The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Soil of the Experimental Field Plot
2.2. Weather Conditions
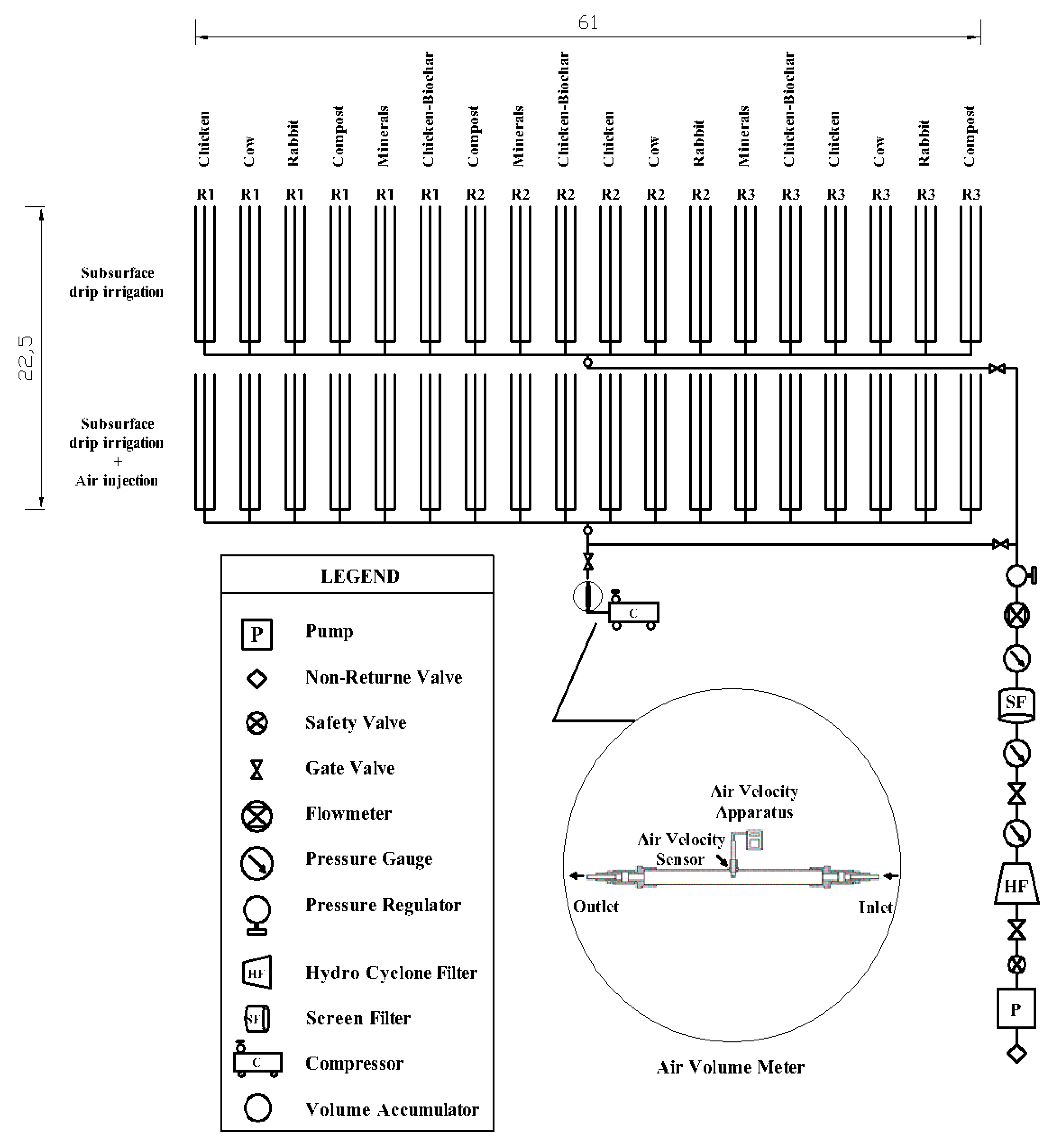
2.3. System Installation and Experimental Treatments
2.4. Nutrient Management
2.5. Air Injection
2.6. Data Recording
2.7. Statistical Analyses
3. Results and Discussion
3.1. Soil Physical Characteristics
3.2. Effect of Irrigation with Organic Fertlizer on Yield
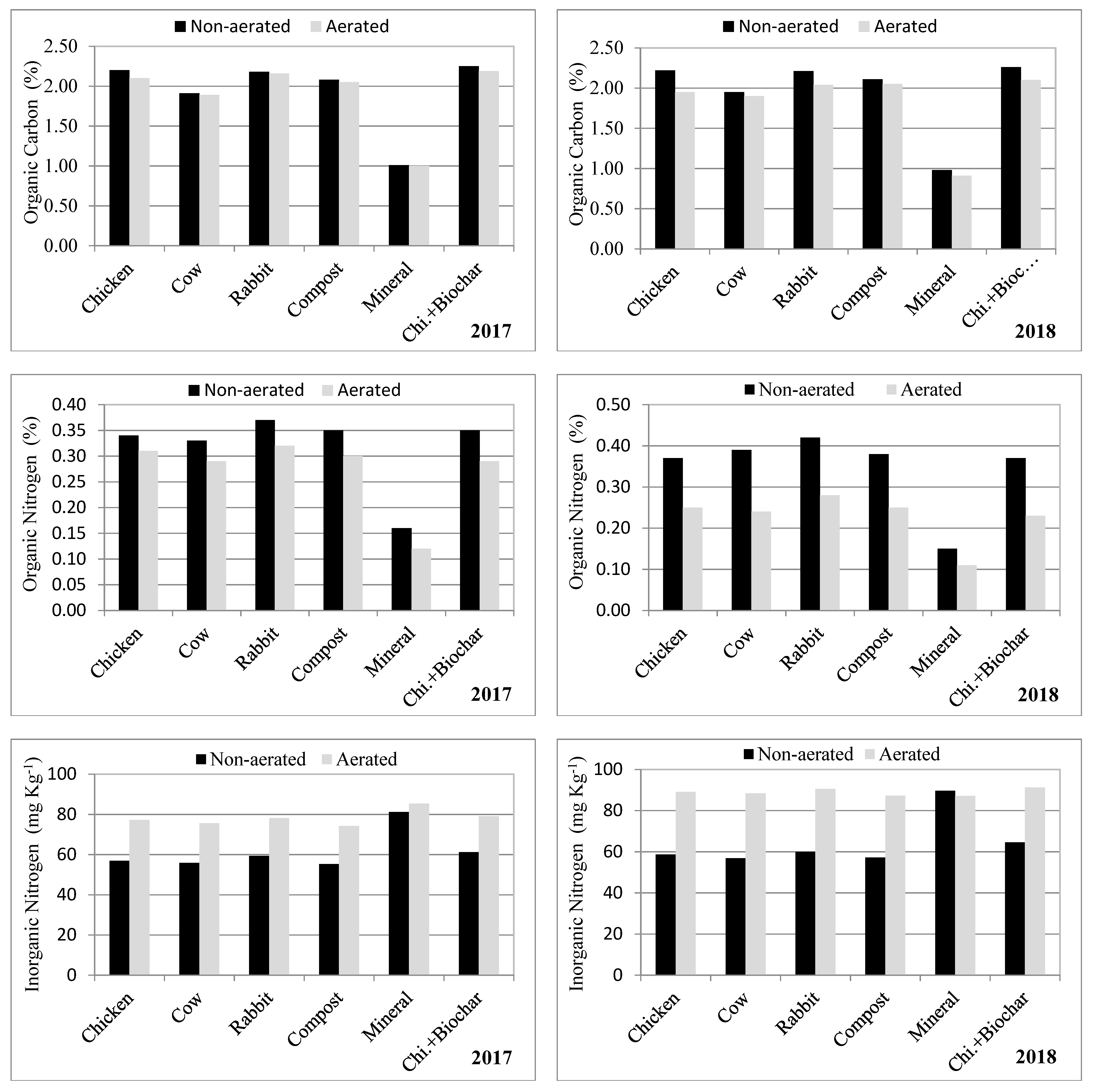
3.3. Soil Chemical Characteristics
3.4. Plant Growth Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-sayed, S.F. Effect of Different Substrates and Nutrient Solutions on Vegetative Growth, Mineral Content, Production and Fruit. Zagazig J. Agric. Res. 2017, 43, 1919–1938. [Google Scholar]
- FAO. Agriculture Organization of the United Nations Statistics Division; FAO: Rome, Italy, 2016. [Google Scholar]
- Yuan, L.; Wenquan, N.; Jingwei, W.; Jian, X. Review on advances of airjection irrigation. Int. J. Agric. Biol. Eng. 2015, 9, 1–10. [Google Scholar]
- Rengasamy, P. Subsoil constraints and agricultural productivity. J. Indian Soc. Soil Sci. 2000, 48, 674–682. [Google Scholar]
- Bhattarai, S.P.; Midmore, D.J. Oxygation of rhizosphere with subsurface aerated irrigation water improves lint yield and performance of cotton on saline heavy clay soil. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; Volume 26. [Google Scholar]
- Ben-Noah, I.; Friedman, S.P. Aeration of clayey soils by injecting air through subsurface drippers: Lysimetric and field experiments. Agric. Water Manag. 2016, 176, 222–233. [Google Scholar] [CrossRef]
- Errebhi, M.; Rosen, C.J.; Gupta, S.C.; Birong, D.E. Potato yield response and nitrate leaching as influenced by nitrogen management. Agron. J. 1998, 90, 10–15. [Google Scholar] [CrossRef]
- Mehrvarz, S.; Chaichi, M.R.; Alikhani, H.A. Effects of phosphate solubilizing microorganisms and phosphorus chemical fertilizer on yield and yield components of barely (Hordeum vulgare L.) department of soil science engineering. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 822–828. [Google Scholar]
- McLaren, R.G.; Cameron, K.C. Soil Sciencesustainable Production and Environmental Protection; Oxford University Press: Mexico, 1996; ISBN 0195583450. [Google Scholar]
- Ferdoushi, S.N.; Farooque, A.M.; Alam, M.S. Effects of organic and inorganic fertilizer management practices and mulch on the growth and yield of potato. J. Agrofor. Environ. 2010, 3, 175–178. [Google Scholar]
- Bhattarai, S.; Pendergast, L.; Midmore, D.J. Oxygation of Subsurface Drip Irrigated Tomato (Lycopersicon esculentum L.) Improves Yield Performance, Tolerance to Salinity and Water Use Efficiency in Normal and Saline Heavy Clay Soil; Sci. Hortic.: Rockhampton, Australia, 2005. [Google Scholar]
- Abuarab, M.; Mostafa, E.; Ibrahim, M. Effect of air injection under subsurface drip irrigation on yield and water use efficiency of corn in a sandy clay loam soil. J. Adv. Res. 2013, 4, 493–499. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Midmore, D.J.; Pendergast, L. Yield, water-use efficiencies and root distribution of soybean, chickpea and pumpkin under different subsurface drip irrigation depths and oxygation treatments in vertisols. Irrig. Sci. 2008, 26, 439–450. [Google Scholar] [CrossRef]
- Niu, W.; Guo, Q.; Zhou, X.; Helmers, M.J. Effect of aeration and soil water redistribution on the air permeability under subsurface drip irrigation. Soil Sci. Soc. Am. J. 2012, 76, 815–820. [Google Scholar] [CrossRef]
- Klute, A. Methods of soil analysis, part 1 physical and mineralogical methods, Arnold Klute ed. Agronomy 1986, 9. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India: New Delh, India, 1967. [Google Scholar]
- Vomocil, J.A. Measurement of soil bulk density and penetrability: A review of methods. Adv. Agron. 1957, 9, 159–175. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; USDA: Denver, CO, USA, 1986; pp. 635–662. [Google Scholar]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Soil organic carbon and total nitrogen stocks as affected by topographic aspect and vegetation in the Bale Mountains, Ethiopia. Geoderma 2006, 135, 335–344. [Google Scholar] [CrossRef]
- Jackson, M.L. Estimation of Phosphorus Content: Soil Chemical Analysis; Printer Hall: New Delhi, India, 1973. [Google Scholar]
- Black, C.A. Methods of Soil Analysis: Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling. Part 2. Chemical and Microbiological Properties; Agronomy: Denver, CO, USA, 1965. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper 1. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Goorahoo, D.; Carstensen, G.; Zoldoske, D.F.; Norum, E.; Mazzei, A. Using air in sub-surface drip irrigation(SDI) to increase yields in bell peppers. Int. Water Irrig. 2002, 22, 39–42. [Google Scholar]
- Heuberger, H.; Livet, J.; Schnitzler, W. Effect of soil aeration on nitrogen availability and growth of selected vegetables-preliminary results. In Proceedings of the International Conference on Environmental Problems Associated with Nitrogen Fertilisation of Field Grown Vegetable Crops, Potsdam, Germany, 30 August–1 September 1999; Volume 563, pp. 147–154. [Google Scholar]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated subsurface irrigation water gives growth and yield benefits to zucchini, vegetable soybean and cotton in heavy clay soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Phillips, S.B.; Warren, J.G.; Keahey, D.A.; Mullins, G.L. Nitrogen Management for White Potato Production. Va. Va. Coop. Ext. 2004, 438. [Google Scholar]
- Bhushan, L.; Ladha, J.K.; Gupta, R.K.; Singh, S.; Tirol-Padre, A.; Saharawat, Y.S.; Gathala, M.; Pathak, H. Saving of water and labor in a rice–wheat system with no-tillage and direct seeding technologies. Agronomy 2007, 99, 1288–1296. [Google Scholar] [CrossRef]
- Marcus, D.M.; Latov, N.; Hsi, B.P.; Gillard, B.K.; Antel, J.; Appel, S.; Callegaro, L.; Daune, G.; Gillard, B.; Latoy, N. Measurement and significance of antibodies against GM1 ganglioside Report of a workshop. J. Neuroimmunol. 1989, 25, 255–259. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Ames, Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Gibbs, J.; Greenway, H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef]
- Everard, J.D.; Drew, M.C. Mechanisms controlling changes in water movement through the roots of Helianthus annuus L. during continuous exposure to oxygen deficiency. J. Exp. Bot. 1989, 40, 95–104. [Google Scholar] [CrossRef]
- Samad, A.; Meisner, C.A.; Saifuzzaman, M.; Ginkel, M.V. Waterlogging tolerance. Application of Physiology in Wheat Breeding; Reynolds, M.P., Ortiz-Monasterio, J.I., McNab, A., Eds.; CIMMYT: Mexico, 2001; pp. 136–144. [Google Scholar]
- Anderson, R.C.; Liberta, A.E.; Dickman, L.A. Interaction of vascular plants and vesicular-arbuscular mycorrhizal fungi across a soil moisture-nutrient gradient. Oecologia 1984, 64, 111–117. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.F.; Hassan, A.H.; El-Mogy, M.M. Impact of Bio- and Organic Fertilizers on Potato Yield, Quality and Tuber Weight Loss After Harvest. Potato Res. 2015, 58, 67–81. [Google Scholar] [CrossRef]
- Huang, B.; Scott Nesmith, D. Soil Aeration Effects on Root Growth and Activity. In Proceedings of the VI Symposium on Stand Establishment and ISHS Seed Symposium, Roanoke, VA, USA, October 1999; Volume 504, pp. 41–52. [Google Scholar]
- Vartapetian, B.B.; Jackson, M.B. Plant adaptations to anaerobic stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Everard, C.O.R.; Everard, J.D. Mongoose rabies in Grenada. Population Dynamics of Rabies in Wildlife; Philip, J.B., Ed.; Academic Press: London, UK, 1985. [Google Scholar]
- Arkin, G.F.; Taylor, H.M. Modifying the Root Environment to Reduce Crop Stress; American Society of Agricultural Engineers: St. Joseph, MO, USA, 1981; ISBN 0916150402. [Google Scholar]
- Bhattarai, S.P.; Su, N.; Midmore, D.J. Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar]
- Rathbone, K.; Fuchs, J.; Anderson, K.; Karthikeyan, R.; Nurhidayat, N. Effects of Pahs on Microbial Activity and Diversity in Freshly Contaminated and Weathered Soils; Kansas State University: Manhattan, NY, USA, 1998; p. 383. [Google Scholar]
- Mundy, G.N.; Jones, H.R.; Mason, W.K. Nitrogen fixation activity by white clover pastures during flood irrigation cycles. Aust. J. Agric. Res. 1988, 39, 409–414. [Google Scholar] [CrossRef]
- Deaker, R.; Kennedy, I.R. Improved potential for nitrogen fixation in Azospirillum brasilense sp7-s associated with wheat nifH expression as a function of oxygen pressure. Acta Biotechnol. 2001, 21, 3–17. [Google Scholar] [CrossRef]
- Abdeldaym, E.A.; Erriquens, F.; Sasanelli, N.; Ceglie, F.G.; Zaccone, C.; Miano, T.; Cocozza, C. Effects of several amendments on organic melon growth and production, Meloidogyne incognita population and soil properties. Sci. Hortic. 2014, 180, 156–160. [Google Scholar] [CrossRef]
- Abdeldaym, E.A.; Traversa, A.; Cocozza, C.; Brunetti, G. Effects of a 2-Year Application of Different Residual Biomasses on Soil Properties and Potato Yield. Clean Soil Air Water 2018, 46. [Google Scholar] [CrossRef]
- Aguilar, E.A.; Turner, D.W.; Gibbs, D.J.; Armstrong, W.; Sivasithamparam, K. Oxygen distribution and movement, respiration and nutrient loading in banana roots (Musa spp. L.) subjected to aerated and oxygen-depleted environments. Plant Soil 2003, 253, 91–102. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by plant roots: An integration of views. Plant Soil 2000, 226, 45–56. [Google Scholar] [CrossRef]
- Armstrong, W. Aeration in higher plants. In Advances in Botanical Research; Elsevier: Hull, UK, 1980; Volume 7, pp. 225–332. ISBN 0065-2296. [Google Scholar]
- Allègre, A.; Silvestre, J.; Morard, P.; Kallerhoff, J.; Pinelli, E. Nitrate reductase regulation in tomato roots by exogenous nitrate: A possible role in tolerance to long-term root anoxia. J. Exp. Bot. 2004, 55, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R.R. Soil Aeration and Temperature. In The Nature and Properties of Soil, 12th ed.; Prentice Hall: New York, NY, USA, 1999; pp. 265–306. [Google Scholar]
- Vasellati, V.; Oesterheld, M.; Medan, D.; Loreti, J. Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann. Bot. 2001, 88, 355–360. [Google Scholar] [CrossRef]

| Soil Depth (cm) | Particle Size Distribution | Texture | Field Capacity (cm3 cm−3) | Wilting Point (cm3 cm−3) | Bulk Density (g cm−3) | ||
|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | |||||
| 0–20 | 35 | 33 | 32 | Clay Loam | 39.18 | 18.31 | 1.28 |
| 20–40 | 31 | 34 | 35 | Clay Loam | 39.02 | 19.31 | 1.3 |
| 40–60 | 29 | 33 | 38 | Clay Loam | 38.72 | 21.4 | 1.33 |
| pH (1:2.5) | ECe (dS m−1) | O.N (%) | N (mg kg−1) | P (mg kg−1) | K (mg kg−1) | Fe (mg kg−1) | Mn (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 7.82 | 2.51 | 0.17 | 41.18 | 58.12 | 512.4 | 5.15 | 22.1 |
| 7.81 | 2.08 | 0.15 | 36.4 | 51.33 | 498.2 | 4.84 | 19.9 |
| 7.83 | 1.98 | 0.14 | 29.58 | 50.08 | 473.3 | 4.4 | 19.2 |
| Year | Climate Parameter | Month | |||||
|---|---|---|---|---|---|---|---|
| October | November | December | January | February | March | ||
| 2016–2017 | Tmin (°C) | 16.0 | 12.2 | 8.2 | 6.3 | 6.6 | 10.1 |
| Tmax (°C) | 30.1 | 25.3 | 21.2 | 19.5 | 21.0 | 24.2 | |
| Tave (°C) | 23.1 | 18.8 | 14.7 | 12.9 | 13.8 | 17.2 | |
| RH (%) | 69 | 75 | 75 | 77 | 72 | 66 | |
| Solar radiation (MJ/m²/day) | 17.1 | 13.4 | 11.2 | 11.7 | 14.7 | 18.3 | |
| 2017–2018 | Tmin. (°C) | 17.6 | 13.7 | 9.8 | 7.9 | 8.9 | 10.9 |
| Tmax (°C) | 29.3 | 24.5 | 19.5 | 18.5 | 20.4 | 23.4 | |
| Tave (°C) | 23.45 | 19.10 | 14.65 | 13.20 | 14.65 | 17.15 | |
| RH (%) | 54 | 57 | 53 | 55 | 51 | 44 | |
| Solar radiation (MJ/m²) | 17.5 | 13.8 | 11.4 | 12.3 | 15.4 | 18.9 | |
| Organic Manure | pH (1:2.5) | ECe (dS m−1) | O.C (%) | N (%) | P (%) | K (%) | Fe (mg kg−1) | Mn (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| Chicken | 6.51 | 2.47 | 19.8 | 1.4 | 0.66 | 0.97 | 648.1 | 140.2 |
| Cow | 7.73 | 2.08 | 16.2 | 1.6 | 0.63 | 0.92 | 634.6 | 142.5 |
| Rabbit | 7.5 | 1.86 | 26.74 | 1.5 | 0.93 | 1.12 | 819.8 | 152.7 |
| Compost | 7.1 | 1.52 | 19.1 | 1.1 | 0.54 | 0.81 | 627.5 | 143.5 |
| Chicken + Biochar | 8.12 | 1.11 | 48.14 | 0.72 | 0.44 | 0.74 | 642.1 | 156.1 |
| Growing Seasons | Fertilizer Applications | Field Capacity (cm3 cm−3) | Wilting Point (cm3cm−3) | H.C (mm hour−1) | Bulk Density (g cm−3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | ||
| 2016–2017 | Mineral | 38.8 d | 38.1 g | 19.3 e | 18.9 f | 3.1 abc | 3.2 ab | 1.3 a | 1.28 bcd |
| Chicken | 42.7 c | 39.2 e | 20.7 d | 18.8 f | 2.9 cde | 3.2 ab | 1.28 bc | 1.23 e | |
| Cow | 39.1 e | 38.1 g | 19.7 e | 18.3 g | 3.0 bcd | 3.3 a | 1.29 b | 1.22 ef | |
| Rabbit | 44.8 a | 39.3 de | 21.6 b | 20.7 d | 2.7 f | 2.9 cde | 1.27 d | 1.22 ef | |
| Compost | 43.2 bc | 39.9 d | 21.1 c | 20.6 d | 2.9 cde | 3 bcd | 1.28 bcd | 1.27 d | |
| Chicken + Biochar | 43.8 bc | 38.2 g | 22.3 a | 20.8 cd | 2.5 g | 2.9 cde | 1.23 e | 1.2 g | |
| LSD 0.05 | 0.607 | 0.379 | 0.170 | 0.012 | |||||
| 2017–2018 | Mineral | 38.6 h | 38 i | 19.2 g | 18.4 h | 3.2 ab | 3.3 a | 1.29 a | 1.27 ab |
| Chicken | 43.3 d | 38.7 h | 21.1 c | 18.4 h | 2.7 de | 3.3 a | 1.27 ab | 1.21 e | |
| Cow | 39.3 f | 37.8 j | 19.6 f | 18.1 i | 2.7 de | 3.1 b | 1.27 ab | 1.21 e | |
| Rabbit | 44.7 a | 39 g | 21.9 b | 20.6 d | 2.6 ef | 2.8 d | 1.24 cd | 1.21 e | |
| Compost | 43.8 c | 39.6 e | 21.2 c | 20.4 e | 2.8 d | 3.1 b | 1.26 bc | 1.24 cd | |
| Chicken + Biochar | 44.1 b | 38.1 i | 22.4 a | 20.6 d | 2.5 g | 3 c | 1.22 de | 1.2 e | |
| LSD 0.05 | 0.134 | 0.120 | 0.108 | 0.021 | |||||
| Growing Seasons | Fertilizer Applications | I (m3 ha−1) | Yield (kg ha−1) | Water Productivity (kg m−3) | |||
|---|---|---|---|---|---|---|---|
| Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | ||
| 2016–2017 | Chicken | 3706.96 h | 3462.84 i | 24,000 d | 34,430 a | 6.47 d | 9.94 a |
| Cow | 3923.13 fg | 3611.07 hi | 24,350 d | 36,250 a | 6.21 d | 10.04 a | |
| Rabbit | 4072.33 ef | 3760.84 gh | 25,480 cd | 33,830 a | 6.26 d | 9.00 b | |
| Compost | 4274.61 cd | 3979.78 ef | 26,150 cd | 34,250 a | 6.12 d | 8.61 b | |
| Mineral | 4507.37 b | 4143.40 de | 27,920 bc | 30,100 b | 6.68 d | 6.74 d | |
| Chicken + Biochar | 4681.11 a | 4329.73 c | 27,650 bc | 33,830 a | 5.91 d | 7.81 c | |
| LSD 0.05 | LSD 0.05 | 168.5 | 2.974 | ||||
| 2017–2018 | Chicken | 3975.72 f | 3674.08 g | 25,000 c | 32,900 ab | 6.29 cde | 8.95 a |
| Cow | 4209.52 e | 3834.95 fg | 24,740 c | 35,000 a | 5.88 cde | 9.13 a | |
| Rabbit | 4371.64 de | 3995.89 f | 25,450 c | 28,700 bc | 5.83 cde | 7.17 bc | |
| Compost | 4590.93 c | 4230.50 de | 25,520 c | 35,000 a | 5.57 de | 8.26 ab | |
| Mineral | 4843.17 b | 4406.51 d | 27,840 bc | 30,080 abc | 5.75 de | 6.83 cd | |
| Chicken + Biochar | 5032.2 a | 4606.83 c | 26,450 c | 28,830 bc | 5.26 e | 6.26 cde | |
| LSD 0.05 | LSD 0.05 | 168.5 | 5.277 | ||||
| Growing Seasons | Fertilizer Applications | pH (1:2.5) | EC (dS m−1) | C/N Ratio | Fe (mg kg−1) | Mn. (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | ||
| 2016–2017 | Mineral | 7.71 g | 7.72 fg | 2.63 c | 2.55 d | 6.47 cde | 6.00 ef | 5.2 bc | 5.1 d ef | 21.22 e | 21.1 e |
| Chicken | 7.76 ef | 7.76 ef | 2.44 e | 2.37 f | 5.79 e | 5.00 g | 5.18 cd | 5.04 f | 23.4 b | 22.9 c | |
| Cow | 7.78 bcde | 7.77 de | 2.03 h | 1.94 i | 5.89 de | 5.26 fg | 5.22 abc | 5.17 cde | 22.35 d | 22.38 d | |
| Rabbit | 7.79 abcde | 7.78 cde | 3.31 a | 3.15 b | 5.94 de | 5.55 efg | 5.28 ab | 5.21 bc | 21.46 e | 21.24 e | |
| Compost | 7.82 a | 7.81 abc | 2.12 g | 2.08 gh | 6.31 cde | 6.53 de | 5.08 ef | 5.05 f | 23.87 a | 23.88 a | |
| Chicken + Biochar | 7.80 abcd | 7.82 ab | 2.52 d | 2.45 e | 6.43 cde | 6.11 ef | 5.31 a | 5.24 abc | 22.05 d | 22.1 d | |
| LSD 0.05 | 0.038 | 0.038 | 0.052 | 0.795 | 0.092 | ||||||
| 2017–2018 | Mineral | 7.70 ef | 7.68 f | 2.72 c | 2.51 d | 6.77 cd | 7.80 bc | 5.33 a | 5.11 c | 23.7 b | 23.55 b |
| Chicken | 7.75 cd | 7.73 de | 2.71 c | 2.47 d | 6.52 cde | 7.92 bc | 5.25 b | 4.98 c | 23.1 b | 23.2 b | |
| Cow | 7.78 abc | 7.77 bcd | 1.98 f | 1.76 g | 6.75 bc | 7.29 cd | 5.20 b | 5.09 c | 24.3 a | 24.4 a | |
| Rabbit | 7.78 abc | 7.76 bcd | 3.36 a | 2.97 b | 6.83 bc | 8.20 bc | 5.33 a | 5.19 b | 23.15 b | 23.17 b | |
| Compost | 7.81 a | 7.79 abc | 2.15 e | 1.89 f | 8.33 a | 8.27 ab | 5.21 b | 5.19 b | 23.44 b | 23.47 b | |
| Chicken + Biochar | 7.81 a | 7.8 ab | 2.53 d | 2.11 e | 7.55 ab | 9.13 a | 5.36 a | 5.21 b | 22.14 c | 22.24 c | |
| LSD 0.05 | 0.041 | 0.041 | 0.092 | 0.872 | 0.064 | ||||||
| Growing Season | Fertilizer Applications | Fresh Plant Weight (g) | Dry Plant Weight (g) | Plant Height (cm) | Number of Branches | Number of Leaves | Fresh Root Weight (g) | Dry Root Weight (g) | Leaf Area (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | Non-Aerated | Aerated | ||
| 2016–2017 | Mineral | 206.02 b | 262.80 ab | 24.90 a | 35.31 a | 46.50 de | 54.17 c | 3.00 bc | 4.34 a | 39.00 de | 55.84 b | 13.78 f | 20.26 bcde | 4.55 c | 8.14 ab | 117.78 bcd | 128.44 b |
| Chicken | 249.34 ab | 257.79 ab | 31.26 a | 30.65 a | 46.17 def | 58.42 b | 3.00 bc | 4.00 ab | 45.50 c | 46.50 c | 16.89 def | 23.00 ab | 4.46 c | 7.68 b | 92.10 de | 111.56 bcde | |
| Cow | 227.71 b | 239.12 ab | 25.99 a | 32.48 a | 44.50 def | 54.72 bc | 2.00 c | 4.00 ab | 41.67 d | 45.84 c | 18.43 cde | 20.80 abcd | 3.95 cd | 8.27 ab | 95.88 cde | 121.70 bc | |
| Rabbit | 249.13 ab | 319.84 a | 28.53 a | 31.99 a | 47.50 d | 67.34 a | 2.50 c | 4.50 a | 46.50 c | 55.50 b | 16.85 def | 24.59 a | 4.16 cd | 8.85 a | 108.33 bcde | 112.20 bcd | |
| Compost | 228.17 b | 318.80 a | 24.98 a | 37.84 a | 42.50 f | 65.50 a | 2.00 c | 4.00 ab | 35.00 f | 60.00 a | 16.93 def | 22.03 abc | 3.26 d | 8.26 ab | 91.24 de | 92.58 de | |
| Chicken + Biochar | 213.99 b | 287.65 ab | 27.32 a | 36.26 a | 43.00 ef | 57.50 bc | 3.00 bc | 4.50 a | 36.00 ef | 62.50 a | 16.13 ef | 24.62 a | 4.57 c | 7.50 b | 84.68 e | 161.48 a | |
| LSD 0.05 | 70.520 | 39.120 | 3.610 | 1.200 | 3.729 | 3.729 | 1.011 | 23.820 | |||||||||
| 2017–2018 | Mineral | 294.78 abcd | 318.21 ab | 25.17 de | 31.06 ab | 51.34 bcd | 62.70 a | 3.50 abc | 4.50 a | 43.00 d | 56.50 b | 21.88 de | 24.74 cd | 5.47 a | 5.66 a | 127.15 bc | 129.14 bc |
| Chicken | 224.21 ef | 253.15 cdef | 20.99 f | 30.26 bc | 43.00 ef | 57.98 ab | 3.67 abc | 3.67 abc | 45.67 d | 51.67 c | 21.19 e | 26.41 bc | 4.46 a | 5.28 a | 119.56 cd | 150.28 ab | |
| Cow | 246.93 def | 348.77 a | 25.21 de | 33.50 a | 42.95 ef | 52.84 bcd | 2.84 bcd | 4.50 a | 37.50 e | 56.00 b | 22.77 de | 26.61 bc | 5.08 a | 6.06 a | 114.20 cd | 152.94 ab | |
| Rabbit | 217.99 f | 301.03 abcd | 22.05 ef | 30.06 bc | 50.00 cde | 62.50 a | 2.50 cd | 4.50 a | 45.17 d | 53.00 bc | 23.68 cde | 29.94 a | 4.54 a | 6.66 a | 125.95 bc | 128.00 bc | |
| Compost | 281.985 bcde | 309.02 abc | 25.91 d | 27.88 bcd | 45.96 def | 56.00 abc | 2.00 d | 4.00 ab | 38.67 e | 46.50 d | 22.84 de | 28.08 ab | 4.59 a | 5.83 a | 96.83 d | 110.62 cd | |
| Chicken + Biochar | 277.03 bcde | 298.29 abcd | 22.16 ef | 27.54 cd | 40.17 f | 57.50 abc | 3.00 bcd | 4.50 a | 32.34 f | 62.50 a | 21.77 de | 24.16 cde | 5.02 a | 6.39 a | 101.83 cd | 162.24 a | |
| LSD 0.05 | 53.240 | 2.991 | 6.989 | 1.088 | 3.948 | 2.859 | 52.430 | 25.900 | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; B. I. El-Sawy, M. The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil. Agronomy 2019, 9, 418. https://doi.org/10.3390/agronomy9080418
Abuarab ME, El-Mogy MM, Hassan AM, Abdeldaym EA, Abdelkader NH, B. I. El-Sawy M. The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil. Agronomy. 2019; 9(8):418. https://doi.org/10.3390/agronomy9080418
Chicago/Turabian StyleAbuarab, Mohamed E., Mohamed M. El-Mogy, Ahmed M. Hassan, Emad A. Abdeldaym, Noha H. Abdelkader, and Mohamed B. I. El-Sawy. 2019. "The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil" Agronomy 9, no. 8: 418. https://doi.org/10.3390/agronomy9080418
APA StyleAbuarab, M. E., El-Mogy, M. M., Hassan, A. M., Abdeldaym, E. A., Abdelkader, N. H., & B. I. El-Sawy, M. (2019). The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil. Agronomy, 9(8), 418. https://doi.org/10.3390/agronomy9080418








