Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MTA Modification and 3D Scaffold Fabrication
2.2. Physicochemical Properties of the CA-Coated MTA Scaffolds
2.3. In Vitro Soaking and Bioactivity Assay
2.4. Cell Proliferation and Morphology
2.5. VEGF Secretion and Adsorption
2.6. Angiogenesis and Osteogenesis Assay
2.7. Rabbit Model of Femoral Bone Defects
2.8. Micro-Computed Tomography (µCT) and Histological Analysis
2.9. Data Analysis
3. Results and Discussion
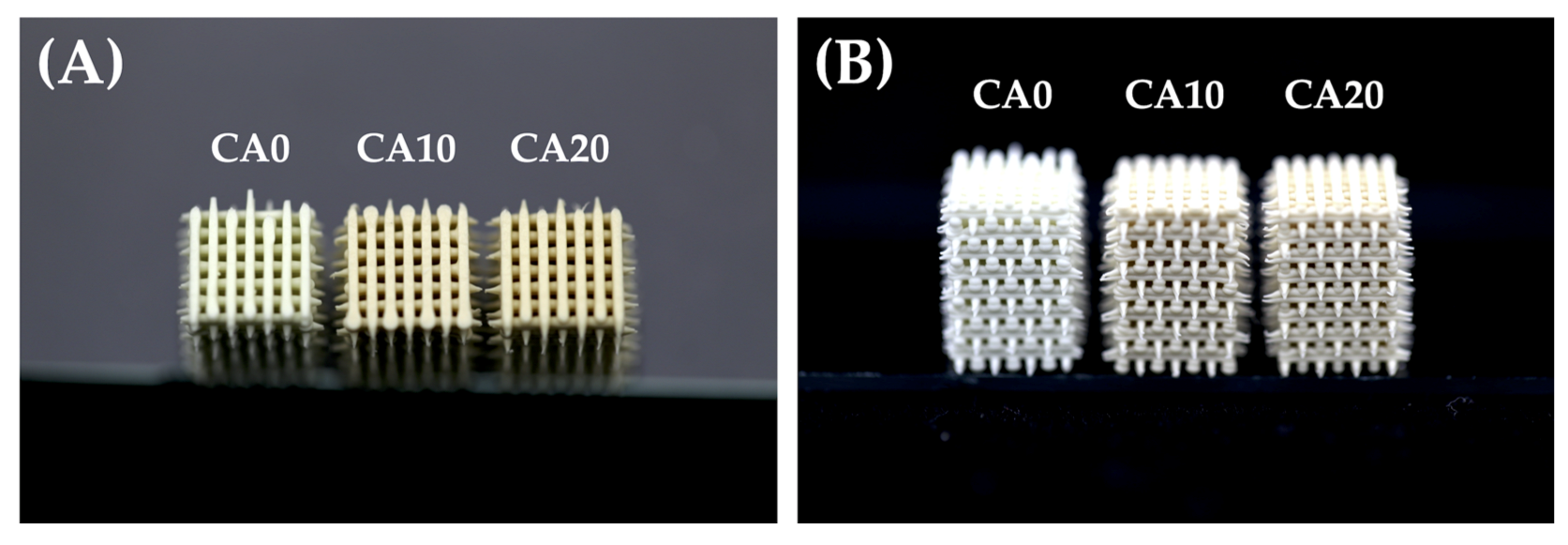
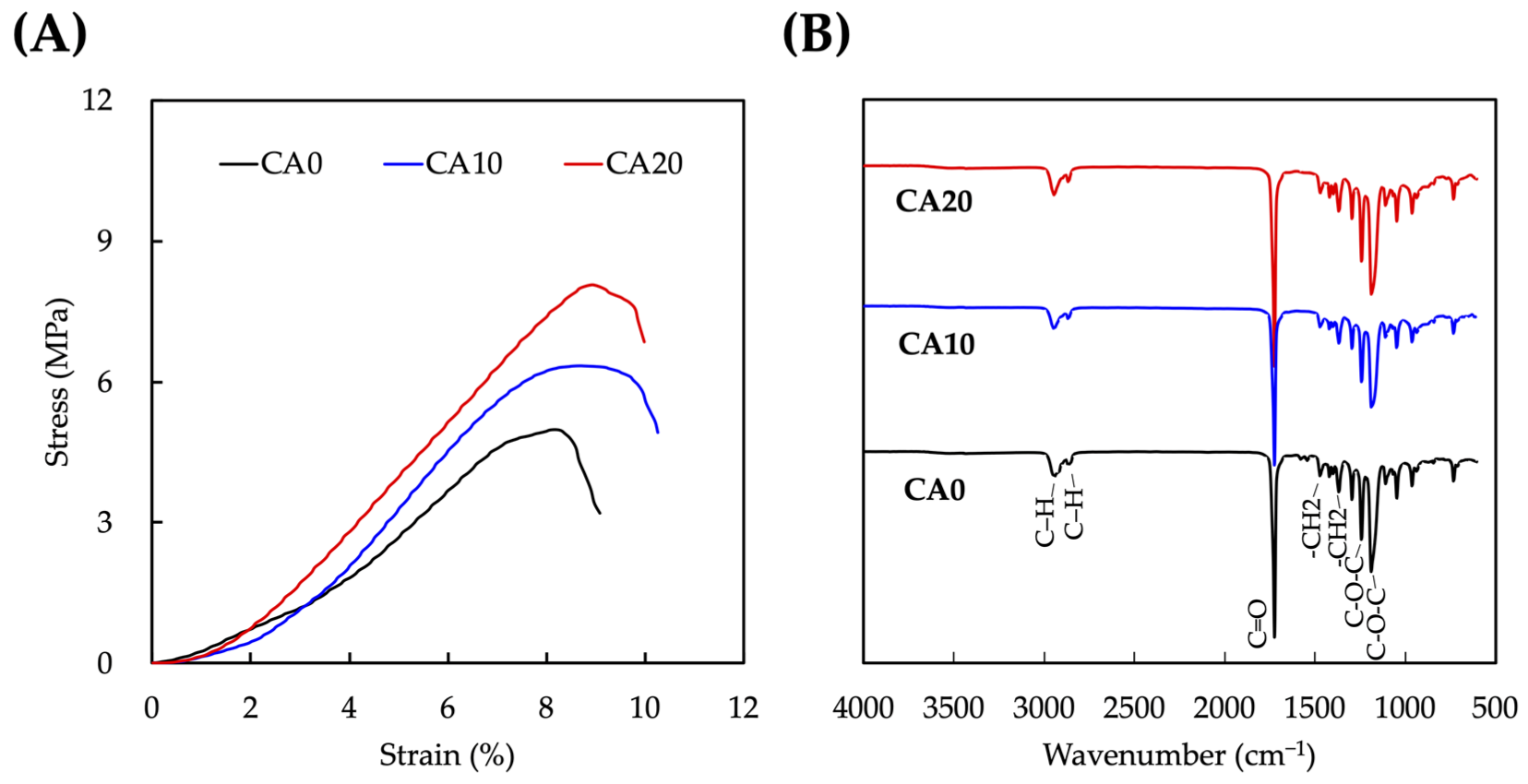
3.1. Physical Properties
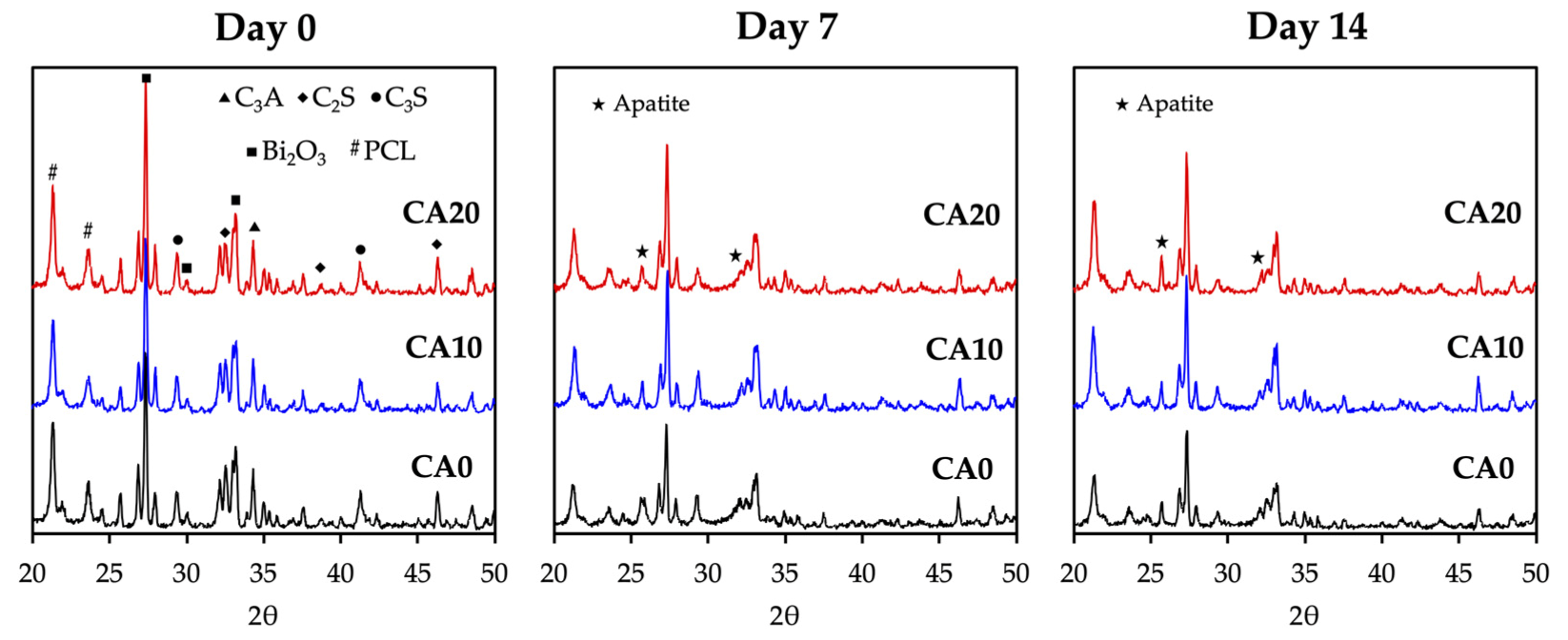
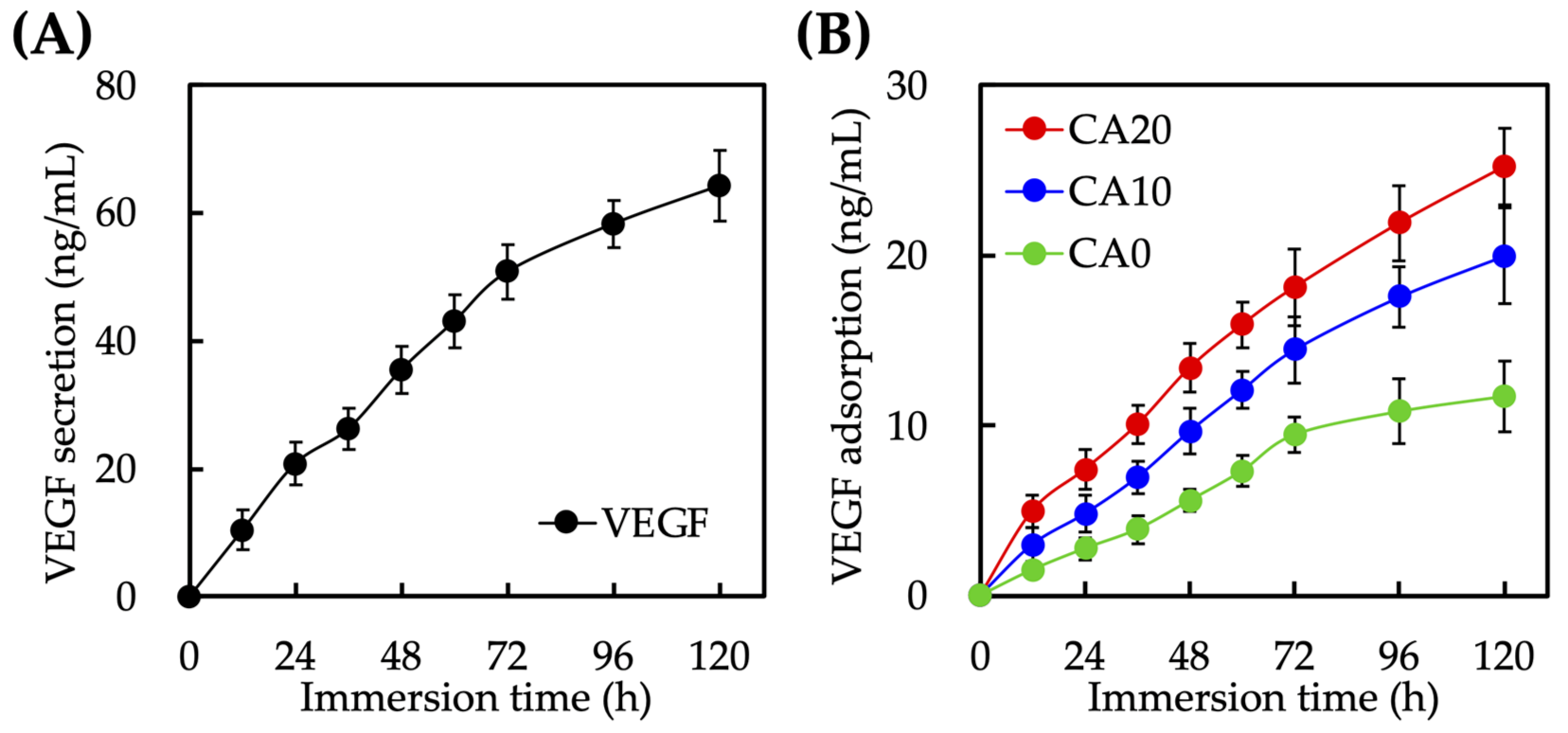
3.2. Immersion Behaviors
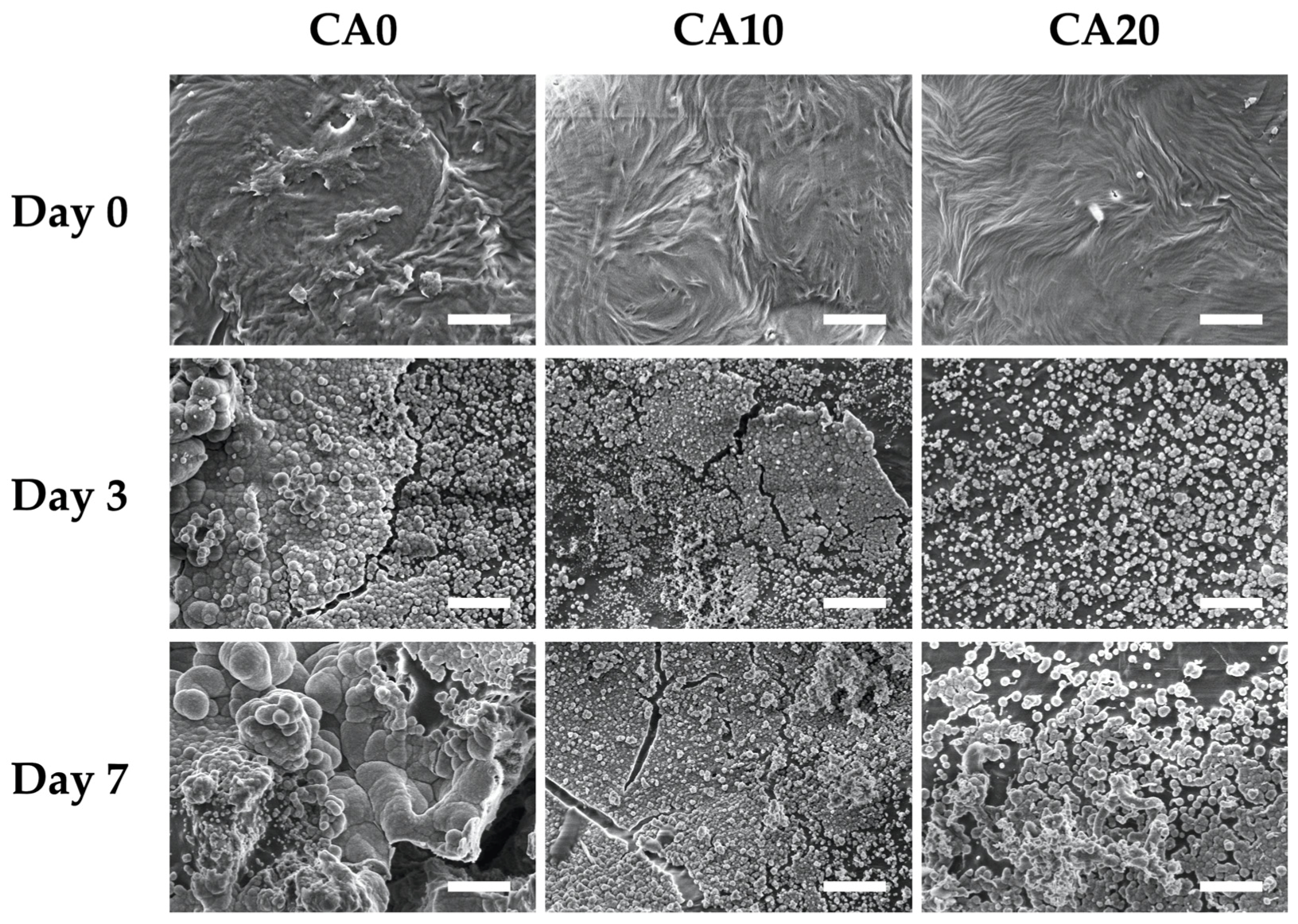
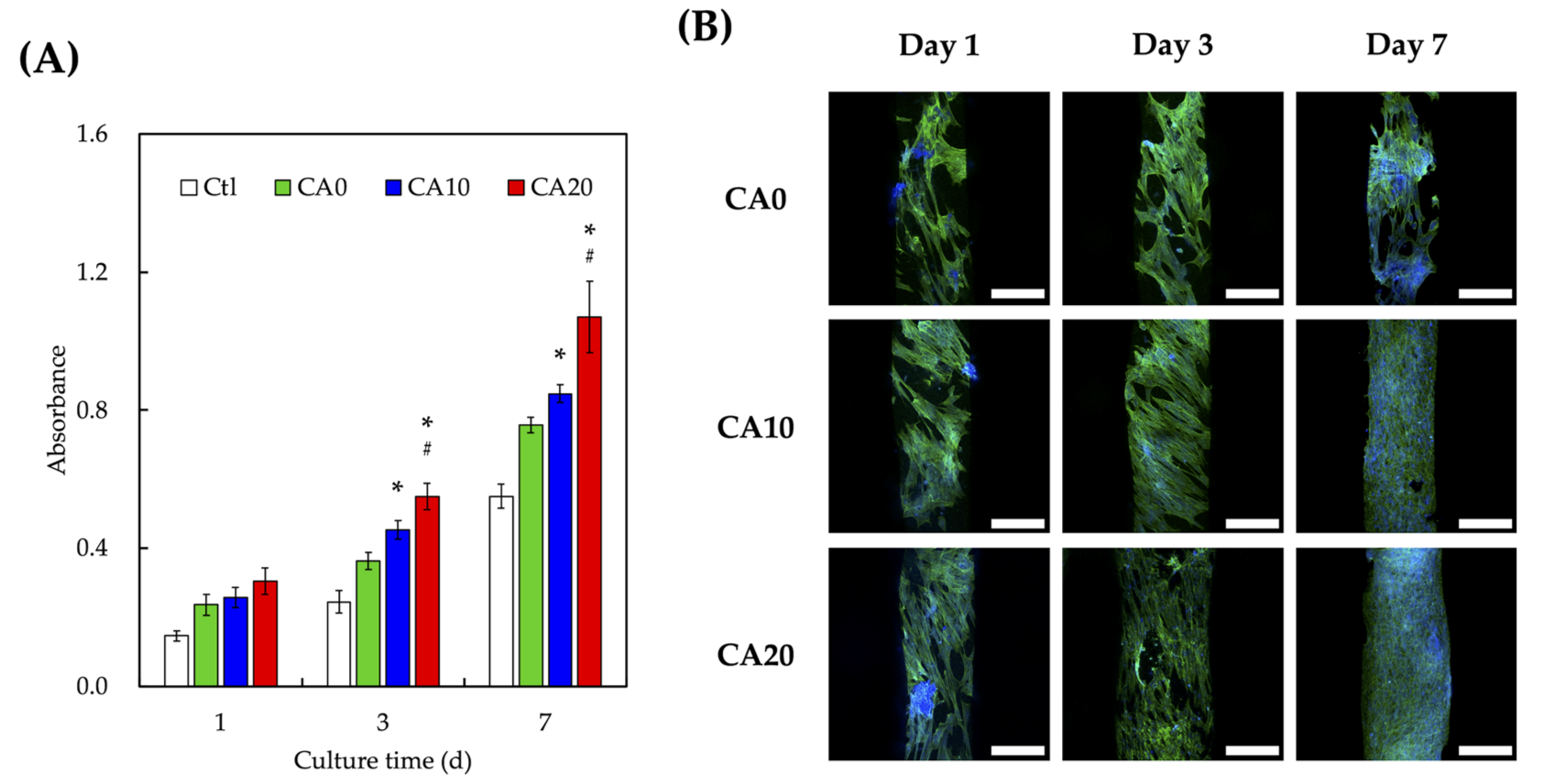
3.3. Biocompatibility
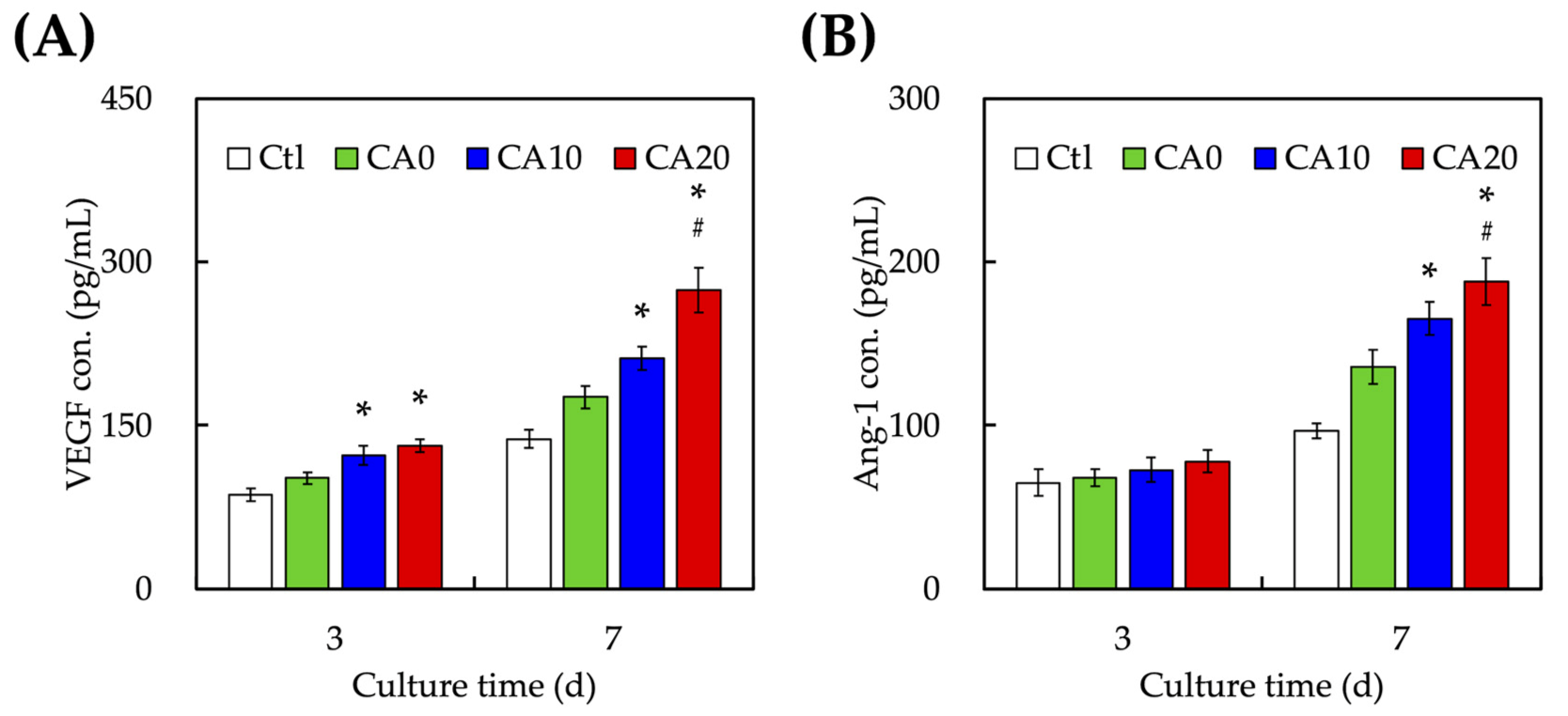
3.4. Angiogenesis
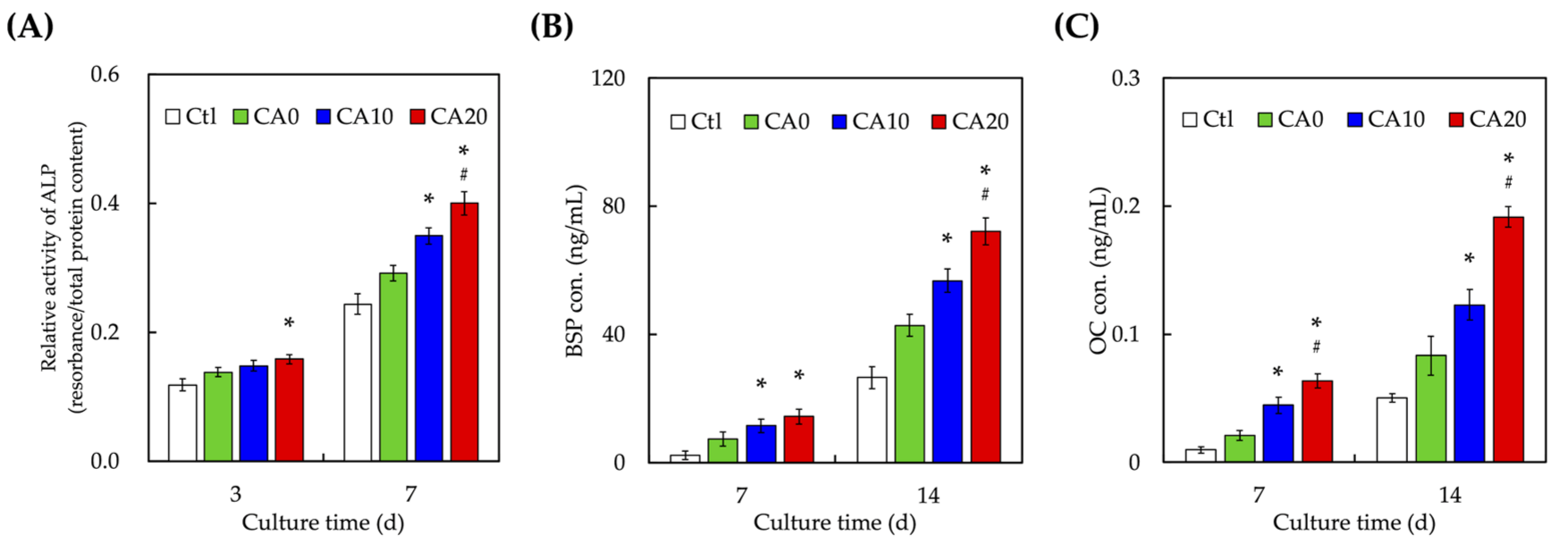
3.5. Osteogenesis
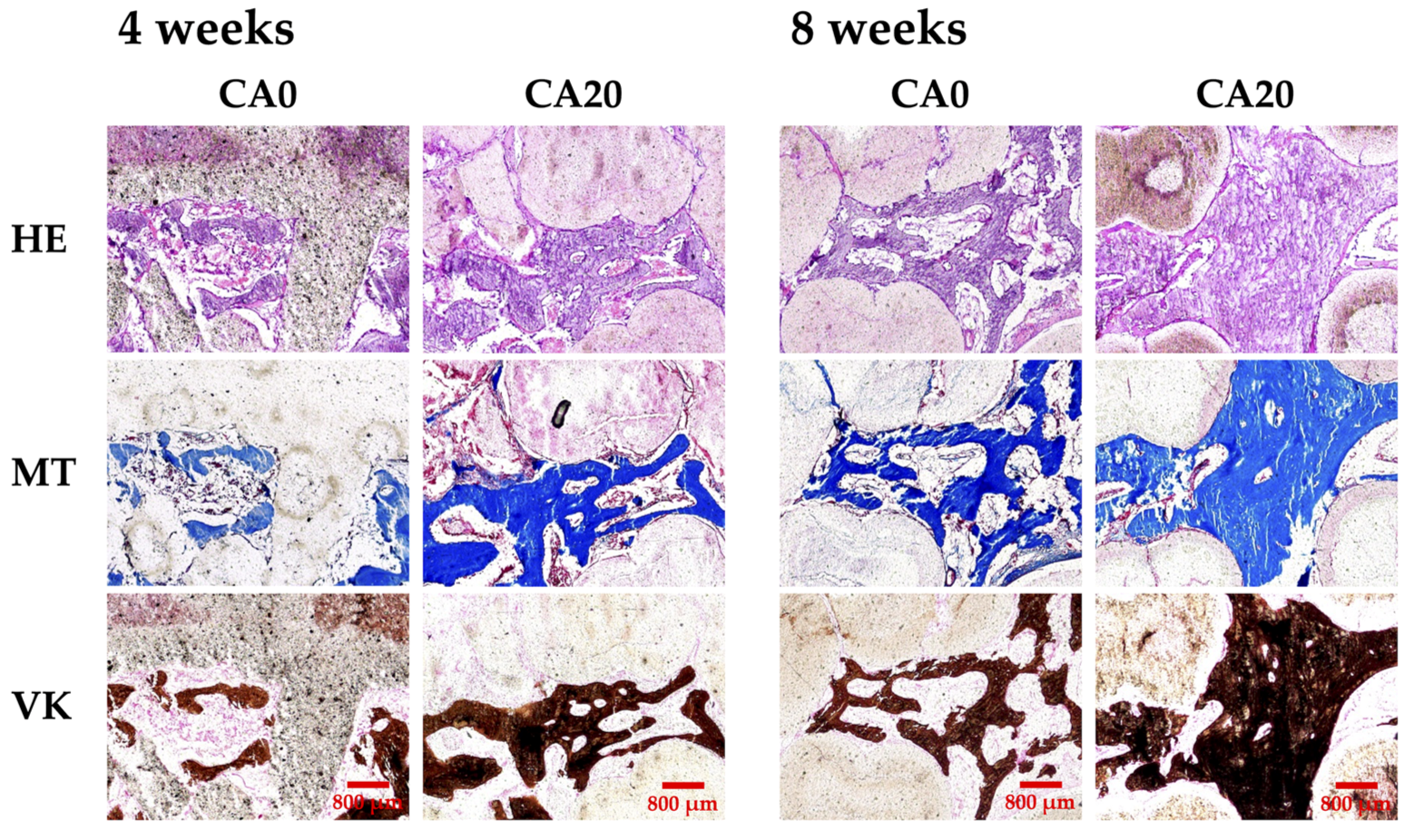
3.6. In Vivo Bone Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, T.T.; Yeh, C.H.; Kao, C.T.; Chen, Y.W.; Huang, T.H.; Yang, J.J.; Shie, M.Y. Antibacterial and odontogenesis efficacy of mineral trioxide aggregate combined with CO2 laser treatment. J. Endod. 2015, 41, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.G.; Ho, C.C.; Hsu, T.T.; Huang, T.H.; Lin, M.J.; Shie, M.Y. Mineral Trioxide Aggregate with mussel-inspired surface nanolayers for stimulating odontogenic differentiation of dental pulp cells. J. Endod. 2018, 44, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Antunes, M.; Falacho, R.I.; Sequeira, D.; Roseiro, L.; Santos, J.M.; Ramos, J.C. Effect of restorative timing on shear bond strength of composite resin/calcium silicate-based cements adhesive interfaces. Clin. Oral Investig. 2021, 25, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.Y.; Wu, H.D.; Hsieh, S.C.; Teng, N.C.; Chen, C.C.; Ke, E.S.; Lin, Y.; Lee, S.Y.; Yang, J.C. Effects of a novel hydration accelerant on the biological and mechanical properties of white mineral trioxide aggregate. J. Endod. 2011, 37, 851–855. [Google Scholar] [CrossRef]
- Santos, J.M.; Marques, J.A.; Diogo, P.; Messias, A.; Sousa, V.; Sequeira, D.; Palma, P.J. Influence of preoperative pulp inflammation in the outcome of full pulpotomy using a dog model. J. Endod. 2021, 47, 1417–1426. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, T.H.; Kao, C.T.; Wu, Y.H.; Chen, W.C.; Shie, M.Y. Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ho, C.C.; Huang, T.H.; Hsu, T.T.; Shie, M.Y. The ionic products from mineral trioxide aggregate–induced odontogenic differentiation of dental pulp cells via activation of the Wnt/β-catenin signaling pathway. J. Endod. 2016, 42, 1062–1069. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The characteristics of Mineral Trioxide Aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Wen, J.; Zhang, X.; Cao, L.; Zeng, D.; Liu, X.; Jiang, X. Novel vascular strategies on polyetheretherketone modification in promoting osseointegration in ovariectomized rats. Mater. Des. 2021, 39, 109526. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Y.; Cheong, S.; Kong, C.; Mazur, F.; Liang, K.; Chandrawwati, R. Modulating nitric oxide-generating activity of zinc oxide by morphology control and surface modification. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112428. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Sala, D.F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural formulations provide antioxidant complement to hyaluronic acid-based topical applications used in wound healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pavasant, P.; Supaphol, P. Preparation and characterization of caffeic acid-grafted electrospun poly(L-lactic acid) fiber mats for biomedical applications. ACS Appl. Mater. Interfaces 2012, 4, 3031–3040. [Google Scholar] [CrossRef]
- Tu, M.G.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Ho, C.C.; Shie, M.Y. Caffeic acid-coated nanolayer on Mineral Trioxide Aggregate potentiate the host immune responses, angiogenesis, and odontogenesis. J. Endod. 2020, 46, 1455–1464. [Google Scholar] [CrossRef]
- Chiang, Z.C.; Li, H.Y.; Chao, A.C.; Su, Y.R. Characterization of the morphology and hydrophilicity of chitosan/caffeic acid hybrid scaffolds. J. Polym. Res. 2011, 18, 2205–2212. [Google Scholar] [CrossRef]
- Hu, M.; Jia, F.; Huang, W.P.; Li, X.; Hu, D.F.; Wang, J.; Ren, K.F.; Fu, G.-S.; Wang, Y.B.; Ji, J. Substrate stiffness differentially impacts autophagy of endothelial cells and smooth muscle cells. Bioact. Mater. 2021, 6, 1413–1422. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Gong, M.M.; Skala, M.C.; Harari, P.M.; Beebe, D.J. Human tumor-lymphatic microfluidic model reveals differential conditioning of lymphatic vessels by breast cancer cells. Adv. Healthc. Mater. 2020, 9, 1900925. [Google Scholar] [CrossRef]
- Li, L.; Qin, S.; Peng, J.; Chen, A.; Nie, Y.; Liu, T.; Song, K. Engineering gelatin-based alginate/carbon nanotubes blend bioink for direct 3D printing of vessel constructs. Int. J. Biol. Macromol. 2020, 145, 262–271. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Yang, G.; Hu, X.; Wang, L.; Liu, B.; Wang, J.; Zhang, S. Delivering proangiogenic factors from 3D-printed polycaprolactone scaffolds for vascularized bone regeneration. Adv. Healthc. Mater. 2020, 9, 2000727. [Google Scholar] [CrossRef]
- Kuttappan, S.; Jo, J.-I.; Sabu, C.K.; Menon, D.; Tabata, Y.; Nair, M.B. Bioinspired nanocomposite fibrous scaffold mediated delivery of ONO-1301 and BMP2 enhance bone regeneration in critical sized defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110591. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.C.; Wang, C.Y.; Lee, K.X.; Yeh, C.L.; Lin, C.P. Assessment of the release of vascular endothelial growth factor from 3D-printed poly-ε-caprolactone/hydroxyapatite/calcium sulfate scaffold with enhanced osteogenic capacity. Polymers 2020, 12, 1455. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111245. [Google Scholar] [CrossRef]
- Hung, C.J.; Hsu, H.I.; Lin, C.C.; Huang, T.H.; Wu, B.C.; Kao, C.T.; Shie, M.Y. The role of integrin αv in proliferation and differentiation of human dental pulp cell response to calcium silicate cement. J. Endod. 2014, 40, 1802–1809. [Google Scholar] [CrossRef]
- Backes, E.H.; Fernandes, E.M.; Diogo, G.S.; Marques, C.F.; Silva, T.H.; Costa, L.C.; Passador, F.R.; Reis, R.L.; Pessan, L.A. Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111928. [Google Scholar] [CrossRef]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng C Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, K.; Ho, C.C.; Kao, C.T.; Ng, H.Y.; Shie, M.Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195, 108982. [Google Scholar] [CrossRef]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink composition and printing parameters for 3D modeling neural tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef]
- Lai, W.Y.; Chen, Y.J.; Lee, K.X.A.; Lin, Y.H.; Liu, Y.W.; Shie, M.Y. Therapeutic effects of the addition of fibroblast growth factor-2 to biodegradable gelatin/magnesium-doped calcium silicate hybrid 3D-printed scaffold with enhanced osteogenic capabilities for critical bone defect restoration. Biomedicines 2021, 9, 712. [Google Scholar] [CrossRef]
- Dabaghi, M.; Saraei, N.; Carpio, M.B.; Nanduri, V.; Ungureanu, J.; Babi, M.; Chandiramohan, A.; Noble, A.; Revill, S.D.; Zhang, B.; et al. A robust protocol for decellularized human lung bioink generation amenable to 2D and 3D lung cell culture. Cells 2021, 10, 1538. [Google Scholar] [CrossRef]
- Horder, H.; Guaza Lasheras, M.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and differentiation of adipose-derived stromal cell spheroids for a 3D breast cancer-adipose tissue model. Cells 2021, 10, 803. [Google Scholar] [CrossRef]
- Chen, K.Y.; Yao, C.H. Repair of bone defects with gelatin-based composites: A review. BioMedicine 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, K.X.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of gingival fibroblast cell-laden collagen/strontium-doped calcium silicate 3D-printed bi-layered scaffold for osteoporotic periodontal regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Wszoła, M.; Nitarska, D.; Cywoniuk, P.; Gomółka, M.; Klak, M. Stem cells as a source of pancreatic cells for production of 3D bioprinted bionic pancreas in the treatment of type 1 diabetes. Cells 2021, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Unalan, I.; Fuggerer, T.; Slavik, B.; Buettner, A.; Boccaccini, A.R. Antibacterial and antioxidant activity of cinnamon essential oil-laden 45S5 bioactive glass/soy protein composite scaffolds for the treatment of bone infections and oxidative stress. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112320. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Wekwejt, M.; Mazur, O.; Zasada, L.; Pałubicka, A.; Olewnik-Kruszkowska, E. The physicochemical and antibacterial properties of chitosan- based materials modified with phenolic acids irradiated by uvc light. Int. J. Mol. Sci. 2021, 22, 6472. [Google Scholar] [CrossRef]
- Kao, C.T.; Chiu, Y.C.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Liu, Y.C.; Shie, M.Y. The synergistic effects of Xu Duan combined Sr-contained calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111629. [Google Scholar] [CrossRef]
- Ling, Z.; He, Y.; Huang, H.; Xie, X.; Li, Q.-L.; Cao, C.Y. Effects of oligopeptide simulating DMP-1/mineral trioxide aggregate/agarose hydrogel biomimetic mineralisation model for the treatment of dentine hypersensitivity. J. Mater. Chem. B 2019, 7, 5825–5833. [Google Scholar] [CrossRef]
- Shiu, J.C.; Ho, M.H.; Yu, S.H.; Chao, A.C.; Su, Y.R.; Chen, W.J.; Chiang, Z.C.; Yang, W.P. Preparation and characterization of caffeic acid grafted chitosan/CPTMS hybrid scaffolds. Carbohyd. Polym. 2010, 79, 724–730. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Cheng, X.; Zheng, Y.; Lyu, M.; Di, P.; Lin, Y. MiR-181d-5p regulates implant surface roughness-induced osteogenic differentiation of bone marrow stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111801. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Camargo, B.; Hauben, E.; De Vleeschauwer, S.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Van Meerbeek, B. Injectable phosphopullulan-functionalized calcium-silicate cement for pulp-tissue engineering: An in-vivo and ex-vivo study. Dent. Mater. 2020, 36, 512–526. [Google Scholar] [CrossRef]
- Zhu, M.; He, H.; Meng, Q.; Zhu, Y.; Ye, X.; Xu, N.; Yu, J. Osteopontin sequence modified mesoporous calcium silicate scaffolds to promote angiogenesis in bone tissue regeneration. J. Mater. Chem. B 2020, 8, 5849–5861. [Google Scholar] [CrossRef]
- Devendran, C.; Carthew, J.; Frith, J.E.; Neild, A. Cell adhesion, morphology, and metabolism variation via acoustic exposure within microfluidic cell handling systems. Adv. Sci. 2019, 6, 1902326. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Zhang, J.; Feng, Z.; Yin, M.; Mo, X. A bilayer vascular scaffold with spatially controlled release of growth factors to enhance in situ rapid endothelialization and smooth muscle regeneration. Mater. Des. 2021, 204, 109649. [Google Scholar] [CrossRef]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef]
- Singh, S.; Wu, B.M.; Dunn, J.C.Y. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials 2011, 32, 2059–2069. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Cao, Q.; Yu, T.; Zhang, J.; Liu, Q.; Yang, X. Growth factors enhanced angiogenesis and osteogenesis on polydopamine coated titanium surface for bone regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar] [CrossRef]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled growth factor release in 3D-printed hydrogels. Adv. Healthc. Mater. 2020, 9, 1900977. [Google Scholar] [CrossRef]
- Teixeira, S.P.B.; Domingues, R.M.A.; Shevchuk, M.; Gomes, M.E.; Peppas, N.A.; Reis, R.L. Biomaterials for sequestration of growth factors and modulation of cell behavior. Adv. Funct. Mater. 2020, 30, 1909011. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhou, G.; Zhang, J.; Su, X.; Huang, Z.; Li, Q.; Wu, Z.; Qiu, G. VEGF-loaded biomimetic scaffolds: A promising approach to improve angiogenesis and osteogenesis in an ischemic environment. RSC Adv. 2017, 7, 4253–4259. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Iaccarino, G.; Fattorusso, R.; Sorriento, D.; Carannante, C.; Capasso, D.; Trimarco, B.; Pedone, C. Targeting angiogenesis: Structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 14215–14220. [Google Scholar] [CrossRef]
- Kakio, S.; Funakoshi-Tago, M.; Kobata, K.; Tamura, H. Coffee induces vascular endothelial growth factor (VEGF) expression in human neuroblastama SH-SY5Y cells. Nutr. Neurosci. 2017, 20, 336–342. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.G.; Hwang, J.H.; Cho, Y.S.; Lee, K.S.; Jeong, H.J.; Park, S.H.; Park, Y.; Cho, Y.S.; Lee, B.K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 949–959. [Google Scholar] [CrossRef]
- Mahapatra, C.; Singh, R.K.; Kim, J.J.; Patel, K.D.; Perez, R.A.; Jang, J.H.; Kim, H.W. Osteopromoting reservoir of stem cells: Bioactive mesoporous nanocarrier/collagen gel through slow-releasing FGF18 and the activated BMP signaling. ACS Appl. Mater. Interfaces 2016, 8, 27573–27584. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid hormone secretion and receptor expression determine the age-related degree of osteogenic differentiation in dental pulp stem cells. J. Pers Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Wang, C.; Lai, J.; Li, K.; Zhu, S.; Lu, B.; Liu, J.; Tang, Y.; Wei, Y. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact. Mater. 2021, 6, 137–145. [Google Scholar] [CrossRef]
- Bin, H.S.; Jeong, J.H.; Choi, U.K. Chlorogenic acid promotes osteoblastogenesis in human adipose tissue-derived mesenchymal stem cells. Food Sci. Biotechnol. 2013, 22, 107–112. [Google Scholar] [CrossRef]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic acid prevents osteoporosis by Shp2/PI3K/Akt pathway in ovariectomized rats. PLoS ONE 2016, 11, 166751. [Google Scholar] [CrossRef]
- Tafazoli Moghadam, E.; Yazdanian, M.; Alam, M.; Tebyanian, H.; Tafazoli, A.; Tahmasebi, E.; Ranjbar, R.; Yazdanian, A.; Seifalian, A. Current natural bioactive materials in bone and tooth regeneration in dentistry: A comprehensive overview. J. Mater. Res. Technol. 2021, 13, 2078–2114. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Santos, J.; Falacho, R.I.; Sequeira, D.; Diogo, P.; Caramelo, F.; Ramos, J.C.; Santos, J.M. Tooth discoloration after regenerative endodontic procedures with calcium silicate-based cements—An ex vivo study. Appl. Sci. 2020, 10, 5793. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tien, N.; Lee, J.-J.; Lee, A.K.-X.; Lin, Y.-H.; Chen, J.-X.; Kuo, T.-Y.; Shie, M.-Y. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells 2021, 10, 2911. https://doi.org/10.3390/cells10112911
Tien N, Lee J-J, Lee AK-X, Lin Y-H, Chen J-X, Kuo T-Y, Shie M-Y. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells. 2021; 10(11):2911. https://doi.org/10.3390/cells10112911
Chicago/Turabian StyleTien, Ni, Jian-Jr Lee, Alvin Kai-Xing Lee, Yen-Hong Lin, Jian-Xun Chen, Ting-You Kuo, and Ming-You Shie. 2021. "Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells" Cells 10, no. 11: 2911. https://doi.org/10.3390/cells10112911
APA StyleTien, N., Lee, J.-J., Lee, A. K.-X., Lin, Y.-H., Chen, J.-X., Kuo, T.-Y., & Shie, M.-Y. (2021). Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells, 10(11), 2911. https://doi.org/10.3390/cells10112911






