Significance of NETs Formation in COVID-19
Abstract
1. Introduction
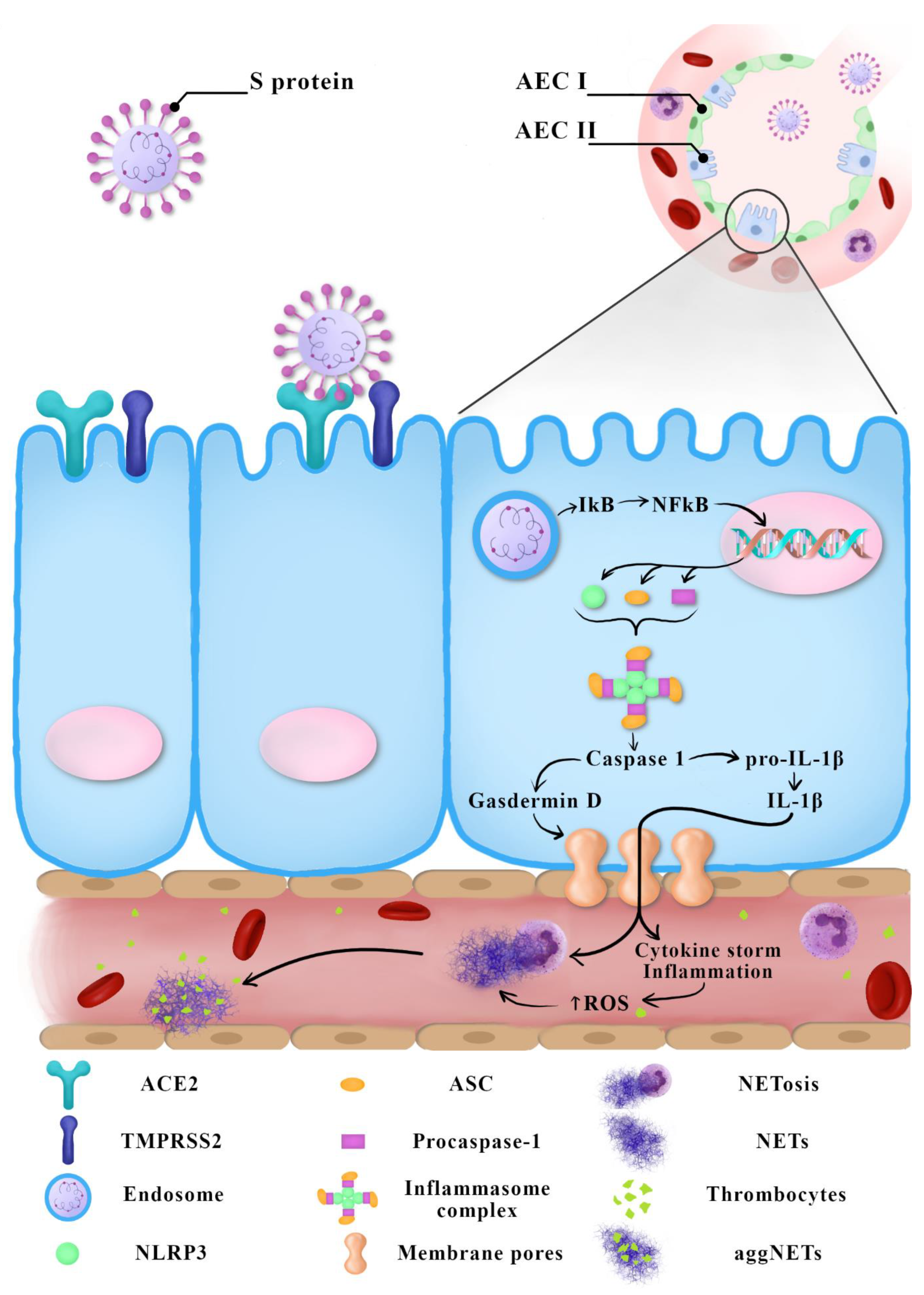
2. Immunopathogenesis of COVID-19
3. Neutrophil Extracellular Traps
- NADPH oxidase engaged in the process of ROS production;
- NE degrading intracellular proteins and initiating the disintegration of the cell’s nucleus;
- Protein arginine deiminase 4 (PAD4), which citrullinates histones to facilitate decondensation and release of chromosomal DNA;
- Gasdermin D, which is responsible for the generation of pores in the cell’s membrane allowing the expulsion of traps beyond the cell wall.
4. NETs and COVID-19 Progression in People with Concurrent Diseases
5. Prospective COVID-19 Therapies Based on the Regulation of NETs Formation
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef]
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R.; Roch, B. SARS-CoV2 may evade innate immune response, causing uncontrolled neutrophil extracellular traps formation and multi-organ failure. Clin. Sci. 2020, 134, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Tomar, B.; Anders, H.J.; Desai, J.; Mulay, S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells 2020, 9, 1383. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body localization of ACE-2: On the trail of the keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.K.; Radic, M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front. Pharmacol. 2020, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Stürzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.L.; Rada, B.; Tandon, E.; Gestal, M.C. NETs and EETs, a whole web of mess. Microorganisms 2020, 8, 1925. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and neutrophils: The relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two potential targets for COVID-19 treatment. Mediators Inflamm. 2020, 2020, 7527953. [Google Scholar] [CrossRef]
- Nakazawa, D.; Ishizu, A. Immunothrombosis in severe COVID-19. EBioMedicine 2020, 59, 102942. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; Dos Reis, M.C.; de Castro, G.M.M.; da Silva Fontes, Y.; Todeschini, A.R.; Freire-de-Lima, L.; Decoté-Ricardo, D.; et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Wu, S.; Chen, S.; Li, Y.; Nong, L.; Lie, P.; Huang, L.; Cheng, L.; Lin, Y.; et al. Definition and risks of cytokine release syndrome in 11 Critically Ill COVID-19 patients with pneumonia: Analysis of disease characteristics. J. Infect. Dis. 2020, 222, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Wong, G.; Cui, J. Bat origin of a new human coronavirus: There and back again. Sci. China Life Sci. 2020, 63, 461–462. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Mozzini, C.; Girelli, D. The role of neutrophil extracellular traps in Covid-19: Only an hypothesis or a potential new field of research? Thromb. Res. 2020, 191, 26–27. [Google Scholar] [CrossRef]
- Dąbrowska, D.; Jabłońska, E.; Garley, M.; Ratajczak-Wrona, W.; Iwaniuk, A. New aspects of the biology of neutrophil extracellular traps. Scand. J. Immunol. 2016, 84, 317–322. [Google Scholar] [CrossRef]
- Santocki, M.; Kolaczkowska, E. On Neutrophil Extracellular Trap (NET) removal: What we know thus far and why so little. Cells 2020, 9, 2079. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [PubMed]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Thomas, G.M.; Martinod, K.; De Meyer, S.F.; Bhandari, A.A.; Wagner, D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 2012, 10, 136–144. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.J. Neutrophils and Neutrophil Extracellular Traps regulate immune responses in health and disease. Cells 2020, 9, 2130. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Kashir, J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med. Hypotheses 2020, 143, 109906. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir. Investig. 2020, 58, 419–420. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Musolino, C. Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection: Pathogenesis and management strategies. Ann. Hematol. 2020, 99, 1953–1965. [Google Scholar] [CrossRef]
- Price, L.C.; McCabe, C.; Garfield, B.; Wort, S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020, 56, 2001608. [Google Scholar] [CrossRef] [PubMed]
- Pujhari, S.; Paul, S.; Ahluwalia, J.; Rasgon, J.L. Clotting disorder in severe acute respiratory syndrome coronavirus 2. Rev. Med. Virol. 2020, e2177. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Z.; Long, Q.; Huang, J.; Hong, T.; Liu, W.; Lin, J. Insights into immunothrombosis: The interplay among Neutrophil Extracellular Trap, von Willebrand factor, and ADAMTS13. Front. Immunol. 2020, 11, 610696. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Thakur, V.; Kaur, P.; Khan, A.; Kulshrestha, S.; Kumar, P. Blood clots in COVID-19 patients: Simplifying the curious mystery. Med. Hypotheses 2020, 110371. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI. Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Thierry, A.R.; Roch, B. Neutrophil extracellular traps and by-products play a key role in COVID-19: Pathogenesis, risk factors, and therapy. J. Clin. Med. 2020, 9, 2942. [Google Scholar] [CrossRef]
- Petito, E.; Falcinelli, E.; Paliani, U.; Cesari, E.; Vaudo, G.; Sebastiano, M.; Cerotto, V.; Guglielmini, G.; Gori, F.; Malvestiti, M.; et al. Neutrophil more than platelet activation associates with thrombotic complications in COVID-19 patients. J. Infect. Dis. 2020, 6, jiaa756. [Google Scholar] [CrossRef]
- Pfeiler, S.; Stark, K.; Massberg, S.; Engelmann, B. Propagation of thrombosis by neutrophils and extracellular nucleosome networks. Haematologica. 2017, 102, 206–213. [Google Scholar] [CrossRef]
- Roberts, K.A.; Colley, L.; Agbaedeng, T.A.; Ellison-Hughes, G.M.; Ross, M.D. Vascular manifestations of COVID-19—thromboembolism and microvascular dysfunction. Front. Cardiovasc. Med. 2020, 7, 598400. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Ding, J.; You, Y.; Dong, Z.; Chehade, H.; Alvero, A.; Mor, Y.; Draghici, S.; Mor, G. Identification of key signaling pathways induced by SARS-CoV2 that underlie thrombosis and vascular injury in COVID-19 patients. J. Leukoc. Biol. 2021, 109, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, É.A.; Galvão, J.G.F.M.; da Silva, J.S.F.; Sales-Neto, J.M.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2020, 90, 107233. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Colling, M.E.; Kanthi, Y. COVID–19-associated coagulopathy: An exploration of mechanisms. Vasc. Med. 2020, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ammollo, C.T.; Semeraro, F.; Xu, J.; Esmon, N.L.; Esmon, C.T. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011, 9, 1795–1803. [Google Scholar] [CrossRef]
- Hidalgo, A. A NET-thrombosis axis in COVID-19. Blood 2020, 136, 1118–1119. [Google Scholar] [CrossRef]
- Ye, W.; Chen, G.; Li, X.; Lan, X.; Ji, C.; Hou, M.; Zhang, D.; Zeng, G.; Wang, Y.; Xu, C.; et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir. Res. 2020, 21, 169. [Google Scholar] [CrossRef]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2020, 1–8. [Google Scholar]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef]
- Wise, J. Covid-19 and thrombosis: What do we know about the risks and treatment? BMJ 2020, 369, m2058. [Google Scholar] [CrossRef]
- Busch, M.H.; Timmermans, S.A.M.E.G.; Nagy, M.; Visser, M.; Huckriede, J.; Aendekerk, J.P.; de Vries, F.; Potjewijd, J.; Jallah, B.; Ysermans, R.; et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation 2020, 142, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.E.; Umapathi, T.; Chua, K.; Chia, Y.W.; Wong, S.W.; Tan, G.W.L.; Chandrasekar, S.; Lum, Y.H.; Vasoo, S.; Dalan, R. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J. Thromb. Thrombolysis 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dalan, R.; Boehm, B.O. The implications of COVID-19 infection on the endothelium: A metabolic vascular perspective. Diabetes Metab. Res. Rev. 2020, e3402. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, S.A.; Dighero-Kemp, B.; Ouédraogo, D.D.; Hensley, L.; Sakandé, J. How NETosis could drive “Post-COVID-19 syndrome” among survivors. Immunol. Lett. 2020, 228, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Dąbrowska, D.; Jabłońska, E.; Garley, M.; Sawicka-Powierza, J.; Nowak, K. The phenomenon of neutrophil extracellular traps in vascular diseases. Arch. Immunol. Ther. Exp. 2018, 66, 273–281. [Google Scholar] [CrossRef]
- Chiang, C.C.; Korinek, M.; Cheng, W.J.; Hwang, T.L. Targeting neutrophils to treat acute respiratory distress syndrome in Coronavirus disease. Front. Pharmacol. 2020, 11, 572009. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Qiu, L.; Zhang, C.; Deng, Q.; Leng, Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating Coronavirus disease 2019 and lung injury. Front. Immunol. 2020, 11, 2056. [Google Scholar] [CrossRef]
- Street, M.E. HMGB1: A possible crucial therapeutic target for COVID-19? Horm. Res. Paediatr. 2020, 93, 73–75. [Google Scholar] [CrossRef]
| Treatment | Target | Action | |
|---|---|---|---|
| Inhibition of NETs formation | NE inhibitors: - Sivelestat - Lonodelestat - Alvelestat - Alafina - CHF6333 | NE | Antiprotease |
| PAD4 inhibitor: - Cl-amidine | PAD4 | Inhibition of histone citrullination | |
| Gasdermin D inhibitor: - Disulfiram | Gasdermin D | Inhibition of pore generation in the cell membrane | |
| Dipyridamole | A2A adenosine receptors | Adenosine uptake inhibitor | |
| Aspirin | NF-κB p65 signaling pathway | Decrease in NETs formation | |
| NETs dissolution induction | Dornase alfa | DNA | DNA degradation |
| Blocking IL-1β activity | IL-1β inhibitors: - Anakinra - Canakinumab - Rilonacept | IL-1β | Disrupting the feedback loop IL-1β/NETs |
| Colchicine | - Neutrophils - IL-1β | - Inhibition of neutrophil recruitment - Blocking the secretion of IL-1β | |
| Anti-inflammatory effect | Glyburide | ATP-sensitive K+ channels | Blocking the activation of the NLRP3 inflammasome |
| Metformin | Alarmin HMGB1 | Suppressing proinflammatory properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiuk, K.; Jabłońska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. https://doi.org/10.3390/cells10010151
Janiuk K, Jabłońska E, Garley M. Significance of NETs Formation in COVID-19. Cells. 2021; 10(1):151. https://doi.org/10.3390/cells10010151
Chicago/Turabian StyleJaniuk, Karolina, Ewa Jabłońska, and Marzena Garley. 2021. "Significance of NETs Formation in COVID-19" Cells 10, no. 1: 151. https://doi.org/10.3390/cells10010151
APA StyleJaniuk, K., Jabłońska, E., & Garley, M. (2021). Significance of NETs Formation in COVID-19. Cells, 10(1), 151. https://doi.org/10.3390/cells10010151





