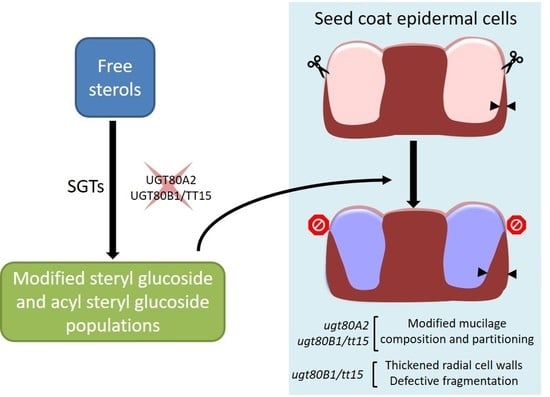
Sterol Glucosyltransferases Tailor Polysaccharide Accumulation in Arabidopsis Seed Coat Epidermal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seed Flotation
2.3. Cytochemical Staining and Immunolabeling of Seed Mucilage
2.4. Scanning Electron Microscopy (SEM) and Measurement of Radial Cell Wall Width
2.5. Extraction of Mucilage Polysaccharides
2.6. Chemical and Physico-Chemical Characterization of Mucilage Extracts
2.7. Analysis of Fluorescent Probe Permeability and Mobility in Inner Mucilage
3. Results
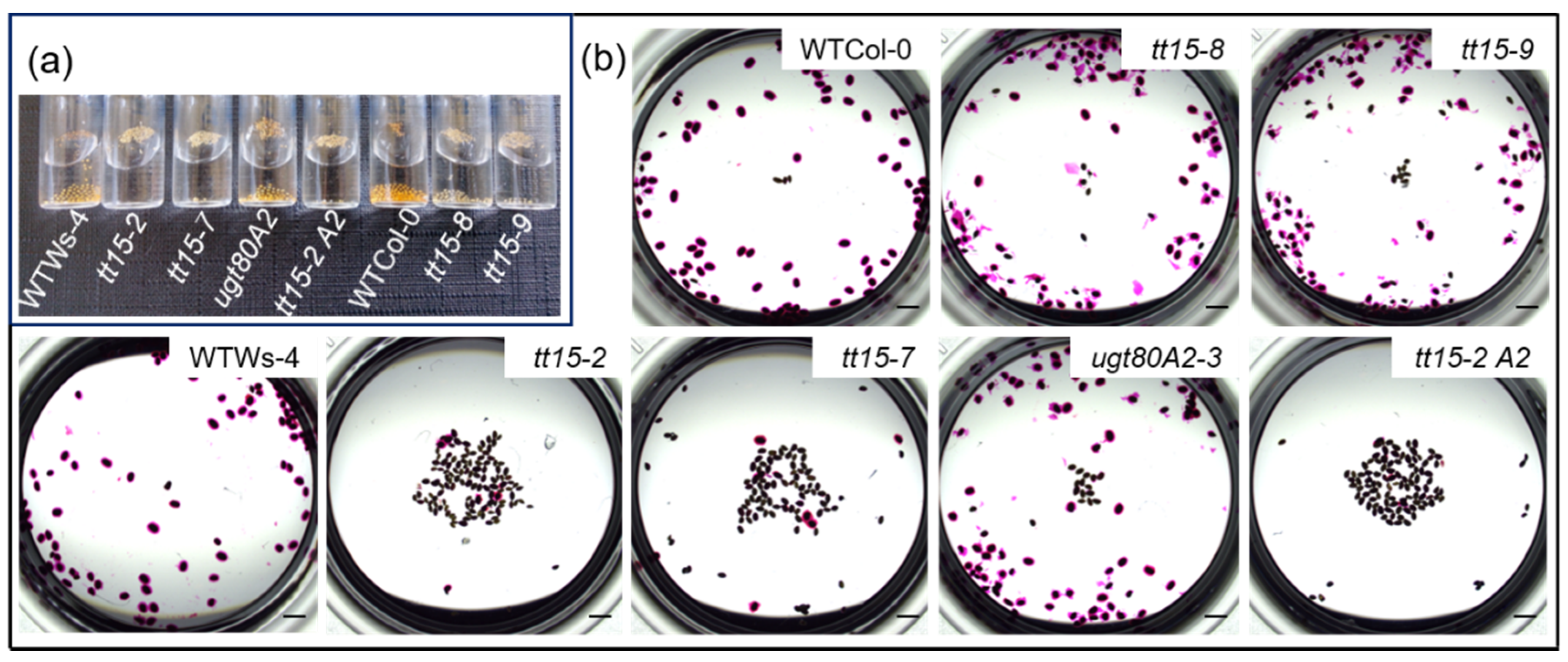
3.1. tt15 Seeds Float Due to Defective Mucilage Release
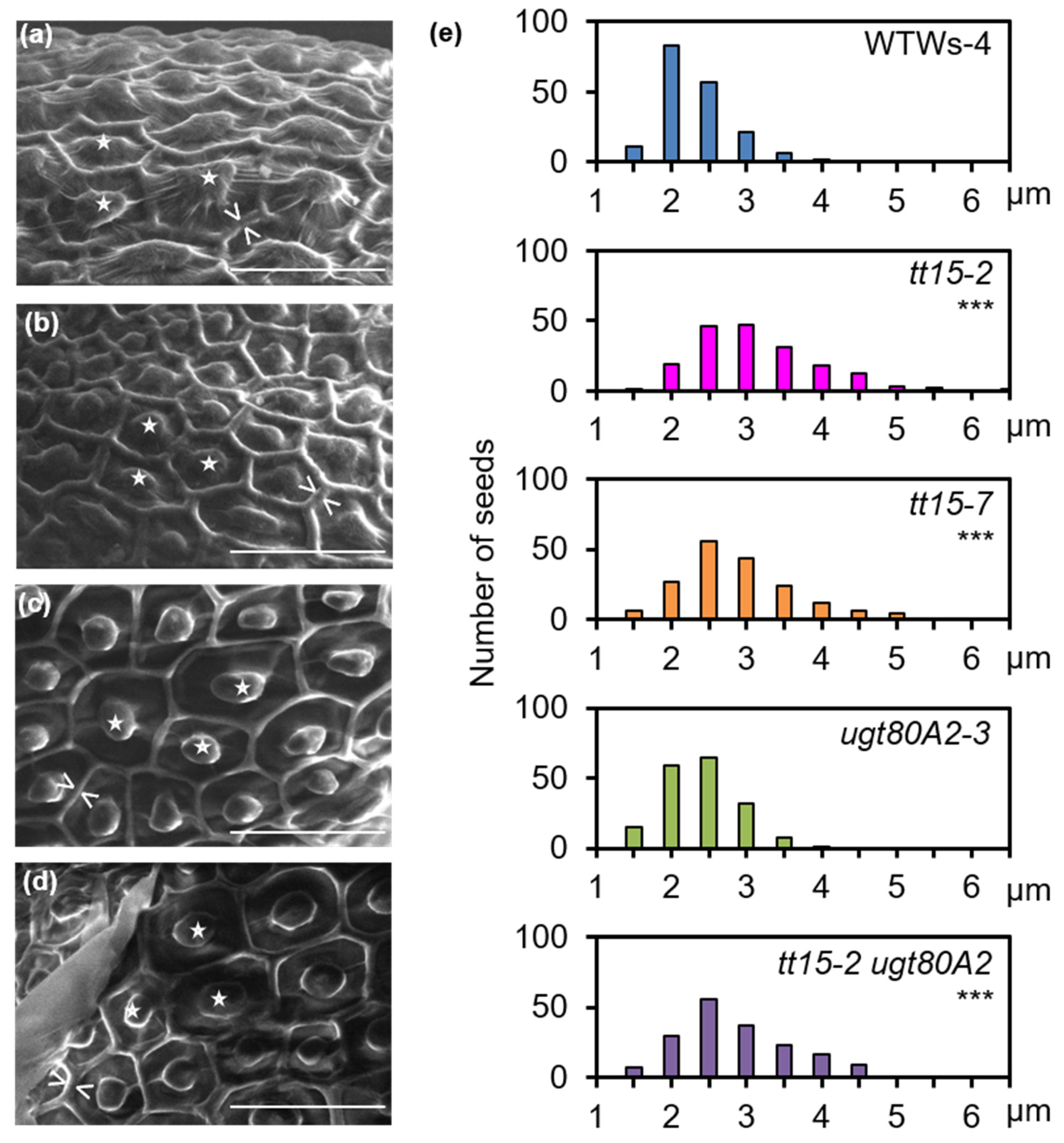
3.2. UGT80B1 Is Necessary for Fragmentation of the Outer Cell Wall of Seed Coat Epidermal Cells
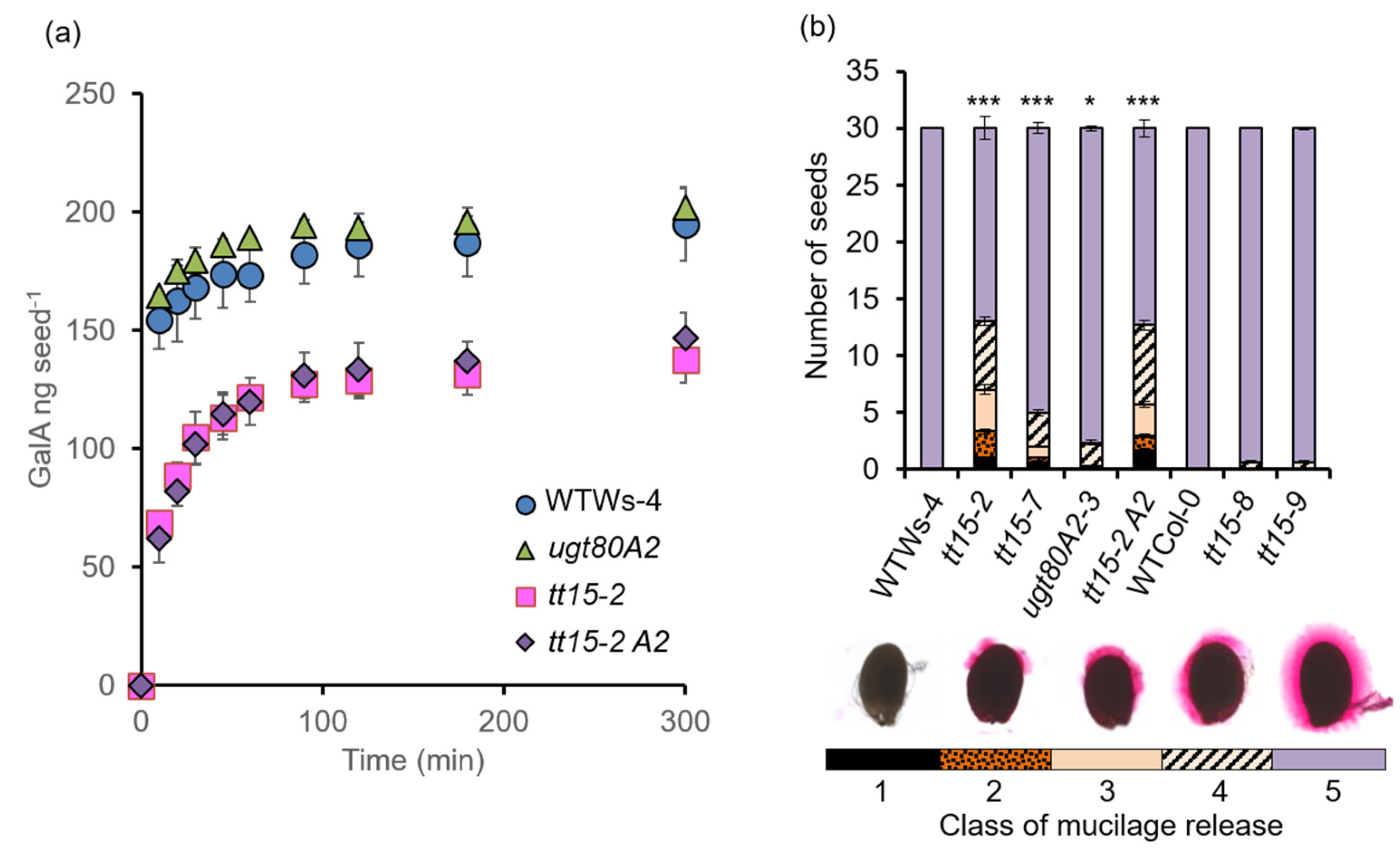
3.3. tt15 Seeds Exhibit Delayed and Heterogeneous Mucilage Release
3.4. HG Methylesterification Is Conserved in Outer Cell Wall Fragments from tt15 Seed Coat Epidermal Cells
3.5. Secondary Thickening of Epidermal Cell Radial Walls Is Increased in tt15 Seeds
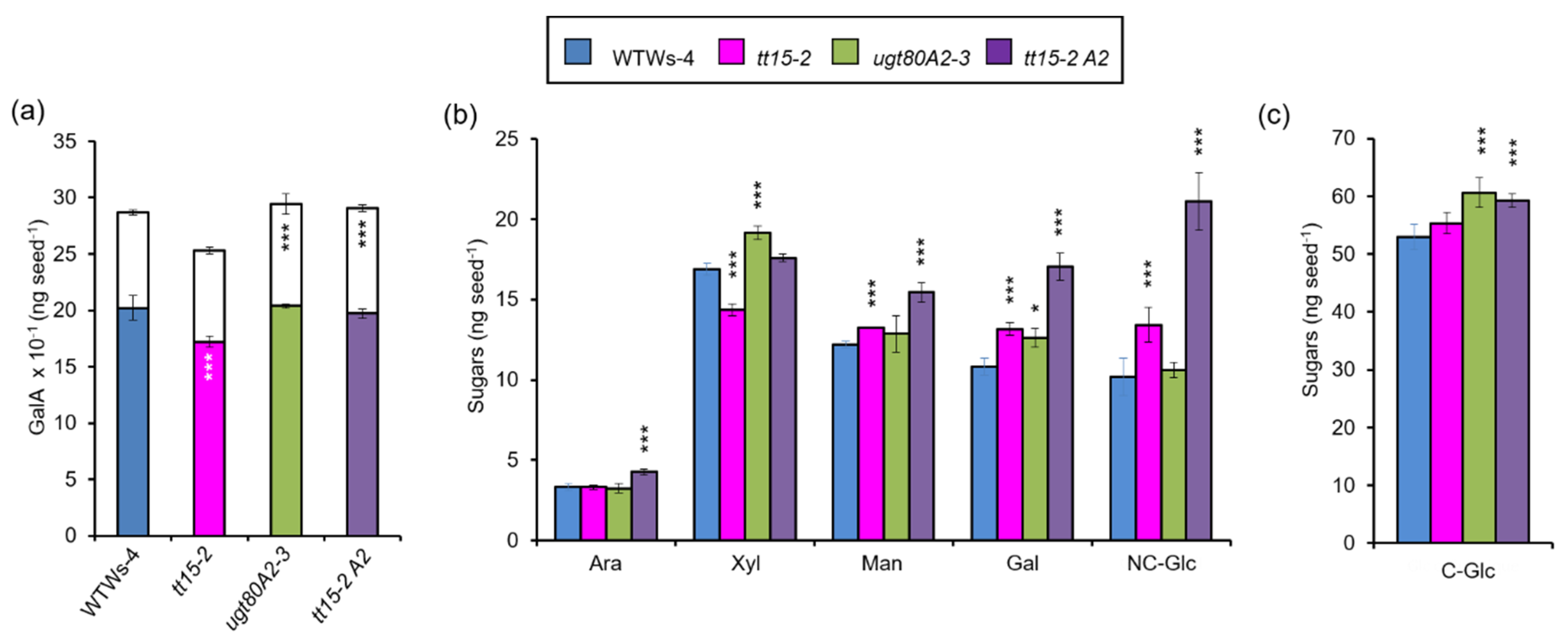
3.6. The Accumulation of Seed Mucilage Polysaccharides Is Modified in Both tt15 and ugt80A2 Mutants
3.7. The Density of the Inner Mucilage Polysaccharide Network Is Altered by Mutation of Sterol Glucosyl Transferases
4. Discussion
4.1. UGT80A2 and UGT80B1 Sterol Glucosyltransferases Influence the Accumulation of Mucilage Polysaccharides
4.2. Steryl Glucosides Produced by UGT80A2 and UGT80B1 Modulate the Density of the Inner Mucilage Polymer Network
4.3. Differences in Seed Size Can Mask Mutation Effects on Seed Constituents
4.4. SG and ASG Synthesized by UGT80B1 Impact Cell Wall Components in Seed Coat Epidermal Cells
4.5. Mucilage Release Phenotypes Caused by TT15 Mutation Exhibit Variable Penetrance and Expressivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, K.; Mongrand, S.; Beney, L.; Simon-Plas, F.; Gerbeau-Pissot, P. Differential effect of plant lipids on membrane organization: Specificities of phytosphingolipids and phytosterols. J. Biol. Chem. 2015, 290, 5810–5825. [Google Scholar] [CrossRef] [Green Version]
- Stucky, D.F.; Arpin, J.C.; Schrick, K. Functional diversification of two UGT80 enzymes required for steryl glucoside synthesis in Arabidopsis. J. Exp. Bot. 2015, 66, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnecke, D.C.; Baltrusch, M.; Buck, F.; Wolter, F.P.; Heinz, E. UDP-glucose:sterol glucosyltransferase: Cloning and functional expression in Escherichia coli. Plant Mol. Biol. 1997, 35, 597–603. [Google Scholar] [CrossRef] [PubMed]
- DeBolt, S.; Scheible, W.R.; Schrick, K.; Auer, M.; Beisson, F.; Bischoff, V.; Bouvier-Nave, P.; Carrol, A.; Hematy, K.; Li, Y.; et al. Mutations in UDP-glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol. 2009, 151, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Focks, N.; Sagasser, M.; Weisshaar, B.; Benning, C. Characterization of tt15, a novel transparent testa mutant of Arabidopsis thaliana (L.) Heynh. Planta 1999, 208, 352–357. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [Green Version]
- Francoz, E.; Lepiniec, L.; North, H.M. Seed coats as an alternative molecular factory: Thinking outside the box. Plant Reprod. 2018, 31, 327–342. [Google Scholar] [CrossRef]
- Beeckman, T.; De Rycke, R.; Viane, R.; Inzé, D. Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 2000, 113, 139–148. [Google Scholar] [CrossRef]
- Molina, I.; Ohlrogge, J.B.; Pollard, M. Deposition and localization of lipid polyester in developing seeds of Brassica napus and Arabidopsis thaliana. Plant J. 2008, 53, 437–449. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Pourcel, L.; Routaboul, J.M.; Kerhoas, L.; Caboche, M.; Lepiniec, L.; Debeaujon, I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 2005, 17, 2966–2980. [Google Scholar] [CrossRef] [Green Version]
- Western, T.L.; Skinner, D.J.; Haughn, G.W. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 2000, 122, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windsor, J.B.; Symonds, V.V.; Mendenhall, J.; Lloyd, A.M. Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 2000, 22, 483–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macquet, A.; Ralet, M.C.; Kronenberger, J.; Marion-Poll, A.; North, H.M. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 2007, 48, 984–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francoz, E.; Ranocha, P.; Le Ru, A.; Martinez, Y.; Fourquaux, I.; Jauneau, A.; Dunand, C.; Burlat, V. Pectin demethylesterification generates platforms that anchor peroxidases to remodel plant cell wall domains. Dev. Cell. 2019, 48, 261–276.e8. [Google Scholar] [CrossRef] [Green Version]
- Saez-Aguayo, S.; Ralet, M.C.; Berger, A.; Botran, L.; Ropartz, D.; Marion-Poll, A.; North, H.M. PECTIN METHYLESTERASE INHIBITOR6 promotes Arabidopsis mucilage release by limiting methylesterification of homogalacturonan in seed coat epidermal cells. Plant Cell 2013, 25, 308–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zauber, H.; Burgos, A.; Zhao, H.; Buros, A.; Garapati, P.; Schulze, W. Plasma membrane lipid–protein interactions affect signaling processes in sterol-biosynthesis mutants in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routaboul, J.M.; Dubos, C.; Beck, G.; Marquis, C.; Bidzinski, P.; Loudet, O.; Lepiniec, L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012, 63, 3749–3764. [Google Scholar] [CrossRef] [Green Version]
- Brunaud, V.; Balzergue, S.; Dubreucq, B.; Aubourg, S.; Samson, F.; Chauvin, S.; Bechtold, N.; Cruaud, C.; DeRose, R.; Pelletier, G.; et al. T-DNA integration into the Arabidopsis genome depends on sequences of pre-insertion sites. EMBO Rep. 2002, 3, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Pook, V.G.; Nair, M.; Ryu, K.; Arpin, J.C.; Schiefelbein, J.; Schrick, K.; DeBolt, S. Positioning of the SCRAMBLED receptor requires UDP-Glc:sterol glucosyltransferase 80B1 in Arabidopsis roots. Sci. Rep. 2017, 7, 5714. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Macquet, A.; Ralet, M.C.; Loudet, O.; Kronenberger, J.; Mouille, G.; Marion-Poll, A.; North, H.M. A naturally occurring mutation in an Arabidopsis accession affects a beta-D-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 2007, 19, 3990–4006. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, S.; Ralet, M.C.; Berger, A.; Diatloff, E.; Bischoff, V.; Gonneau, M.; Marion-Poll, A.; North, H.M. CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol. 2011, 156, 1725–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralet, M.C.; Tranquet, O.; Poulain, D.; Moise, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Blake, A.W.; Benians, T.A.; Lee, K.J.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; King, J.; Cooper, C.; Roberts, K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 1990, 181, 512–521. [Google Scholar] [CrossRef]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Cambert, M.; Berger, A.; Sallé, C.; Esling, S.; Charif, D.; Cadoret, T.; Ralet, M.-C.; North, H.M.; Rondeau-Mouro, C. Datasets of seed mucilage traits for Arabidopsis thaliana natural accessions with atypical outer mucilage. Sci. Data 2021, 8, 79. [Google Scholar] [CrossRef]
- Schols, H.A.; Geraeds, C.C.J.M.; Searle-van Leeuwen, M.J.F.; Kormelink, F.J.M.; Voragen, A.C.J. Rhamnogalacturonase: A novel enzyme that degrades the hairy regions of pectins. Carbohydr. Res. 1990, 206, 105–115. [Google Scholar] [CrossRef]
- Zhao, X.; Qioa, L.; Wu, A.-M. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci. Rep. 2017, 7, 40672. [Google Scholar] [CrossRef] [Green Version]
- Thibault, J.-F. Automatisation du dosage des substances pectiques par la méthode au métahydroxydiphényle. Lebensm. Wiss. Technol. 1979, 12, 247–251. [Google Scholar]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Saez-Aguayo, S.; Parra-Rojas, J.P.; Sepúlveda-Orellana, P.; Celiz-Balboa, J.; Arenas-Morales, V.; Sallé, C.; Salinas-Grenet, H.; Largo-Gosens, A.; North, H.M.; Ralet, M.-C.; et al. Transport of UDP-Rha by URGT2, URGT4 and URGT6 modulates rhamnogalacturonan-1 length. Plant Physiol. 2021, 185, 914–933. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Schmidt, M.H.; Berger, A.; Yang, B.; Ebert, B.; Scheller, H.V.; North, H.M.; Usadel, B.; Gunl, M. MUCILAGE-RELATED10 Produces galactoglucomannan that maintains pectin and cellulose architecture in Arabidopsis seed mucilage. Plant Physiol. 2015, 169, 403–420. [Google Scholar] [CrossRef]
- Marquer, C.; Fruchart-Gaillard, C.; Mourier, G.; Grandjean, O.; Girard, E.; le Maire, M.; Brown, S.; Serven, D. Influence of MT7 toxin on the oligomerization state of the M1 muscarinic receptor. Biol. Cell 2010, 102, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez-Aguayo, S.; Rondeau-Mouro, C.; Macquet, A.; Kronholm, I.; Ralet, M.C.; Berger, A.; Salle, C.; Poulain, D.; Granier, F.; Botran, L.; et al. Local evolution of seed flotation in Arabidopsis. PLoS Genet. 2014, 10, e1004221. [Google Scholar] [CrossRef] [PubMed]
- Voiniciuc, C.; Gunl, M.; Schmidt, M.H.; Usadel, B. Highly Branched Xylan Made by IRREGULAR XYLEM14 and MUCILAGE-RELATED21 links mucilage to Arabidopsis seeds. Plant Physiol. 2015, 169, 2481–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, H.M.; Berger, A.; Saez-Aguayo, S.; Ralet, M.C. Understanding polysaccharide production and properties using seed coat mutants: Future perspectives for the exploitation of natural variants. Ann. Bot. 2014, 114, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Arsovski, A.A.; Villota, M.M.; Rowland, O.; Subramaniam, R.; Western, T.L. MUM ENHANCERS are important for seed coat mucilage production and mucilage secretory cell differentiation in Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 2601–2612. [Google Scholar] [CrossRef] [Green Version]
- Kunieda, T.; Shimada, T.; Kondo, M.; Nishimura, M.; Nishitani, K.; Hara-Nishimura, I. Spatiotemporal secretion of PEROXIDASE36 is required for seed coat mucilage extrusion in Arabidopsis. Plant Cell 2013, 25, 1355–1367. [Google Scholar] [CrossRef] [Green Version]
- Rautengarten, C.; Usadel, B.; Neurnetzler, L.; Hartmann, J.; Buessis, D.; Altmann, T. A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J. 2008, 54, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Powell, D.A.; Gidley, M.J.; Rees, D.A. Conformations and interactions of pectins. I. Polymorphism between gel and solid states of calcium polygalacturonate. J. Mol. Biol. 1982, 155, 507–516. [Google Scholar] [CrossRef]
- Bhargava, A.; Ahad, A.; Wang, S.; Mansfield, S.D.; Haughn, G.W.; Douglas, C.J.; Ellis, B.E. The interacting MYB75 and KNAT7 transcription factors modulate secondary cell wall deposition both in stems and seed coat in Arabidopsis. Planta 2013, 237, 1199–1211. [Google Scholar] [CrossRef]
- Fabrissin, I.; Cueff, G.; Berger, A.; Granier, F.; Sallé, C.; Poulain, D.; Ralet, M.-C.; North, H.M. Natural variation reveals a key role for rhamnogalacturonan I in seed outer mucilage and underlying genes. Plant Physiol. 2019, 181, 1498–1518. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Crepeau, M.J.; Vigouroux, J.; Tran, J.; Berger, A.; Sallé, C.; Granier, F.; Botran, L.; North, H.M. Xylans provide the structural driving force for mucilage adhesion to the Arabidopsis seed coat. Plant Physiol. 2016, 171, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Shi, D.; Li, J.; Kong, Y.; Yu, Y.; Chai, G.; Hu, R.; Wang, J.; Hahn, M.G.; Zhou, G. CELLULOSE SYNTHASE-LIKE A2, a glucomannan synthase, is involved in maintaining adherent mucilage structure in Arabidopsis seed. Plant Physiol. 2014, 164, 1842–1856. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.S.; Crépeau, M.-J.; Ralet, M.-C.; Seifert, G.J.; North, H.M. Dissecting seed mucilage adherence mediated by FEI2 and SOS5. Front. Plant Sci. 2016, 7, 1073. [Google Scholar] [CrossRef] [Green Version]
- Willats, W.G.; McCartney, L.; Knox, J.P. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 2001, 213, 37–44. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; McFarlane, H.E.; Xu, S.; Divi, U.K.; Forward, B.; Western, T.L.; Kieber, J.J. Cellulose synthesis via the FEI2 RLK/SOS5 pathway and CELLULOSE SYNTHASE 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J. 2011, 68, 941–953. [Google Scholar] [CrossRef]
- Mendu, V.; Griffiths, J.S.; Persson, S.; Stork, J.; Downie, A.B.; Voiniciuc, C.; Haughn, G.W.; DeBolt, S. Subfunctionalization of cellulose synthases in seed coat epidermal cells mediates secondary radial wall synthesis and mucilage attachment. Plant Physiol. 2011, 157, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Estrada, K.; Castillo, N.; Lara, J.A.; Arró, M.; Boronat, A.; Ferrer, A.; Altabella, T. Tomato UDP-Glucose Sterol Glycosyltransferases: A family of developmental and stress regulated genes that encode cytosolic and membrane-associated forms of the enzyme. Front. Plant Sci. 2017, 8, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Kawagoe, Y.; Hogan, P.; Delmer, D. Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 2002, 295, 147–150. [Google Scholar] [CrossRef]
- Šola, K.; Gilchrist, E.J.; Ropartz, D.; Wang, L.; Feussner, I.; Mansfield, S.D.; Ralet, M.C.; Haughn, G.W. RUBY, a putative galactose oxidase, influences pectin properties and promotes cell-to-cell adhesion in the seed coat epidermis of Arabidopsis. Plant Cell 2019, 31, 809–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Pei, S.; Lu, M.; Kong, Y.; Zhou, G.; Hu, R. KNAT7 regulates xylan biosynthesis in Arabidopsis seed-coat mucilage. J. Exp. Bot. 2020, 71, 4125–4139. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; Fujioka, S.; Takatsuto, S.; Stierhof, Y.D.; Stransky, H.; Yoshida, S.; Jürgens, G. A link between sterol biosynthesis, the cell wall, and cellulose in Arabidopsis. Plant J. 2004, 38, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Thiedig, K.; Nordholt, N.; Schmidt, N.; Huep, G.; Sagasser, M.; Weisshaar, B. Update on transparent testa mutants from Arabidopsis thaliana: Characterisation of new alleles from an isogenic collection. Planta 2014, 240, 955–970. [Google Scholar] [CrossRef]
- Gendre, D.; McFarlane, H.E.; Johnson, E.; Mouille, G.; Sjödin, A.; Oh, J.; Levesque-Tremblay, G.; Watanabe, Y.; Samuels, L.; Bhalerao, R.P. Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 2013, 25, 2633–2646. [Google Scholar] [CrossRef] [Green Version]
- Kulich, I.; Cole, R.; Drdová, E.; Cvrcková, F.; Soukup, A.; Fowler, J.; Zárský, V. Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol. 2010, 188, 615–625. [Google Scholar] [CrossRef]






| Genotype | Biological Replicate 1 | Biological Replicate 2 |
|---|---|---|
| Wild type Ws-4 1 | 0.0306 (±0.0003) | 0.0228 (±0.0002) |
| tt15-2 ugt80A2-3 1 | 0.0329 (±0.0003) *** | 0.0291 (±0.0006) * |
| tt15-2 ugt80A2-3 2 | 0.0341 (±0.0005) *** | 0.0240 (±0.0003) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, A.; Ralet, M.-C.; Akary, E.; Sallé, C.; Grandjean, O.; Debeaujon, I.; North, H.M. Sterol Glucosyltransferases Tailor Polysaccharide Accumulation in Arabidopsis Seed Coat Epidermal Cells. Cells 2021, 10, 2546. https://doi.org/10.3390/cells10102546
Berger A, Ralet M-C, Akary E, Sallé C, Grandjean O, Debeaujon I, North HM. Sterol Glucosyltransferases Tailor Polysaccharide Accumulation in Arabidopsis Seed Coat Epidermal Cells. Cells. 2021; 10(10):2546. https://doi.org/10.3390/cells10102546
Chicago/Turabian StyleBerger, Adeline, Marie-Christine Ralet, Elodie Akary, Christine Sallé, Olivier Grandjean, Isabelle Debeaujon, and Helen M. North. 2021. "Sterol Glucosyltransferases Tailor Polysaccharide Accumulation in Arabidopsis Seed Coat Epidermal Cells" Cells 10, no. 10: 2546. https://doi.org/10.3390/cells10102546
APA StyleBerger, A., Ralet, M.-C., Akary, E., Sallé, C., Grandjean, O., Debeaujon, I., & North, H. M. (2021). Sterol Glucosyltransferases Tailor Polysaccharide Accumulation in Arabidopsis Seed Coat Epidermal Cells. Cells, 10(10), 2546. https://doi.org/10.3390/cells10102546