A New Induction Method for the Controlled Differentiation of Human-Induced Pluripotent Stem Cells Using Frozen Sections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acute Liver Injury
2.2. Preparation of Frozen Sections
2.3. Cell Culture and Differentiation of Human iPSCs
2.4. RT-PCR
2.5. Immunocytochemistry
2.6. Differentiation-Inducing Effects
3. Results
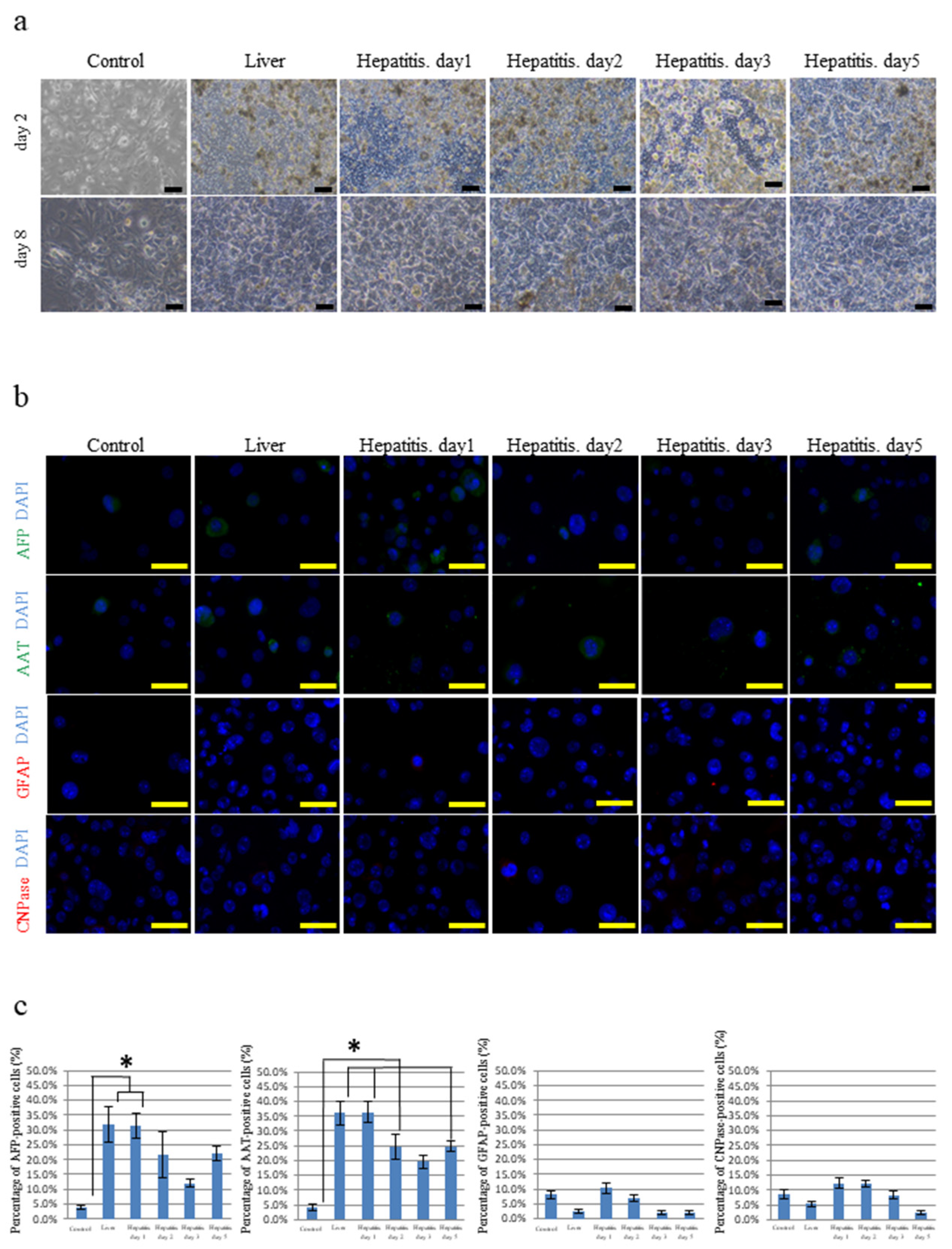
3.1. Differentiation of iPSCs Cultured on Frozen Sections of the Liver
3.2. Differentiation of hOF-iPCS Cultured on Frozen Sections of the Brain and the Spinal Cord
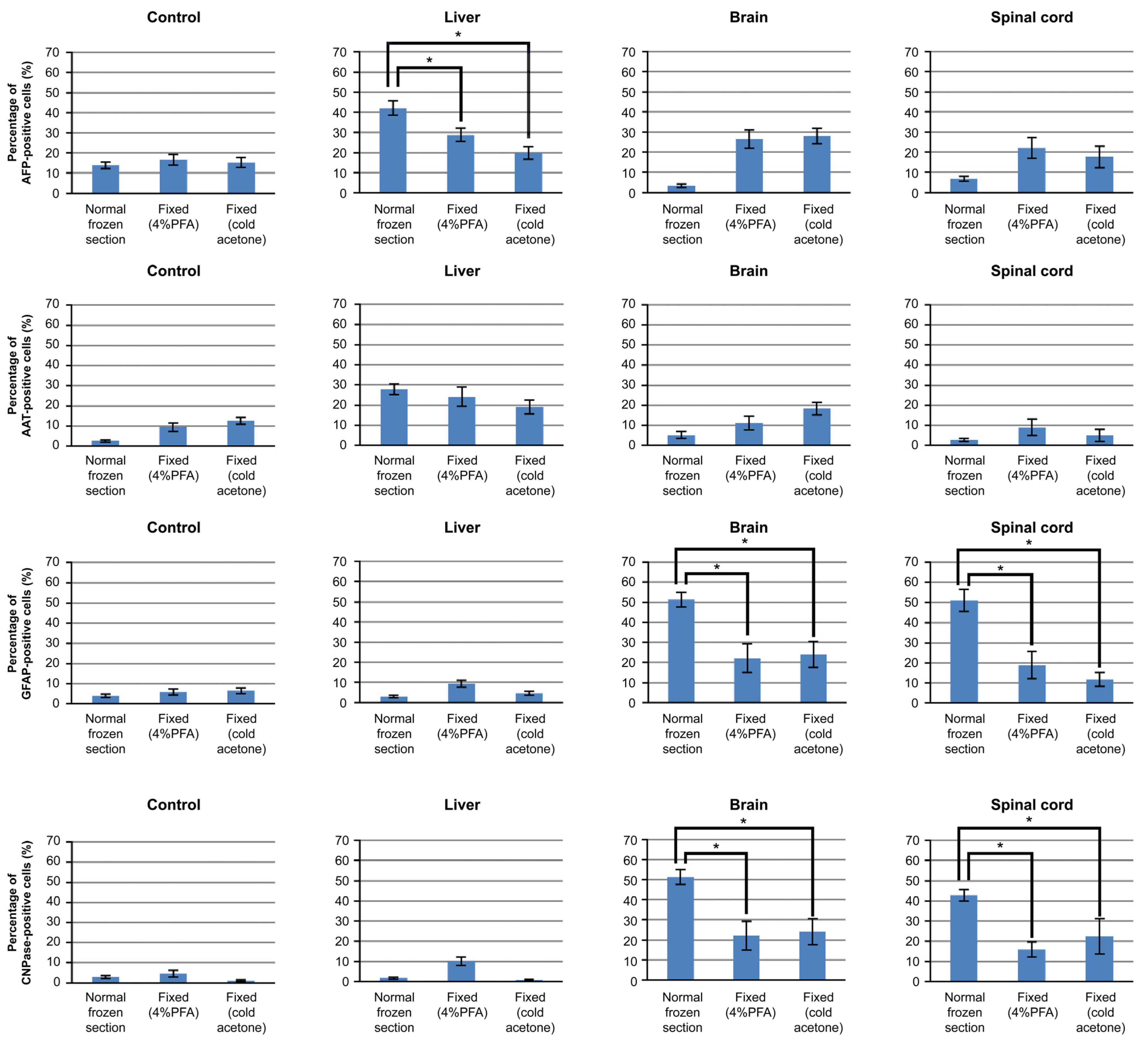
3.3. Differentiation of iPSCs Cultured on Frozen Sections
3.4. Mechanism of iPSCs Cultured on Frozen Sections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Ogawa, M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993, 81, 2844–2853. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimeault, M.; Hauke, R.; Batra, S.K. Stem cells: A revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin. Pharmacol. Ther. 2007, 82, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Kahler, D.J.; Ahmad, F.S.; Ritz, A.; Hua, H.; Moroziewicz, D.N.; Sproul, A.A.; Dusenberry, C.R.; Shang, L.; Paull, D.; Zimmer, M.; et al. Improved methods for reprogramming human dermal fibroblasts using fluorescence activated cell sorting. PLoS ONE 2013, 8, e59867. [Google Scholar] [CrossRef]
- Aoki, T.; Ohnishi, H.; Oda, Y.; Tadokoro, M.; Sasao, M.; Kato, H.; Hattori, K.; Ohgushi, H. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng. Part A 2010, 16, 2197–2206. [Google Scholar] [CrossRef]
- Eminli, S.; Utikal, J.; Arnold, K.; Jaenisch, R.; Hochedlinger, K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells 2008, 26, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.I.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, K.; Tsuji, D.; Kudoh, K.; Satomura, K.; Muto, T.; Itoh, K.; Noma, T. Generation of human induced pluripotent stem cells from oral mucosa. J. Biosci. Bioeng. 2010, 110, 345–350. [Google Scholar] [CrossRef]
- Choi, K.D.; Yu, J.; Smuga-Otto, K.; Salvagiotto, G.; Rehrauer, W.; Vodyanik, M.; Thomson, J.; Slukvin, I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 2009, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Taura, D.; Sone, M.; Homma, K.; Oyamada, N.; Takahashi, K.; Tamura, N.; Yamanaka, S.; Nakao, K. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1100–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [Green Version]
- Karumbayaram, S.; Novitch, B.G.; Patterson, M.; Umbach, J.A.; Richter, L.; Lindgren, A.; Conway, A.E.; Clark, A.T.; Goldman, S.A.; Plath, K.; et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 2009, 27, 806–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Hirami, Y.; Osakada, F.; Takahashi, K.; Okita, K.; Yamanaka, S.; Ikeda, H.; Yoshimura, N.; Takahashi, M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 2009, 458, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Lamba, D.A.; McUSic, A.; Hirata, R.K.; Wang, P.R.; Russell, D.; Reh, T.A. Generation, purification, and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef]
- Senju, S.; Haruta, M.; Matsumura, K.; Matsunaga, Y.; Fukushima, S.; Ikeda, T.; Takamatsu, K.; Irie, A.; Nishimura, Y. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011, 18, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagimachi, M.D.; Niwa, A.; Tanaka, T.; Honda-Ozaki, F.; Nishimoto, S.; Murata, Y.; Yasumi, T.; Ito, J.; Tomida, S.; Oshima, K.; et al. Robust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions. PLoS ONE 2013, 8, e59243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Jiang, W.; Liu, M.; Sui, X.; Yin, X.; Chen, S.; Shi, Y.; Deng, H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009, 19, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi-Aoi, M.; Ohnuki, M.; Takahashi, K.; Okita, K.; Noma, H.; Sawamura, Y.; Teramoto, I.; Narita, M.; Sato, Y.; Ichisaka, T.; et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20569–20574. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Y.; Li, W.; Lv, Z.; Liu, L.; Tong, M.; Hai, T.; Hao, J.; Guo, C.L.; Ma, Q.W.; Wang, L.; et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009, 461, 86–90. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ishii, H.; Nagano, H.; Haraguchi, N.; Dewi, D.L.; Kano, Y.; Nishikawa, S.; Tanemura, M.; Mimori, K.; Tanaka, F.; et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011, 8, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, C.H.; Moon, J.I.; Chung, Y.G.; Chang, M.Y.; Han, B.S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wu, S.; Joo, J.Y.; Zhu, S.; Han, D.W.; Lin, T.; Trauger, S.; Bien, G.; Yao, S.; Zhu, Y.; et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009, 4, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, T.; Yuasa, S.; Oda, M.; Egashira, T.; Yae, K.; Kusumoto, D.; Nakata, H.; Tohyama, S.; Hashimoto, H.; Kodaira, M.; et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010, 7, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.M.; Cooper, J.B. Lab-specific gene expression signatures in pluripoftent stem cells. Cell Stem Cell 2010, 7, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Bennett, S.A.; Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh. Migr. 2012, 6, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiwara, M.; Aoi, T.; Okita, K.; Takahashi, R.; Inoue, H.; Takayama, N.; Endo, H.; Eto, K.; Toguchida, J.; Uemoto, S.; et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent. Proc. Natl. Acad. Sci. USA 2012, 109, 12538–12543. [Google Scholar] [CrossRef] [Green Version]
- Peters, D.T.; Henderson, C.A.; Warren, C.R.; Friesen, M.; Xia, F.; Becker, C.E.; Musunuru, K.; Cowan, C.A. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development 2016, 143, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Mahajani, S.; Raina, A.; Fokken, C.; Kügler, S.; Bähr, M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019, 10, 898. [Google Scholar] [CrossRef]

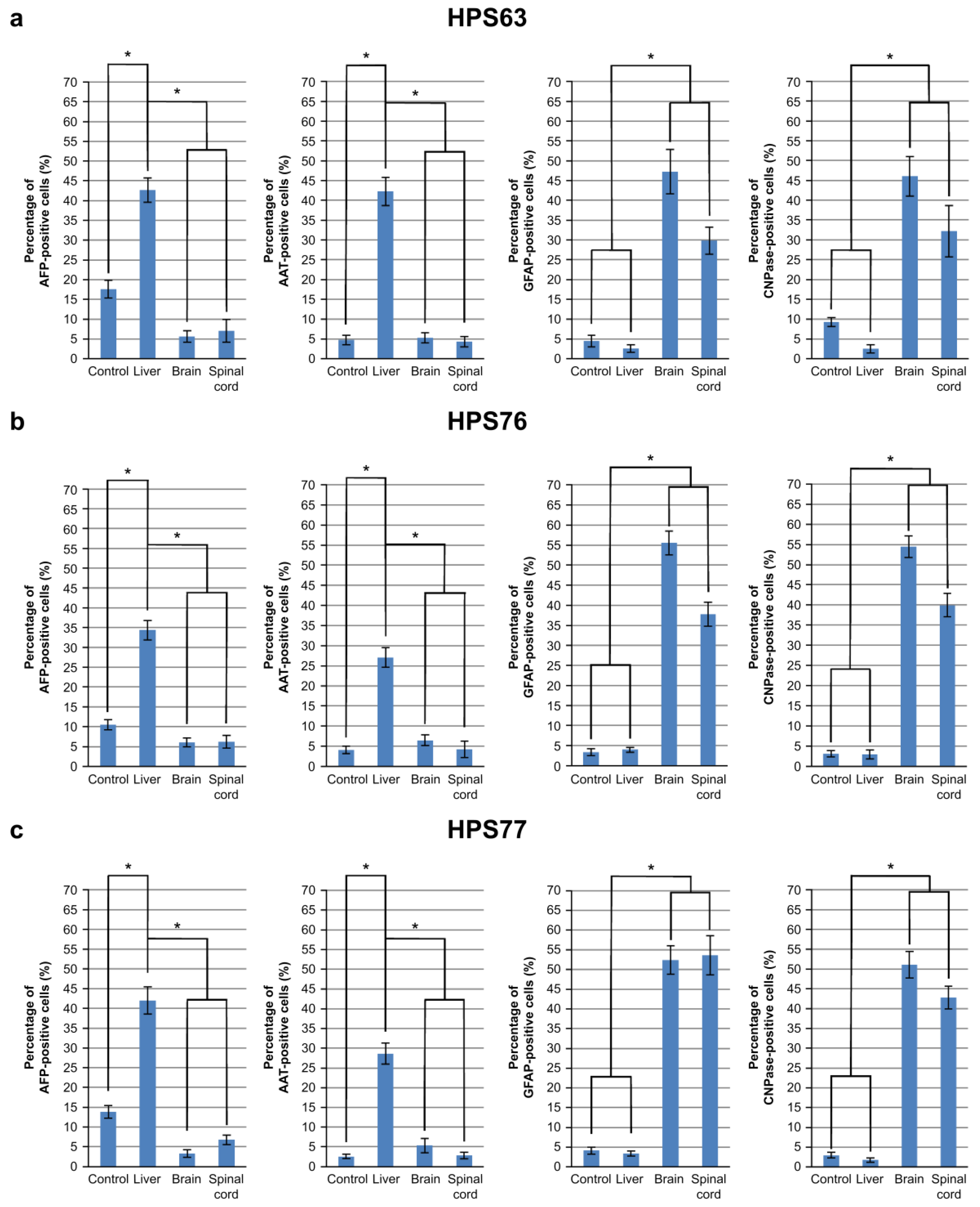
| hOF-iPSCs | HPS63 | HPS76 | HPS77 | ||
|---|---|---|---|---|---|
| Origin | oral mucosa | skin | skin | dental pulp | |
| Gene expression | retrovirus vector | retrovirus vector | episomal vector | episomal vector | |
| Foreign gene | OCT3/4, SOX2, KLF4, C-MYC | OCT3/4, SOX2, KLF4, C-MYC | OCT3/4, SOX2, KLF4, L-MYC, LIN28, P53 SHRNA | OCT3/4, SOX2, KLF4, L-MYC, LIN28, P53 SHRNA | |
| AFP | Control | 4.03% | 17.59% | 10.53% | 13.82% |
| Liver | 31.88% | 42.67% | 34.35% | 42.01% | |
| Brain | 4.15% | 5.65% | 6.07% | 3.31% | |
| Spinal cord | 3.70% | 7.07% | 6.25% | 6.80% | |
| AAT | Control | 4.19% | 4.72% | 4.09% | 2.51% |
| Liver | 36.19% | 42.20% | 27.07% | 27.82% | |
| Brain | 4.24% | 5.25% | 6.47% | 5.17% | |
| Spinal cord | 5.80% | 4.32% | 4.22% | 2.69% | |
| GFAP | Control | 8.21% | 4.48% | 3.34% | 4.00% |
| Liver | 5.37% | 2.57% | 3.99% | 3.03% | |
| Brain | 51.64% | 47.24% | 55.51% | 51.30% | |
| Spinal cord | 39.57% | 29.83% | 37.75% | 51.01% | |
| CNPase | Control | 8.59% | 9.25% | 3.10% | 2.89% |
| Liver | 5.37% | 2.51% | 2.89% | 1.62% | |
| Brain | 67.66% | 46.02% | 54.39% | 51.63% | |
| Spinal cord | 64.39% | 32.18% | 39.95% | 42.72% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadokoro, S.; Tokuyama-Toda, R.; Tatehara, S.; Ide, S.; Umeki, H.; Miyoshi, K.; Noma, T.; Satomura, K. A New Induction Method for the Controlled Differentiation of Human-Induced Pluripotent Stem Cells Using Frozen Sections. Cells 2021, 10, 2827. https://doi.org/10.3390/cells10112827
Tadokoro S, Tokuyama-Toda R, Tatehara S, Ide S, Umeki H, Miyoshi K, Noma T, Satomura K. A New Induction Method for the Controlled Differentiation of Human-Induced Pluripotent Stem Cells Using Frozen Sections. Cells. 2021; 10(11):2827. https://doi.org/10.3390/cells10112827
Chicago/Turabian StyleTadokoro, Susumu, Reiko Tokuyama-Toda, Seiko Tatehara, Shinji Ide, Hirochika Umeki, Keiko Miyoshi, Takafumi Noma, and Kazuhito Satomura. 2021. "A New Induction Method for the Controlled Differentiation of Human-Induced Pluripotent Stem Cells Using Frozen Sections" Cells 10, no. 11: 2827. https://doi.org/10.3390/cells10112827
APA StyleTadokoro, S., Tokuyama-Toda, R., Tatehara, S., Ide, S., Umeki, H., Miyoshi, K., Noma, T., & Satomura, K. (2021). A New Induction Method for the Controlled Differentiation of Human-Induced Pluripotent Stem Cells Using Frozen Sections. Cells, 10(11), 2827. https://doi.org/10.3390/cells10112827








