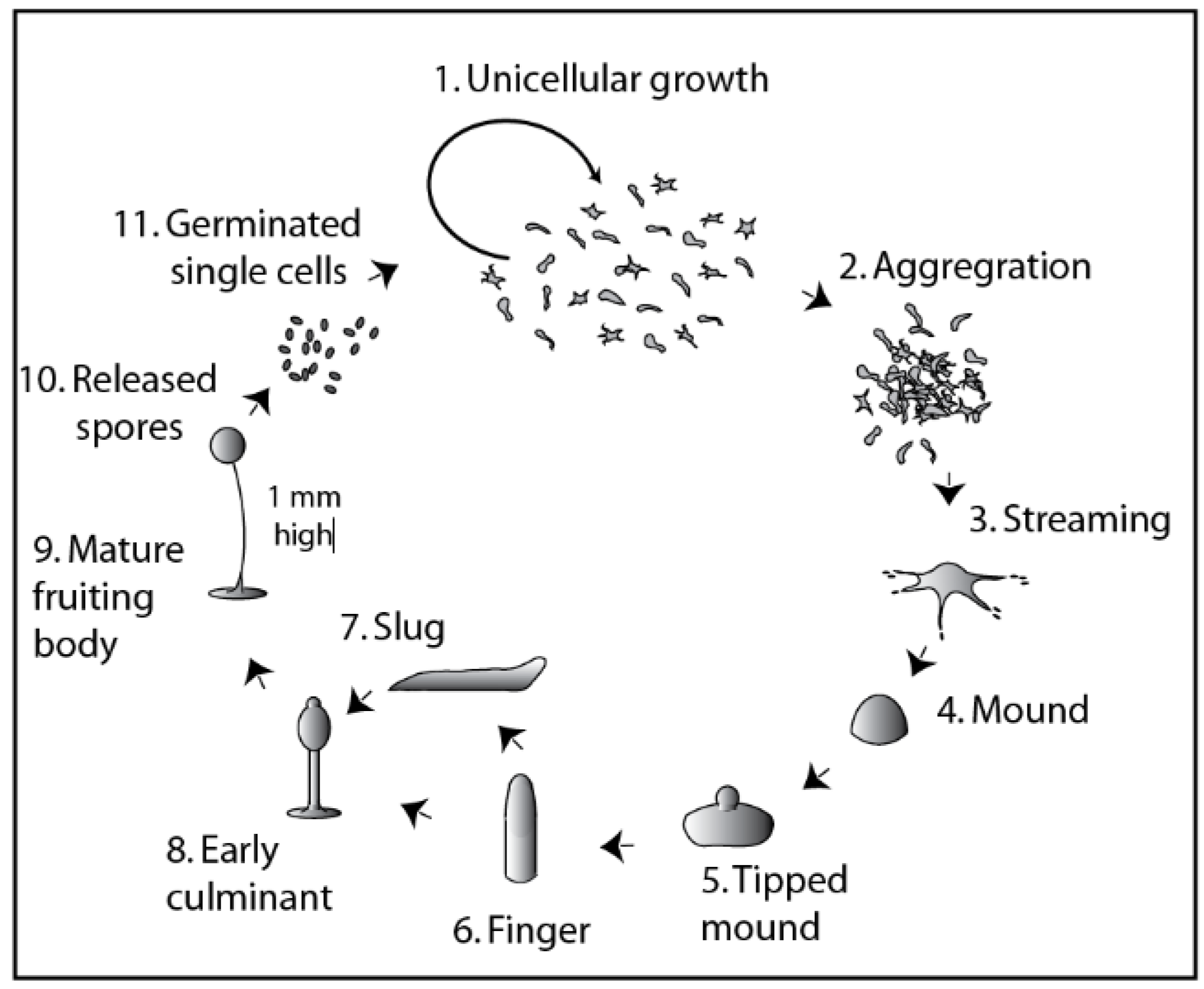
2. Understanding Cell Fate Decisions
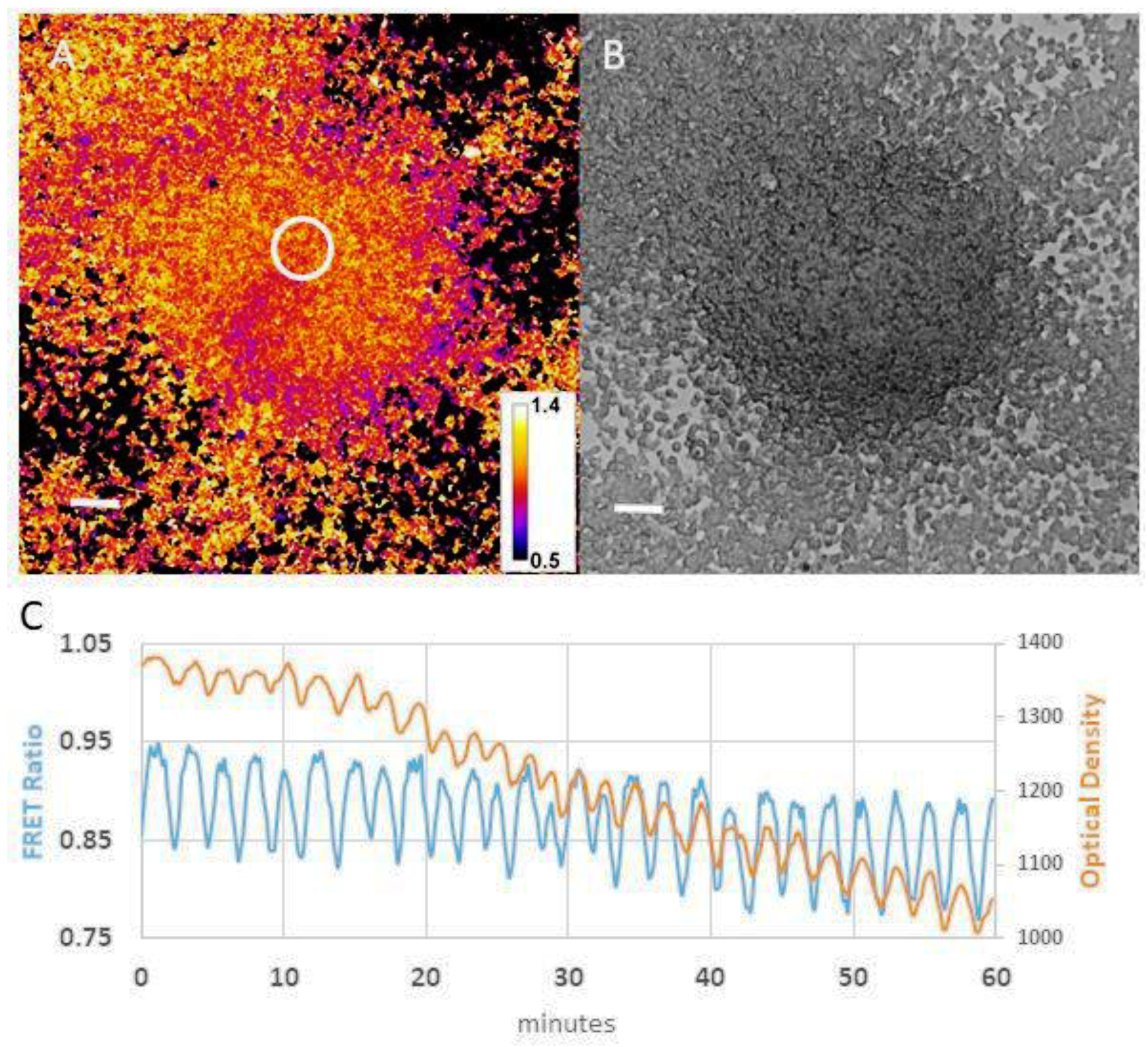
3. Self-Generated Chemotactic Gradients
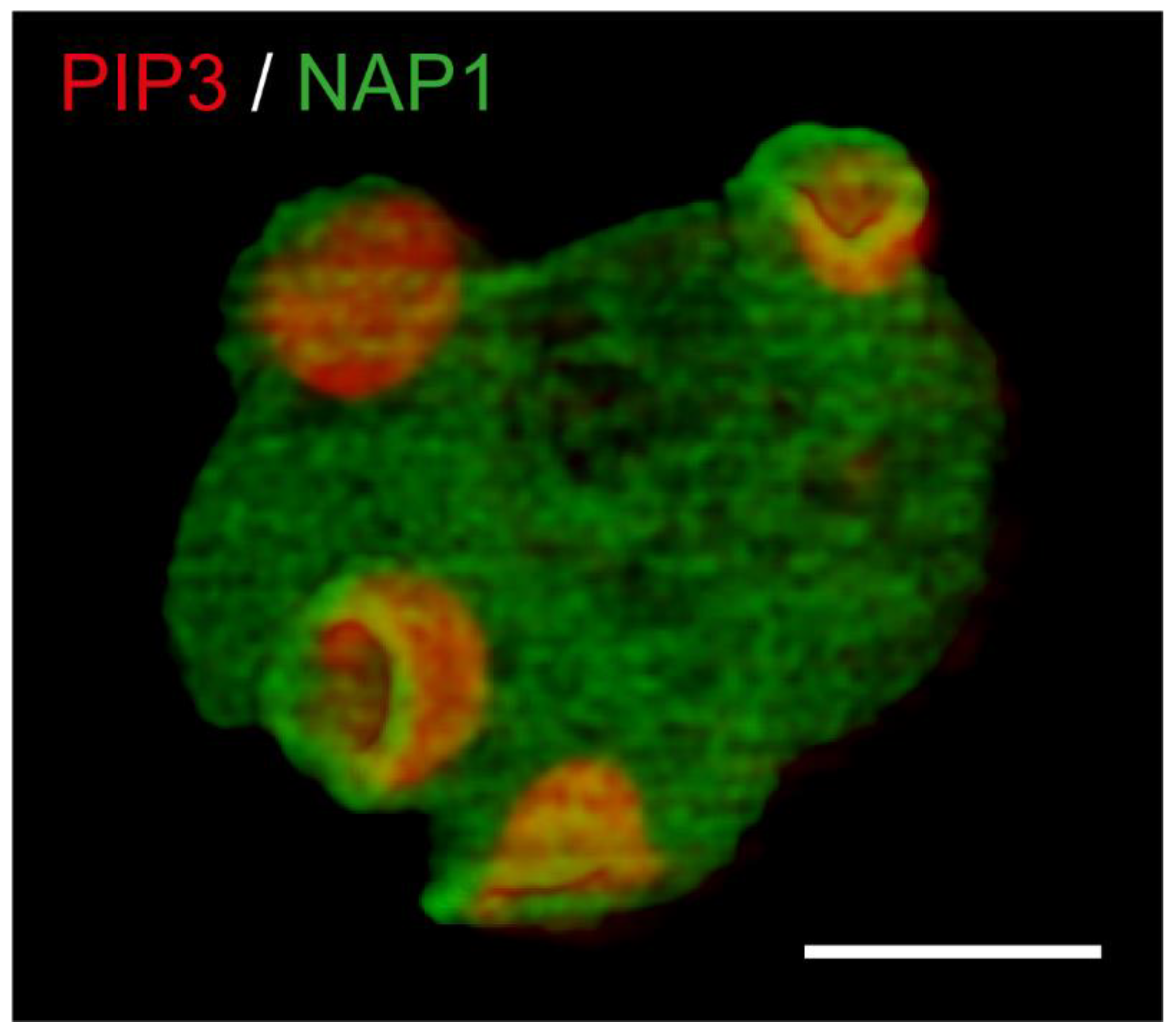
4. Macropinocytosis
5. Targeting Histone Post-Translational Modifications
6. Evolutionary Membrane Biology
7. Cellular Mechanisms Underlying Multicellular Morphogenesis
8. Molecular Medicine and Drug Discovery
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Albani, A.; Mangano, M.G.; Buatois, L.A.; Bengtson, S.; Riboulleau, A.; Bekker, A.; Konhauser, K.; Lyons, T.; Rollion-Bard, C.; Bankole, O.; et al. Organism motility in an oxygenated shallow-marine environment 2.1 billion years ago. Proc. Natl. Acad. Sci. USA 2019, 116, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Heidel, A.J.; Lawal, H.M.; Felder, M.; Schilde, C.; Helps, N.R.; Tunggal, B.; Rivero, F.; John, U.; Schleicher, M.; Eichinger, L.; et al. Phylogeny-wide analysis of social amoeba genomes highlights ancient origins for complex intercellular communication. Genome Res. 2011, 21, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Raper, K.B. Stalk formation in Dictyostelium. Science 1950, 112, 450. [Google Scholar] [CrossRef] [PubMed]
- Raper, K.B.; Smith, N.R. The Growth of Dictyostelium discoideum upon Pathogenic Bacteria. J. Bacteriol. 1939, 38, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Raper, K.B. Dictyostelium discoideum, a new species of slime mold from decaying forest leaves. J. Agric. Res. 1935, 50, 135–147. [Google Scholar]
- Bonner, J.T. Evidence for the formation of cell aggregates by chemotaxis in the development of the slime mold Dictyostelium discoideum. J. Exp. Zool. 1947, 106, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.T.; Eldredge, D., Jr. A note on the rate of morphogenetic movement in the slime mold, Dictyostelium discoideum. Growth 1945, 9, 287–297. [Google Scholar]
- Douglas, T.E.; Brock, D.A.; Adu-Oppong, B.; Queller, D.C.; Strassmann, J.E. Collection and cultivation of dictyostelids from the wild. Methods Mol. Biol. 2013, 983, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, R.; Tyree, T.; Gomer, R.H.; Rappel, W.J. Cell dispersal by localized degradation of a chemoattractant. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Fisher, P.R.; Annesley, S.J. Slug phototaxis, thermotaxis, and spontaneous turning behavior. Methods Mol. Biol. 2006, 346, 137–170. [Google Scholar] [CrossRef]
- Chisholm, R.L.; Gaudet, P.; Just, E.M.; Pilcher, K.E.; Fey, P.; Merchant, S.N.; Kibbe, W.A. dictyBase, the model organism database for Dictyostelium discoideum. Nucleic Acids Res. 2006, 34, D423–D427. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Boeckeler, K.; Graf, R.; Muller-Taubenberger, A.; Li, Z.; Isberg, R.R.; Wessels, D.; Soll, D.R.; Alexander, H.; Alexander, S. Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol. Med. 2006, 12, 415–424. [Google Scholar] [CrossRef]
- Muller-Taubenberger, A.; Kortholt, A.; Eichinger, L. Simple system–substantial share: The use of Dictyostelium in cell biology and molecular medicine. Eur. J. Cell Biol. 2013, 92, 45–53. [Google Scholar] [CrossRef]
- Pearce, X.G.; Annesley, S.J.; Fisher, P.R. The Dictyostelium model for mitochondrial biology and disease. Int. J. Dev. Biol. 2019, 63, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Vincent, O.; Anton-Esteban, L.; Bueno-Arribas, M.; Tornero-Ecija, A.; Navas, M.A.; Escalante, R. The WIPI Gene Family and Neurodegenerative Diseases: Insights From Yeast and Dictyostelium Models. Front. Cell Dev. Biol. 2021, 9, 737071. [Google Scholar] [CrossRef]
- Huber, R.J. Molecular networking in the neuronal ceroid lipofuscinoses: Insights from mammalian models and the social amoeba Dictyostelium discoideum. J. Biomed. Sci. 2020, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Sharma, D.; Williams, R.S. Non-Catalytic Roles of Presenilin Throughout Evolution. J. Alzheimers Dis. 2016, 52, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Varas, M.A.; Riquelme-Barrios, S.; Valenzuela, C.; Marcoleta, A.E.; Berrios-Pasten, C.; Santiviago, C.A.; Chavez, F.P. Inorganic Polyphosphate Is Essential for Salmonella Typhimurium Virulence and Survival in Dictyostelium discoideum. Front. Cell Infect. Microbiol. 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Bosmani, C.; Barisch, C.; Raykov, L.; Lefrancois, L.H.; Cardenal-Munoz, E.; Lopez-Jimenez, A.T.; Soldati, T. Eat Prey, Live: Dictyostelium discoideum As a Model for Cell-Autonomous Defenses. Front. Immunol. 2017, 8, 1906. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, W.; Li, P.; Yang, M.; Wu, C.; Eichinger, L. The Role of ATG16 in Autophagy and The Ubiquitin Proteasome System. Cells 2018, 8, 2. [Google Scholar] [CrossRef]
- Dominguez-Martin, E.; Cardenal-Munoz, E.; King, J.S.; Soldati, T.; Coria, R.; Escalante, R. Methods to Monitor and Quantify Autophagy in the Social Amoeba Dictyostelium discoideum. Cells 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, S.I.; Buttery, N.J.; Thompson, C.R.; Purugganan, M.D. Sociogenomics of self vs. non-self cooperation during development of Dictyostelium discoideum. BMC Genomics 2014, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Pears, C.; Brustel, J.; Lakin, N. Dictyostelium discoideum as a model to assess genome stability through DNA repair. Front. Cell Dev. Biol. 2021, 7, 9–752175. [Google Scholar] [CrossRef]
- Gruenheit, N.; Baldwin, A.; Stewart, B.; Jaques, S.; Keller, T.; Parkinson, K.; Salvidge, W.; Baines, R.; Brimson, C.; Wolf, J.B.; et al. Mutant resources for functional genomics in Dictyostelium discoideum using REMI-seq technology. BMC Biol. 2021, 19, 172. [Google Scholar] [CrossRef]
- Sekine, R.; Kawata, T.; Muramoto, T. CRISPR/Cas9 mediated targeting of multiple genes in Dictyostelium. Sci. Rep. 2018, 8, 8471. [Google Scholar] [CrossRef]
- Chubb, J.R.; Ford, H.Z.; Antolovic, V. Distributed and centralized control during differentiation. Dev. cell 2021, 56, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Devreotes, P.N.; Bhattacharya, S.; Edwards, M.; Iglesias, P.A.; Lampert, T.; Miao, Y. Excitable Signal Transduction Networks in Directed Cell Migration. Annu. Rev. Cell Dev. Biol. 2017, 33, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Miermont, A.; Antolovic, V.; Lenn, T.; Nichols, J.M.E.; Millward, L.J.; Chubb, J.R. The fate of cells undergoing spontaneous DNA damage during development. Development 2019, 146, dev174268. [Google Scholar] [CrossRef]
- Chubb, J.R.; Trcek, T.; Shenoy, S.M.; Singer, R.H. Transcriptional pulsing of a developmental gene. Curr. Biol. 2006, 16, 1018–1025. [Google Scholar] [CrossRef]
- Corrigan, A.M.; Tunnacliffe, E.; Cannon, D.; Chubb, J.R. A continuum model of transcriptional bursting. eLife 2016, 5, e13051. [Google Scholar] [CrossRef]
- Tunnacliffe, E.; Corrigan, A.M.; Chubb, J.R. Promoter-mediated diversification of transcriptional bursting dynamics following gene duplication. Proc. Natl. Acad. Sci. USA 2018, 115, 8364–8369. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, E.; Chubb, J.R. What Is a Transcriptional Burst? Trends Genet. 2020, 36, 288–297. [Google Scholar] [CrossRef]
- Wilkins, A.; Khosla, M.; Fraser, D.J.; Spiegelman, G.B.; Fisher, P.R.; Weeks, G.; Insall, R.H. Dictyostelium RasD is required for normal phototaxis, but not differentiation. Genes Dev. 2000, 14, 1407–1413. [Google Scholar] [PubMed]
- Fey, P.; Dodson, R.J.; Basu, S.; Chisholm, R.L. One stop shop for everything Dictyostelium: DictyBase and the Dicty Stock Center in 2012. Methods Mol. Biol. 2013, 983, 59–92. [Google Scholar] [CrossRef]
- Zigmond, S.H. Mechanisms of sensing chemical gradients by polymorphonuclear leukocytes. Nature 1974, 249, 450–452. [Google Scholar] [CrossRef]
- Insall, R.; Andrew, N. Chemotaxis in Dictyostelium: How to walk straight using parallel pathways. Curr. Opin. Microbiol. 2007, 10, 578–581. [Google Scholar] [CrossRef]
- Insall, R. The interaction between pseudopods and extracellular signalling during chemotaxis and directed migration. Curr. Opin. Cell Biol. 2013, 25, 526–531. [Google Scholar] [CrossRef]
- Tweedy, L.; Insall, R.H. Self-Generated Gradients Yield Exceptionally Robust Steering Cues. Front. Cell Dev. Biol 2020, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Susanto, O.; Koh, Y.W.H.; Morrice, N.; Tumanov, S.; Thomason, P.A.; Nielson, M.; Tweedy, L.; Muinonen-Martin, A.J.; Kamphorst, J.J.; Mackay, G.M.; et al. LPP3 mediates self-generation of chemotactic LPA gradients by melanoma cells. J. Cell Sci. 2017, 130, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Van Haastert, P.J.; Jastorff, B.; Pinas, J.E.; Konijn, T.M. Analogs of cyclic AMP as chemoattractants and inhibitors of Dictyostelium chemotaxis. J. Bacteriol. 1982, 149, 99–105. [Google Scholar] [CrossRef]
- Franke, J.; Kessin, R.H. The cyclic nucleotide phosphodiesterases of Dictyostelium discoideum: Molecular genetics and biochemistry. Cell Signal. 1992, 4, 471–478. [Google Scholar] [CrossRef]
- Bernstein, R.L.; Tabler, M.; Vestweber, D.; Van Driel, R. Extracellular folate deaminase of Dictyostelium discoideum. Biochim. Biophys. Acta. 1981, 677, 295–302. [Google Scholar] [CrossRef]
- Tweedy, L.; Thomason, P.A.; Paschke, P.I.; Martin, K.; Machesky, L.M.; Zagnoni, M.; Insall, R.H. Seeing around corners: Cells solve mazes and respond at a distance using attractant breakdown. Science 2020, 369, e13051. [Google Scholar] [CrossRef]
- Skoge, M.; Wong, E.; Hamza, B.; Bae, A.; Martel, J.; Kataria, R.; Keizer-Gunnink, I.; Kortholt, A.; Van Haastert, P.J.; Charras, G.; et al. A Worldwide Competition to Compare the Speed and Chemotactic Accuracy of Neutrophil-Like Cells. PLoS ONE 2016, 11, e0154491. [Google Scholar] [CrossRef]
- King, J.S.; Kay, R.R. The origins and evolution of macropinocytosis. Philos. Trans. R Soc. Lond. B Biol. Sci 2019, 374, 20180158. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Swanson, J.A.; King, J.S. The breadth of macropinocytosis research. Philos. Trans. R Soc. Lond. B Biol. Sci 2019, 374, 20180146. [Google Scholar] [CrossRef]
- Veltman, D.M.; Williams, T.D.; Bloomfield, G.; Chen, B.C.; Betzig, E.; Insall, R.H.; Kay, R.R. A plasma membrane template for macropinocytic cups. eLife 2016, 5, e20085. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.; Traynor, D.; Sander, S.P.; Veltman, D.M.; Pachebat, J.A.; Kay, R.R. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife 2015, 4, e04940. [Google Scholar] [CrossRef]
- Buckley, C.M.; Pots, H.; Gueho, A.; Vines, J.H.; Munn, C.J.; Phillips, B.A.; Gilsbach, B.; Traynor, D.; Nikolaev, A.; Soldati, T.; et al. Coordinated Ras and Rac Activity Shapes Macropinocytic Cups and Enables Phagocytosis of Geometrically Diverse Bacteria. Curr. Biol. 2020, 30, 2912–2926. [Google Scholar] [CrossRef] [PubMed]
- Junemann, A.; Filic, V.; Winterhoff, M.; Nordholz, B.; Litschko, C.; Schwellenbach, H.; Stephan, T.; Weber, I.; Faix, J. A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis. Proc. Natl. Acad. Sci. USA 2016, 113, E7464–E7473. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Chao, X.; Singh, S.P.; Thomason, P.; Yan, Y.; Dong, M.; Li, L.; Insall, R.H.; Cai, H. Leep1 interacts with PIP3 and the Scar/WAVE complex to regulate cell migration and macropinocytosis. J. Cell Biol. 2021, 220, e202010096. [Google Scholar] [CrossRef]
- Buckley, C.M.; Gopaldass, N.; Bosmani, C.; Johnston, S.A.; Soldati, T.; Insall, R.H.; King, J.S. WASH drives early recycling from macropinosomes and phagosomes to maintain surface phagocytic receptors. Proc. Natl. Acad. Sci. USA 2016, 113, E5906–E5915. [Google Scholar] [CrossRef]
- Novohradska, S.; Ferling, I.; Hillmann, F. Exploring Virulence Determinants of Filamentous Fungal Pathogens through Interactions with Soil Amoebae. Front. Cell Infect. Microbiol. 2017, 7, 497. [Google Scholar] [CrossRef]
- Stevense, M.; Chubb, J.R.; Muramoto, T. Nuclear organization and transcriptional dynamics in Dictyostelium. Dev. Growth Differ. 2011, 53, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Yamashita, K.; Hasegawa, A.; Ogasawara, T.; Iriki, H.; Muramoto, T. Knock-in and precise nucleotide substitution using near-PAMless engineered Cas9 variants in Dictyostelium discoideum. Sci. Rep. 2021, 11, 11163. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, T.; Muller, I.; Thomas, G.; Melvin, A.; Chubb, J.R. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr. Biol. 2010, 20, 397–406. [Google Scholar] [CrossRef]
- Chubb, J.R.; Bloomfield, G.; Xu, Q.; Kaller, M.; Ivens, A.; Skelton, J.; Turner, B.M.; Nellen, W.; Shaulsky, G.; Kay, R.R.; et al. Developmental timing in Dictyostelium is regulated by the Set1 histone methyltransferase. Dev. Biol. 2006, 292, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.L.; Hazzalin, C.A.; Mahadevan, L.C. Enhanced histone acetylation and transcription: A dynamic perspective. Mol. Cell 2006, 23, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.W.; Chubb, J.R.; Muramoto, T.; Pears, C.J.; Mahadevan, L.C. Dynamic acetylation of lysine-4-trimethylated histone H3 and H3 variant biology in a simple multicellular eukaryote. Nucleic. Acids. Res. 2012, 40, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Hsu, D.W.; Pears, C.J. Methylation-directed acetylation of histone H3 regulates developmental sensitivity to histone deacetylase inhibition. Nucleic. Acids. Res. 2021, 49, 3781–3795. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Couto, C.A.; Hsu, D.W.; Teo, R.; Rakhimova, A.; Lempidaki, S.; Pears, C.J.; Lakin, N.D. Nonhomologous end-joining promotes resistance to DNA damage in the absence of an ADP-ribosyltransferase that signals DNA single strand breaks. J. Cell Sci. 2013, 126, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.A.; Wang, H.Y.; Green, J.C.; Kiely, R.; Siddaway, R.; Borer, C.; Pears, C.J.; Lakin, N.D. PARP regulates nonhomologous end joining through retention of Ku at double-strand breaks. J. Cell Biol. 2011, 194, 367–375. [Google Scholar] [CrossRef]
- Rakhimova, A.; Ura, S.; Hsu, D.W.; Wang, H.Y.; Pears, C.J.; Lakin, N.D. Site-specific ADP-ribosylation of histone H2B in response to DNA double strand breaks. Sci. Rep. 2017, 7, 43750. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.P.; Wilkins, C.; Demidchik, V.; Davies, J.M.; Glover, B.J. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J. Exp. Bot. 2010, 61, 439–451. [Google Scholar] [CrossRef]
- Lavell, A.; Froehlich, J.E.; Baylis, O.; Rotondo, A.D.; Benning, C. A predicted plastid rhomboid protease affects phosphatidic acid metabolism in Arabidopsis thaliana. Plant. J. 2019, 99, 978–987. [Google Scholar] [CrossRef]
- Saita, S.; Tatsuta, T.; Lampe, P.A.; Konig, T.; Ohba, Y.; Langer, T. PARL partitions the lipid transfer protein STARD7 between the cytosol and mitochondria. EMBO J. 2018, 37, e97909. [Google Scholar] [CrossRef]
- Rafiq, M.; Paleckyte, A.; Thompson, E. Dictyostelium rhomboid proteases and implications for mitochondrial disease. Access Microbiol. 2019, 1. [Google Scholar] [CrossRef]
- Weinrich, T.W.; Kam, J.H.; Ferrara, B.T.; Thompson, E.P.; Mitrofanis, J.; Jeffery, G. A day in the life of mitochondria reveals shifting workloads. Sci. Rep. 2019, 9, 13898. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, B.T.; Thompson, E.P. A method for visualizing fluorescence of flavonoid therapeutics in vivo in the model eukaryote Dictyostelium discoideum. Biotechniques 2019, 66, 65–71. [Google Scholar] [CrossRef]
- Kessin, R. Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity (Developmental and Cell Biology Series); Cambridge University Press: Cambridge, UK, 2001. [Google Scholar] [CrossRef]
- Weeks, G.; Weijer, C.J. The Dictyostelium cell cycle and its relationship to differentiation. (Minireview). FEMS Microbiol. Lett. 1994, 124, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Konijn, T.M.; van de Meene, J.G.C.; Chang, Y.Y.; Barkley, D.S.; Bonner, J.T. Identification of adenosine-3′,5′-monophosphate as the bacterial attractant for myxamoebae of Dictyostelium discoideum. J. Bacteriol. 1969, 99, 510–512. [Google Scholar] [CrossRef]
- Tomchik, K.J.; Devreotes, P.N. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: A demonstration by isotope dilution-fluorography technique. Science 1981, 212, 443–446. [Google Scholar] [CrossRef]
- Weijer, C.J. Dictyostelium morphogenesis. Curr. Opin. Genet. Dev. 2004, 14, 392–398. [Google Scholar] [CrossRef]
- Singer, G.; Araki, T.; Weijer, C.J. Oscillatory cAMP cell-cell signalling persists during multicellular Dictyostelium development. Commun. Biol. 2019, 2, 139. [Google Scholar] [CrossRef]
- Bretschneider, T.; Othmer, H.G.; Weijer, C.J. Progress and perspectives in signal transduction, actin dynamics, and movement at the cell and tissue level: Lessons from Dictyostelium. Interface Focus 2016, 6, 20160047. [Google Scholar] [CrossRef]
- Belotti, Y.; McGloin, D.; Weijer, C.J. Analysis of barotactic and chemotactic guidance cues on directional decision-making of Dictyostelium discoideum cells in confined environments. Proc. Natl. Acad. Sci. USA 2020, 117, 25553–25559. [Google Scholar] [CrossRef]
- Fujimori, T.; Nakajima, A.; Shimada, N.; Sawai, S. Tissue self-organization based on collective cell migration by contact activation of locomotion and chemotaxis. Proc. Natl. Acad. Sci. USA 2019, 116, 4291–4296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pal, D.S.; Biswas, D.; Iglesias, P.A.; Devreotes, P.N. Reverse fountain flow of phosphatidylinositol-3,4-bisphosphate polarizes migrating cells. EMBO J. 2021, 40, e105094. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, G.J.; Wang, J.; Weijer, C.J. SILAC-based proteomic quantification of chemoattractant-induced cytoskeleton dynamics on a second to minute timescale. Nat. Commun. 2014, 5, 3319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J., Avoli, M., Rogawski, M., Olsen, R., Delgado-Escueta, A., Eds.; Oxford University Press: Oxford, UK, Oxford Medicine Online; Available online: https://oxfordmedicine.com/view/10.1093/med/9780199746545.001.0001/med-9780199746545 (accessed on 3 October 2021).
- Loscher, W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef] [PubMed]
- Bromley, R.; Weston, J.; Adab, N.; Greenhalgh, J.; Sanniti, A.; McKay, A.J.; Tudur Smith, C.; Marson, A.G. Treatment for epilepsy in pregnancy: Neurodevelopmental outcomes in the child. Cochrane Database Syst. Rev. 2014, CD010236. [Google Scholar] [CrossRef]
- Jentink, J.; Loane, M.A.; Dolk, H.; Barisic, I.; Garne, E.; Morris, J.K.; de Jong-van den Berg, L.T. Valproic acid monotherapy in pregnancy and major congenital malformations. N. Engl. J. Med. 2010, 362, 2185–2193. [Google Scholar] [CrossRef]
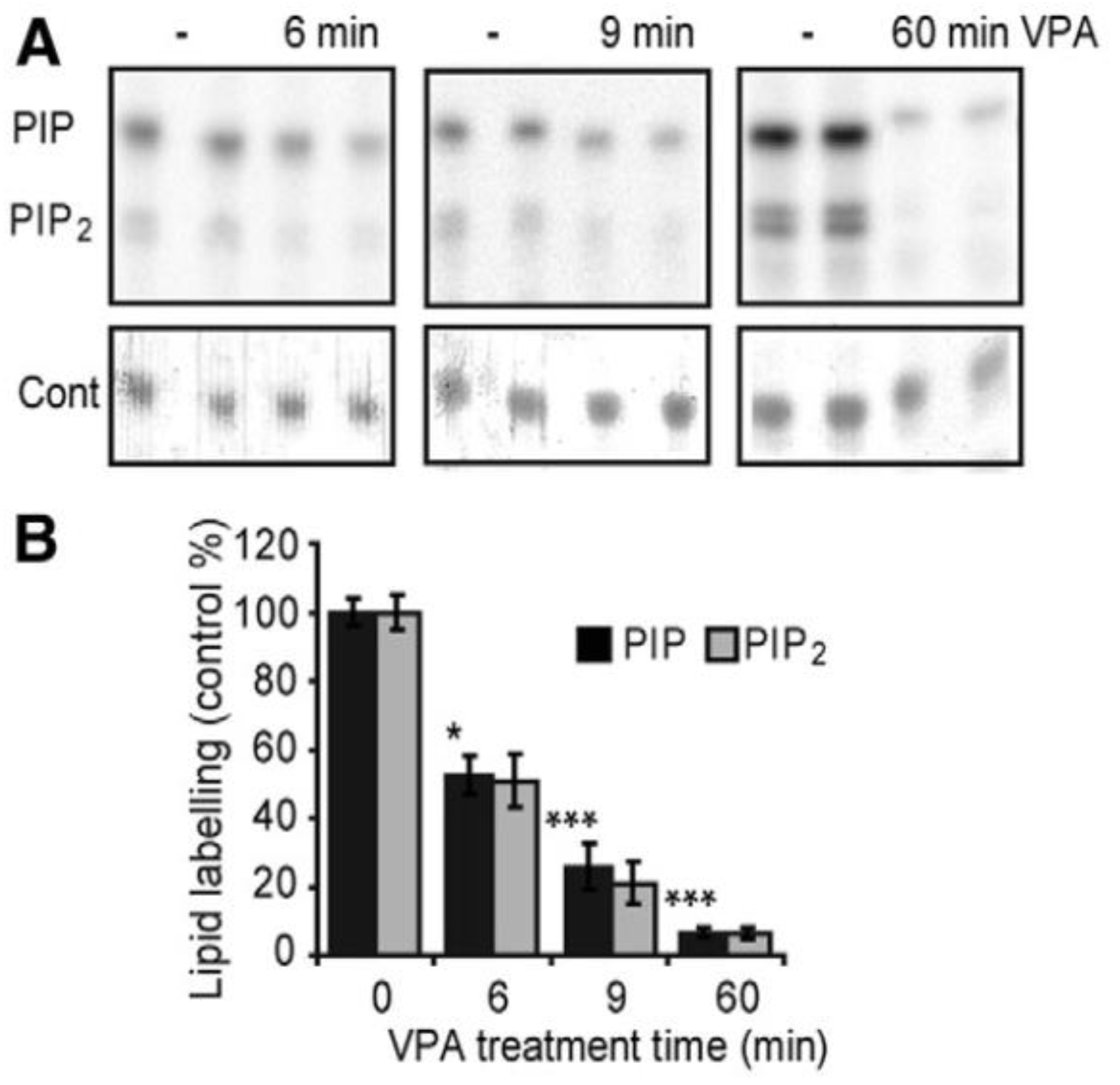
- Xu, X.; Muller-Taubenberger, A.; Adley, K.E.; Pawolleck, N.; Lee, V.W.; Wiedemann, C.; Sihra, T.S.; Maniak, M.; Jin, T.; Williams, R.S. Attenuation of phospholipid signaling provides a novel mechanism for the action of valproic acid. Eukaryot. Cell 2007, 6, 899–906. [Google Scholar] [CrossRef]
- Chang, P.; Orabi, B.; Deranieh, R.M.; Dham, M.; Hoeller, O.; Shimshoni, J.A.; Yagen, B.; Bialer, M.; Greenberg, M.L.; Walker, M.C.; et al. The antiepileptic drug valproic acid and other medium-chain fatty acids acutely reduce phosphoinositide levels independently of inositol in Dictyostelium. Dis. Model. Mech. 2012, 5, 115–124. [Google Scholar] [CrossRef]
- Chang, P.; Walker, M.C.; Williams, R.S. Seizure-induced reduction in PIP3 levels contributes to seizure-activity and is rescued by valproic acid. Neurobiol. Dis. 2014, 62, 296–306. [Google Scholar] [CrossRef]
- Chang, P.; Terbach, N.; Plant, N.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013, 69, 105–114. [Google Scholar] [CrossRef]
- Chang, P.; Zuckermann, A.M.; Williams, S.; Close, A.J.; Cano-Jaimez, M.; McEvoy, J.P.; Spencer, J.; Walker, M.C.; Williams, R.S. Seizure control by derivatives of medium chain fatty acids associated with the ketogenic diet show novel branching-point structure for enhanced potency. J. Pharmacol. Exp. Ther. 2015, 352, 43–52. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.D.; Chen, P.E.; Walker, M.C.; Williams, R.S.B. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.C.; Dooves, S.; Lugara, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, R.S.B.; Chubb, J.R.; Insall, R.; King, J.S.; Pears, C.J.; Thompson, E.; Weijer, C.J. Moving the Research Forward: The Best of British Biology Using the Tractable Model System Dictyostelium discoideum. Cells 2021, 10, 3036. https://doi.org/10.3390/cells10113036
Williams RSB, Chubb JR, Insall R, King JS, Pears CJ, Thompson E, Weijer CJ. Moving the Research Forward: The Best of British Biology Using the Tractable Model System Dictyostelium discoideum. Cells. 2021; 10(11):3036. https://doi.org/10.3390/cells10113036
Chicago/Turabian StyleWilliams, Robin S. B., Jonathan R. Chubb, Robert Insall, Jason S. King, Catherine J. Pears, Elinor Thompson, and Cornelis J. Weijer. 2021. "Moving the Research Forward: The Best of British Biology Using the Tractable Model System Dictyostelium discoideum" Cells 10, no. 11: 3036. https://doi.org/10.3390/cells10113036
APA StyleWilliams, R. S. B., Chubb, J. R., Insall, R., King, J. S., Pears, C. J., Thompson, E., & Weijer, C. J. (2021). Moving the Research Forward: The Best of British Biology Using the Tractable Model System Dictyostelium discoideum. Cells, 10(11), 3036. https://doi.org/10.3390/cells10113036





