Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Animal Model
2.3. Tissue Preparation
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Immunofluorescence (IF) Staining
2.6. Exploratory Behavior Tests
2.7. Chemical Composition of Maca
2.8. Statistical Analysis
3. Results and Discussion
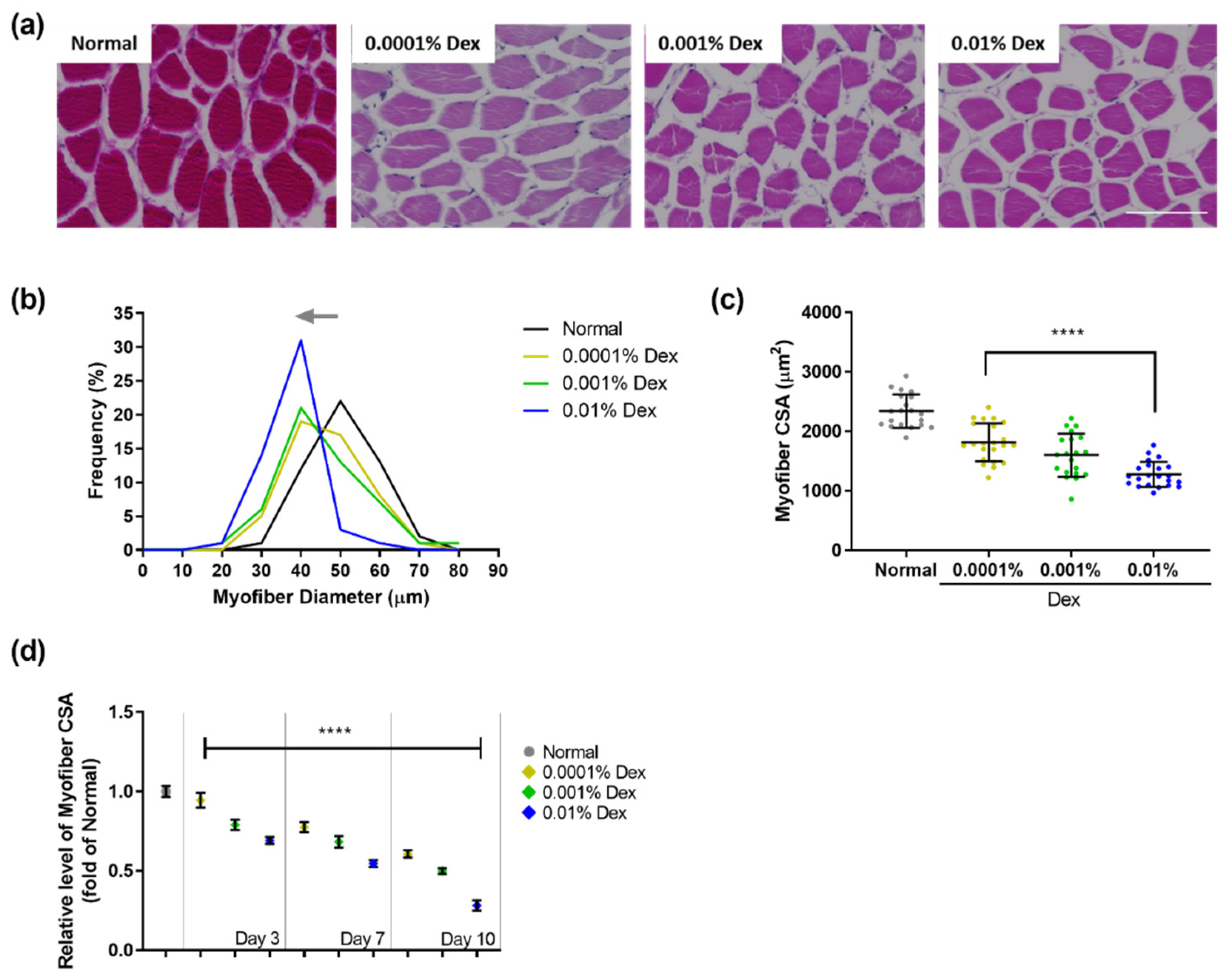
3.1. Muscle Atrophy in Zebrafish Treated with Dexamethasone
3.2. Characterization of the Polysaccharides Derived from Maca
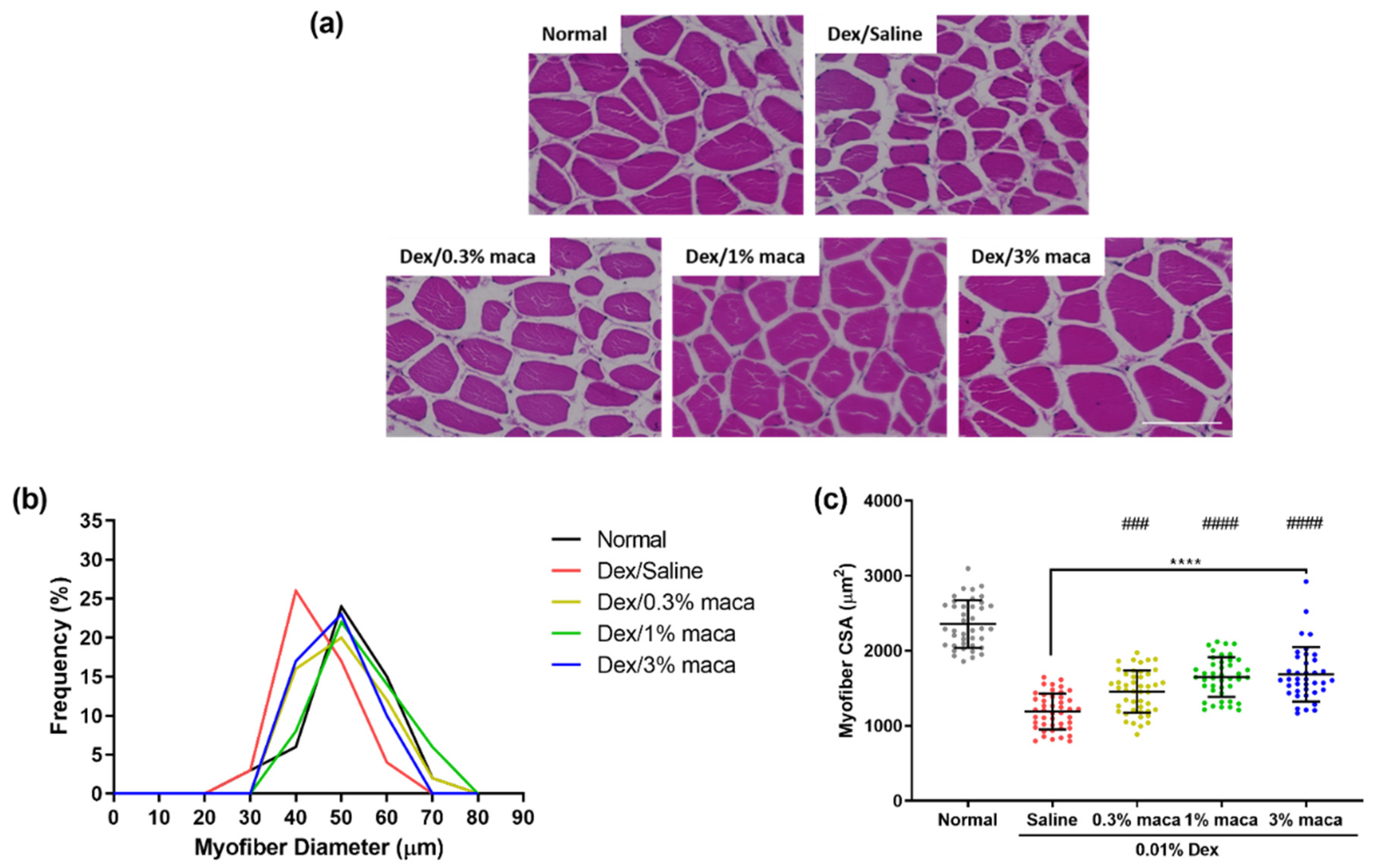
3.3. Effect of Maca in Myofibers of Zebrafish with Muscle Atrophy
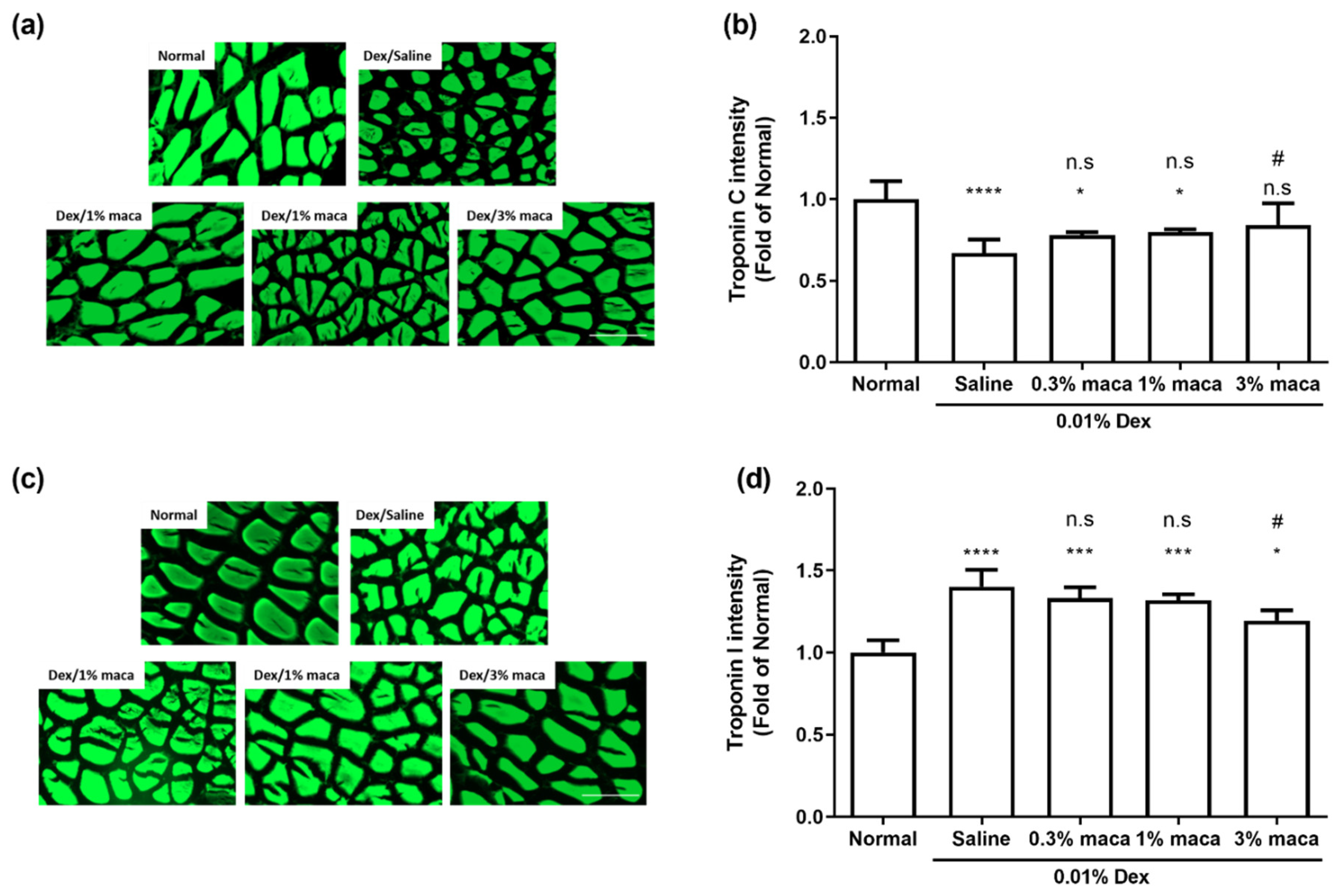
3.4. Effect of Maca on Muscle Contractile Proteins in Zebrafish with Muscle Atrophy
3.5. Effect of Maca on Exploratory Behavior in Zebrafish with Muscle Atrophy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [Green Version]
- Andres-Mateos, E.; Brinkmeier, H.; Burks, T.N.; Mejias, R.; Files, D.C.; Steinberger, M.; Soleimani, A.; Marx, R.; Simmers, J.L.; Lin, B. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 2013, 5, 80–91. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, K.F.; McGee, S.L. Epigenetic regulation of skeletal muscle metabolism. Clin. Sci. 2016, 130, 1051–1063. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Kandarian, S.C.; Jackman, R.W. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 155–165. [Google Scholar] [CrossRef]
- Nowak, M.; Suenkel, B.; Porras, P.; Migotti, R.; Schmidt, F.; Kny, M.; Zhu, X.; Wanker, E.E.; Dittmar, G.; Fielitz, J. DCAF8, a novel MuRF1 interaction partner, promotes muscle atrophy. J. Cell Sci. 2019, 132, jcs233395. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Kubota, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch. Biochem. Biophys. 2019, 664, 157–166. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin attenuates dexamethasone-induced muscle atrophy by improving mitochondrial function via the PGC-1α pathway. Cell. Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, R.; Paredes, F.; Parra, V.; Gatica, D.; Vásquez-Trincado, C.; Quiroga, C.; Bravo-Sagua, R.; López-Crisosto, C.; Rodriguez, A.E.; Oyarzún, A.P. Dexamethasone-induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle 2014, 13, 2281–2295. [Google Scholar] [CrossRef] [Green Version]
- Cea, L.A.; Balboa, E.; Puebla, C.; Vargas, A.A.; Cisterna, B.A.; Escamilla, R.; Regueira, T.; Sáez, J.C. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1891–1899. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.-M.; Thissen, J.-P. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, L.M.; West, R.C.; Hayes, C.S.; Waddell, D.S. Dual-specificity phosphatase 29 is induced during neurogenic skeletal muscle atrophy and attenuates glucocorticoid receptor activity in muscle cell culture. Am. J. Physiol.-Cell Physiol. 2020, 319, C441–C454. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.A.; Mayer, J.L.; De Kloet, E.R.; Joëls, M.; Lucassen, P.J. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur. J. Neurosci. 2007, 26, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Cattaneo, A.; Luoni, A.; Musaelyan, K.; Zunszain, P.A.; Milanesi, E.; Rybka, J.; Berry, A.; Cirulli, F.; Thuret, S. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 2013, 38, 872–883. [Google Scholar] [CrossRef]
- Meyer, M.; Deniselle, M.C.G.; Hunt, H.; de Kloet, E.R.; de Nicola, A.F. The selective glucocorticoid receptor modulator CORT108297 restores faulty hippocampal parameters in Wobbler and corticosterone-treated mice. J. Steroid Biochem. Mol. Biol. 2014, 143, 40–48. [Google Scholar] [CrossRef]
- Melby, J.C. Clinical pharmacology of systemic corticosteroids. Annu. Rev. Pharmacol. Toxicol. 1977, 17, 511–527. [Google Scholar] [CrossRef]
- Dietrich, J.; Rao, K.; Pastorino, S.; Kesari, S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert Rev. Clin. Pharmacol. 2011, 4, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Dixit, K.S.; Kumthekar, P.U. Optimal management of corticosteroids in patients with intracranial malignancies. Curr. Treat. Options Oncol. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ku, S.-K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-K.; Choi, J.-W.; Choi, Y.H.; Nam, T.-J. Pyropia yezoensis Protein Supplementation Prevents Dexamethasone-Induced Muscle Atrophy in C57BL/6 Mice. Mar. Drugs 2018, 16, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebacher, F.; Little, A.G.; James, R.S. Skeletal muscle contractile function predicts activity and behaviour in zebrafish. J. Exp. Biol. 2015, 218, 3878–3884. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, M.; Chatzopoulou, A.; Spaink, H. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 75–82. [Google Scholar] [CrossRef]
- Sireeni, J.; Bakker, N.; Jaikumar, G.; Obdam, D.; Slabbekoorn, H.; Tudorache, C.; Schaaf, M. Profound effects of glucocorticoid resistance on anxiety-related behavior in zebrafish adults but not in larvae. Gen. Comp. Endocrinol. 2020, 292, 113461. [Google Scholar] [CrossRef]
- Nguyen, M.; Yang, E.; Neelkantan, N.; Mikhaylova, A.; Arnold, R.; Poudel, M.K.; Stewart, A.M.; Kalueff, A.V. Developing ‘integrative’zebrafish models of behavioral and metabolic disorders. Behav. Brain Res. 2013, 256, 172–187. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; McNeil, B.; Harvey, L.M. Maca: An Andean crop with multi-pharmacological functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, J.I.; Cho, J.Y.; Lee, S.H.; Kim, T.S.; Yeo, I.H.; Chun, H.S. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J. Funct. Foods 2012, 4, 568–573. [Google Scholar] [CrossRef]
- Morikane, D.; Zang, L.; Nishimura, N. Evaluation of the Percutaneous Absorption of Drug Molecules in Zebrafish. Molecules 2020, 25, 3974. [Google Scholar] [CrossRef]
- Zhang, F.; Qin, W.; Zhang, J.-P.; Hu, C.-Q. Antibiotic toxicity and absorption in zebrafish using liquid chromatography-tandem mass spectrometry. PLoS ONE 2015, 10, e0124805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Le, E.; Schwartzkopf, M.; Arner, A. Relation between skeletal muscle strength and ubiquitin ligase expression in muscle atrophy models in zebrafish larvae (Danio Rerio). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Villano, C.M.; Cooper, K.R.; White, L.A. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 2007, 100, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Ryan, B.; Medriano, C.D.; Cho, Y.; Kim, H.; Chung, I.-Y.; Seok, K.-S.; Song, K.G.; Hong, S.W.; Park, Y.; Kim, S. Sub-lethal pharmaceutical hazard tracking in adult zebrafish using untargeted LC–MS environmental metabolomics. J. Hazard. Mater. 2017, 339, 63–72. [Google Scholar] [CrossRef]
- Khor, Y.M.; Soga, T.; Parhar, I.S. Caffeine neuroprotects against dexamethasone-induced anxiety-like behaviour in the Zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2013, 181, 310–315. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Adams, G.R.; Caiozzo, V.J.; Baldwin, K.M. Skeletal muscle unweighting: Spaceflight and ground-based models. J. Appl. Physiol. 2003, 95, 2185–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Abreu, M.S.; Friend, A.J.; Demin, K.A.; Amstislavskaya, T.G.; Bao, W.; Kalueff, A.V. Zebrafish models: Do we have valid paradigms for depression? J. Pharmacol. Toxicol. Methods 2018, 94, 16–22. [Google Scholar] [CrossRef]
- Xin, N.; Jiang, Y.; Liu, S.; Zhou, Y.; Cheng, Y. Effects of prednisolone on behavior and hypothalamic–pituitary–interrenal axis activity in zebrafish. Environ. Toxicol. Pharmacol. 2020, 75, 103325. [Google Scholar] [CrossRef]
- Schoonheim, P.J.; Chatzopoulou, A.; Schaaf, M.J. The zebrafish as an in vivo model system for glucocorticoid resistance. Steroids 2010, 75, 918–925. [Google Scholar] [CrossRef]
- Barry, S.C.; Gallagher, C.G. Corticosteroids and skeletal muscle function in cystic fibrosis. J. Appl. Physiol. 2003, 95, 1379–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decramer, M.; Lacquet, L.M.; Fagard, R.; Rogiers, P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am. J. Respir. Crit. Care Med. 1994, 150, 11–16. [Google Scholar] [CrossRef]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xue, C.; Chang, Y.; Liu, G. Chain conformation, rheological and charge properties of fucoidan extracted from sea cucumber Thelenota ananas: A semi-flexible coil negative polyelectrolyte. Food Chem. 2017, 237, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Caicai, K.; Limin, H.; Liming, Z.; Zhiqiang, Z.; Yongwu, Y. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.L.M.; Delgadillo, I.; Čopíková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.; Shao, X.; Jin, D.; Zheng, D.; Zhao, T.; Zhu, H.; Zhang, L. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process. Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Meng, Q.; Wang, L.; Xiong, W.; Zhang, L. Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp.(maca). Int. J. Biol. Macromol. 2017, 95, 1305–1311. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Farah, C.S.; Reinach, F.C. The troponin complex and regulation of muscle contraction. FASEB J. 1995, 9, 755–767. [Google Scholar] [CrossRef]
- El-Saleh, S.C.; Warber, K.D.; Potter, J.D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J. Muscle Res. Cell Motil. 1986, 7, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Schmidt, W.; Fritz, K.S.; Jeong, M.Y.; Cammarato, A.; Foster, D.B.; Biesiadecki, B.J.; McKinsey, T.A.; Woulfe, K.C. Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J. Mol. Cell. Cardiol. 2020, 139, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Rao, V.; Cheng, Y.; Lindert, S.; Wang, D.; Oxenford, L.; McCulloch, A.D.; McCammon, J.A.; Regnier, M. PKA phosphorylation of cardiac troponin I modulates activation and relaxation kinetics of ventricular myofibrils. Biophys. J. 2014, 107, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mañas-García, L.; Denhard, C.; Mateu, J.; Duran, X.; Gea, J.; Barreiro, E. Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis. Cells 2021, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Labugger, R.; Collier, C.; Brison, R.J.; Iscoe, S.; Van Eyk, J.E. Fast and slow skeletal troponin I in serum from patients with various skeletal muscle disorders: A pilot study. Clin. Chem. 2005, 51, 966–972. [Google Scholar] [CrossRef] [Green Version]
- Knapton, A.D.; Espandiari, P.; Morales, A.; Moussazadeh, M.; Robins, T.; Herman, E. The Utility of Monitoring Skeletal Muscle Troponin I to Detect Drug-Induced Skeletal Muscle Injury; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Chang, H.; Lei, T.; Ma, X.; Zhang, J.; Wang, H.; Zhang, X.; Gao, Y.-F. Muscle-specific activation of calpain system in hindlimb unloading rats and hibernating Daurian ground squirrels: A comparison between artificial and natural disuse. J. Comp. Physiol. B 2018, 188, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Sztal, T.E.; Ruparelia, A.A.; Williams, C.; Bryson-Richardson, R.J. Using touch-evoked response and locomotion assays to assess muscle performance and function in zebrafish. J. Vis. Exp. 2016, 116, e54431. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Altringham, J.D.; Ellerby, D.J. Fish swimming: Patterns in muscle function. J. Exp. Biol. 1999, 202, 3397–3403. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, B.; Je, J.-G.; Jeon, Y.-J.; Yang, H.-W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells 2021, 10, 2879. https://doi.org/10.3390/cells10112879
Ryu B, Je J-G, Jeon Y-J, Yang H-W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells. 2021; 10(11):2879. https://doi.org/10.3390/cells10112879
Chicago/Turabian StyleRyu, Bomi, Jun-Geon Je, You-Jin Jeon, and Hye-Won Yang. 2021. "Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii)" Cells 10, no. 11: 2879. https://doi.org/10.3390/cells10112879
APA StyleRyu, B., Je, J.-G., Jeon, Y.-J., & Yang, H.-W. (2021). Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells, 10(11), 2879. https://doi.org/10.3390/cells10112879






