Identification of Tropical Plant Extracts That Extend Yeast Chronological Life Span
Abstract
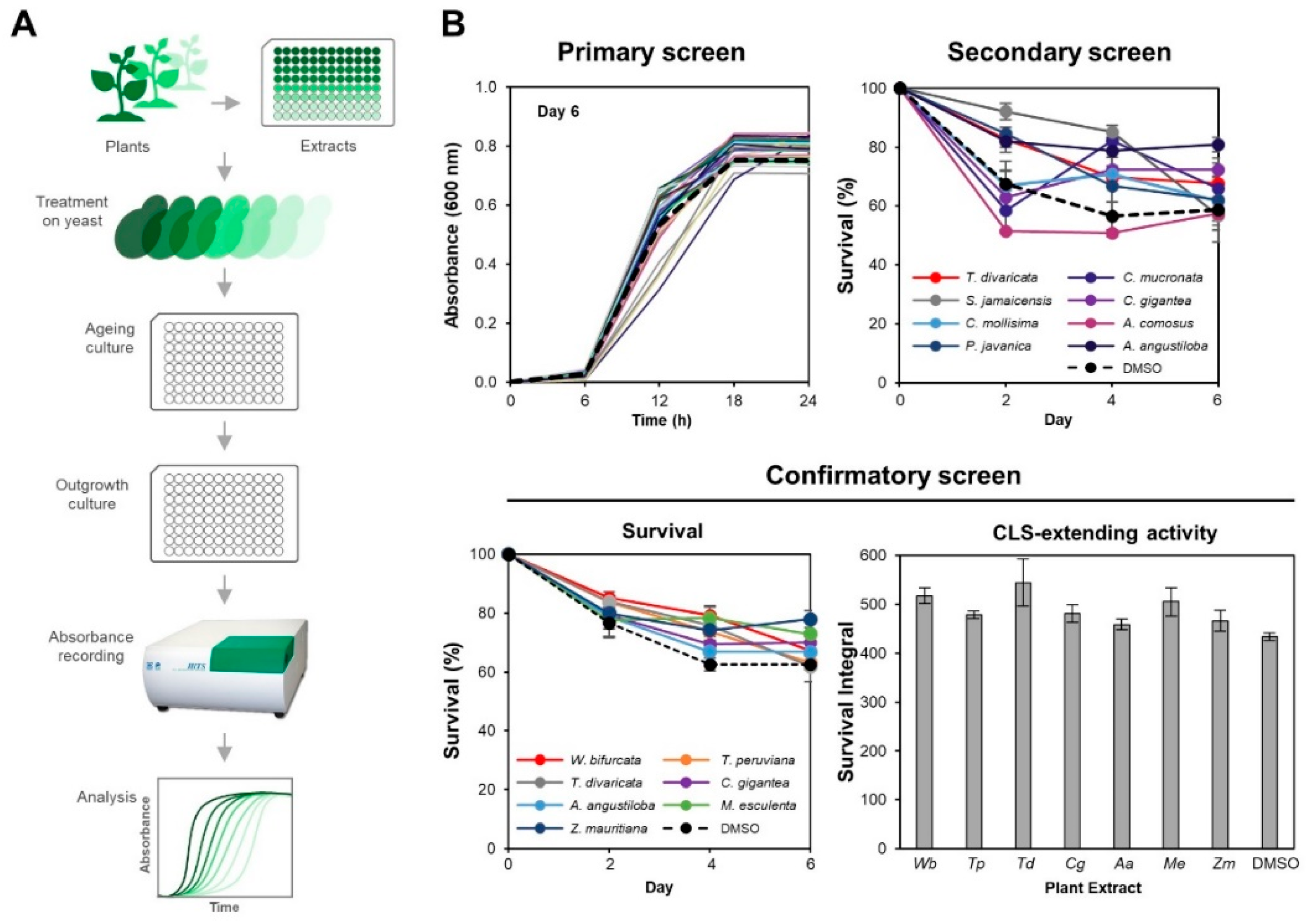
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Media and Growth Conditions
2.2. Preparation of Plant Extract (PE) Library
2.3. Chronological Life Span Assay
2.4. Dose-Dependent Effects of Plant Extracts on Yeast Chronological Life Span
2.5. Stress Assay
3. Results
3.1. Seven PEs Extend Chronological Life Span in Yeast
3.2. M. esculenta and W. bifurcata Extracts Provide Dose-Dependent Effects in Extending Yeast CLS
3.3. M. esculenta and W. bifurcata Extracts Contribute to Oxidative Stress Tolerance during Chronological Ageing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakatta, E.G.; Mitchell, J.H.; Pomerance, A.; Rowe, G.G. Human aging: Changes in structure and function. J. Am. Coll. Cardiol. 1987, 10, 42A–47A. [Google Scholar] [CrossRef] [Green Version]
- Chodzko-Zajko, W.J. The physiology of aging: Structural changes and functional consequences. Implications for research and clinical practice in the exercise and activity sciences. Quest 1996, 48, 311–329. [Google Scholar] [CrossRef]
- Viña, J.; Borrás, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodero, S.; Fernández-Morera, J.L.; Menéndez-Torre, E.; Calvanese, V.; Fernández, A.F.; Fraga, M.F. Aging genetics and aging. Aging Dis. 2011, 2, 186–195. [Google Scholar]
- Lee, J.W.; Ong, E.B.B. Genomic instability and cellular senescence: Lessons from the budding yeast. Front. Cell Dev. Biol. 2021, 8, 619126. [Google Scholar] [CrossRef]
- Murakami, C.J.; Burtner, C.R.; Kennedy, B.K.; Kaeberlein, M. A method for high-throughput quantitative analysis of yeast chronological life span. J. Gerontol. A Biol. Sci. Med. Sc. 2008, 63A, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio-Marques, B.; Burhans, W.C.; Ludovico, P. Longevity pathways and maintenance of the proteome: The role of autophagy and mitophagy during yeast ageing. Microb. Cell 2014, 1, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Aging Cell 2003, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Polymenis, M.; Kennedy, B.K. Chronological and replicative lifespan in yeast: Do they meet in the middle? Cell Cycle 2012, 11, 3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Talmale, S.A.; Bhujade, A.M.; Patil, M.B. Phytochemical analysis of stem bark and root bark of Zizyphus mauritiana. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 526–535. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Guan, Y.; Rui, X.; Zhang, Y.; Dong, M.; Ma, W. An aqueous polyphenol extract from Rosa rugosa tea has antiaging effects on Caenorhabditis elegans. J. Food Biochem 2019, 43, e12796. [Google Scholar] [CrossRef]
- Leite, N.R.; Araújo, L.C.A.D.; Rocha, P.D.S.D.; Agarrayua, D.A.; Ávila, D.S.; Carollo, C.A.; Silva, D.B.; Estevinho, L.M.; de Picoli Souza, K.; dos Santos, E.L. Baru pulp (Dipteryx alata Vogel): Fruit from the Brazilian savanna protects against oxidative stress and increases the life expectancy of Caenorhabditis elegans via SOD-3 and DAF-16. Biomolecules 2020, 10, 1106. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Dakik, P.; Rodriguez, M.E.L.; Junio, J.A.B.; Mitrofanova, D.; Medkour, Y.; Tafakori, T.; Taifour, T.; Lutchman, V.; Samson, E.; Arlia-Ciommo, A.; et al. Discovery of fifteen new geroprotective plant extracts and identification of cellular processes they affect to prolong the chronological lifespan of budding yeast. Oncotarget 2020, 11, 2192–2213. [Google Scholar] [CrossRef] [PubMed]
- Lutchman, V.; Medkour, Y.; Samson, E.; Arlia-Ciommo, A.; Dakik, P.; Cortes, B.; Feldman, R.; Mohtashami, S.; McAuley, M.; Chancharoen, M.; et al. Discovery of plant extracts that greatly delay yeast chronological aging and have different effects on longevity-defining cellular processes. Oncotarget 2016, 7, 16542–16566. [Google Scholar] [CrossRef] [Green Version]
- Powers III, R.W.; Kaeberlein, M.; Caldwell, S.D.; Kennedy, B.K.; Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006, 20, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, 0139–0149. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, J.; Dickson, R.C. Down-regulating sphingolipid synthesis increases yeast lifespan. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierman, M.B.; Maqani, N.; Strickler, E.; Li, M.; Smith, J.S. Caloric restriction extends yeast chronological life span by optimizing the Snf1 (AMPK) signaling pathway. Mol. Cell Biol. 2017, 37, e00562-16. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B exerts antiaging effects in yeast by regulating autophagy and oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 4517091. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Singh, R. Longevity Genes: A Blueprint for Aging; Atzmon, G., Ed.; Springer: New York, NY, USA, 2015; pp. 73–87. [Google Scholar]
- Lutchman, V.; Dakik, P.; McAuley, M.; Cortes, B.; Ferraye, G.; Gontmacher, L.; Graziano, D.; Moukhariq, F.-Z.; Simard, É.; Titorenko, V.I. Six plant extracts delay yeast chronological aging through different signaling pathways. Oncotarget 2016, 7, 50845–50863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, M.M.Y.; Lee, J.W.; Samian, M.R.; Watanabe, N.; Osada, H.; Ong, E.B.B. Comparison of microplate- and bottle-based methods to age yeast for chronological life span assays. J. Microbiol. Methods 2019, 167, 105743. [Google Scholar] [CrossRef]
- Stirpe, M.; Palermo, V.; Bianchi, M.M.; Silvestri, R.; Falcone, C.; Tenore, G.; Novellino, E.; Mazzoni, C. Annurca apple (M. pumila Miller cv Annurca) extracts act against stress and ageing in S. cerevisiae yeast cells. BMC Complement. Altern Med. 2017, 17, 200. [Google Scholar] [CrossRef]
- Sunthonkun, P.; Palajai, R.; Somboon, P.; Suan, C.L.; Ungsurangsri, M.; Soontorngun, N. Life-span extension by pigmented rice bran in the model yeast Saccharomyces cerevisiae. Sci. Rep. 2019, 9, 18061. [Google Scholar] [CrossRef] [Green Version]
- Sengab, A.E.B.; El naggar, D.M.Y.; Elgindi, M.R.; Elsaid, M.B. Biological studies of isolated triterpenoids and phenolic compounds identified from Wodyetia bifurcata family Arecaceae. J. Pharmacogn. Phytochem. 2015, 3, 67–73. [Google Scholar]
- Hassan, M.M.; Saha, A.K.; Khan, S.A.; Islam, A.; Mahabub-Uz-Zaman, M.; Ahmed, S.S.U. Studies on the antidiarrhoeal, antimicrobial and cytotoxic activities of ethanol-extracted leaves of yellow oleander (Thevetia Peruviana). Open Vet. J. 2011, 1, 28–31. [Google Scholar] [PubMed]
- Gezahegn, Z.; Akhtar, M.S.; Woyessa, D.; Tariku, Y. Antibacterial potential of Thevetia Peruviana leaf extracts against food associated bacterial pathogens. J. Coast. Life Med. 2015, 3, 150–157. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Verpoorte, R.; Svendsen, A.B.; Leeuwenberg, A.J.M.; Bisset, N.G. Tabernaemontana L. (Apocynaceae): A review of its taxonomy, phytochemistry, ethnobotany and pharmacology. J. Ethnopharmacol. 1984, 10, 1–156. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. Reversible acetylcholinesterase inhibitory effect of Tabernaemontana divaricata extract on synaptic transmission in rat CA1 hippocampus. Indian J. Med. Res. 2010, 131, 411–417. [Google Scholar] [PubMed]
- Islam, M.S.; Islam, N.; Ahsan, M.K.; Mahdi, S.H.A. Bio-efficacy of Tabernaemontana Divaricata (L.) leaf and stem bark extract against Callosobruchus chinensis L. Sch. Acad. J. Biosci. 2018, 6, 247–251. [Google Scholar] [CrossRef]
- Kumar, G.; Karthik, L.; Rao, K.V.B. Antibacterial activity of aqueous extract of Calotropis gigantea leaves—An in vitro study. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 141–144. [Google Scholar]
- Kumar, G.; Karthik, L.; Rao, K.V.B. In vitro anti-Candida activity of Calotropis gigantea. J. Pharm. Res. 2010, 3, 539–542. [Google Scholar]
- Singh, N.; Jain, N.K.; Kannojia, P.; Garud, N.; Pathak, A.K.; Mehta, S.C. In vitro antioxidant activity of Calotropis gigantea hydroalcohlic leaves extract. Der Pharm. Lett. 2010, 2, 95–100. [Google Scholar]
- Wong, S.; Lim, Y.; Abdullah, N.; Nordin, F. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Pharmacogn. Res. 2011, 3, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Ku, W.-F.; Tan, S.-J.; Low, Y.-Y.; Komiyama, K.; Kam, T.-S. Angustilobine and andranginine type indole alkaloids and an uleine–secovallesamine bisindole alkaloid from Alstonia angustiloba. Phytochemistry 2011, 72, 2212–2218. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Udino, L.; Philibert, L.; Bocage, B.; Archimede, H. In vitro effects of Cassava (Manihot esculenta) leaf extracts on four development stages of Haemonchus Contortus. Vet. Parasitol. 2010, 173, 85–92. [Google Scholar] [CrossRef]
- Yusuf, U.F.; Okechukwu, P.N. Anti-inflammatory, analgesic and anti—Pyretic activity of cassava leaves extract. Asia J. Pharm. Clin. Res. 2013, 6, 89–92. [Google Scholar]
- Mbahi, M.A.; Mbahi, A.M.; Umar, I.A.; Ameh, D.A.; Joseph, I.; Amos, P.I. Phytochemical screening and antimicrobial activity of the pulp extract and fractions of Ziziphus mauritiana. Biochem. Anal. Biochem. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Dutta, R.P.; Patil, M.B. Therapeutic potential of root and stem bark of wild medicinal plant Ziziphus mauritiana (Lamk.) against silica induced toxicity in Wistar albino rats. J. Ethnopharmacol. 2018, 224, 111–118. [Google Scholar] [CrossRef]
- Verbeke, P.; Fonager, J.; Clark, B.F.C.; Rattan, S.I.S. Heat shock response and ageing: Mechanisms and applications. Cell Biol. Int. 2001, 25, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Chen, C.; Hou, J.; Xin, W.; Mori, A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim. Biophys. Acta Mol. Cell Res. 2000, 1498, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.S.; Mirian, A.I.S.; Rodrigo, M.F.; Mariana, A.B.; Tamara, R.M.; Mariene, H.D.; Claudia, M.D.S.; Juliana, M.F.; Angelita, D.C. Antioxidants and chlorophyll in cassava leaves at three plant ages. Afr. J. Agric. Res. 2013, 8, 3724–3730. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Abu Bakar, M.F.; Abdul Karim, F.; Perisamy, E. Comparison of phytochemicals and antioxidant properties of different fruit parts of selected Artocarpus species from Sabah, Malaysia. Sains Malays. 2015, 44, 355–363. [Google Scholar] [CrossRef]
- Abdelhakim, I.A.; Abdel-baky, A.M.; Bishay, D.W. In vitro evaluation of antioxidant activity of Caryota mitis Lour. Leaves extracts. J. Pharmacogn. Phytochem. 2017, 6, 2559–2562. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Wang, M.; Meng, D.; Zhang, P.; Wang, X.; Du, G.; Brennan, C.; Li, S.; Ho, C.-T.; Zhao, H. Antioxidant protection of nobiletin, 5-demethylnobiletin, tangeretin, and 5-demethyltangeretin from citrus peel in Saccharomyces Cerevisiae. J. Agric. Food Chem. 2018, 66, 3155–3160. [Google Scholar] [CrossRef] [PubMed]


| PE (Organ) | Phytochemical | Biological Activity | Reference |
|---|---|---|---|
| Wodyetia bifurcate (leaf) | Methanol and butanol extracts contain triterpene, flavonoids, benzenoid and polyphenol | Weak cytotoxicity against human liver Hep-G2 cancer cell line | [30] |
| Thevetia peruviana (leaf) | Contains alkaloids, cardiac glycosides, flavonoids, polyphenols, saponins and tannins | Antibacterial activity against foodborne microorganisms; treatment for a diarrhoea-induced rat model | [31,32] |
| Tabernaemontana divaricate (leaf) | Diverse in alkaloids and non-alkaloids | Mild insecticides against crop pests; folk medicine; mimics the effect of acetylcholinesterase inhibitors towards Alzheimer’s disease | [33,34,35] |
| Calotropis gigantean (leaf) | Hydro-alcoholic extracts contain flavonoid, tannins, alkaloids, and steroids | Traditional medicine; antifungal against Candida sp.; antibacterial activity towards Escherichia coli., Pseudomonas aeruginosa and Bacillus cereus; antiproliferative effect against human cancer cell lines (MCF7, MDA-MB-231, and HeLa); antioxidant activity | [36,37,38,39] |
| Alstonia angustiloba (leaf) | Ethanol extracts contain alkaloids | Weak antiproliferative against human cancer cell lines (MCF7, MDA-MB-231, and HeLa) | [39,40] |
| Manihot esculenta (leaf) | Ethanol extracts contain terpenoids, flavonoids, carotenoids and tannins | In vivo anti-inflammatory on rat model with paw oedema; contains antipyretic activity, which mimics the effect of paracetamol; anthelmintic activity against gastrointestinal parasite Haemonchus contortus | [41,42] |
| Ziziphus mauritiana (stem) | Various solvent extraction contains alkaloids, flavonoids, tannins, carotenoids, saponins, steroids, triterpenoids and anthraquinones | Traditional medicine; in vivo anti-ulcers against aspirin and ethanol ulcers in mice model; detoxification of silica-induced toxicity in the liver of Albino rats; antibacterial against pathogens such as Salmonella typhi, Bacillus subtilis and Staphylococcus aureus | [12,43,44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwong, M.M.Y.; Lee, J.W.; Samian, M.R.; Wahab, H.A.; Watanabe, N.; Ong, E.B.B. Identification of Tropical Plant Extracts That Extend Yeast Chronological Life Span. Cells 2021, 10, 2718. https://doi.org/10.3390/cells10102718
Kwong MMY, Lee JW, Samian MR, Wahab HA, Watanabe N, Ong EBB. Identification of Tropical Plant Extracts That Extend Yeast Chronological Life Span. Cells. 2021; 10(10):2718. https://doi.org/10.3390/cells10102718
Chicago/Turabian StyleKwong, Mandy Mun Yee, Jee Whu Lee, Mohammed Razip Samian, Habibah A. Wahab, Nobumoto Watanabe, and Eugene Boon Beng Ong. 2021. "Identification of Tropical Plant Extracts That Extend Yeast Chronological Life Span" Cells 10, no. 10: 2718. https://doi.org/10.3390/cells10102718
APA StyleKwong, M. M. Y., Lee, J. W., Samian, M. R., Wahab, H. A., Watanabe, N., & Ong, E. B. B. (2021). Identification of Tropical Plant Extracts That Extend Yeast Chronological Life Span. Cells, 10(10), 2718. https://doi.org/10.3390/cells10102718







