Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences
Abstract
:1. Introduction
2. Atrial Cardiomyopathy
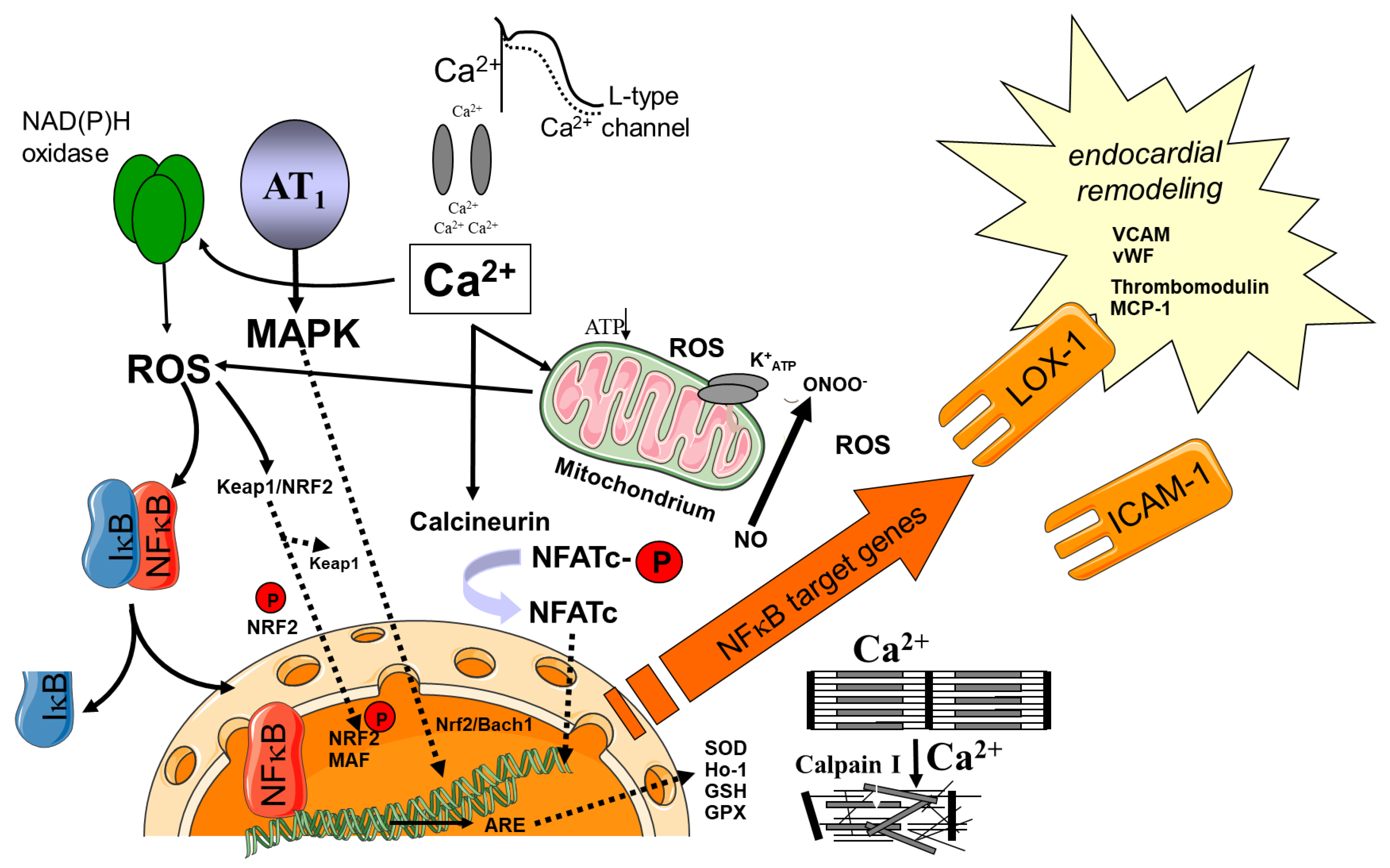
3. Oxidative Stress as Central Mediator for Atrial Electrical and Structural Remodeling in AF
4. Sources of ROS/RNS
4.1. Redox-Regulated Signaling Pathways
4.2. Role of Cellular Inflammatory Pathways
4.3. Role of Adipose Tissue/Obesity and Diabetes
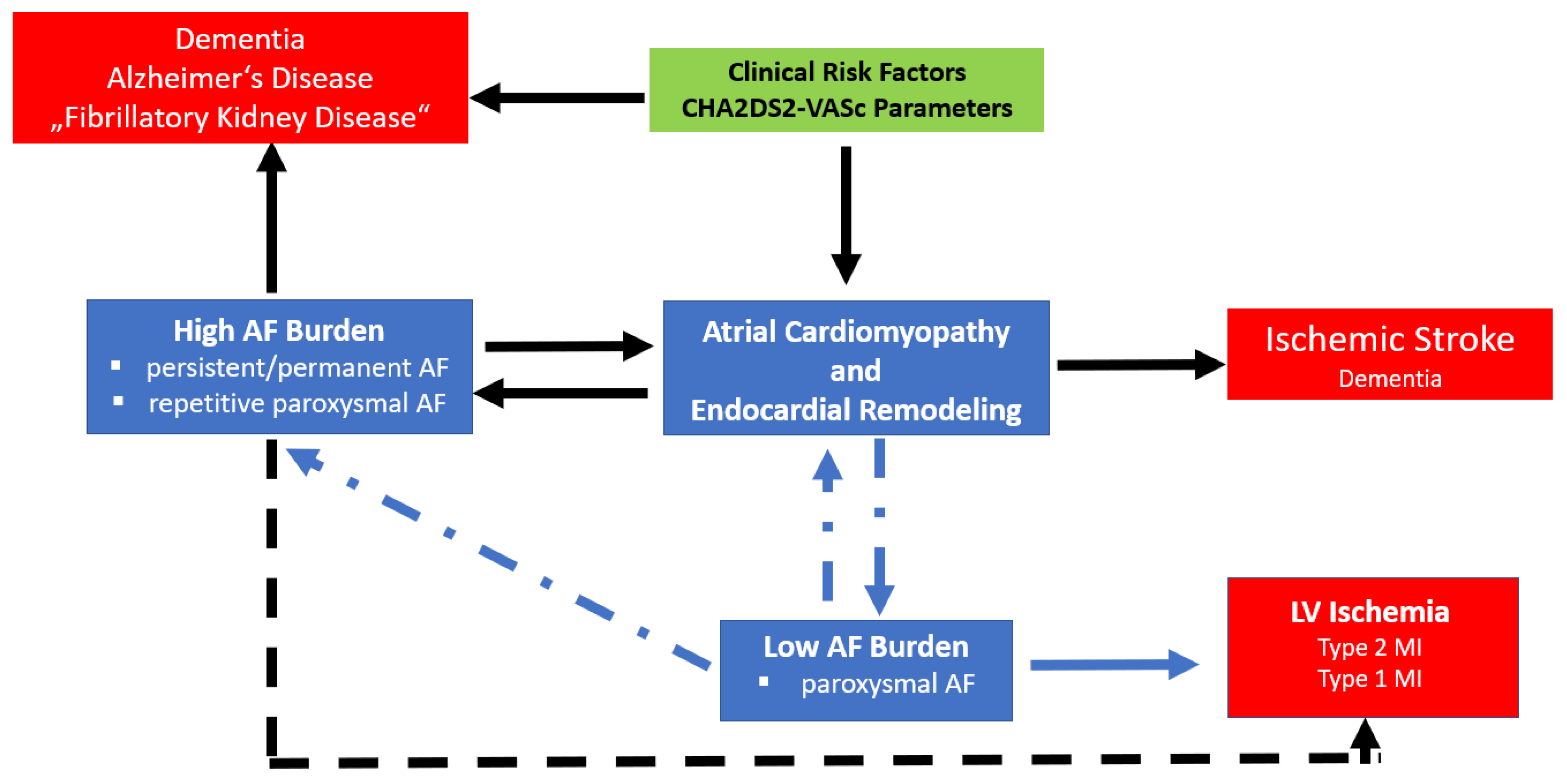
5. Atrial Endocardial Remodeling as a Cause of Thrombogenesis and Stroke
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Honeycutt, C.; Langberg, J.J. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation 1996, 94, 2968–2974. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. EP Eur. 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Felgendreher, M.; Scholz, B.; Wolke, C.; Schulte, J.S.; Fehrmann, E.; Wardelmann, E.; Seidl, M.D.; Lendeckel, U.; Himmler, K.; et al. CREM-transgene mice: An animal model of atrial fibrillation and thrombogenesis. Thromb. Res. 2018, 163, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef]
- Chilukoti, R.K.; Mostertz, J.; Bukowska, A.; Aderkast, C.; Felix, S.B.; Busch, M.; Volker, U.; Goette, A.; Wolke, C.; Homuth, G.; et al. Effects of irbesartan on gene expression revealed by transcriptome analysis of left atrial tissue in a porcine model of acute rapid pacing in vivo. Int. J. Cardiol. 2013, 168, 2100–2108. [Google Scholar] [CrossRef]
- Goette, A.; Bukowska, A.; Dobrev, D.; Pfeiffenberger, J.; Morawietz, H.; Strugala, D.; Wiswedel, I.; Rohl, F.W.; Wolke, C.; Bergmann, S.; et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur. Heart J. 2009, 30, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Torp-Pedersen, C.; Goette, A.; Nielsen, P.B.; Potpara, T.; Fauchier, L.; John Camm, A.; Arbelo, E.; Boriani, G.; Skjoeth, F.; Rumsfeld, J.; et al. ‘Real-world’ observational studies in arrhythmia research: Data sources, methodology, and interpretation. A position document from European Heart Rhythm Association (EHRA), endorsed by Heart Rhythm Society (HRS), Asia-Pacific HRS (APHRS), and Latin America HRS (LAHRS). EP Eur. 2020, 22, 831–832. [Google Scholar] [CrossRef]
- Shah, A.K.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Oxidative Stress as A Mechanism for Functional Alterations in Cardiac Hypertrophy and Heart Failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef]
- Singal, P.K.; Khaper, N.; Farahmand, F.; Bello-Klein, A. Oxidative stress in congestive heart failure. Curr. Cardiol. Rep. 2000, 2, 206–211. [Google Scholar] [CrossRef]
- Singal, P.K.; Khaper, N.; Palace, V.; Kumar, D. The role of oxidative stress in the genesis of heart disease. Cardiovasc. Res. 1998, 40, 426–432. [Google Scholar] [CrossRef]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Kang, Y.J. Oxidative stress and diabetic cardiomyopathy: A brief review. Cardiovasc. Toxicol. 2001, 1, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, M.; Chen, C.; Mai, M.; Huang, J.; Zhu, P. Roles of Reactive Oxygen Species in Cardiac Differentiation, Reprogramming, and Regenerative Therapies. Oxidative Med. Cell. Longev. 2020, 2020, 2102841. [Google Scholar] [CrossRef]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Bonetto, V.; Fratelli, M. Thiol-disulfide balance: From the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005, 7, 964–972. [Google Scholar] [CrossRef]
- Goette, A.; Bukowska, A.; Lillig, C.H.; Lendeckel, U. Oxidative Stress and Microcirculatory Flow Abnormalities in the Ventricles during Atrial Fibrillation. Front. Physiol. 2012, 3, 236. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Yang, K.C.; Dudley, S.C., Jr. Oxidative stress and atrial fibrillation: Finding a missing piece to the puzzle. Circulation 2013, 128, 1724–1726. [Google Scholar] [CrossRef] [Green Version]
- Negi, S.; Sovari, A.A.; Dudley, S.C., Jr. Atrial fibrillation: The emerging role of inflammation and oxidative stress. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 262–268. [Google Scholar] [CrossRef]
- Mihm, M.J.; Yu, F.; Carnes, C.A.; Reiser, P.J.; McCarthy, P.M.; Van Wagoner, D.R.; Bauer, J.A. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 2001, 104, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Carnes, C.A.; Chung, M.K.; Nakayama, T.; Nakayama, H.; Baliga, R.S.; Piao, S.; Kanderian, A.; Pavia, S.; Hamlin, R.L.; McCarthy, P.M.; et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ. Res. 2001, 89, E32–E38. [Google Scholar] [CrossRef]
- Schild, L.; Bukowska, A.; Gardemann, A.; Polczyk, P.; Keilhoff, G.; Tager, M.; Dudley, S.C.; Klein, H.U.; Goette, A.; Lendeckel, U. Rapid pacing of embryoid bodies impairs mitochondrial ATP synthesis by a calcium-dependent mechanism—A model of in vitro differentiated cardiomyocytes to study molecular effects of tachycardia. Biochim. Biophys. Acta 2006, 1762, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Bukowska, A.; Schild, L.; Keilhoff, G.; Hirte, D.; Neumann, M.; Gardemann, A.; Neumann, K.H.; Rohl, F.W.; Huth, C.; Goette, A.; et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp. Biol. Med. 2008, 233, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Ben Abraham, R.; Matza, M.; Marmor, S.; Rudick, V.; Frolkis, I.; Shapira, I.; Weinbroum, A.A. Electromechanical impairment of human auricle and rat myocardial strip subjected to exogenous oxidative stress. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2003, 23, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Dhalla, N.S.; Temsah, R.M. Sarcoplasmic reticulum and cardiac oxidative stress: An emerging target for heart disease. Expert Opin. Ther. Targets 2001, 5, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Loh, S.H.; Jin, J.S.; Tsai, C.S.; Chao, C.M.; Tsai, Y.; Chen, W.H.; Cheng, T.H.; Chuang, C.C.; Lin, C.I. Possible underlying mechanism for hydrogen peroxide-induced electromechanical suppression in human atrial myocardium. J. Pharmacol. Sci. 2003, 91, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Polina, I.; Jansen, H.J.; Li, T.; Moghtadaei, M.; Bohne, L.J.; Liu, Y.; Krishnaswamy, P.; Egom, E.E.; Belke, D.D.; Rafferty, S.A.; et al. Loss of insulin signaling may contribute to atrial fibrillation and atrial electrical remodeling in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2020, 117, 7990–8000. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef]
- Rosa, C.M.; Xavier, N.P.; Henrique Campos, D.; Fernandes, A.A.; Cezar, M.D.; Martinez, P.F.; Cicogna, A.C.; Gimenes, C.; Gimenes, R.; Okoshi, M.P.; et al. Diabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive rats. Cardiovasc. Diabetol. 2013, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sensi, F.; Costantino, S.; Limbruno, U.; Paneni, F. Atrial fibrillation in the cardiometabolic patient. Minerva Med. 2019, 110, 157–167. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.D.; Hong, L.; Sridhar, A.; Menon, A.; Perike, S.; Zhang, M.; da Silva, I.B.; Yan, J.; Bonini, M.G.; Ai, X.; et al. Ion Channel and Structural Remodeling in Obesity-Mediated Atrial Fibrillation. Circulation. Arrhythmia Electrophysiol. 2020, 13, e008296. [Google Scholar] [CrossRef]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of Mitochondrial Dysfunction and Monoamine Oxidase Contribution to Oxidative Stress in Human Diabetic Hearts. Oxidative Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.H.; Lee, S.H.; Su, C.P.; Wei, Y.H. Oxidative damage to mitochondrial DNA in atrial muscle of patients with atrial fibrillation. Free. Radic. Biol. Med. 2003, 35, 1310–1318. [Google Scholar] [CrossRef]
- Rennison, J.H.; Li, L.; Lin, C.R.; Lovano, B.S.; Castel, L.; Wass, S.Y.; Cantlay, C.C.; McHale, M.; Gillinov, A.M.; Mehra, R.; et al. Atrial fibrillation rhythm is associated with marked changes in metabolic and myofibrillar protein expression in left atrial appendage. Pflügers Arch. Eur. J. Physiol. 2021, 473, 461–475. [Google Scholar] [CrossRef]
- Ausma, J.; Wijffels, M.; Thone, F.; Wouters, L.; Allessie, M.; Borgers, M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 1997, 96, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lim, D.S.; Lee, J.H.; Shim, W.J.; Ro, Y.M.; Park, G.H.; Becker, K.G.; Cho-Chung, Y.S.; Kim, M.K. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp. Mol. Med. 2003, 35, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.; Tsai, Y.T.; Lee, C.Y.; Chang, C.Y.; Tsai, C.S.; Cheng, T.H.; Lin, C.I. Antiarrhythmic effects of dehydroevodiamine in isolated human myocardium and cardiomyocytes. J. Ethnopharmacol. 2014, 153, 753–762. [Google Scholar] [CrossRef]
- Bukowska, A.; Hammwohner, M.; Sixdorf, A.; Schild, L.; Wiswedel, I.; Rohl, F.W.; Wolke, C.; Lendeckel, U.; Aderkast, C.; Bochmann, S.; et al. Dronedarone prevents microcirculatory abnormalities in the left ventricle during atrial tachypacing in pigs. Br. J. Pharmacol. 2012, 166, 964–980. [Google Scholar] [CrossRef] [Green Version]
- Steenman, M. Insight into atrial fibrillation through analysis of the coding transcriptome in humans. Biophys. Rev. 2020, 12, 817–826. [Google Scholar] [CrossRef]
- Haas Bueno, R.; Recamonde-Mendoza, M. Meta-analysis of Transcriptomic Data Reveals Pathophysiological Modules Involved with Atrial Fibrillation. Mol. Diagn. Ther. 2020, 24, 737–751. [Google Scholar] [CrossRef]
- De Souza, A.I.; Cardin, S.; Wait, R.; Chung, Y.L.; Vijayakumar, M.; Maguy, A.; Camm, A.J.; Nattel, S. Proteomic and metabolomic analysis of atrial profibrillatory remodeling in congestive heart failure. J. Mol. Cell. Cardiol. 2010, 49, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.; Li, Q.; Gao, X.; Sugano, E.; Tomita, H.; Yang, L.; Shi, S. Thioredoxin 2 Offers Protection against Mitochondrial Oxidative Stress in H9c2 Cells and against Myocardial Hypertrophy Induced by Hyperglycemia. Int. J. Mol. Sci. 2017, 18, 1958. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006, 34, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ravens, U.; Liu, G.S.; Vandeplassche, G.; Borgers, M. Protection of human, rat, and guinea-pig atrial muscle by mioflazine, lidoflazine, and verapamil against the destructive effects of high concentrations of Ca2+. Cardiovasc. Drugs Ther. Spons. Int. Soc. Cardiovasc. Pharmacother. 1992, 6, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free. Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wieckowski, M.R.; Lebiedzinska, M.; Wojtczak, L. Mitochondrial fatty acid oxidation and oxidative stress: Lack of reverse electron transfer-associated production of reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.P.; Mak, I.T.; Walter, M.F.; Tulenko, T.N.; Mason, P.E. Antioxidant and cytoprotective activities of the calcium channel blocker mibefradil. Biochem. Pharmacol. 1998, 55, 1843–1852. [Google Scholar] [CrossRef]
- Rivard, L.; Sinno, H.; Shiroshita-Takeshita, A.; Schram, G.; Leung, T.K.; Nattel, S. The pharmacological response of ischemia-related atrial fibrillation in dogs: Evidence for substrate-specific efficacy. Cardiovasc. Res. 2007, 74, 104–113. [Google Scholar] [CrossRef]
- Sinno, H.; Derakhchan, K.; Libersan, D.; Merhi, Y.; Leung, T.K.; Nattel, S. Atrial ischemia promotes atrial fibrillation in dogs. Circulation 2003, 107, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Qi, X.Y.; Wakili, R.; Comtois, P.; Chartier, D.; Harada, M.; Iwasaki, Y.K.; Romeo, P.; Maguy, A.; Dobrev, D.; et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation 2011, 123, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.K.; Lai, M.S.; Chen, Y.C.; Cheng, C.C.; Huang, J.H.; Chen, S.A.; Chen, Y.J.; Lin, C.I. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin. Sci. 2012, 122, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afanas’ev, I. ROS and RNS signaling in heart disorders: Could antioxidant treatment be successful? Oxidative Med. Cell. Longev. 2011, 2011, 293769. [Google Scholar] [CrossRef] [Green Version]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaimes, E.A.; Galceran, J.M.; Raij, L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998, 54, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Yasunari, K.; Maeda, K.; Nakamura, M.; Yoshikawa, J. Pressure promotes angiotensin II--mediated migration of human coronary smooth muscle cells through increase in oxidative stress. Hypertension 2002, 39, 433–437. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Gall, N.P.; Grieve, D.J.; Chen, M.; Shah, A.M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 2002, 40, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forro, L.; Schlegel, W.; Krause, K.H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shimizu, H.; Siu, K.L.; Mahajan, A.; Chen, J.N.; Cai, H. NADPH Oxidase 4 Induces Cardiac Arrhythmic Phenotype in Zebrafish. J. Biol. Chem. 2014, 289, 23200–23208. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.; Clempus, R.; Lassegue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.X.; Ren, K.; Guan, Y.; Wang, Y.T.; Shan, Z.L. Protective effects of apocynin on atrial electrical remodeling and oxidative stress in a rabbit rapid atrial pacing model. Chin. J. Physiol. 2014, 57, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mighiu, A.S.; Recalde, A.; Ziberna, K.; Carnicer, R.; Tomek, J.; Bub, G.; Brewer, A.C.; Verheule, S.; Shah, A.M.; Simon, J.N.; et al. Inducibility, but not stability, of atrial fibrillation is increased by NOX2 overexpression in mice. Cardiovasc. Res. 2021, cvab019. [Google Scholar] [CrossRef]
- Sakabe, M.; Fujiki, A.; Sakamoto, T.; Nakatani, Y.; Mizumaki, K.; Inoue, H. Xanthine oxidase inhibition prevents atrial fibrillation in a canine model of atrial pacing-induced left ventricular dysfunction. J. Cardiovasc. Electrophysiol. 2012, 23, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Efird, J.T.; Davies, S.W.; O’Neal, W.T.; Darden, T.M.; Thayne, K.A.; Katunga, L.A.; Kindell, L.C.; Ferguson, T.B.; Anderson, C.A.; et al. Monoamine oxidase is a major determinant of redox balance in human atrial myocardium and is associated with postoperative atrial fibrillation. J. Am. Heart Assoc. 2014, 3, e000713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: Implications for the antiarrhythmic effect of statins. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielis, J.F.; Lin, J.Y.; Wingler, K.; Van Schil, P.E.; Schmidt, H.H.; Moens, A.L. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free. Radic. Biol. Med. 2011, 50, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Frohlich, L.G.; Kotsonis, P.; Frey, A.; Bommel, H.M.; Wink, D.A.; Pfleiderer, W.; Schmidt, H.H. Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J. Biol. Chem. 1999, 274, 24921–24929. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C., Jr.; Harrison, D.G.; Langberg, J.J. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: Potential mechanisms for atrial thrombosis and stroke. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef] [Green Version]
- Minamino, T.; Kitakaze, M.; Sato, H.; Asanuma, H.; Funaya, H.; Koretsune, Y.; Hori, M. Plasma levels of nitrite/nitrate and platelet cGMP levels are decreased in patients with atrial fibrillation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3191–3195. [Google Scholar] [CrossRef]
- Nikitovic, D.; Zacharis, E.A.; Manios, E.G.; Malliaraki, N.E.; Kanoupakis, E.M.; Sfiridaki, K.I.; Skalidis, E.I.; Margioris, A.N.; Vardas, P.E. Plasma Levels of Nitrites/Nitrates in Patients with Chronic Atrial Fibrillation are Increased after Electrical Restoration of Sinus Rhythm. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2002, 7, 171–176. [Google Scholar] [CrossRef]
- Goette, A.; Hammwohner, M.; Bukowska, A.; Scalera, F.; Martens-Lobenhoffer, J.; Dobrev, D.; Ravens, U.; Weinert, S.; Medunjanin, S.; Lendeckel, U.; et al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int. J. Cardiol. 2012, 154, 141–146. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Liang, Z.; Chen, W.; Xia, W.; Song, Y. Variance of DDAH/PRMT/ADMA pathway in atrial fibrillation dogs. Biochem. Biophys. Res. Commun. 2008, 377, 884–888. [Google Scholar] [CrossRef]
- Feng, Q.; Lu, X.; Fortin, A.J.; Pettersson, A.; Hedner, T.; Kline, R.L.; Arnold, J.M. Elevation of an endogenous inhibitor of nitric oxide synthesis in experimental congestive heart failure. Cardiovasc. Res. 1998, 37, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, M.; Osanai, T.; Kamada, T.; Matsunaga, T.; Ishizaka, H.; Hanada, H.; Okumura, K. High plasma level of asymmetric dimethylarginine in patients with acutely exacerbated congestive heart failure: Role in reduction of plasma nitric oxide level. Heart Vessel. 2003, 18, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sekiguchi, A.; Kato, T.; Tsuneda, T.; Iwasaki, Y.K.; Sagara, K.; Iinuma, H.; Sawada, H.; Aizawa, T. Angiotensin type 1 receptor blockade prevents endocardial dysfunction of rapidly paced atria in rats. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2007, 8, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; No, C.W.; Goo, S.H.; Cha, T.J. An Angiotensin receptor blocker prevents arrhythmogenic left atrial remodeling in a rat post myocardial infarction induced heart failure model. J. Korean Med. Sci. 2013, 28, 700–708. [Google Scholar] [CrossRef]
- Bukowska, A.; Rocken, C.; Erxleben, M.; Rohl, F.W.; Hammwohner, M.; Huth, C.; Ebert, M.P.; Lendeckel, U.; Goette, A. Atrial expression of endothelial nitric oxide synthase in patients with and without atrial fibrillation. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2010, 19, e51–e60. [Google Scholar] [CrossRef]
- Kaludercic, N.; Carpi, A.; Nagayama, T.; Sivakumaran, V.; Zhu, G.; Lai, E.W.; Bedja, D.; De Mario, A.; Chen, K.; Gabrielson, K.L.; et al. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid. Redox Signal. 2014, 20, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Kaludercic, N.; Takimoto, E.; Nagayama, T.; Feng, N.; Lai, E.W.; Bedja, D.; Chen, K.; Gabrielson, K.L.; Blakely, R.D.; Shih, J.C.; et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010, 106, 193–202. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free. Radic. Biol. Med. 2013, 61C, 473–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, A.; Rokita, A.G.; Guan, X.; Chen, B.; Koval, O.M.; Voigt, N.; Neef, S.; Sowa, T.; Gao, Z.; Luczak, E.D.; et al. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013, 128, 1748–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luczak, E.D.; Anderson, M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 2014, 73, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schondube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Dudley, S.C., Jr. Redox regulation, NF-kappaB, and atrial fibrillation. Antioxid. Redox Signal. 2009, 11, 2265–2277. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.L.; Dudley, S.C., Jr. Tandem promoters and developmentally regulated 5′- and 3′-mRNA untranslated regions of the mouse Scn5a cardiac sodium channel. J. Biol. Chem. 2005, 280, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.L.; Sanyal, S.; Pfahnl, A.E.; Jiao, Z.; Allen, J.; Liu, H.; Dudley, S.C., Jr. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. American journal of physiology. Cell Physiol. 2008, 294, C372–C379. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998, 392, 933–936. [Google Scholar] [CrossRef]
- Kunsch, C.; Medford, R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999, 85, 753–766. [Google Scholar] [CrossRef]
- Burstein, B.; Libby, E.; Calderone, A.; Nattel, S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008, 117, 1630–1641. [Google Scholar] [CrossRef] [Green Version]
- Adam, O.; Lavall, D.; Theobald, K.; Hohl, M.; Grube, M.; Ameling, S.; Sussman, M.A.; Rosenkranz, S.; Kroemer, H.K.; Schafers, H.J.; et al. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J. Am. Coll. Cardiol. 2010, 55, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Sulciner, D.J.; Irani, K.; Yu, Z.X.; Ferrans, V.J.; Goldschmidt-Clermont, P.; Finkel, T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol. Cell. Biol. 1996, 16, 7115–7121. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Yuan, J.; Liu, G.; Ling, Z.; Zeng, H.; Chen, Y.; Zhang, Y.; She, Q.; Zhou, X. Angiotensin receptor blockers and statins could alleviate atrial fibrosis via regulating platelet-derived growth factor/Rac1/nuclear factor-kappa B Axis. Int. J. Med. Sci. 2013, 10, 812–824. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Chiang, L.L.; Lin, C.H.; Shih, C.H.; Liao, Y.T.; Hsu, M.J.; Chen, B.C. Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur. J. Pharmacol. 2007, 560, 101–109. [Google Scholar] [CrossRef]
- Tyrrell, R. Redox regulation and oxidant activation of heme oxygenase-1. Free. Radic. Res. 1999, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriakidis, S.; Andreakos, E.; Monaco, C.; Foxwell, B.; Feldmann, M.; Paleolog, E. VEGF expression in human macrophages is NF-kappaB-dependent: Studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J. Cell Sci. 2003, 116, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.M.; Seth, P.; Durham, L.; Diaz, F.; Boursiquot, R.; Ransohoff, R.M.; Major, E.O. Astrocyte differentiation selectively upregulates CCL2/monocyte chemoattractant protein-1 in cultured human brain-derived progenitor cells. Glia 2006, 53, 81–91. [Google Scholar] [CrossRef]
- Bonello, S.; Zahringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Gorlach, A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. CMLS 2006, 63, 2560–2570. [Google Scholar] [CrossRef]
- Bloom, D.A.; Jaiswal, A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003, 278, 44675–44682. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Huang, L.; Liu, J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review). Exp. Ther. Med. 2021, 22, 678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ku, C.H.; Siow, R.C. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free. Radic. Biol. Med. 2013, 64, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Raghavan, P.; Thomou, T.; Boucher, J.; Robida-Stubbs, S.; Macotela, Y.; Russell, S.J.; Kirkland, J.L.; Blackwell, T.K.; Kahn, C.R. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012, 16, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Tucsek, Z.; Sosnowska, D.; Toth, P.; Gautam, T.; Podlutsky, A.; Csiszar, A.; Losonczy, G.; Valcarcel-Ares, M.N.; Sonntag, W.E.; et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2013, 68, 877–891. [Google Scholar] [CrossRef] [Green Version]
- Wiesen, J.L.; Tomasi, T.B. Dicer is regulated by cellular stresses and interferons. Mol. Immunol. 2009, 46, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Santulli, G.; Iaccarino, G.; De Luca, N.; Trimarco, B.; Condorelli, G. Atrial fibrillation and microRNAs. Front. Physiol. 2014, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 2010, 122, 2378–2387. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yang, B. MicroRNAs and atrial fibrillation: New fundamentals. Cardiovasc. Res. 2011, 89, 710–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chen, G.X.; Liang, M.Y.; Qin, H.; Rong, J.; Yao, J.P.; Wu, Z.K. Atrial fibrillation alters the microRNA expression profiles of the left atria of patients with mitral stenosis. BMC Cardiovasc. Disord. 2014, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Qin, H.; Chen, G.X.; Liang, M.Y.; Rong, J.; Yao, J.P.; Wu, Z.K. Comparative expression profiles of microRNA in left and right atrial appendages from patients with rheumatic mitral valve disease exhibiting sinus rhythm or atrial fibrillation. J. Transl. Med. 2014, 12, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldan, V.; Arroyo, A.B.; Salloum-Asfar, S.; Manzano-Fernandez, S.; Garcia-Barbera, N.; Marin, F.; Vicente, V.; Gonzalez-Conejero, R.; Martinez, C. Prognostic role of MIR146A polymorphisms for cardiovascular events in atrial fibrillation. Thromb. Haemost. 2014, 112, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Sadek, H.A. The cardiac hypoxic niche: Emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc. Diagn. Ther. 2012, 2, 278–289. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Frantz, G.; LeCouter, J.; Dillard-Telm, L.; Pham, T.; Draksharapu, A.; Giordano, T.; Peale, F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am. J. Pathol. 2003, 162, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Adam, O.; Theobald, K.; Lavall, D.; Grube, M.; Kroemer, H.K.; Ameling, S.; Schafers, H.J.; Bohm, M.; Laufs, U. Increased lysyl oxidase expression and collagen cross-linking during atrial fibrillation. J. Mol. Cell. Cardiol. 2011, 50, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 2003, 278, 14387–14393. [Google Scholar] [CrossRef] [Green Version]
- Maki, J.M.; Rasanen, J.; Tikkanen, H.; Sormunen, R.; Makikallio, K.; Kivirikko, K.I.; Soininen, R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 2002, 106, 2503–2509. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Alcudia, J.F.; Martinez-Gonzalez, J.; Raposo, B.; Navarro, M.A.; Badimon, L. Lysyl oxidase (LOX) down-regulation by TNFalpha: A new mechanism underlying TNFalpha-induced endothelial dysfunction. Atherosclerosis 2008, 196, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Smith-Mungo, L.I.; Kagan, H.M. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol. J. Int. Soc. Matrix Biol. 1998, 16, 387–398. [Google Scholar] [CrossRef]
- Li, W.; Nellaiappan, K.; Strassmaier, T.; Graham, L.; Thomas, K.M.; Kagan, H.M. Localization and activity of lysyl oxidase within nuclei of fibrogenic cells. Proc. Natl. Acad. Sci. USA 1997, 94, 12817–12822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.; Martinez-Gonzalez, J.; Raposo, B.; Alcudia, J.F.; Guadall, A.; Badimon, L. Regulation of lysyl oxidase in vascular cells: Lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc. Res. 2008, 79, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, H.M.; Cruikshank, W.W.; Narasimhan, N.; Kagan, H.M.; Center, D.M. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. J. Int. Soc. Matrix Biol. 1995, 14, 727–731. [Google Scholar] [CrossRef]
- Li, W.; Liu, G.; Chou, I.N.; Kagan, H.M. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J. Cell. Biochem. 2000, 78, 550–557. [Google Scholar] [CrossRef]
- Goette, A. Is left atrial strain the pathophysiological link between transplanted stem cells and atrial fibrillation? Int. J. Cardiol. 2021, 339, 60–61. [Google Scholar] [CrossRef]
- Goette, A.; Jentsch-Ullrich, K.; Lendeckel, U.; Rocken, C.; Agbaria, M.; Auricchio, A.; Mohren, M.; Franke, A.; Klein, H.U. Effect of atrial fibrillation on hematopoietic progenitor cells: A novel pathophysiological role of the atrial natriuretic peptide? Circulation 2003, 108, 2446–2449. [Google Scholar] [CrossRef] [Green Version]
- Galea, R.; Cardillo, M.T.; Caroli, A.; Marini, M.G.; Sonnino, C.; Narducci, M.L.; Biasucci, L.M. Inflammation and C-reactive protein in atrial fibrillation: Cause or effect? Tex. Heart Inst. J. 2014, 41, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Suthahar, N.; Meijers, W.C.; Sillje, H.H.W.; de Boer, R.A. From Inflammation to Fibrosis-Molecular and Cellular Mechanisms of Myocardial Tissue Remodeling and Perspectives on Differential Treatment Opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef] [Green Version]
- Vonderlin, N.; Siebermair, J.; Kaya, E.; Kohler, M.; Rassaf, T.; Wakili, R. Critical inflammatory mechanisms underlying arrhythmias. Herz 2019, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C.; Bellocci, F.; Morgante, E.; Russo, M.A.; Maseri, A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997, 96, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chen, Y.C.; Chen, Y.J.; Chang, S.L.; Tai, C.T.; Wongcharoen, W.; Yeh, H.I.; Lin, C.I.; Chen, S.A. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007, 80, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Wakili, R.; Voigt, N.; Kaab, S.; Dobrev, D.; Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Investig. 2011, 121, 2955–2968. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.D.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Kim, N.; Jung, Y.; Nam, M.; Sun Kang, M.; Lee, M.K.; Cho, Y.; Choi, E.K.; Hwang, G.S.; Soo Kim, H. Angiotensin II affects inflammation mechanisms via AMPK-related signalling pathways in HL-1 atrial myocytes. Sci. Rep. 2017, 7, 10328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goette, A.; Lendeckel, U. Electrophysiological effects of angiotensin II. Part I: Signal transduction and basic electrophysiological mechanisms. EP Eur. 2008, 10, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Psychari, S.N.; Apostolou, T.S.; Sinos, L.; Hamodraka, E.; Liakos, G.; Kremastinos, D.T. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am. J. Cardiol. 2005, 95, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Aulin, J.; Siegbahn, A.; Hijazi, Z.; Ezekowitz, M.D.; Andersson, U.; Connolly, S.J.; Huber, K.; Reilly, P.A.; Wallentin, L.; Oldgren, J. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am. Heart J. 2015, 170, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.; Martin, D.O.; Marrouche, N.F.; Shaaraoui, M.; Chung, M.K.; Almahameed, S.; Schweikert, R.A.; Saliba, W.I.; Natale, A. C reactive protein concentration and recurrence of atrial fibrillation after electrical cardioversion. Heart 2005, 91, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Dai, L.; Song, Z.; Li, H.; Shu, M. Association between C-reactive protein and atrial fibrillation recurrence after catheter ablation: A meta-analysis. Clin. Cardiol. 2013, 36, 548–554. [Google Scholar] [CrossRef]
- Yo, C.H.; Lee, S.H.; Chang, S.S.; Lee, M.C.; Lee, C.C. Value of high-sensitivity C-reactive protein assays in predicting atrial fibrillation recurrence: A systematic review and meta-analysis. BMJ Open 2014, 4, e004418. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Sullivan, L.M.; Pencina, M.J.; Meigs, J.B.; Tofler, G.H.; Selhub, J.; Jacques, P.F.; Wolf, P.A.; et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010, 121, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.S.; Barnes, M.E.; Miyasaka, Y.; Cha, S.S.; Bailey, K.R.; Verzosa, G.C.; Seward, J.B.; Gersh, B.J. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: A longitudinal cohort study of 21 years. Eur. Heart J. 2008, 29, 2227–2233. [Google Scholar] [CrossRef]
- Wanahita, N.; Messerli, F.H.; Bangalore, S.; Gami, A.S.; Somers, V.K.; Steinberg, J.S. Atrial fibrillation and obesity—Results of a meta-analysis. Am. Heart J. 2008, 155, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B., Sr.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Tanabe, N.; Watanabe, T.; Darbar, D.; Roden, D.M.; Sasaki, S.; Aizawa, Y. Metabolic syndrome and risk of development of atrial fibrillation: The Niigata preventive medicine study. Circulation 2008, 117, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Habib, R.H. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation 2005, 112, 3247–3255. [Google Scholar] [CrossRef] [Green Version]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur. Heart J. 2009, 30, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef] [Green Version]
- Tsao, H.M.; Hu, W.C.; Wu, M.H.; Tai, C.T.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Wu, T.J.; et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Goette, A.; Lendeckel, U.; Kuchenbecker, A.; Bukowska, A.; Peters, B.; Klein, H.U.; Huth, C.; Rocken, C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 2007, 93, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clement, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805. [Google Scholar] [CrossRef] [Green Version]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodeling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Puceat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef]
- Goette, A.; Patscheke, M.; Henschke, F.; Hammwohner, M. COVID-19-Induced Cytokine Release Syndrome Associated with Pulmonary Vein Thromboses, Atrial Cardiomyopathy, and Arterial Intima Inflammation. TH Open 2020, 4, e271–e279. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Batal, O.; Schoenhagen, P.; Shao, M.; Ayyad, A.E.; Van Wagoner, D.R.; Halliburton, S.S.; Tchou, P.J.; Chung, M.K. Left atrial epicardial adiposity and atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2010, 3, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, K.; Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, M.; Mano, H.; Sonoda, K.; Hiro, T.; Nikaido, M.; et al. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ. Arrhythmia Electrophysiol. 2012, 5, 676–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Yong, H.S.; Lim, H.E.; Na, J.O.; Choi, C.U.; Choi, J.I.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Park, S.W.; et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011, 22, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Hammwohner, M.; Bukowska, A.; Mahardika, W.; Goette, A. Clinical importance of atrial cardiomyopathy. Int. J. Cardiol. 2019, 287, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kumagai, K.; Minami, K.; Nakano, M.; Inoue, H.; Oshima, S. Location of epicardial adipose tissue affects the efficacy of a combined dominant frequency and complex fractionated atrial electrogram ablation of atrial fibrillation. Heart Rhythm. 2015, 12, 257–265. [Google Scholar] [CrossRef]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef] [Green Version]
- Bruins, P.; te Velthuis, H.; Yazdanbakhsh, A.P.; Jansen, P.G.; van Hardevelt, F.W.; de Beaumont, E.M.; Wildevuur, C.R.; Eijsman, L.; Trouwborst, A.; Hack, C.E. Activation of the complement system during and after cardiopulmonary bypass surgery: Postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997, 96, 3542–3548. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Takeishi, Y.; Hirono, O.; Itoh, M.; Matsui, M.; Nakamura, K.; Tamada, Y.; Kubota, I. C-reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessel. 2005, 20, 45–49. [Google Scholar] [CrossRef]
- Ozcan, K.S.; Gungor, B.; Altay, S.; Osmonov, D.; Ekmekci, A.; Ozpamuk, F.; Kemaloglu, T.; Yildirim, A.; Tayyareci, G.; Erdinler, I. Increased level of resistin predicts development of atrial fibrillation. J. Cardiol. 2014, 63, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gungor, H.; Ayik, M.F.; Kirilmaz, B.; Ertugay, S.; Gul, I.; Yildiz, B.S.; Nalbantgil, S.; Zoghi, M. Serum resistin level: As a predictor of atrial fibrillation after coronary artery bypass graft surgery. Coron. Artery. Dis. 2011, 22, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.; Almeida, M.E.F. Biochemical and immunological changes in obesity. Arch. Biochem. Biophys. 2021, 708, 108951. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Chen, Y.C.; Chen, J.H.; Chen, S.A.; Chen, Y.J. Adipocytes modulate the electrophysiology of atrial myocytes: Implications in obesity-induced atrial fibrillation. Basic Res. Cardiol. 2012, 107, 293. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L. Fat infiltration in the heart. Heart 2001, 85, 253. [Google Scholar] [CrossRef]
- Samanta, R.; Pouliopoulos, J.; Thiagalingam, A.; Kovoor, P. Role of adipose tissue in the pathogenesis of cardiac arrhythmias. Heart Rhythm. 2016, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Filion, K.B.; Konety, S.; Alonso, A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am. J. Cardiol. 2011, 108, 56–62. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.M. Influence of Inflammation and Atherosclerosis in Atrial Fibrillation. Curr. Atheroscler. Rep. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Van Wagoner, D.R. Oxidant and Inflammatory Mechanisms and Targeted Therapy in Atrial Fibrillation: An Update. J. Cardiovasc. Pharmacol. 2015, 66, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, D.R. Oxidative stress and inflammation in atrial fibrillation: Role in pathogenesis and potential as a therapeutic target. J. Cardiovasc. Pharmacol. 2008, 52, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Kypson, A.P.; Rodriguez, E.; Anderson, C.A.; Lehr, E.J.; Neufer, P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009, 54, 1891–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Z.; Zhao, Y.; Jiang, N.; Qiu, J.; Yang, Y.; Li, J.; Liang, X.; Wang, X.; Tse, G.; et al. Alogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function and Biogenesis in Diabetic Rabbits. J. Am. Heart Assoc. 2017, 6, e005945. [Google Scholar] [CrossRef]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.X.; Wronska, A.; Marks, A.R. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2012, 111, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 2014, 164, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.M.; Zhang, W.; Wang, L.P.; Li, G.R.; Deng, X.L. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflügers Arch. Eur. J. Physiol. 2012, 464, 613–621. [Google Scholar] [CrossRef]
- Oldfield, M.D.; Bach, L.A.; Forbes, J.M.; Nikolic-Paterson, D.; McRobert, A.; Thallas, V.; Atkins, R.C.; Osicka, T.; Jerums, G.; Cooper, M.E. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J. Clin. Investig. 2001, 108, 1853–1863. [Google Scholar] [CrossRef]
- Goette, A.; Arndt, M.; Rocken, C.; Spiess, A.; Staack, T.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 2000, 101, 2678–2681. [Google Scholar] [CrossRef] [Green Version]
- Goette, A.; Bukowska, A.; Lendeckel, U.; Erxleben, M.; Hammwohner, M.; Strugala, D.; Pfeiffenberger, J.; Rohl, F.W.; Huth, C.; Ebert, M.P.; et al. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation 2008, 117, 732–742. [Google Scholar] [CrossRef]
- Goette, A.; Lendeckel, U.; Klein, H.U. Signal transduction systems and atrial fibrillation. Cardiovasc. Res. 2002, 54, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Goette, A.; Staack, T.; Rocken, C.; Arndt, M.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 2000, 35, 1669–1677. [Google Scholar] [CrossRef] [Green Version]
- Barton, M.; Carmona, R.; Ortmann, J.; Krieger, J.E.; Traupe, T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int. J. Biochem. Cell Biol. 2003, 35, 826–837. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Torres, N.; Tovar, A.R. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013, 24, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Nyui, N.; Tamura, K.; Hibi, K.; Yamaguchi, S.; Nakamaru, M.; Ishigami, T.; Yabana, M.; Kihara, M.; Inoue, S.; et al. Plasma angiotensinogen concentrations in obese patients. Am. J. Hypertens. 1997, 10, 629–633. [Google Scholar] [CrossRef]
- Ailhaud, G. Adipose tissue as a secretory organ: From adipogenesis to the metabolic syndrome. Comptes Rendus Biol. 2006, 329, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Acuna, M.J.; Cifuentes, M.; Rojas, C.V. The anti-adipogenic effect of angiotensin II on human preadipose cells involves ERK1,2 activation and PPARG phosphorylation. J. Endocrinol. 2010, 206, 75–83. [Google Scholar] [CrossRef]
- Janke, J.; Schupp, M.; Engeli, S.; Gorzelniak, K.; Boschmann, M.; Sauma, L.; Nystrom, F.H.; Jordan, J.; Luft, F.C.; Sharma, A.M. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activation. J. Hypertens. 2006, 24, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Mogi, M.; Horiuchi, M. Role of renin-angiotensin-aldosterone system in adipose tissue dysfunction. Mol. Cell Endocrinol. 2013, 378, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Ura, N.; Higashiura, K.; Murakami, H.; Tanaka, M.; Moniwa, N.; Yoshida, D.; Shimamoto, K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 2003, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mathai, M.L.; Naik, S.; Sinclair, A.J.; Weisinger, H.S.; Weisinger, R.S. Selective reduction in body fat mass and plasma leptin induced by angiotensin-converting enzyme inhibition in rats. Int. J. Obes. 2008, 32, 1576–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, E.L.; de Picoli Souza, K.; da Silva, E.D.; Batista, E.C.; Martins, P.J.; D’Almeida, V.; Pesquero, J.B. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem. Pharmacol. 2009, 78, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Luo, R.; Zhao, Z.; Wu, Y.; Ban, D. Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Exp. Clin. Endocrinol. Diabetes 2010, 118, 258–265. [Google Scholar] [CrossRef]
- Weisinger, R.S.; Stanley, T.K.; Begg, D.P.; Weisinger, H.S.; Spark, K.J.; Jois, M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol. Behav. 2009, 98, 192–197. [Google Scholar] [CrossRef]
- Furuhashi, M.; Ura, N.; Takizawa, H.; Yoshida, D.; Moniwa, N.; Murakami, H.; Higashiura, K.; Shimamoto, K. Blockade of the renin-angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J. Hypertens. 2004, 22, 1977–1982. [Google Scholar] [CrossRef]
- Bukowska, A.; Schild, L.; Bornfleth, P.; Peter, D.; Wiese-Rischke, C.; Gardemann, A.; Isermann, B.; Walles, T.; Goette, A. Activated clotting factor X mediates mitochondrial alterations and inflammatory responses via protease-activated receptor signaling in alveolar epithelial cells. Eur. J. Pharmacol. 2020, 869, 172875. [Google Scholar] [CrossRef]
- Bukowska, A.; Hammwohner, M.; Corradi, D.; Mahardhika, W.; Goette, A. Atrial thrombogenesis in atrial fibrillation: Results from atrial fibrillation models and AF-patients. Herzschrittmacherther. Elektrophysiol. 2018, 29, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Fabritz, L.; Crijns, H.; Guasch, E.; Goette, A.; Hausler, K.G.; Kotecha, D.; Lewalter, T.; Meyer, C.; Potpara, T.S.; Rienstra, M.; et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: The 7th AFNET/EHRA Consensus Conference. EP Eur. 2021, 23, 329–344. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Camen, S.; Knebel, F.; Hagendorff, A.; Bavendiek, U.; Bohm, M.; Doehner, W.; Endres, M.; Groschel, K.; Goette, A.; et al. Expert opinion paper on cardiac imaging after ischemic stroke. Clin. Res. Cardiol. 2021, 110, 938–958. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Eckardt, L.; Valgimigli, M.; Lewalter, T.; Laeis, P.; Reimitz, P.E.; Smolnik, R.; Zierhut, W.; Tijssen, J.G.; Vranckx, P. Clinical risk predictors in atrial fibrillation patients following successful coronary stenting: ENTRUST-AF PCI sub-analysis. Clin. Res. Cardiol. 2021, 110, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Lip, G.Y.H.; Jin, J.; Heidbuchel, H.; Cohen, A.A.; Ezekowitz, M.; Merino, J.L. Differences in Thromboembolic Complications Between Paroxysmal and Persistent Atrial Fibrillation Patients Following Electrical Cardioversion (From the ENSURE-AF Study). Am. J. Cardiol. 2020, 131, 27–32. [Google Scholar] [CrossRef]
- Gorenek, B.; Pelliccia, A.; Benjamin, E.J.; Boriani, G.; Crijns, H.J.; Fogel, R.I.; Van Gelder, I.C.; Halle, M.; Kudaiberdieva, G.; Lane, D.A.; et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). EP Eur. 2017, 19, 190–225. [Google Scholar] [CrossRef]
- Kotecha, D.; Breithardt, G.; Camm, A.J.; Lip, G.Y.H.; Schotten, U.; Ahlsson, A.; Arnar, D.; Atar, D.; Auricchio, A.; Bax, J.; et al. Integrating new approaches to atrial fibrillation management: The 6th AFNET/EHRA Consensus Conference. EP Eur. 2018, 20, 395–407. [Google Scholar] [CrossRef]
- Rocken, C.; Peters, B.; Juenemann, G.; Saeger, W.; Klein, H.U.; Huth, C.; Roessner, A.; Goette, A. Atrial amyloidosis: An arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002, 106, 2091–2097. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goette, A.; Lendeckel, U. Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences. Cells 2021, 10, 2605. https://doi.org/10.3390/cells10102605
Goette A, Lendeckel U. Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences. Cells. 2021; 10(10):2605. https://doi.org/10.3390/cells10102605
Chicago/Turabian StyleGoette, Andreas, and Uwe Lendeckel. 2021. "Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences" Cells 10, no. 10: 2605. https://doi.org/10.3390/cells10102605
APA StyleGoette, A., & Lendeckel, U. (2021). Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences. Cells, 10(10), 2605. https://doi.org/10.3390/cells10102605






