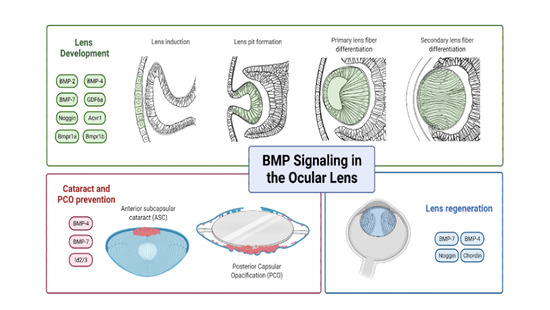
Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology
Abstract
1. Introduction
2. Bone Morphogenetics Proteins (BMPs)
2.1. Synthesis of BMPs
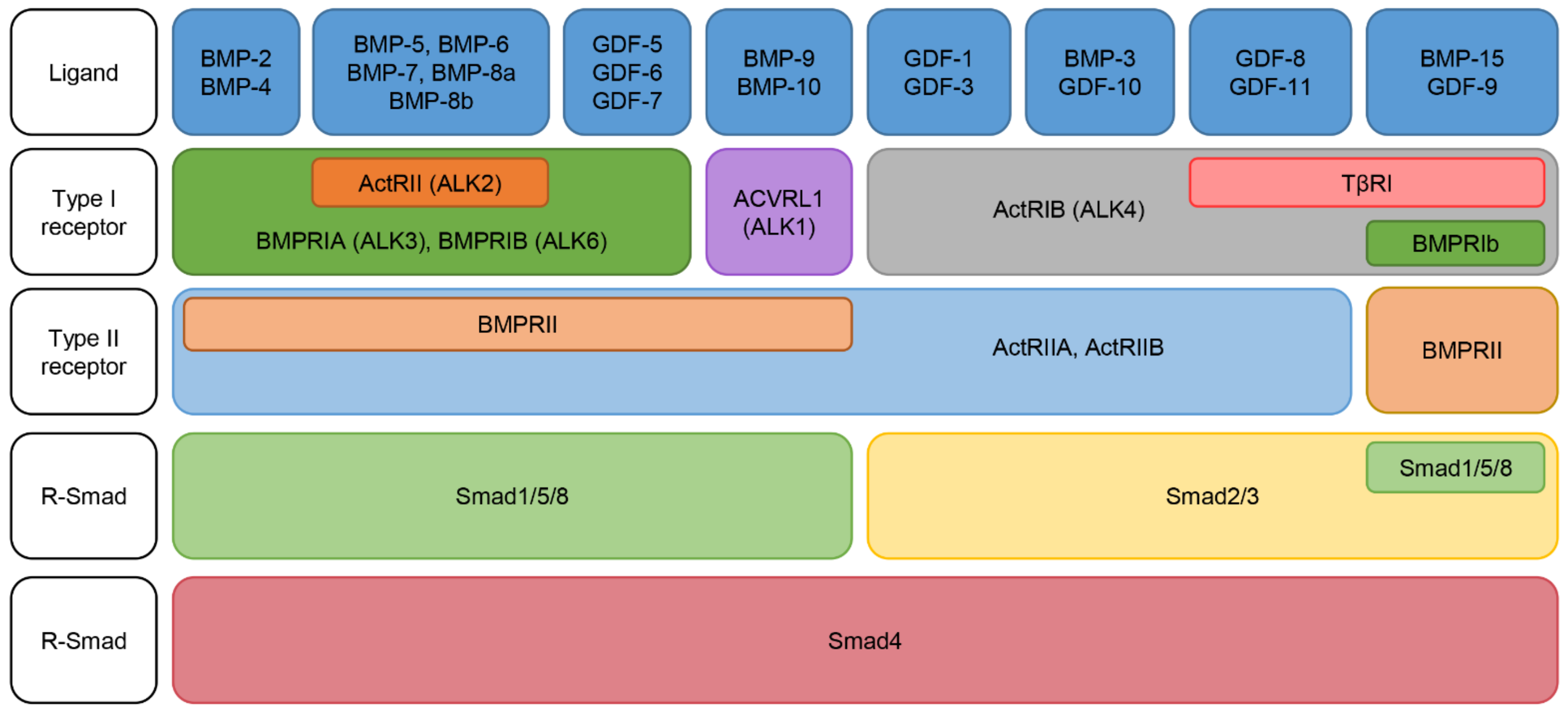
2.2. Classification of BMPs
2.3. BMP Receptors: Specificity and Activation
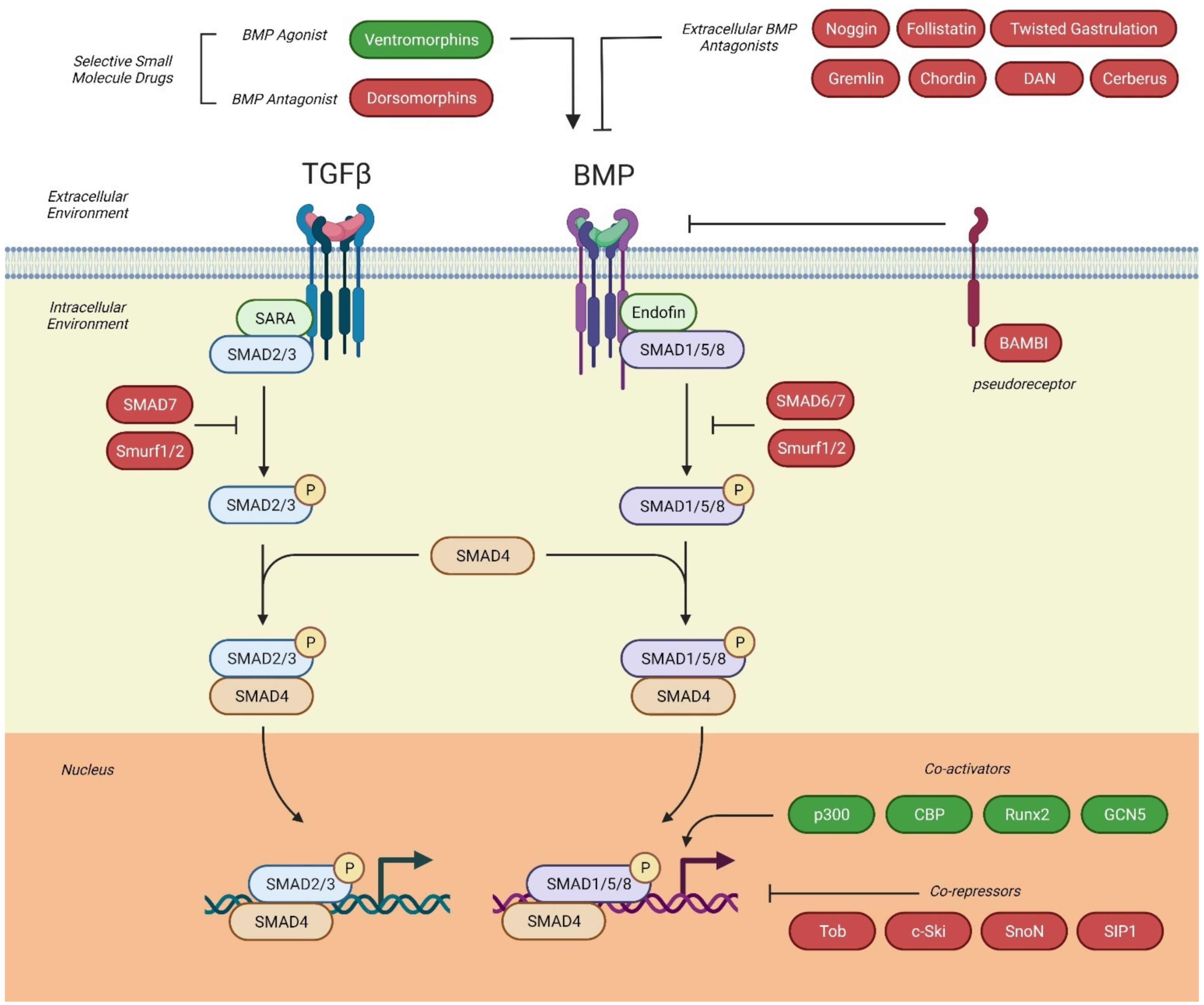
2.4. BMP Intracellular Signaling Pathways
2.4.1. Canonical Signaling Pathway
2.4.2. Non-Canonical Signaling Pathway
2.5. Antagonists of BMP-Signaling
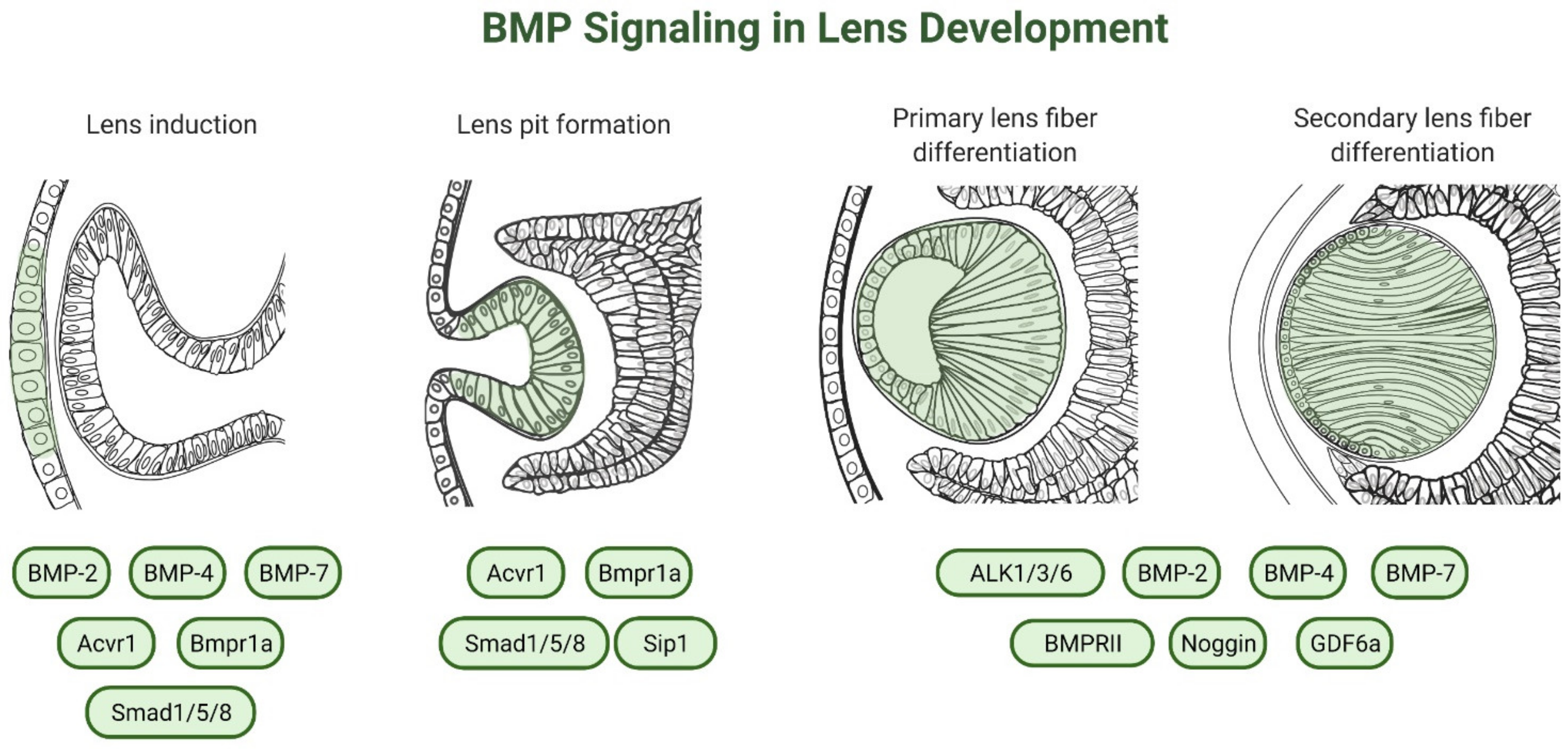
3. Role of BMP-Signaling in Lens Development
3.1. Lens Specification
3.2. Lens Induction
3.3. Lens Placode Invagination
3.4. Lens Fiber Differentiation
3.4.1. Role of FGF in Lens Fiber Differentiation
3.4.2. Role of BMP Ligands in Lens Fiber Differentiation
3.4.3. Role of BMP Antagonists in Lens Fiber Differentiation
3.4.4. Role of BMP Receptors in Lens Fiber Differentiation
3.4.5. Synergistic Roles of FGFs and BMPs in Lens Fiber Differentiation
3.5. Gap Junction-Mediated Intercellular Communication in Lens Cells
4. Genetic Mutations in BMPs
5. BMPs in Lens Regeneration
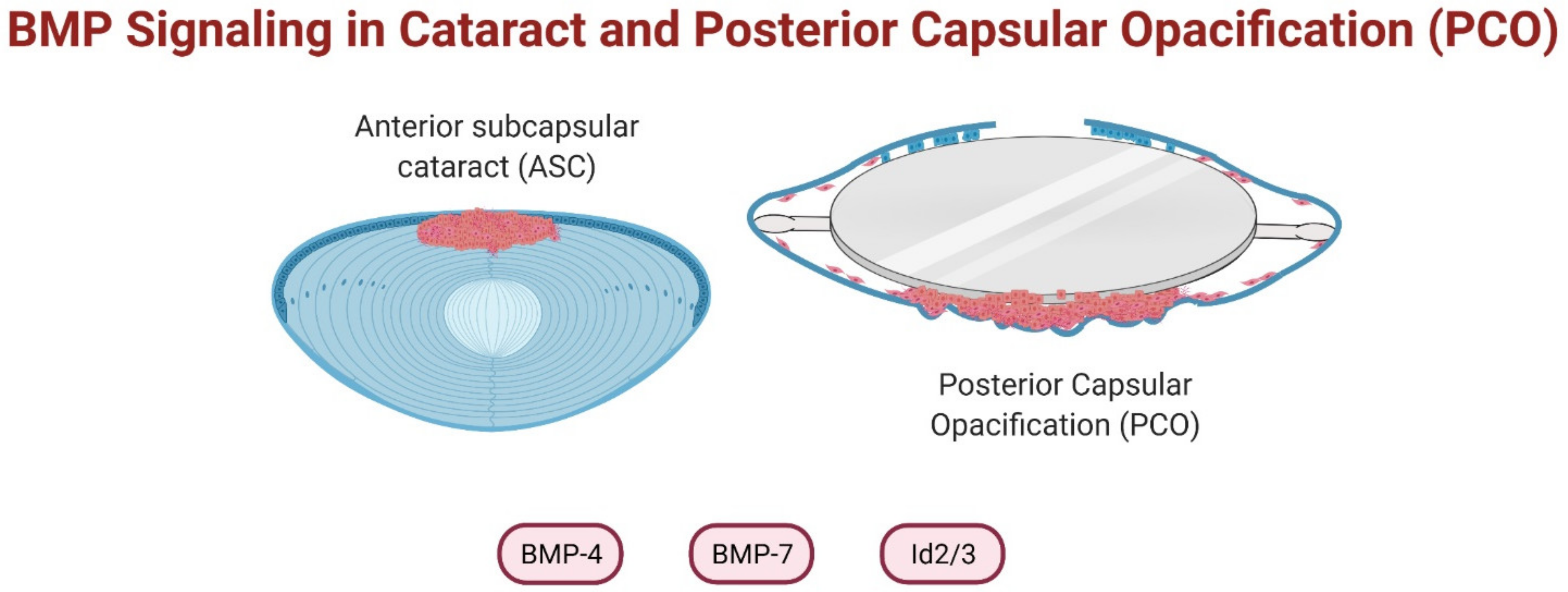
6. BMPs in Cataract Prevention
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.J.; Iannazzi, J.A.; Taylor, R.C.; Hewick, R.M.; Rosen, V.; Wang, E.A.; Wozney, J.M. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc. Natl. Acad. Sci. USA 1990, 87, 9843–9847. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Rueger, D.C.; Drier, E.A.; Corbett, C.; Ridge, R.J.; Sampath, T.K.; Oppermann, H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990, 9, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Schnegelsberg, P.N.; Jin, D.F.; Clifford, G.M.; Warren, F.D.; Drier, E.A.; Oppermann, H. Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesis. J. Biol. Chem. 1992, 267, 25220–25227. [Google Scholar] [CrossRef]
- Hemmati-Brivanlou, A.; Thomsen, G.H. Ventral mesodermal patterning in Xenopus embryos: Expression patterns and activities of BMP-2 and BMP-4. Dev. Genet. 1995, 17, 78–89. [Google Scholar] [CrossRef]
- Zou, H.; Niswander, L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 1996, 272, 738–741. [Google Scholar] [CrossRef]
- Stewart, A.; Guan, H.; Yang, K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J. Cell. Physiol. 2010, 223, 658–666. [Google Scholar] [CrossRef]
- Kobayashi, T.; Lyons, K.M.; McMahon, A.P.; Kronenberg, H.M. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc. Natl. Acad. Sci. USA 2005, 102, 18023–18027. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Reddi, A.H. BMPs: From bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005, 16, 249–250. [Google Scholar] [CrossRef]
- Hocking, J.C.; McFarlane, S. Expression of Bmp ligands and receptors in the developing Xenopus retina. Int. J. Dev. Biol. 2007, 51, 161–165. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Xiang, L.-X.; Shao, J.-Z. Bone morphogenetic protein. Biochem. Biophys. Res. Commun. 2007, 362, 550–553. [Google Scholar] [CrossRef]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell. Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Jones, W.K.; Richmond, E.A.; White, K.; Sasak, H.; Kusmik, W.; Smart, J.; Oppermann, H.; Rueger, D.C.; Tucker, R.F. Osteogenic protein-1 (OP-1) expression and processing in Chinese hamster ovary cells: Isolation of a soluble complex containing the mature and pro-domains of OP-1. Growth Factors 1994, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef]
- Wordinger, R.J.; Clark, A.F. Bone morphogenetic proteins and their receptors in the eye. Exp. Biol. Med. 2007, 232, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012, 586, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Hoang, B.; Thomas, J.T.; Vukicevic, S.; Luyten, F.P.; Ryba, N.J.; Kozak, C.A.; Reddi, A.H.; Moos, M., Jr. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J. Biol. Chem. 1994, 269, 28227–28234. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vail, A.L.; Grasser, W.A.; Brown, T.A.; Xu, H.; Vukicevic, S.; Ke, H.Z.; Qi, H.; Owen, T.A.; Thompson, D.D. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J. Biol. Chem. 1998, 273, 13760–13767. [Google Scholar] [CrossRef]
- Kessler, E.; Takahara, K.; Biniaminov, L.; Brusel, M.; Greenspan, D.S. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science 1996, 271, 360–362. [Google Scholar] [CrossRef]
- Daluiski, A.; Engstrand, T.; Bahamonde, M.E.; Gamer, L.W.; Agius, E.; Stevenson, S.L.; Cox, K.; Rosen, V.; Lyons, K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001, 27, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Bhargav, D.; Wei, A.; Williams, L.A.; Tao, H.; Ma, D.D.F.; Diwan, A.D. BMP-13 emerges as a potential inhibitor of bone formation. Int. J. Biol. Sci. 2009, 5, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bradley, A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 1996, 122, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic Analysis of the Roles of BMP2, BMP4, and BMP7 in Limb Patterning and Skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Patel, S.R.; Mishina, Y.; Manley, N.R. Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev. Biol. 2010, 339, 141–154. [Google Scholar] [CrossRef]
- Luo, G.; Hofmann, C.; Bronckers, A.L.; Sohocki, M.; Bradley, A.; Karsenty, G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995, 9, 2808–2820. [Google Scholar] [CrossRef]
- Le Dréau, G.; Garcia-Campmany, L.; Rabadán, M.A.; Ferronha, T.; Tozer, S.; Briscoe, J.; Martí, E. Canonical BMP7 activity is required for the generation of discrete neuronal populations in the dorsal spinal cord. Development 2012, 139, 259–268. [Google Scholar] [CrossRef]
- Kim, R.Y.; Robertson, E.J.; Solloway, M.J. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev. Biol. 2001, 235, 449–466. [Google Scholar] [CrossRef]
- Simon, M.; Maresh, J.G.; Harris, S.E.; Hernandez, J.D.; Arar, M.; Olson, M.S.; Abboud, H.E. Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am. J. Physiol. 1999, 276, F382–F389. [Google Scholar] [CrossRef]
- Helder, M.N.; Ozkaynak, E.; Sampath, K.T.; Luyten, F.P.; Latin, V.; Oppermann, H.; Vukicevic, S. Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J. Histochem. Cytochem. 1995, 43, 1035–1044. [Google Scholar] [CrossRef]
- Bosukonda, D.; Shih, M.S.; Sampath, K.T.; Vukicevic, S. Characterization of receptors for osteogenic protein-1/bone morphogenetic protein-7 (OP-1/BMP-7) in rat kidneys. Kidney Int. 2000, 58, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Hurtig, M.; Rueger, D.C. OP-1/BMP-7 in cartilage repair. Int. Orthop. 2007, 31, 773–781. [Google Scholar] [CrossRef]
- Söderström, S.; Ebendal, T. Localized expression of BMP and GDF mRNA in the rodent brain. J. Neurosci. Res. 1999, 56, 482–492. [Google Scholar] [CrossRef]
- Meynard, D.; Kautz, L.; Darnaud, V.; Canonne-Hergaux, F.; Coppin, H.; Roth, M.P. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 2009, 41, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulos, B., Jr.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009, 41, 482–487. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Yamashita, H.; Sampath, T.K.; Reddi, A.H.; Estevez, M.; Riddle, D.L.; Ichijo, H.; Heldin, C.H.; Miyazono, K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994, 269, 16985–16988. [Google Scholar] [CrossRef]
- Souchelnytskyi, S.; ten Dijke, P.; Miyazono, K.; Heldin, C.H. Phosphorylation of Ser165 in TGF-beta type I receptor modulates TGF-beta1-induced cellular responses. EMBO J. 1996, 15, 6231–6240. [Google Scholar] [CrossRef]
- Nohe, A.; Keating, E.; Knaus, P.; Petersen, N.O. Signal transduction of bone morphogenetic protein receptors. Cell. Signal. 2004, 16, 291–299. [Google Scholar] [CrossRef]
- Yu, P.B.; Beppu, H.; Kawai, N.; Li, E.; Bloch, K.D. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J. Biol. Chem. 2005, 280, 24443–24450. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Nickel, J.; Sebald, W. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J. 2000, 19, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Nishitoh, H.; Ichijo, H.; Kimura, M.; Matsumoto, T.; Makishima, F.; Yamaguchi, A.; Yamashita, H.; Enomoto, S.; Miyazono, K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 1996, 271, 21345–21352. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.; Zelman-Femiak, M.; Boergermann, J.H.; Paschkowsky, S.; Kreuzaler, P.A.; Fratzl, P.; Harms, G.S.; Knaus, P. SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors. J. Biol. Chem. 2012, 287, 39492–39504. [Google Scholar] [CrossRef]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef]
- Hartung, A.; Bitton-Worms, K.; Rechtman, M.M.; Wenzel, V.; Boergermann, J.H.; Hassel, S.; Henis, Y.I.; Knaus, P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol. Cell. Biol. 2006, 26, 7791–7805. [Google Scholar] [CrossRef]
- Hassel, S.; Schmitt, S.; Hartung, A.; Roth, M.; Nohe, A.; Petersen, N.; Ehrlich, M.; Henis, Y.I.; Sebald, W.; Knaus, P. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J. Bone Jt. Surg. Am. 2003, 85, 44–51. [Google Scholar] [CrossRef]
- Miyazono, K.; Kusanagi, K.; Inoue, H. Divergence and convergence of TGF-beta/BMP signaling. J. Cell. Physiol. 2001, 187, 265–276. [Google Scholar] [CrossRef]
- Gazzerro, E.; Canalis, E. Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 2006, 7, 51–65. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Tang, W.B.; Ling, G.H.; Sun, L.; Liu, F.Y. Smad anchor for receptor activation (SARA) in TGF-beta signaling. Front. Biosci. 2010, 2, 857–860. [Google Scholar] [CrossRef]
- Shi, W.; Chang, C.; Nie, S.; Xie, S.; Wan, M.; Cao, X. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J. Cell Sci. 2007, 120, 1216–1224. [Google Scholar] [CrossRef]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005, 16, 251–263. [Google Scholar] [CrossRef]
- Hata, A.; Lagna, G.; Massagué, J.; Hemmati-Brivanlou, A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998, 12, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Murakami, G.; Watabe, T.; Takaoka, K.; Miyazono, K.; Imamura, T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol. Biol. Cell 2003, 14, 2809–2817. [Google Scholar] [CrossRef]
- Kishigami, S.; Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005, 16, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, J.; Lu, Y.; Duan, X.; Sun, W. Activation of the PI3K/Akt pathway mediates bone morphogenetic protein 2-induced invasion of pancreatic cancer cells Panc-1. Pathol. Oncol. Res. 2011, 17, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Rocher, C.; Singla, D.K. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS ONE 2013, 8, e84009. [Google Scholar] [CrossRef]
- Lauzon, M.A.; Drevelle, O.; Daviau, A.; Faucheux, N. Effects of BMP-9 and BMP-2 on the PI3K/Akt Pathway in MC3T3-E1 Preosteoblasts. Tissue Eng. Part A 2016, 22, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Chen, Y.G. Controlling TGF-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [PubMed]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J. Investig. Derm. 2003, 120, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Avsian-Kretchmer, O.; Hsueh, A.J. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 2004, 18, 1–12. [Google Scholar] [CrossRef]
- Yanagita, M. BMP antagonists: Their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005, 16, 309–317. [Google Scholar] [CrossRef]
- Zimmerman, L.B.; De Jesús-Escobar, J.M.; Harland, R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 1996, 86, 599–606. [Google Scholar] [CrossRef]
- Gazzerro, E.; Gangji, V.; Canalis, E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J. Clin. Investig. 1998, 102, 2106–2114. [Google Scholar] [CrossRef]
- Zhu, W.; Kim, J.; Cheng, C.; Rawlins, B.A.; Boachie-Adjei, O.; Crystal, R.G.; Hidaka, C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 2006, 39, 61–71. [Google Scholar] [CrossRef]
- Haudenschild, D.R.; Palmer, S.M.; Moseley, T.A.; You, Z.; Reddi, A.H. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004, 64, 8276–8284. [Google Scholar] [CrossRef][Green Version]
- Krause, C.; Guzman, A.; Knaus, P. Noggin. Int. J. Biochem. Cell Biol. 2011, 43, 478–481. [Google Scholar] [CrossRef]
- Song, K.; Krause, C.; Shi, S.; Patterson, M.; Suto, R.; Grgurevic, L.; Vukicevic, S.; van Dinther, M.; Falb, D.; Ten Dijke, P.; et al. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J. Biol. Chem. 2010, 285, 12169–12180. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Greenspan, D.S. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J. Cell Biol. 2006, 175, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Mardaryev, A.N.; Lewis, C.J.; Sharov, A.A.; Botchkareva, N.V. MicroRNA-21 is an important downstream component of BMP signalling in epidermal keratinocytes. J. Cell Sci. 2011, 124, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Pardali, E.; Sánchez-Duffhues, G.; ten Dijke, P. BMP signaling in vascular diseases. FEBS Lett. 2012, 586, 1993–2002. [Google Scholar] [CrossRef]
- Corradini, E.; Babitt, J.L.; Lin, H.Y. The RGM/DRAGON family of BMP co-receptors. Cytokine Growth Factor Rev. 2009, 20, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massagué, J.; Niehrs, C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [CrossRef]
- Reza, H.M.; Yasuda, K. Lens differentiation and crystallin regulation: A chick model. Int. J. Dev. Biol. 2004, 48, 805–817. [Google Scholar] [CrossRef]
- Gunhaga, L. The lens: A classical model of embryonic induction providing new insights into cell determination in early development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1193–1203. [Google Scholar] [CrossRef]
- McAvoy, J.W. Induction of the Eye Lens. Differentiation 1980, 17, 137–149. [Google Scholar] [CrossRef]
- Ogino, H.; Yasuda, K. Sequential activation of transcription factors in lens induction. Dev. Growth Differ. 2000, 42, 437–448. [Google Scholar] [CrossRef]
- Boswell, B.A.; Overbeek, P.A.; Musil, L.S. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev. Biol. 2008, 324, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Lovicu, F.J.; McAvoy, J.W. Growth factor regulation of lens development. Dev. Biol. 2005, 280, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Hogan, B.L. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998, 12, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Wawersik, S.; Purcell, P.; Rauchman, M.; Dudley, A.T.; Robertson, E.J.; Maas, R. BMP7 acts in murine lens placode development. Dev. Biol. 1999, 207, 176–188. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Q.; Hung, F.C.; Overbeek, P.A. BMP signaling is required for development of the ciliary body. Development 2002, 129, 4435–4442. [Google Scholar]
- Sjödal, M.; Edlund, T.; Gunhaga, L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev. Cell 2007, 13, 141–149. [Google Scholar] [CrossRef]
- French, C.R.; Erickson, T.; French, D.V.; Pilgrim, D.B.; Waskiewicz, A.J. Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development. Dev. Biol. 2009, 333, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Huang, J.; Dattilo, L.K.; Kaartinen, V.; Mishina, Y.; Deng, C.X.; Umans, L.; Zwijsen, A.; Roberts, A.B.; Beebe, D.C. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev. Biol. 2009, 335, 305–316. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Filas, B.; Gunhaga, L.; Beebe, D.C. Negative and positive auto-regulation of BMP expression in early eye development. Dev. Biol. 2015, 407, 256–264. [Google Scholar] [CrossRef]
- Hung, F.C.; Zhao, S.; Chen, Q.; Overbeek, P.A. Retinal ablation and altered lens differentiation induced by ocular overexpression of BMP7. Vis. Res. 2002, 42, 427–438. [Google Scholar] [CrossRef][Green Version]
- Faber, S.C.; Robinson, M.L.; Makarenkova, H.P.; Lang, R.A. Bmp signaling is required for development of primary lens fiber cells. Development 2002, 129, 3727–3737. [Google Scholar] [CrossRef]
- Belecky-Adams, T.L.; Adler, R.; Beebe, D.C. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development 2002, 129, 3795–3802. [Google Scholar] [CrossRef]
- De Iongh, R.U.; Chen, Y.; Kokkinos, M.I.; McAvoy, J.W. BMP and activin receptor expression in lens development. Mol. Vis. 2004, 10, 566–576. [Google Scholar] [PubMed]
- Jarrin, M.; Pandit, T.; Gunhaga, L. A balance of FGF and BMP signals regulates cell cycle exit and Equarin expression in lens cells. Mol. Biol. Cell 2012, 23, 3266–3274. [Google Scholar] [CrossRef]
- Pan, Y.; Woodbury, A.; Esko, J.D.; Grobe, K.; Zhang, X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 2006, 133, 4933–4944. [Google Scholar] [CrossRef]
- Pandit, T.; Jidigam, V.K.; Gunhaga, L. BMP-induced L-Maf regulates subsequent BMP-independent differentiation of primary lens fibre cells. Dev. Dyn. 2011, 240, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.A.; Rajagopal, R.; Dattilo, L.K.; Beebe, D.C. The tumor suppressor gene Trp53 protects the mouse lens against posterior subcapsular cataracts and the BMP receptor Acvr1 acts as a tumor suppressor in the lens. Dis. Model. Mech. 2011, 4, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Jidigam, V.K.; Srinivasan, R.C.; Patthey, C.; Gunhaga, L. Apical constriction and epithelial invagination are regulated by BMP activity. Biol. Open 2015, 4, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Boswell, B.A.; Musil, L.S. Synergistic interaction between the fibroblast growth factor and bone morphogenetic protein signaling pathways in lens cells. Mol. Biol. Cell 2015, 26, 2561–2572. [Google Scholar] [CrossRef]
- Boswell, B.A.; Lein, P.J.; Musil, L.S. Cross-talk between fibroblast growth factor and bone morphogenetic proteins regulates gap junction-mediated intercellular communication in lens cells. Mol. Biol. Cell 2008, 19, 2631–2641. [Google Scholar] [CrossRef]
- Boswell, B.A.; Le, A.C.; Musil, L.S. Upregulation and maintenance of gap junctional communication in lens cells. Exp. Eye Res. 2009, 88, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Grogg, M.W.; Call, M.K.; Okamoto, M.; Vergara, M.N.; Del Rio-Tsonis, K.; Tsonis, P.A. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature 2005, 438, 858–862. [Google Scholar] [CrossRef]
- Kurata, T.; Nakabayashi, J.; Yamamoto, T.S.; Mochii, M.; Ueno, N. Visualization of endogenous BMP signaling during Xenopus development. Differentiation 2001, 67, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Day, R.C.; Beck, C.W. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC Dev. Biol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Brennan, L.; Bouhassira, E.E.; Kantorow, M.; Cvekl, A. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB J. 2010, 24, 3274–3283. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Valcourt, U.; Bergstrom, R.; Heldin, C.H.; Moustakas, A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell. Biol. 2004, 24, 4241–4254. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Flanders, K.C.; Ohnishi, Y.; Nakajima, Y.; Muragaki, Y.; Ooshima, A. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am. J. Physiol. Cell Physiol. 2006, 290, C282–C289. [Google Scholar] [CrossRef][Green Version]
- Shu, D.Y.; Wojciechowski, M.C.; Lovicu, F.J. Bone Morphogenetic Protein-7 Suppresses TGFβ2-Induced Epithelial-Mesenchymal Transition in the Lens: Implications for Cataract Prevention. Investig. Ophthalmol. Vis. Sci. 2017, 58, 781–796. [Google Scholar] [CrossRef]
- Shu, D.Y.; Ng, K.; Wishart, T.F.L.; Chui, J.; Lundmark, M.; Flokis, M.; Lovicu, F.J. Contrasting roles for BMP-4 and ventromorphins (BMP agonists) in TGFbeta-induced lens EMT. Exp. Eye Res. 2021, 206, 108546. [Google Scholar] [CrossRef]
- Du, B.; Zheng, J.-L.; Huang, L.-Y.; Zhang, H.; Wang, Q.; Hong, Y.-R.; Zhang, X.-m.; Li, X.-R.; Dong, L.-J. Protective Effect and Mechanism of Bone Morphogenetic Protein-4 on Apoptosis of Human Lens Epithelium Cells under Oxidative Stress. BioMed Res. Int. 2021, 2021, 8109134. [Google Scholar] [CrossRef]
- Cvekl, A.; Zhang, X. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet. 2017, 33, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; de Santa Barbara, P.; Roberts, D.J.; Whitman, M. Endogenous patterns of BMP signaling during early chick development. Dev. Biol. 2002, 244, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.C.; Schubert, F.R.; Schoenwolf, G.C.; Lumsden, A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 2002, 245, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Bailey, A.P.; Bronner-Fraser, M.; Streit, A. Segregation of lens and olfactory precursors from a common territory: Cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev. Biol. 2004, 271, 403–414. [Google Scholar] [CrossRef]
- Dudley, A.T.; Robertson, E.J. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 1997, 208, 349–362. [Google Scholar] [CrossRef]
- Patthey, C.; Gunhaga, L. Signaling pathways regulating ectodermal cell fate choices. Exp. Cell Res. 2014, 321, 11–16. [Google Scholar] [CrossRef]
- Kawauchi, S.; Takahashi, S.; Nakajima, O.; Ogino, H.; Morita, M.; Nishizawa, M.; Yasuda, K.; Yamamoto, M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 1999, 274, 19254–19260. [Google Scholar] [CrossRef]
- Beebe, D.; Garcia, C.; Wang, X.; Rajagopal, R.; Feldmeier, M.; Kim, J.Y.; Chytil, A.; Moses, H.; Ashery-Padan, R.; Rauchman, M. Contributions by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 2004, 48, 845–856. [Google Scholar] [CrossRef]
- Kamachi, Y.; Sockanathan, S.; Liu, Q.; Breitman, M.; Lovell-Badge, R.; Kondoh, H. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 1995, 14, 3510–3519. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.M.; Hogan, B.L.; Robertson, E.J. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 1995, 50, 71–83. [Google Scholar] [CrossRef]
- Jena, N.; Martín-Seisdedos, C.; McCue, P.; Croce, C.M. BMP7 null mutation in mice: Developmental defects in skeleton, kidney, and eye. Exp. Cell Res. 1997, 230, 28–37. [Google Scholar] [CrossRef]
- Cvekl, A.; Ashery-Padan, R. The cellular and molecular mechanisms of vertebrate lens development. Development 2014, 141, 4432–4447. [Google Scholar] [CrossRef]
- Wrenn, J.T.; Wessells, N.K. An ultrastructural study of lens invagination in the mouse. J. Exp. Zool. 1969, 171, 359–367. [Google Scholar] [CrossRef]
- Kakrana, A.; Yang, A.; Anand, D.; Djordjevic, D.; Ramachandruni, D.; Singh, A.; Huang, H.; Ho, J.W.K.; Lachke, S.A. iSyTE 2.0: A database for expression-based gene discovery in the eye. Nucleic Acids Res. 2018, 46, D875–D885. [Google Scholar] [CrossRef] [PubMed]
- McAvoy, J.W.; Chamberlain, C.G. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 1989, 107, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lovicu, F.J.; McAvoy, J.W. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF). Exp. Eye Res. 1989, 49, 479–494. [Google Scholar] [CrossRef]
- Lovicu, F.J.; McAvoy, J.W. The age of rats affects the response of lens epithelial explants to fibroblast growth factor. An ultrastructural analysis. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2269–2278. [Google Scholar]
- Chow, R.L.; Roux, G.D.; Roghani, M.; Palmer, M.A.; Rifkin, D.B.; Moscatelli, D.A.; Lang, R.A. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development 1995, 121, 4383–4393. [Google Scholar] [CrossRef]
- Robinson, M.L.; MacMillan-Crow, L.A.; Thompson, J.A.; Overbeek, P.A. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development 1995, 121, 3959–3967. [Google Scholar] [CrossRef] [PubMed]
- Stolen, C.M.; Griep, A.E. Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev. Biol. 2000, 217, 205–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, H.; Yang, T.; Madakashira, B.P.; Thiels, C.A.; Bechtle, C.A.; Garcia, C.M.; Zhang, H.; Yu, K.; Ornitz, D.M.; Beebe, D.C.; et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 2008, 318, 276–288. [Google Scholar] [CrossRef]
- Trousse, F.; Esteve, P.; Bovolenta, P. Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 2001, 21, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Vinader, L.M.; van Genesen, S.T.; de Jong, W.W.; Lubsen, N.H. Influence of hormones and growth factors on lens protein composition: The effect of dexamethasone and PDGF-AA. Mol. Vis. 2003, 9, 723–729. [Google Scholar] [PubMed]
- Musil, L.S. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. J. Membr. Biol. 2012, 245, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Pandit, T.; Jidigam, V.K.; Patthey, C.; Gunhaga, L. Neural retina identity is specified by lens-derived BMP signals. Development 2015, 142, 1850–1859. [Google Scholar] [CrossRef]
- Maruyama-Koide, Y.; Mikawa, S.; Sasaki, T.; Sato, K. Bone morphogenetic protein-4 and bone morphogenetic protein receptors expressions in the adult rat eye. Eur. J. Histochem. 2017, 61, 2797. [Google Scholar] [CrossRef]
- Yamada, H.; Obata, H.; Kaji, Y.; Yamashita, H. Expression of transforming growth factor-beta superfamily receptors in developing rat eyes. Jpn. J. Ophthalmol. 1999, 43, 290–294. [Google Scholar] [CrossRef]
- Lam, P.T.; Padula, S.L.; Hoang, T.V.; Poth, J.E.; Liu, L.; Liang, C.; LeFever, A.S.; Wallace, L.M.; Ashery-Padan, R.; Riggs, P.K.; et al. Considerations for the use of Cre recombinase for conditional gene deletion in the mouse lens. Hum. Genom. 2019, 13, 10. [Google Scholar] [CrossRef]
- Mu, H.; Ohta, K.; Kuriyama, S.; Shimada, N.; Tanihara, H.; Yasuda, K.; Tanaka, H. Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech. Dev. 2003, 120, 143–155. [Google Scholar] [CrossRef]
- Goodenough, D.A. The crystalline lens. A system networked by gap junctional intercellular communication. Semin. Cell Biol. 1992, 3, 49–58. [Google Scholar] [CrossRef]
- Paul, D.L.; Ebihara, L.; Takemoto, L.J.; Swenson, K.I.; Goodenough, D.A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991, 115, 1077–1089. [Google Scholar] [CrossRef]
- White, T.W.; Bruzzone, R.; Goodenough, D.A.; Paul, D.L. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol. Biol. Cell 1992, 3, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Ponnam, S.P.; Ramesha, K.; Tejwani, S.; Ramamurthy, B.; Kannabiran, C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. BMJ Case Rep. 2009, 2009, bcr0620091995. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.T.; Rae, J.L.; Baldo, G.J. Physiological properties of the normal lens. Physiol. Rev. 1997, 77, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R.; van Marle, J.; Prescott, A.R.; Quinlan, R.A. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp. Eye Res. 1999, 69, 45–56. [Google Scholar] [CrossRef]
- Vrensen, G.; Van Marle, J.; Van Veen, H.; Willekens, B. Membrane architecture as a function of lens fibre maturation: A freeze fracture and scanning electron microscopic study in the human lens. Exp. Eye Res. 1992, 54, 433–446. [Google Scholar] [CrossRef]
- Le, A.C.; Musil, L.S. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 2001, 154, 197–216. [Google Scholar] [CrossRef]
- Reis, L.M.; Semina, E.V. Conserved genetic pathways associated with microphthalmia, anophthalmia, and coloboma. Birth Defects Res. C Embryo Today 2015, 105, 96–113. [Google Scholar] [CrossRef]
- Bakrania, P.; Efthymiou, M.; Klein, J.C.; Salt, A.; Bunyan, D.J.; Wyatt, A.; Ponting, C.P.; Martin, A.; Williams, S.; Lindley, V.; et al. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: Overlap between the BMP4 and hedgehog signaling pathways. Am. J. Hum. Genet. 2008, 82, 304–319. [Google Scholar] [CrossRef]
- Reis, L.M.; Tyler, R.C.; Schilter, K.F.; Abdul-Rahman, O.; Innis, J.W.; Kozel, B.A.; Schneider, A.S.; Bardakjian, T.M.; Lose, E.J.; Martin, D.M.; et al. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum. Genet. 2011, 130, 495–504. [Google Scholar] [CrossRef]
- Wyatt, A.W.; Osborne, R.J.; Stewart, H.; Ragge, N.K. Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum. Mutat. 2010, 31, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995, 9, 2795–2807. [Google Scholar] [CrossRef]
- Hanel, M.L.; Hensey, C. Eye and neural defects associated with loss of GDF6. BMC Dev. Biol. 2006, 6, 43. [Google Scholar] [CrossRef][Green Version]
- Asai-Coakwell, M.; French, C.R.; Berry, K.M.; Ye, M.; Koss, R.; Somerville, M.; Mueller, R.; van Heyningen, V.; Waskiewicz, A.J.; Lehmann, O.J. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am. J. Hum. Genet. 2007, 80, 306–315. [Google Scholar] [CrossRef]
- Chassaing, N.; Causse, A.; Vigouroux, A.; Delahaye, A.; Alessandri, J.L.; Boespflug-Tanguy, O.; Boute-Benejean, O.; Dollfus, H.; Duban-Bedu, B.; Gilbert-Dussardier, B.; et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin. Genet. 2014, 86, 326–334. [Google Scholar] [CrossRef]
- Williamson, K.A.; FitzPatrick, D.R. The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur. J. Med. Genet. 2014, 57, 369–380. [Google Scholar] [CrossRef]
- Ye, M.; Berry-Wynne, K.M.; Asai-Coakwell, M.; Sundaresan, P.; Footz, T.; French, C.R.; Abitbol, M.; Fleisch, V.C.; Corbett, N.; Allison, W.T.; et al. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum. Mol. Genet. 2010, 19, 287–298. [Google Scholar] [CrossRef]
- Freitas, G.P.; Lopes, H.B.; Souza, A.T.P.; Gomes, M.P.O.; Quiles, G.K.; Gordon, J.; Tye, C.; Stein, J.L.; Stein, G.S.; Lian, J.B.; et al. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Hutchinson, L.D.; Bozatzi, P.; Macartney, T.; Sapkota, G.P. Generation of Endogenous BMP Transcriptional Reporter Cells Through CRISPR/Cas9 Genome Editing. Methods Mol. Biol. 2019, 1891, 29–35. [Google Scholar] [CrossRef][Green Version]
- Henry, J.J.; Tsonis, P.A. Molecular and cellular aspects of amphibian lens regeneration. Prog. Retin. Eye Res. 2010, 29, 543–555. [Google Scholar] [CrossRef]
- Henry, J.J.; Thomas, A.G.; Hamilton, P.W.; Moore, L.; Perry, K.J. Cell signaling pathways in vertebrate lens regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 75–98. [Google Scholar] [CrossRef]
- Gu, Y.; Yao, K.; Fu, Q. Lens regeneration: Scientific discoveries and clinical possibilities. Mol. Biol. Rep. 2021, 48, 4911–4923. [Google Scholar] [CrossRef]
- Colucci, V. Sulla rigenerazione parziale dell’occhio nei tritoni: Istogenesi e sviluppo. Mem. R. Accad. Sci. Ist. Bologna 1890, 51, 167–203. [Google Scholar]
- Wolff, G. Entwicklungsphysiologische Studien. I. Regen. Urodelenlinse. Arch. Entw. Mechan. 1895, 1, 380–390. [Google Scholar]
- Henry, J.J. The cellular and molecular bases of vertebrate lens regeneration. Int. Rev. Cytol. 2003, 228, 195–265. [Google Scholar] [CrossRef]
- Grogg, M.W.; Call, M.K.; Tsonis, P.A. Signaling during lens regeneration. Semin. Cell Dev. Biol. 2006, 17, 753–758. [Google Scholar] [CrossRef][Green Version]
- Barbosa-Sabanero, K.; Hoffmann, A.; Judge, C.; Lightcap, N.; Tsonis, P.A.; Del Rio-Tsonis, K. Lens and retina regeneration: New perspectives from model organisms. Biochem. J. 2012, 447, 321–334. [Google Scholar] [CrossRef][Green Version]
- De Robertis, E.M.; Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004, 20, 285–308. [Google Scholar] [CrossRef]
- Freeman, G. Lens Regeneration from the Cornea in Xenopus Laevis. J. Exp. Zool. 1963, 154, 39–65. [Google Scholar] [CrossRef]
- Filoni, S.; Bosco, L.; Cioni, C. The role of neural retina in lens regeneration from cornea in larval Xenopus laevis. Acta Embryol. Morphol. Exp. 1982, 3, 15–28. [Google Scholar]
- Bosco, L.; Testa, O.; Venturini, G.; Willems, D. Lens fibre transdifferentiation in cultured larval Xenopus laevis outer cornea under the influence of neural retina-conditioned medium. Cell. Mol. Life Sci. 1997, 53, 921–928. [Google Scholar] [CrossRef]
- De Iongh, R.U.; Wederell, E.; Lovicu, F.J.; McAvoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef]
- Shu, D.Y.; Ong, K.; Lovicu, F.J. Histopathology of Subcapsular Cataract in a Patient with Atopic Dermatitis. Optom. Vis. Sci. 2017, 94, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Lovicu, F.J. Enhanced EGF receptor-signaling potentiates TGFβ-induced lens epithelial-mesenchymal transition. Exp. Eye Res. 2019, 185, 107693. [Google Scholar] [CrossRef]
- Shu, D.Y.; Wojciechowski, M.; Lovicu, F.J. ERK1/2-mediated EGFR-signaling is required for TGFβ-induced lens epithelial-mesenchymal transition. Exp. Eye Res. 2019, 178, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, M.C.; Mahmutovic, L.; Shu, D.Y.; Lovicu, F.J. ERK1/2 signaling is required for the initiation but not progression of TGFβ-induced lens epithelial to mesenchymal transition (EMT). Exp. Eye Res. 2017, 159, 98–113. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, J.; Guo, Q.; Yang, H. E-cadherin involvement in human lens epithelial cell transdifferentiation may be associated with N-cadherin. Mol. Med. Rep. 2017, 16, 5031–5035. [Google Scholar] [CrossRef]
- Taylor, M.A.; Parvani, J.G.; Schiemann, W.P. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia 2010, 15, 169–190. [Google Scholar] [CrossRef]
- Shirai, K.; Tanaka, S.I.; Lovicu, F.J.; Saika, S. The murine lens: A model to investigate in vivo epithelial-mesenchymal transition. Dev. Dyn. 2018, 247, 340–345. [Google Scholar] [CrossRef]
- Liu, J.; Hales, A.M.; Chamberlain, C.G.; McAvoy, J.W. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Investig. Ophthalmol. Vis. Sci. 1994, 35, 388–401. [Google Scholar]
- Shin, E.H.H.; Basson, M.A.; Robinson, M.L.; McAvoy, J.W.; Lovicu, F.J. Sprouty is a negative regulator of transforming growth factor β-induced epithelial-to-mesenchymal transition and cataract. Mol. Med. 2012, 18, 861–873. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Tamiya, S.; Anderson, I.; Duncan, G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2301–2308. [Google Scholar]
- Hales, A.M.; Chamberlain, C.G.; Dreher, B.; McAvoy, J.W. Intravitreal injection of TGFbeta induces cataract in rats. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3231–3236. [Google Scholar]
- Lovicu, F.J.; Schulz, M.W.; Hales, A.M.; Vincent, L.N.; Overbeek, P.A.; Chamberlain, C.G.; McAvoy, J.W. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br. J. Ophthalmol. 2002, 86, 220–226. [Google Scholar] [CrossRef]
- Robertson, J.V.; Nathu, Z.; Najjar, A.; Dwivedi, D.; Gauldie, J.; West-Mays, J.A. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol. Vis. 2007, 13, 457–469. [Google Scholar]
- Weiskirchen, R.; Meurer, S.K.; Gressner, O.A.; Herrmann, J.; Borkham-Kamphorst, E.; Gressner, A.M. BMP-7 as antagonist of organ fibrosis. Front. Biosci. 2009, 14, 4992–5012. [Google Scholar] [CrossRef]
- Meng, X.M.; Chung, A.C.; Lan, H.Y. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin. Sci. 2013, 124, 243–254. [Google Scholar] [CrossRef]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, J.; Jiang, D.; Wu, X. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition in human renal proximal tubular epithelial cells. J. Nephrol. 2009, 22, 403–410. [Google Scholar]
- Motazed, R.; Colville-Nash, P.; Kwan, J.T.; Dockrell, M.E. BMP-7 and proximal tubule epithelial cells: Activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm. Res. 2008, 25, 2440–2446. [Google Scholar] [CrossRef]
- Wang, S.; Hirschberg, R. Bone morphogenetic protein-7 signals opposing transforming growth factor beta in mesangial cells. J. Biol. Chem. 2004, 279, 23200–23206. [Google Scholar] [CrossRef]
- Izumi, N.; Mizuguchi, S.; Inagaki, Y.; Saika, S.; Kawada, N.; Nakajima, Y.; Inoue, K.; Suehiro, S.; Friedman, S.L.; Ikeda, K. BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L120–L126. [Google Scholar] [CrossRef]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar] [CrossRef]
- Murray, L.A.; Hackett, T.L.; Warner, S.M.; Shaheen, F.; Argentieri, R.L.; Dudas, P.; Farrell, F.X.; Knight, D.A. BMP-7 does not protect against bleomycin-induced lung or skin fibrosis. PLoS ONE 2008, 3, e4039. [Google Scholar] [CrossRef]
- Hackett, T.L.; Warner, S.M.; Stefanowicz, D.; Shaheen, F.; Pechkovsky, D.V.; Murray, L.A.; Argentieri, R.; Kicic, A.; Stick, S.M.; Bai, T.R.; et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am. J. Respir. Crit. Care Med. 2009, 180, 122–133. [Google Scholar] [CrossRef]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Zhao, J.; Pscherer, S.; Freude, T.; Dooley, S.; Kolk, A.; Stöckle, U.; Nussler, A.K.; Hube, R. Transforming growth factor β 1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): Possible mechanism for the failure of BMP therapy? BMC Med. 2012, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Antebi, Y.E.; Linton, J.M.; Klumpe, H.; Bintu, B.; Gong, M.; Su, C.; McCardell, R.; Elowitz, M.B. Combinatorial Signal Perception in the BMP Pathway. Cell 2017, 170, 1184–1196.e24. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Experimental Model | BMPs Investigated |
|---|---|---|
| Lens Induction | ||
| Luo et al. (1995) [27] | In vivo mouse | BMP-7 |
| Furuta et al. (1998) [83] | In vivo mouse | BMP-4 |
| Wawersik et al. (1999) [84] | In vivo mouse | BMP-7 |
| Zhao et al. (2002) [85] | In vivo mouse | BMP-7, noggin |
| Sjödal et al. (2007) [86] | In vivo chick | BMP-4 |
| French et al. (2009) [87] | In vivo zebrafish | BMP-4, GDF6a |
| Rajagopal et al. (2009) [88] | In vivo mouse | BMP receptor Acvr1 and Bmpr1a |
| Huang et al. (2015) [89] | In vivo chick, in vivo mouse | BMP-7, Acvr1, Bmpr1a |
| Lens Fiber Differentiation | ||
| Hung et al. (2002) [90] | In vivo mouse | BMP-7 |
| Faber et al. (2002) [91] | In vivo mouse | Bmpr1b |
| Belecky-Adams et al. (2002) [92] | In vivo chick | BMP-2, BMP-4, BMP-7, noggin |
| de Iongh et al. (2004) [93] | In vivo mouse, rat lenses | ActRIIA, ActRIIB, BmprII, ALK3 |
| Jarrin et al. (2004) [94] | In vivo chick | Noggin |
| Pan et al. (2006) [95] | In vivo mouse | BMP-4 |
| Boswell et al. (2008) [81] | In vitro embryonic chick | BMP-2, BMP-4, BMP-7, noggin |
| Rajagopal et al. (2009) [88] | In vivo mouse | BMP receptor Acvr1 |
| Pandit et al. (2011) [96] | In vitro in vivo chick | BMP-4 |
| Wiley et al. (2011) [97] | In vivo mouse | BMP receptor Acvr1 |
| Jidigam et al. (2015) [98] | In vivo chick | BMP-4, BMP-7 |
| Boswell et al. (2015) [99] | In vitro embryonic chick | BMP-2, BMP-4, BMP-7, noggin |
| Gap-junction Mediated Communication | ||
| Boswell et al. (2008) [100] | In vitro embryonic chick | BMP-2, BMP-4, BMP-7, noggin |
| Boswell et al. (2009) [101] | In vitro embryonic chick | BMP-4 |
| Lens Regeneration | ||
| Grogg et al. (2005) [102] | In vivo newt | BMP-4, BMP-7, chordin, Bmpr1a |
| Kurata et al. (2001) [103] | Xenopus | BMP-4 |
| Day and Beck (2011) [104] | Xenopus | Noggin, Nipsnap1 |
| Yang et al. (2010) [105] | Human embryonic stem cells | BMP-4, BMP-7, noggin |
| Cataract Prevention | ||
| Kowanetz et al. (2004) [106] | Mouse epithelial cell line | BMP-7, Id2, Id3 |
| Saika et al. (2006) [107] | In vivo mouse | BMP-7, Id2, Id3 |
| Shu et al. (2017) [108] | In vitro rat lens explant | BMP-7, Id2, Id3 |
| Shu et al. (2021) [109] | In vitro rat lens explant | BMP-4, ventromorphins |
| Du et al. (2021) [110] | HLE-B3 human lens cell line | BMP-4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, D.Y.; Lovicu, F.J. Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology. Cells 2021, 10, 2604. https://doi.org/10.3390/cells10102604
Shu DY, Lovicu FJ. Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology. Cells. 2021; 10(10):2604. https://doi.org/10.3390/cells10102604
Chicago/Turabian StyleShu, Daisy Y., and Frank J. Lovicu. 2021. "Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology" Cells 10, no. 10: 2604. https://doi.org/10.3390/cells10102604
APA StyleShu, D. Y., & Lovicu, F. J. (2021). Insights into Bone Morphogenetic Protein—(BMP-) Signaling in Ocular Lens Biology and Pathology. Cells, 10(10), 2604. https://doi.org/10.3390/cells10102604