NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective
Abstract
1. Introduction
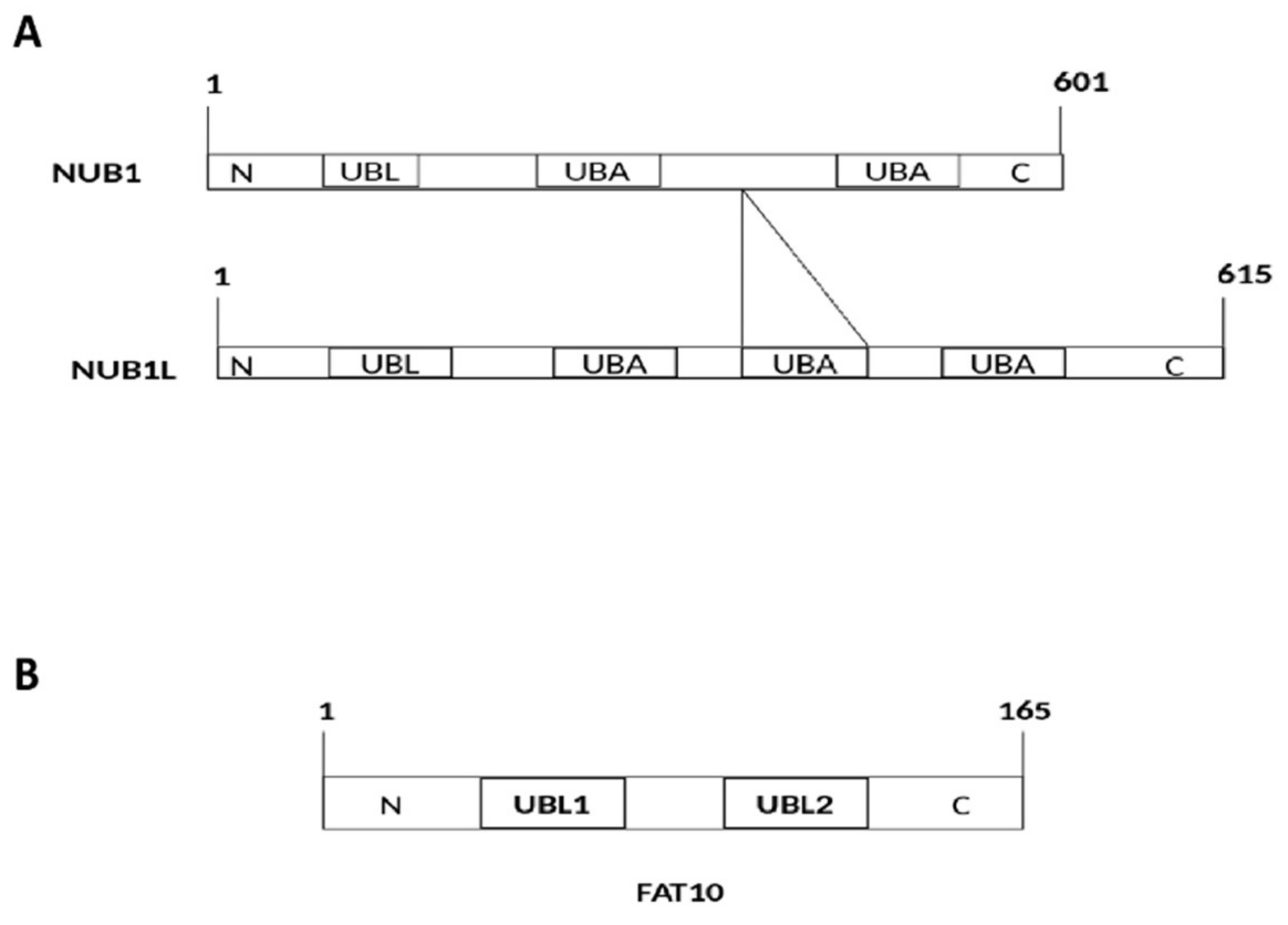
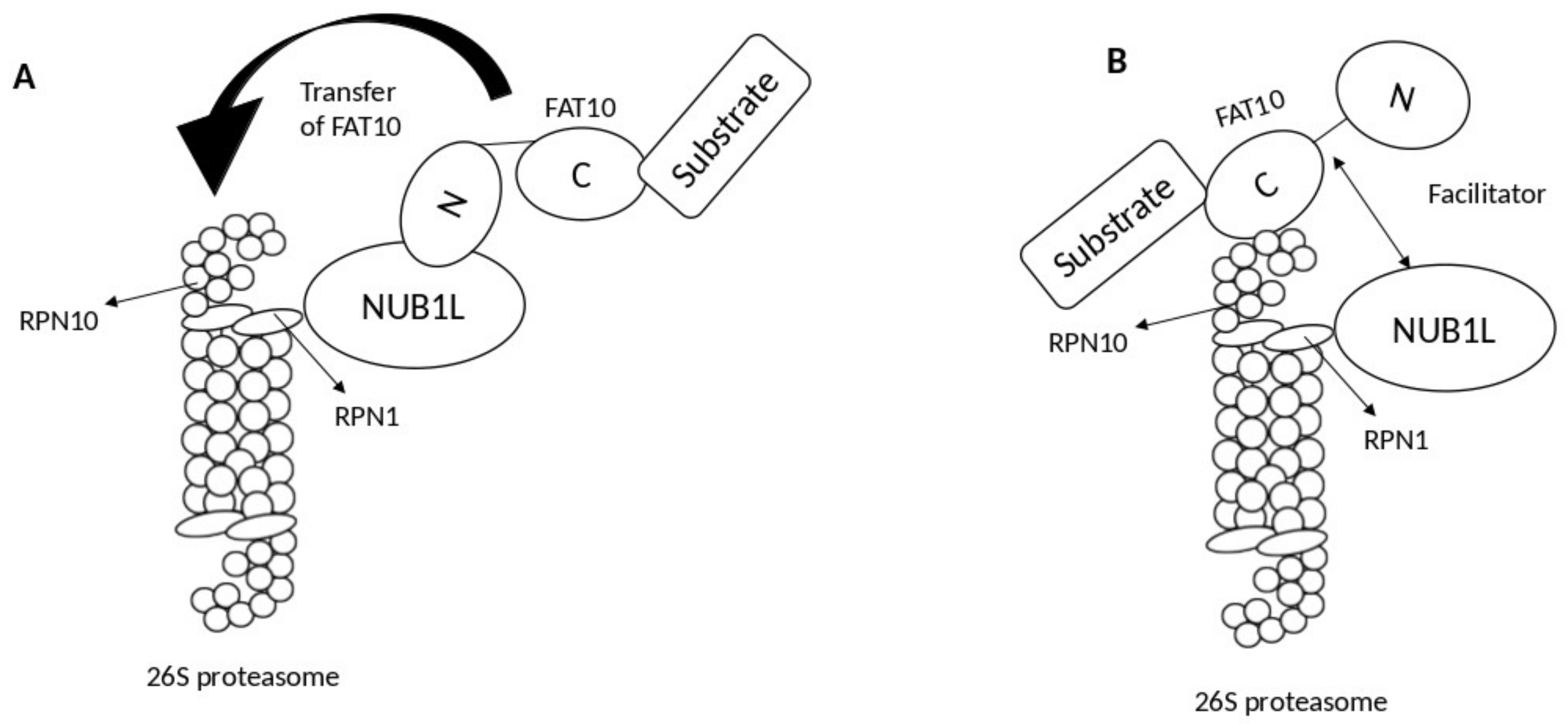
2. Interaction of FAT10 and NUB1
3. NUB1 Protein Actions in Cancer
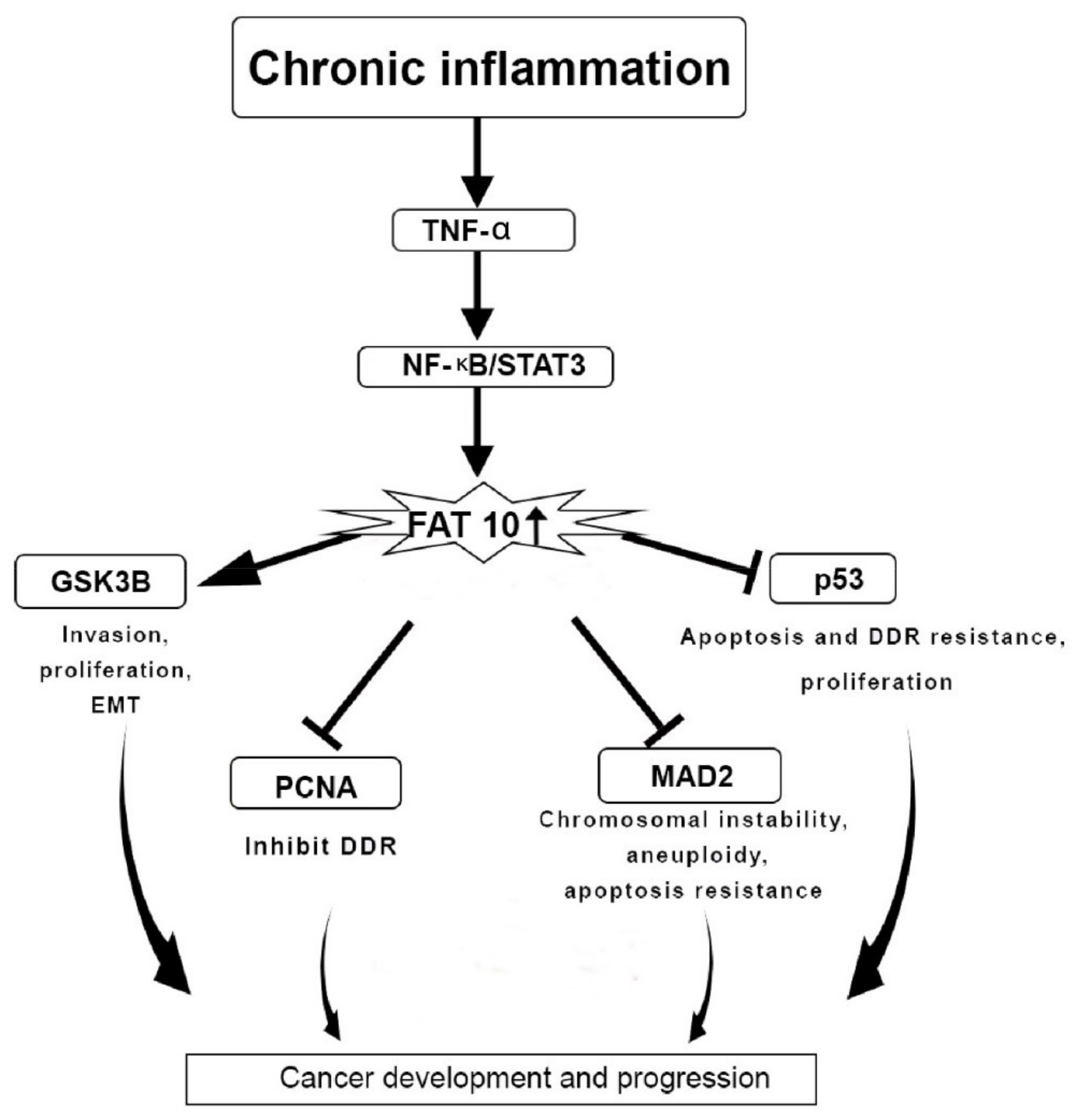
4. FAT10 Protein Actions in Cancer
5. Use of NUB1 and FAT10 as Biomarkers in a Clinical Setting
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hipp, M.S.; Raasi, S.; Groettrup, M.; Schmidtke, G. NEDD8 ultimate buster-1L interacts with the ubiquitin-like protein FAT10 and accelerates its degradation. J. Biol. Chem. 2004, 279, 16503–16510. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Cai, W.; Parimoo, S.; Schwarz, D.C.; Lennon, G.G.; Weissman, S.M. Identification of seven new human MHC class I region genes around the HLA-F locus. Immunogenetics 1996, 44, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Theng, S.S.; Wang, W.; Mah, W.C.; Chan, C.; Zhuo, J.; Gao, Y.; Qin, H.; Lim, L.; Chong, S.S.; Song, J.; et al. Disruption of FAT10-MAD2 binding inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2014, 111, E5282–E5291. [Google Scholar] [CrossRef] [PubMed]
- Groettrup, M.; Pelzer, C.; Schmidtke, G.; Hofmann, K. Activating the ubiquitin family: UBA6 challenges the field. Trends Biochem. Sci. 2008, 33, 230–237. [Google Scholar] [CrossRef]
- Schmidtke, G.; Aichem, A.; Groettrup, M. FAT10ylation as a signal for proteasomal degradation. Biochim. Biophys. Acta. Mol. Cell Res. 2014, 1843, 97–102. [Google Scholar] [CrossRef]
- Aichem, A.; Anders, S.; Catone, N.; Rößler, P.; Stotz, S.; Berg, A.; Schwab, R.; Scheuermann, S.; Bialas, J.; Schütz-Stoffregen, M.C.; et al. The structure of the ubiquitin-like modifier FAT10 reveals an alternative targeting mechanism for proteasomal degradation. Nat. Commun. 2018, 9, 3321. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Gu, X.; Zhu, T.; Yang, S.; Sun, W. LMO2 blocks the UBA6-USE1 interaction and downstream FAT10ylation by targeting the ubiquitin fold domain of UBA6. Biochem. Biophys. Res. Commun. 2016, 478, 1442–1448. [Google Scholar] [CrossRef]
- Gavin, J.M.; Chen, J.J.; Liao, H.; Rollins, N.; Yang, X.; Xu, Q.; Ma, J.; Loke, H.K.; Lingaraj, T.; Brownell, J.E.; et al. Mechanistic studies on activation of ubiquitin and di-ubiquitin-like protein, FAT10, by ubiquitin-like modifier activating enzyme 6, Uba6. J. Biol. Chem. 2012, 287, 15512–15522. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Sun, Q.; Chen, Z.J. E1-L2 activates both ubiquitin and FAT10. Mol. Cell 2007, 27, 1014–1023. [Google Scholar] [CrossRef]
- Pelzer, C.; Kassner, I.; Matentzoglu, K.; Singh, R.K.; Wollscheid, H.P.; Scheffner, M.; Schmidtke, G.; Groettrup, M. UBE1L2, a novel E1 enzyme specific for ubiquitin. J. Biol. Chem. 2007, 282, 23010–23014. [Google Scholar] [CrossRef]
- Aichem, A.; Catone, N.; Groettrup, M. Investigations into the auto-FAT10ylation of the bispecific E2 conjugating enzyme UBA6-specific E2 enzyme 1. FEBS J. 2014, 281, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, G.; Kalveram, B.; Groettrup, M. Degradation of FAT10 by the 26S proteasome is independent of ubiquitylation but relies on NUB1L. FEBS Lett. 2009, 583, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Zhao, J.; Zhang, Y.-H.; Song, A.-X.; Hu, H.-Y. NEDD8 ultimate buster-1 long (NUB1L) protein promotes transfer of NEDD8 to proteasome for degradation through the P97UFD1/NPL4 complex. J. Biol. Chem. 2013, 288, 31339–31349. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Aichem, A.; Schmidtke, G.; Kreft, S.G.; Groettrup, M. FAT10 and NUB1L bind to the VWA domain of Rpn10 and Rpn1 to enable proteasome-mediated proteolysis. Nat. Commun. 2012, 3, 749. [Google Scholar] [CrossRef]
- Schmidtke, G.; Kalveram, B.; Weber, E.; Bochtler, P.; Lukasiak, S.; Hipp, M.S.; Groettrup, M. The UBA domains of NUB1L are required for binding but not for accelerated degradation of the ubiquitin-like modifier FAT10. J. Biol. Chem. 2006, 281, 20045–20054. [Google Scholar] [CrossRef]
- Kito, K.; Yeh, E.T.; Kamitani, T. NUB1, a NEDD8-interacting protein, is induced by interferon and down-regulates the NEDD8 expression. J. Biol. Chem. 2001, 276, 20603–20609. [Google Scholar] [CrossRef]
- Hosono, T.; Tanaka, T.; Tanji, K.; Nakatani, T.; Kamitani, T. NUB1, an interferon-inducible protein, mediates anti-proliferative actions and apoptosis in renal cell carcinoma cells through cell-cycle regulation. Br. J. Cancer 2010, 102, 873–882. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, P.; Zhang, Z.; An, W.; Zhang, C.; Pan, S.; Tan, Y.; Xu, H. Overexpression of negative regulator of ubiquitin-like proteins 1 (NUB1) inhibits proliferation and invasion of gastric cancer cells through upregulation of p27Kip1 and inhibition of epithelial-mesenchymal transition. Pathol. Res. Pract. 2020, 216, 153002. [Google Scholar] [CrossRef]
- Masuda, T.A.; Inoue, H.; Sonoda, H.; Mine, S.; Yoshikawa, Y.; Nakayama, K.; Nakayama, K.; Mori, M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: Modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002, 62, 3819–3825. [Google Scholar]
- Tanaka, T.; Nakatani, T.; Kamitani, T. Inhibition of NEDD8-conjugation pathway by novel molecules: Potential approaches to anticancer therapy. Mol. Oncol. 2012, 6, 267–275. [Google Scholar] [CrossRef]
- Kamitani, T.; Kito, K.; Nguyen, H.P.; Yeh, E.T. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J. Biol. Chem. 1997, 272, 28557–28562. [Google Scholar] [CrossRef]
- He, S.; Cao, Y.; Xie, P.; Dong, G.; Zhang, L. The Nedd8 non-covalent binding region in the Smurf HECT domain is critical to its ubiquitn ligase function. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lu, B. NUB1 suppression of Huntington toxicity: Mechanistic insights. Res. Rep. Biochem. 2015, 5, 129–136. [Google Scholar]
- Aichem, A.; Groettrup, M. The ubiquitin-like modifier FAT10 in cancer development. Int. J. Biochem. Cell Biol. 2016, 79, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Ren, J.; Cheong, I.S.; Ban, K.H.; Ooi, L.L.; Yong Tan, S.; Kan, A.; Nuchprayoon, I.; Jin, R.; Lee, K.H.; et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene 2003, 22, 2592–2603. [Google Scholar] [CrossRef]
- Fiebiger, B.M.; Pfister, H.; Behrends, U.; Mautner, J. Polyubiquitination of lysine-48 is an essential but indirect signal for MHC class I antigen processing. Eur. J. Immunol. 2015, 45, 716–727. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, K.; Hu, J.; Yan, C.; Li, M.; Yu, X.; Liu, X.; Lei, J.; Guo, W.; Wu, L.; et al. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying β-catenin degradation. Cancer Res. 2014, 74, 5287–5300. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Ge, J.; Yan, C.; Huang, Z.; Hu, J.; Wen, C.; Li, M.; Huang, D.; Qiu, Y.; et al. The Ubiquitin-like Protein FAT10 Stabilizes eEF1A1 Expression to Promote Tumor Proliferation in a Complex Manner. Cancer Res. 2016, 76, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ouyang, Q.; Wei, W.; Yang, S.; Zhang, Y.; Yang, W. FAT10 promotes the invasion and migration of breast cancer cell through stabilization of ZEB2. Biochem. Biophys. Res. Commun. 2018, 506, 563–570. [Google Scholar] [CrossRef]
- Dong, D.; Jiang, W.; Lei, J.; Chen, L.; Liu, X.; Ge, J.; Che, B.; Xi, X.; Shao, J. Ubiquitin-like protein FAT10 promotes bladder cancer progression by stabilizing survivin. Oncotarget 2016, 7, 81463–81473. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xiong, H.; Chen, L.; Liu, X.; Zou, S.; Guan, J.; Wang, K. GRP78 Promotes Hepatocellular Carcinoma proliferation by increasing FAT10 expression through the NF-κB pathway. Exp. Cell Res. 2018, 365, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Yun, Z.; Zhang, X.; Gong, F.; Wang, Y.; Ji, S.; Leng, L. Ubiquitin-like protein FAT10 regulates DNA damage repair via modification of proliferating cell nuclear antigen. Mol. Med. Rep. 2018, 17, 7487–7496. [Google Scholar] [CrossRef]
- Xirodimas, D.P.; Saville, M.K.; Bourdon, J.-C.; Hay, R.T.; Lane, D.P. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 2004, 118, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xirodimas, D. NUB1 promotes cytoplasmic localization of p53 through cooperation of the NEDD8 and ubiquitin pathways. Oncogene 2010, 29, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhu, L.; Meng, Q.W.; Wang, L.; Chen, X.S.; Zhao, Y.B.; Xing, Y.; Wang, X.Y.; Cai, L. FAT10 is associated with the malignancy and drug resistance of non-small-cell lung cancer. Onco Targets Ther. 2016, 9, 4397–4409. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Zhang, Y.; Zhang, P.; Pan, C.; Xu, C.; Wan, W.; Wu, Z.; Zhang, J.; Zhang, L. Upregulation of p-Smad2 contributes to FAT10-induced oncogenic activities in glioma. Tumor Biol. 2016, 37, 8621–8631. [Google Scholar] [CrossRef]
- Chen, H.A.; Su, C.M.; Hsieh, H.Y.; Tung, C.L.; Hsu, C.D.; Wang, Y.H.; Shen, C.H. Clinical significance of survivin expression in patients with urothelial carcinoma. Dis. Markers 2014, 2014, 574985. [Google Scholar] [CrossRef]
- Azuhata, T.; Scott, D.; Takamizawa, S.; Wen, J.; Davidoff, A.; Fukuzawa, M.; Sandler, A. The inhibitor of apoptosis protein survivin is associated with high-risk behavior of neuroblastoma. J. Pediatr. Surg. 2001, 36, 1785–1791. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Y.; Lee, A.S. Beyond the endoplasmic reticulum: Atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 2011, 434, 181–188. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Gao, Y.; Mehta, S.B.; Lee, C.G. FAT10 mediates the effect of TNF-α in inducing chromosomal instability. J. Cell Sci. 2011, 124, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.K.; Yoo, J.Y. NFκB and STAT3 synergistically activate the expression of FAT10, a gene counteracting the tumor suppressor p53. Mol. Oncol. 2014, 8, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-kB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef]
- Guo, K.; Kang, N.X.; Li, Y.; Sun, L.; Gan, L.; Cui, F.J.; Gao, M.D.; Liu, K.Y. Regulation of HSP27 on NF-kB pathway activation may be involved in metastatic hepatocellular carcinoma cells apoptosis. BMC Cancer 2009, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Lukasiak, S.; Schiller, C.; Oehlschlaeger, P.; Schmidtke, G.; Krause, P.; Legler, D.F.; Autschbach, F.; Schirmacher, P.; Breuhahn, K.; Groettrup, M. Proinflammatory cytokines cause FAT10 upregulation in cancers of liver and colon. Oncogene 2008, 27, 6068–6074. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Z.; Liang, J.; Cao, C.; Sun, J.; Ding, Y.; Wu, D. As an independent prognostic factor, FAT10 promotes hepatitis B virus-related hepatocellular carcinoma progression via Akt/GSK3β pathway. Oncogene 2014, 33, 909–920. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Gong, P.; Canaan, A.; Wang, B.; Leventhal, J.; Snyder, A.; Nair, V.; Cohen, C.D.; Kretzler, M.; D’Agati, V.; Weissman, S.; et al. The ubiquitin-like protein FAT10 mediates NF-kB activation. J. Am. Soc. Nephrol. 2010, 21, 316–326. [Google Scholar] [CrossRef]
- Zhang, D.W.; Jeang, K.T.; Lee, C.G. p53 negatively regulates the expression of FAT10, a gene upregulated in various cancers. Oncogene 2006, 25, 2318–2327. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Z.; Cui, Y.; Yuan, H.; Wang, F. Silencing FAT10 inhibits metastasis of osteosarcoma. Int. J. Oncol. 2016, 49, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, Y.; Zhang, D.; Wang, J.; Wang, G.; Lee, C.G.L. Investigating the Promoter of FAT10 Gene in HCC Patients. Genes 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.P. Hepatocellular carcinoma and the ubiquitin-proteasome system. Biochim. Biophys. Acta.Mol. Basis Dis. 2008, 1782, 775–784. [Google Scholar] [CrossRef]
- Yuan, R.; Jiang, C.; Hong, K.; Yu, X.; Wu, L.; Liu, T.; Liu, X.; Tang, X.; Cai, H.; Shao, J. Genetic variation in the Fat10 gene is associated with risk of hepatocellular carcinoma in a Chinese population. Asian Pac. J. Cancer Prev 2011, 12, 2117–2122. [Google Scholar]
- Lim, C.B.; Zhang, D.; Lee, C.G. FAT10, a gene up-regulated in various cancers, is cell-cycle regulated. Cell Div. 2006, 1, 20. [Google Scholar] [CrossRef]
- Jia, Y.; French, B.; Tillman, B.; French, S. Different roles of FAT10, FOXO1, and ADRA2A in hepatocellular carcinoma tumorigenesis in patients with alcoholic steatohepatitis (ASH) vs. non-alcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 2018, 105, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Buzzanco, A.; Gomez, A.; Rodriguez, E.; French, B.A.; Tillman, B.A.; Chang, S.; Ganapathy, E.; Junrungsee, S.; Zarrinpar, A.; Agopian, V.G.; et al. Digital quantitation of HCC-associated stem cell markers and protein quality control factors using tissue arrays of human liver sections. Exp. Mol. Pathol. 2014, 97, 399–410. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Oliva, J.; Li, J.; French, B.; French, S. SAMe prevents the induction of the immunoproteasome and preserves the 26S proteasome in the DDC-induced MDB mouse model. Exp. Mol. Pathol. 2010, 88, 353–362. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, D.; Zhou, D.; Jiao, F.; Yang, W.; Huan, Y. Induction of anti-tumor immunity by dendritic cells transduced with FAT10 recombinant adenovirus in mice. Cell. Immunol. 2015, 293, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tu, Y.; Mao, X.; He, S.; Wang, L.; Fu, G.; Zong, J.; Zhang, Y. Increased expression of FAT10 is correlated with progression and prognosis of human glioma. Pathol. Oncol. Res. 2012, 18, 833–839. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Chang, B.D.; Fang, J.; Roninson, I.B. Retinoid-induced growth arrest of breast carcinoma cells involves co-activation of multiple growth-inhibitory genes. Cancer Biol. Ther. 2002, 1, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, S.; Bercovich, B.; Ciechanover, A. FAT10 is a proteasomal degradation signal that is itself regulated by ubiquitination. Mol. Biol. Cell 2012, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, O.; Felberbaum, R.; Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 2006, 22, 159–180. [Google Scholar] [CrossRef]
- Welchman, R.L.; Gordon, C.; Mayer, R.J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 2005, 6, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, S.; Pyrowolakis, G. Ubiquitin and its kin: How close are the family ties? Trends Cell Biol. 2000, 10, 335–342. [Google Scholar] [CrossRef]
- Liu, Y.C.; Pan, J.; Zhang, C.; Fan, W.; Collinge, M.; Bender, J.R.; Weissman, S.M. A MHC-encoded ubiquitin-like protein (FAT10) binds noncovalently to the spindle assembly checkpoint protein MAD2. Proc. Natl. Acad. Sci. USA 1999, 96, 4313–4318. [Google Scholar] [CrossRef]
- Ren, J.; Kan, A.; Leong, S.H.; Ooi, L.L.; Jeang, K.T.; Chong, S.S.; Kon, O.L.; Lee, C.G. FAT10 plays a role in the regulation of chromosomal stability. J. Biol. Chem. 2006, 281, 11769–11779. [Google Scholar] [CrossRef]
- Broustas, C.G.; Lieberman, H.B. DNA damage response genes and the development of cancer metastasis. Radiat Res. 2014, 181, 111–130. [Google Scholar] [CrossRef]
- Tan, K.; Pezzella, F.; Harris, A.; Acuto, O. PO-479 NUB1 as a prognostic marker in breast cancer: A retrospective, integrated genomic, transcriptomic, and protein analysis. ESMO Open 2018, 3, A417–A418. [Google Scholar] [CrossRef]
- Podust, V.N.; Brownell, J.E.; Gladysheva, T.B.; Luo, R.-S.; Wang, C.; Coggins, M.B.; Pierce, J.W.; Lightcap, E.S.; Chau, V. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc. Natl. Acad. Sci. USA 2000, 97, 4579–4584. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-H.; Liu, Y.-D.; Yu, G.; Li, N.; Sun, X.; Yang, J. Increased FAT10 expression is related to poor prognosis in pancreatic ductal adenocarcinoma. Tumor Biol. 2014, 35, 5167–5171. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Jin, X.; Jiao, C.-H.; Xu, Q.-W.; Wang, Z.-W.; Chen, Y.-L. FAT10 level in human gastric cancer and its relation with mutant p53 level, lymph node metastasis and TNM staging. World J. Gastroenterol. WJG 2009, 15, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Remarks | References |
|---|---|---|
| Hepatocellular carcinoma (HCC) | GRP78 protein increases FAT10 protein expression via direct activation on the NF-κB pathway. | [29] |
| Breast cancer | FAT10 protein induces pro-metastasis effect with the help of ZEB2 overexpression. | [30] |
| Bladder cancer | FAT10 protein non-covalently binds to Survivin protein to inhibit ubiquitin-mediated degradation. | [31] |
| B-cell non-Hodgkin lymphomas | FAT10 protein non-covalently binds to MAD2 protein to maintain mitosis. | [32] |
| Colorectal cancer, HCC, Gastric cancer | FAT10 protein disrupts the DNA damage repair response via modification of PCNA protein. | [33,34,35] |
| NSCLC | FAT10 causes NSCLS malignancy via interaction with NF-κB signalling pathway. | [36] |
| Glioma | FAT10 protein increases phosphorylation of SMAD2 protein, which triggers FAT10 induced oncogenic activities. | [37] |
| Neuroblastoma | FAT10 protein stabilises the survivin protein via non-covalent binding. | [38,39] |
| Type | Human Sample Types | Sample Size | Antibody Clone and Host Species | Method of Detection and Biomarker Type | Findings | References |
|---|---|---|---|---|---|---|
| Anti-NUB1 and -NUB1L | Gastric cancer patients | 116 | Ab38438 (Rabbit polyclonal) | Immunohistochemistry/ Prognostic | Reduced NUB1 level associated to poor prognosis of gastric cancer. p < 0.05; HR: 0.33 (0.20–0.54) | [18] |
| Breast cancer patients | 114 | 4H2 (Mouse Monoclonal Antibody) | Immunohistochemistry/ Prognostic | Low cytoplasmic NUB1 protein level exerts poorer overall survival. p = 0.048, HR: 1.779 (1.006–3.346) | [69] | |
| Anti-FAT10 antibody | Bladder cancer samples | 133 | MBS4750652 (Rabbit Polyclonal Antibody) | Immunohistochemistry/ Prognostic | Higher FAT10 expression in bladder cancer tissues had poorer survival than those with lower FAT10 expression. p = 0.002; HR:? | [31] |
| Non-small cell lung carcinoma (NSCLC) samples | 45 | sc-133199 (mouse monoclonal antibody) | Immunohistochemistry/ Prognostic | High FAT10 expression confers quick chemoresistance than the lower FAT10 expression group. p = 0.001; HR:? | [36] | |
| Breast cancer tissues | 120 | ab168680 (Mouse polyclonal antibody) | Immunohistochemistry/ Prognostic | FAT10 overexpression leads to poor prognostic factor for poorer outcomes of patients with breast cancer. p < 0.05; HR:1.563 (1.232–2.531) | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.; Abdul Hamid, N.; Chan, M.C.; Ismail, F.; Tan, G.C.; Pezzella, F.; Tan, K.-L. NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective. Cells 2021, 10, 2176. https://doi.org/10.3390/cells10092176
Arshad M, Abdul Hamid N, Chan MC, Ismail F, Tan GC, Pezzella F, Tan K-L. NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective. Cells. 2021; 10(9):2176. https://doi.org/10.3390/cells10092176
Chicago/Turabian StyleArshad, Maria, Nazefah Abdul Hamid, Mun Chiang Chan, Fuad Ismail, Geok Chin Tan, Francesco Pezzella, and Ka-Liong Tan. 2021. "NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective" Cells 10, no. 9: 2176. https://doi.org/10.3390/cells10092176
APA StyleArshad, M., Abdul Hamid, N., Chan, M. C., Ismail, F., Tan, G. C., Pezzella, F., & Tan, K.-L. (2021). NUB1 and FAT10 Proteins as Potential Novel Biomarkers in Cancer: A Translational Perspective. Cells, 10(9), 2176. https://doi.org/10.3390/cells10092176








