Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results
Abstract
:1. Introduction
2. Determination of Relative Biological Effectiveness or Compound Biological Effectiveness for Melanoma Control In Vitro and In Vivo
2.1. Effect of BNCT on Cultured Melanoma Cells Grown in Monolayer
2.2. RBE of BNCT for Melanoma Control In Vivo
3. Radiobiological Basis for Human Melanoma Control by Single Irradiation
4. RBE of BNCT for Normal Tissue (Skin) Damage
4.1. RBE of BNCT for Animal Skin Damage
4.2. Tolerable Dose to the Human Skin of a Single Irradiation (Estimated from the Literature)
4.3. RBE of BNCT for Human Skin Damage
5. Pharmacokinetics of BPA in Humans
6. Clinical Results of BNCT for Malignant Cutaneous Melanoma
6.1. Japanese Group
6.2. Argentine Group
6.3. Other Groups
7. Discussion
7.1. RBE Values of BNCT Radiation and CBE of 10B (n, α)7Li Reaction Using BPA
7.2. Optimal Dose Estimation by Single Irradiation for Melanoma Control
7.3. Pharmacokinetics of BPA in Human Patients
7.4. Clinical Results
7.5. BNCT of Cancer as Clinical Practice
8. Conclusions
Funding
Institutional Review Board Statements
Informed Consent Statements
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asbury, A.K.; Ojemann, R.G.; Nielsen, S.L.; Sweet, W.H. Neuropathologic Study of Fourteen Cases of Malignant Brain Tumor Treated by Boron-10 Slow Neutron Capture Radiation. J. Neuropathol. Exp. Neurol. 1972, 31, 278–303. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, H.; Kamano, S.; Amano, K.; Hojo, S.; Sano, K.; Egawa, S.; Yasukochi, H. Clinical experience of boron-neutron capture therapy for gliomas: A comparison with conventional chemo-immuno-radiotherapy. In Boron Neutron Capture Therapy for Tumors; Hatanaka, H., Ed.; Nishimura: Niigata, Japan, 1986; pp. 349–379. ISBN 4-89013-052-7. [Google Scholar]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T. New thermal neutron capture therapy for malignant melanoma: Melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigment. Cell Res. 1989, 2, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single neutron capture treatment with melanoma-seeking 10B-compound. Lancet 1989, 334, 388–389. [Google Scholar] [CrossRef]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef]
- Aihara, T.; Morita, N.; Kamitani, N.; Kumada, H.; Ono, K.; Hiratsuka, J.; Harada, T. Boron neutron capture therapy for advanced salivary gland carcinoma in head and neck. Int. J. Clin. Oncol. 2014, 19, 437–444. [Google Scholar] [CrossRef]
- Mishima, Y. Neutron capture treatment of malignant melanoma using 10B-chlorpromazine compound. In Pigment Cell, Volume 1: Mechanisms in Pigmentation; McGovern, V.J., Russell, P., Eds.; S. Karger: Basel, Switzerland, 1973; pp. 215–221. ISSN 03010139. [Google Scholar]
- Yoshino, K.; Kakihana, H.; Okamoto, M.; Mori, Y. Chemical behavior of dopaborate and 10B-p-boronophenylalanine. In Neutron Capture Therapy; Hatanaka, H., Ed.; Nishimura: Niigata, Japan, 1986; pp. 55–60. ISBN 4-89013-069-1. [Google Scholar]
- Yoshino, K.; Mishima, Y.; Kimura, M.; Hiratsuka, J.; Mori, Y.; Ito, S.; Kakihana, H. Capture of p-boronophenylalanine in malignant melanoma cells by complex formation with melanin monomers, DOPA, DHI and DHICA. BPA trapping mechanism. In Advances in Neutron Capture Therapy; Larsson, B., Crawford, J., Weinreich, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume II, pp. 234–238. ISBN 0-444-82781-1. [Google Scholar]
- Nakanishi, T.; Ichihashi, M.; Mishima, Y.; Matsuzawa, T.; Fukuda, H. Thermal neuron capture therapy of malignant melanoma: In vitro radiobiological analysis. Int. J. Radiat. Biol. 1980, 37, 573–580. [Google Scholar] [CrossRef]
- Ichihashi, M.; Nakanishi, T.; Mishima, Y. Specific killing effect of 10B-paraboronophenylalanine in thermal neutron capture therapy of malignant melanoma: In vitro radiobiological evaluation. J. Investig. Dermatol. 1982, 78, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Kanda, K. Analytical calculation of boron-10 dosage in cell nucleus for neutron capture therapy. Radiat. Res. 1982, 91, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kanda, K. Microanalysis of ppm-order 10B concentration in tissue for neutron capture therapy by prompt gamma-ray spectrometry. Nucl. Inst. Method. 1982, 204, 525–531. [Google Scholar] [CrossRef]
- Fukuda, H.; Kobayashi, T.; Matsuzawa, T.; Kanda, K.; Ichihashi, M.; Mishima, Y. RBE of a thermal neutron beam and the 10B(n, α) 7Li reaction on cultured B-16 melanoma cells. Int. J. Radiat. Biol. 1987, 51, 167–175. [Google Scholar] [CrossRef]
- Taniyama, K.; Fugiwara, H.; Kuno, T.; Saito, N.; Shunto, H.; Sakaue, M.; Tanaka, C. Acute and subacute toxicity of 10B-paraboronophenylalanine. Pigment. Cell Res. 1989, 2, 291–296. [Google Scholar] [CrossRef]
- Yoshino, K.; Suzuki, K.; Mori, Y.; Kakihana, H. Improvement of solubility of p-boronophenylalanine by complex formation with monosaccarides. Strahlenther Oncol. 1989, 165, 127–129. [Google Scholar]
- Fukuda, H.; Kobayashi, T.; Hiratsuka, J.; Karashima, H.; Honda, C.; Yamamura, K.; Ichihashi, M.; Kanda, K.; Mishima, Y. Estimation of absorbed dose in the covering skin of human melanoma treated by neutron capture therapy. Pigment. Cell Res. 1989, 2, 365–369. [Google Scholar] [CrossRef]
- Fukuda, H.; Hiratsuka, J.; Honda, C.; Kobayashi, T.; Yoshino, K.; Karashima, H.; Takahashi, J.; Abe, Y.; Kanda, K.; Ichihashi, M. Boron neutron capture therapy of malignant melanoma using 10B-paraboronophenylalanine with special reference to evaluation of radiation dose and damage to the normal skin. Radiat. Res. 1994, 138, 435–442. [Google Scholar] [CrossRef]
- Mishima, Y. Selective thermal neutron capture therapy of cancer cells using their specific metabolic activities-Melanoma as prototype. In Cancer Neutron. Capture Therapy; Mishima, Y., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 1–26. ISBN 0-306-45307-x. [Google Scholar]
- Hiratsuka, J.; Fukuda, H.; Kobayashi, T.; Kanda, K.; Honda, C.; Ichihashi, M.; Mishima, Y. Human melanoma treated by boron neutron capture therapy: Comparison of the clinical response with the predicted response. Radiat. Med. 1996, 14, 257–263. [Google Scholar]
- Fukuda, H.; Mishima, Y.; Hiratsuka, J.; Honda, C.; Wadabayashi, N.; Kobayashi, T.; Yoshino, K.; Karashima, H.; Takahashi, J.; Abe, Y.; et al. BNCT of malignant melanoma—Radiobiological analysis and data comparison with conventional radiotherapy. In Cancer Neutron. Capture Therapy; Mishima, Y., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 663–671. ISBN 0-306-45307-x. [Google Scholar]
- Fukuda, H.; Ando, K.; Honda, C.; Ichihashi, M.; Mishima, Y.; Hiratsuka, J.; Kobayashi, T.; Kanda, K. Tolerance limits of the normal skin treated by single thermal neutron capture therapy. In Progress in Neutron Capture Therapy; Allen, B.J., Moore, D.E., Harrington, B.V., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 511–514. ISBN 0-306-44104-7. [Google Scholar]
- Fukuda, H.; Hiratsuka, J.; Kobayashi, T.; Sakurai, Y.; Yoshino, K.; Karashima, H.; Turu, K.; Araki, K.; Mishima, Y.; Ichihashi, M. Boron neutron capture therapy (BNCT) for malignant melanoma with special reference to absorbed doses to the normal skin and tumor. Australas. Phys. Eng. Sci. Med. 2003, 26, 97–103. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Kamitani, N.; Tanaka, R.; Tokiya, R.; Yoden, E.; Sakurai, Y.; Suzuki, M. Long-term outcome of cutaneous melanoma patients treated with boron neutron capture therapy (BNCT). J. Radiat. Res. 2020, 61, 945–951. [Google Scholar] [CrossRef]
- Kitao, K. A method for calculating the absorbed dose near-interface from 10B(n, α)7Li reaction. Radiat. Res. 1975, 61, 204–221. [Google Scholar] [CrossRef]
- Rossini, A.E.; Dagrosa, M.A.; Purtu, A.; Martin, G.S.; Thorp, S.; Casa, M.; Juvenal, A.J.; Pisarev, M.A. Assessment of biological effectiveness of boron neutron capture therapy in primary and metastatic melanoma cell lines. Int. J. Radiat. Biol. 2015, 91, 81–89. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Kono, M.; Mishima, Y. RBEs of thermal neutron capture therapy and 10B(n, α)7Li reaction on melanoma-bearing hamsters. Pigment. Cell Res. 1989, 2, 352–355. [Google Scholar] [CrossRef]
- Hall, E.J. 4. Model tumor system. In Radiobiology for the Radiobiologist, 3rd ed.; Hall, E.J., Ed.; Lippincott: Philadelphia, PA, USA, 1988; pp. 70–89. ISBN 0-397-50848-4. [Google Scholar]
- Coderre, J.A.; Kalef-Ezara, J.A.; Fairchild, R.G.; Micca, P.L.; Reinstein, L.E.; Glass, J.D. Boron neutron capture therapy of murine melanoma. Cancer Res. 1988, 48, 6313–6316. [Google Scholar]
- Coderre, J.A.; Slatkin, D.N.; Micca, O.L.; Ciallella, J. Boron neutron capture therapy of a murine melanoma with p-boronophenylalanine: Dose-response analysis using a morbidity index. Radiat. Res. 1991, 128, 177–185. [Google Scholar] [CrossRef]
- Coderre, J.A.; Maker, M.S.; Micca, P.L.; Nawrocky, M.M.; Liu, H.B.; Joel, D.D.; Slatkin, D.N.; Amols, I.A. Derivation of relative biological effectiveness for the high-LET radiations produced during boron neutron capture irradiation of the 9L rat gliosarcoma in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1121–1129. [Google Scholar] [CrossRef]
- Suzuki, M.; Masunaga, S.; Kinashi, Y.; Takagaki, M.; Sakuarai, Y.; Kobayashi, T.; Ono, K. The effects of boron neutron capture therapy on liver tumors and normal hepatocytes in mice. Jpn. J. Cancer Res. 2000, 91, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Overgaad, J. The role of radiotherapy in recurrent and metastatic malignant melanoma: A clinical radiobiological study. Radiother. Oncol. 1986, 12, 867–872. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Fukuda, H.; Kobayashi, T.; Karashima, H.; Yoshino, K.; Imajo, Y.; Mishima, Y. The relative biological effectiveness of 10B-neutron capture therapy for early skin reaction in the hamster. Radiat. Res. 1991, 128, 186–191. [Google Scholar] [CrossRef]
- Morris, G.M.; Coderre, J.A.; Hopewell, J.W.; Micca, P.L.; Rezvani, M. Response of rat skin to boron neutron capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother. Oncol. 1994, 32, 144–153. [Google Scholar] [CrossRef]
- Douglas, B.G. Implication of the quadratic cell survival curve and human skin radiation “tolerance dose” on fractionation and super-fractionation dose selection. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1135–1142. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Walter, H.M.D. Effects of different ionizing radiations on human cells in tissue culture III. Experiments with cyclotron-accelerated alpha-particles and deuterons. Radiat. Res. 1963, 18, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Overgaard, J.; Bassler, N. In vitro RBE-LET dependence for multiple particle types. Acta Oncol. 2011, 50, 757–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, H.; Honda, C.; Wadabayashi, N.; Kobayashi, T.; Yoshino, K.; Hiratsuka, J.; Takahashi, J.; Akaizawa, T.; Abe, Y.; Ishihashi, M.; et al. Pharmacokinetics of 10B-p-boronophenylalanine in tumours, skin and blood of melanoma patients: A study of boron neutron capture therapy for malignant melanoma. Melanoma Res. 1999, 9, 75–83. [Google Scholar] [CrossRef]
- Fukuda, H.; Hiratsuka, J. Pharmacokinetics of 10B-p-boronophenylalanine (BPA) in the blood and tumors in human patients: A critical review with special reference to tumor-to-blood (T/B) ratios using resected tumor samples. Appl. Radiat. Isot. 2020, 166, 109308. [Google Scholar] [CrossRef]
- Elowitz, E.H.; Richard, M.B.; Coderre, C.A.; Darrel, D.J.; Manjeet, C.; Arjun, D.C. Biodistribution of p-boronophenylalanine in patients with glioblastoma multiforme for use in in boron neutron capture therapy. Neurosurgery 1998, 42, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wittig, A.; Collette, L.; Appleman, K.; Buhrmann, S.; Jackel, M.C.; Jockel, K.; Schmid, K.S.; Ortman, U.; Moss, R.; Sauwerwein, W.A. Eortic 11001: Distribution of two 10B-compounds in patients with squamous cell carcinoma of head and neck, a translational research/phase 1 trial. J. Cell Mol. Med. 2009, 13, 1653–1665. [Google Scholar] [CrossRef]
- Lieberman, S.J.; Dagrosa, A.; Rebagliati, J.; Bonomi, M.R.; Roth, B.M.; Turjanski, L.; Castiglia, S.I.; Gonzalez, S.J.; Menendez, P.R.; Carrini, R.; et al. Biodistribution studies of boronophenylalanine-fructose in melanoma and brain tumor patients in Argentine. Appl. Radiat. Isot. 2004, 61, 1095–1100. [Google Scholar] [CrossRef]
- Capala, J.; Stenstam, B.H.; af Rosenschold, R.M.; Giusti, V.; Persson, C.; Wallin, E.; Brun, A.; Franzen, L.; Carlsson, J.; Salford, L. Boron neutron capture therapy for glioblastoma multiforme: Clinical studies in Sweden. J. Neuro Oncol. 2003, 62, 135–144. [Google Scholar] [CrossRef]
- Zhang, Z.; Yong, Z.; Jin, C.; Song, Z.; Zhu, S.; Liu, T.; Chen, Y.; Chong, Y.; Chen, X.; Zhou, Y. Biodistribution studies of boronophenylalanine in different types of skin melanoma. Appl. Radiat. Isot. 2020, 163, 109215. [Google Scholar] [CrossRef]
- Ono, K.; Masunaga, S.-I.; Kinashi, Y. Neutron irradiation under continuous BPA. In Advances in Neuron Capture Therapy 2006; Nakagawa, Y., Kobayashi, T., Fukuda, H., Eds.; International Society for Neutron Capture: Kagawa, Japan, 2006; pp. 27–30. ISBN 4-9903242-0-X. [Google Scholar]
- Morita, N.; Hiratsuka, J.; Kuwabara, C.; Aihara, T.; Ono, K.; Fukuda, H.; Kumada, H.; Harada, T.; Imajo, Y. Successful BNCT for patients with cutaneous and mucosal melanomas: Report of 4 cases. In Advances in Neuron Capture Therapy 2006; Nakagawa, Y., Kobayashi, T., Fukuda, H., Eds.; International Society for Neutron Capture: Kagawa, Japan, 2006; pp. 18–20. ISBN 4-9903242-0-X. [Google Scholar]
- Menéndez, P.R.; Roth, B.M.C.; Pereira, M.D.; Casal, M.R.; González, S.J.; Feld, D.B.; Santa Cruz, G.A.; Kessler, J.; Longhino, J.; Blaumann, H.; et al. BNCT for skin melanoma in extremities: Updated Argentine clinical results. Appl. Radiat. Isot. 2009, 67, s50–s53. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.M.; Zamenhof, R.; Madoc-Jones, H.; Solares, G.; Kiger, S.; Reley, K.; Chung, C.; Roger, G.; Harling, O. Clinical follow-up of patients with melanoma of the extremity treated in a phase I boron neutron capture therapy protocol. In Advances in Neutron Capture Therapy; Larsson, B., Crawford, J., Weinreich, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 1, pp. 60–64. ISBN 0-444-82781-1. [Google Scholar]
- Yong, Z.; Song, Z.; Zhou, Y.; Liu, T.; Zhang, Z.; Zhao, Y.; Chen, Y.; Jin, C.; Chen, X.; Lu, J.; et al. Boron neutron capture therapy for malignant melanoma: First clinical case report in China. Chin. J. Cancer Res. 2016, 28, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Smith, D.R.; Patel, H.; Chandra, S.; Morrison, G.H.; Hopewell, J.W.; Rezvani, M.; Micca, P.L.; Coderre, J.A. Boron microlocalization in oral mucosal tissue: Implications for boron neutron capture therapy. Br. J. Cancer 2000, 82, 1764–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coderre, J.A.; Morris, G.M. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef] [PubMed]

| Tumor Line | BNCTbeam | 14N (n, p)14C | 10B (n, α)7Li | Endpoint | Reference |
|---|---|---|---|---|---|
| B-16 (vitro) | 2.55 | - | 3.3 | D0 ratio | Fukuda, 1987 [15] |
| M8 (vitro) | 1.3 | 1.38 | 2.1 | Ratio at SF 0.001 | Rossini, 2014 [27] |
| Me-J (vitro) | 1.5 | 1.75 | 3.0 | Ratio at SF 0.001 | Rossini, 2014 [27] |
| Green’s melanoma (vivo) | 2.22 | 3.0 | 2.5 | Growth delay time | Hiratsuka, 1989 [28] |
| Harding–Passey (vivo) | 2.0 | - | - | Growth delay time | Coderre, 1988 [30] |
| B-16 (vivo) | 2.0 | (2.0) 1 | (2.3) 1 | Morbidity index | Coderre, 1991 [31] |
| 9L-gliosarcoma (vivo) | 2.3 | 3.2 | 3.8 | Ratio at SF 0.001 | Coderre, 1993 [32] |
| SCC VII (vivo) | 2.79 | - | 4.22 | D0 ratio | Suzuki, 2000 [33] |
| System | BNCTbeam | 14N (n, p)14C | 10B (n, α)7Li | End Point | Reference |
|---|---|---|---|---|---|
| Hamster | 2.16 ± 0.06 | 2.9 ± 0.04 | 2.4 ± 0.06 | Moist desquamation | Hiratsuka, 1991 [35] |
| Fisher rat | - | 3.50 ± 0.23 | 3.74 ± 0.70 | Moist desquamation | Morris, 1994 [36] |
| Human | - | 2.5 | 2.5 | Moist desquamation | Fukuda, 1994 [19] |
| Group | BPA Dose (mg/kg) | Infusion Time (h) | Number of Patients | Maximum Value (μg/g) | T1/2 (1st) (h) | T1/2 (2nd) (h) | Reference |
|---|---|---|---|---|---|---|---|
| Japan | 500 iv | 2–3 | 9 | 36.9 ± 6.9 | 0.8 | 6.7 | Fukuda, 2020 [41] |
| 180 ± 14.9 iv | 3–5 | 8 | 9.4 ± 2.6 | 2.8 | 9.2 | Fukuda, 1999 [40] | |
| 174 ± 7.0 iv +30 sc | 3–5 | 7 | 7.4 ± 2.1 | 3.3 | 9.0 | ||
| 85 iv | 2–3 | 7 | 6.8 ± 1.2 | 3.7 | 10.0 | ||
| USA | 250 iv | 2 | 3 | 22.1 ± 3.4 | 1.2 | 8.2 | Elowitz, 1998 [42] |
| 210 iv | 2 | 3 | 17.3 ± 3.7 | n.a | n.a | ||
| 170 iv | 2 | 4 | 14.2 ± 2.2 | n.a | n.a | ||
| 130 iv | 2 | 5 | 13.1 ± 1.9 | n.a | n.a | ||
| Germany | 100 iv | 1 | 3 | 9.5 ± 0.8 | 0.7 | 7.3 | Wittig, 2009 [43] |
| Argentina | 300 iv | 1.5 | 3 | 22.1–25.3 | 0.3 | 11.0 | Lieberman, 2004 [44] |
| 100 iv | 1–1.5 | 3 | 5.5–9.8 | n.a | n.a | ||
| Sweden | 900 iv | 6 | 18 | 24–50 | 1.4 | 12 | Capala, 2003 [45] |
| Group | BPA dose (mg/kg) | Infusion Time (h) | Skin/Blood (Number of pts) | Tumor/Blood (Number of pts) | Hours after the End of Infusion | Reference |
|---|---|---|---|---|---|---|
| Japan | 85, 170 iv | 3–5 | 1.31 ± 0.22 (15) | 3.40 ± 0.83 (11) | 1.0–5.5 | Fukuda, 1999 [40] |
| Argentina | 100, 300 | 1.5 | - | 2.1 ± 0.4 (3) | 0.75–4.5 | Lieberman, 2004 [44] |
| China | 350 | 1.5 | 1.33 ± 0.36 (2) | 2.54 ± 1.61 (2) | 0.5, 1.5 | Zhang, 2020 [46] |
| 90 | 1.5 | 1.05 (1) | 1.48 (1) | 0.75 |
| Reference (Period of Clinical Study) | Stage | BPA dose (mg/kg) | Number of Patients | Tumor Response | Long-Term Survival |
|---|---|---|---|---|---|
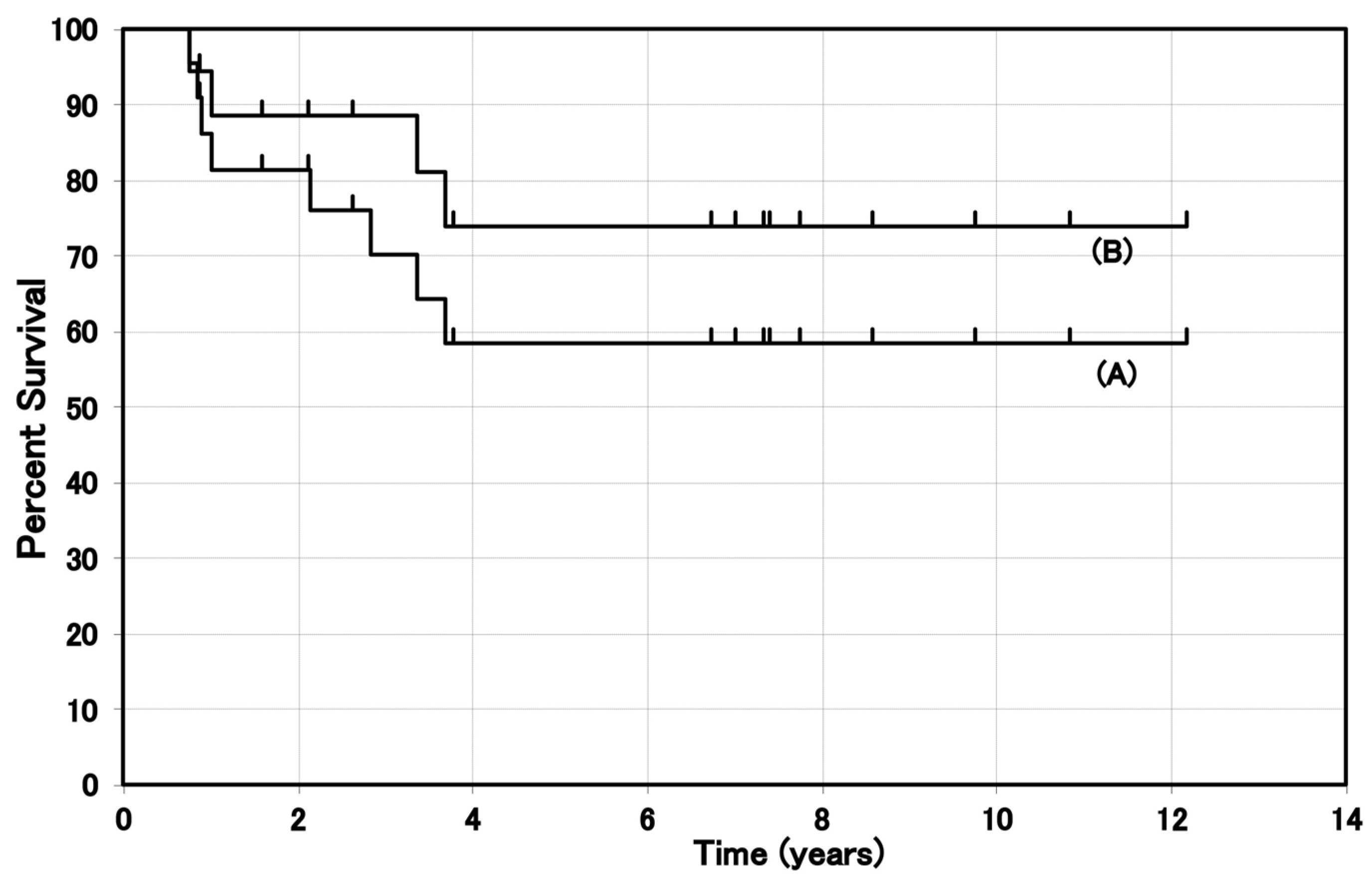
| Fukuda, 2003 [24] (1987–2002) | II–IV | 170–210 iv | 22 | CR 68.2%, PR 23.0% | 5-year survival 58% # |
| Busse, 2006 [46] (1994–1996) | Recurrent melanoma | 400 (oral) | 3 | CR 25%, PR 75% | Not available |
| Menendez, 2009 [49] (2003–2007) | IV Multiple metastases | 300 iv | 7 | Lesion-based CR + PR 69.3% | 36 months, 3 dead |
| Hiratsuka, 2020 [25] (2003–2014) | T1-2N0M0 | 500 iv | 8 | CR 75%, PR 25% | Survived 5.5–12.6 years, 3 dead |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, H. Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results. Cells 2021, 10, 2881. https://doi.org/10.3390/cells10112881
Fukuda H. Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results. Cells. 2021; 10(11):2881. https://doi.org/10.3390/cells10112881
Chicago/Turabian StyleFukuda, Hiroshi. 2021. "Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results" Cells 10, no. 11: 2881. https://doi.org/10.3390/cells10112881
APA StyleFukuda, H. (2021). Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results. Cells, 10(11), 2881. https://doi.org/10.3390/cells10112881





