Ground Truthing and Physiological Validation of Vis-NIR Spectral Indices for Early Diagnosis of Nitrogen Deficiency in cv. Barbera (Vitis vinifera L.) Grapevines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Layout
2.2. Vine Measurements
2.3. Grape Composition
2.4. Statistical Analysis
3. Results
3.1. Leaf Nutrition, Vegetative Growth, Yield Components, and Grape Composition
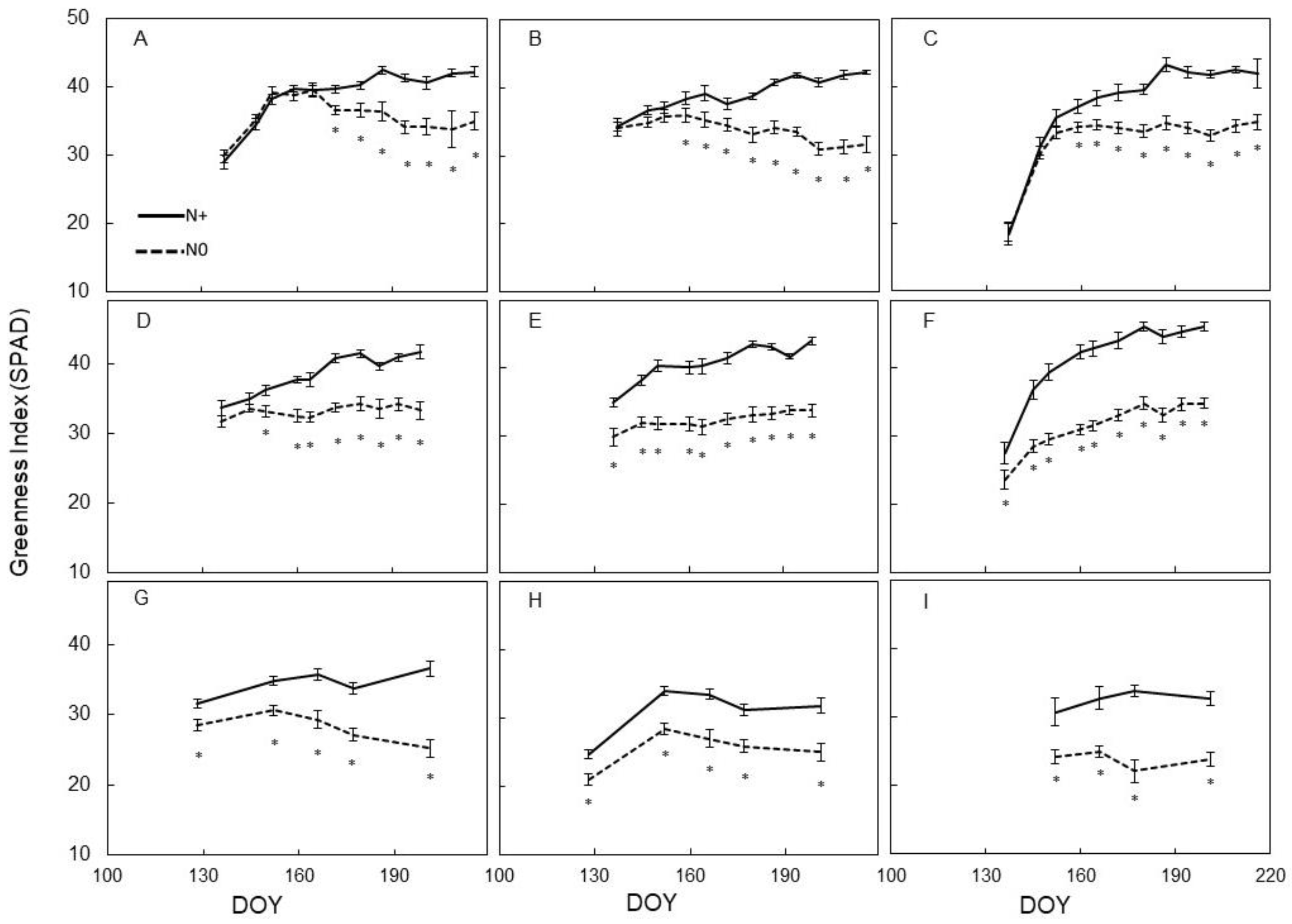
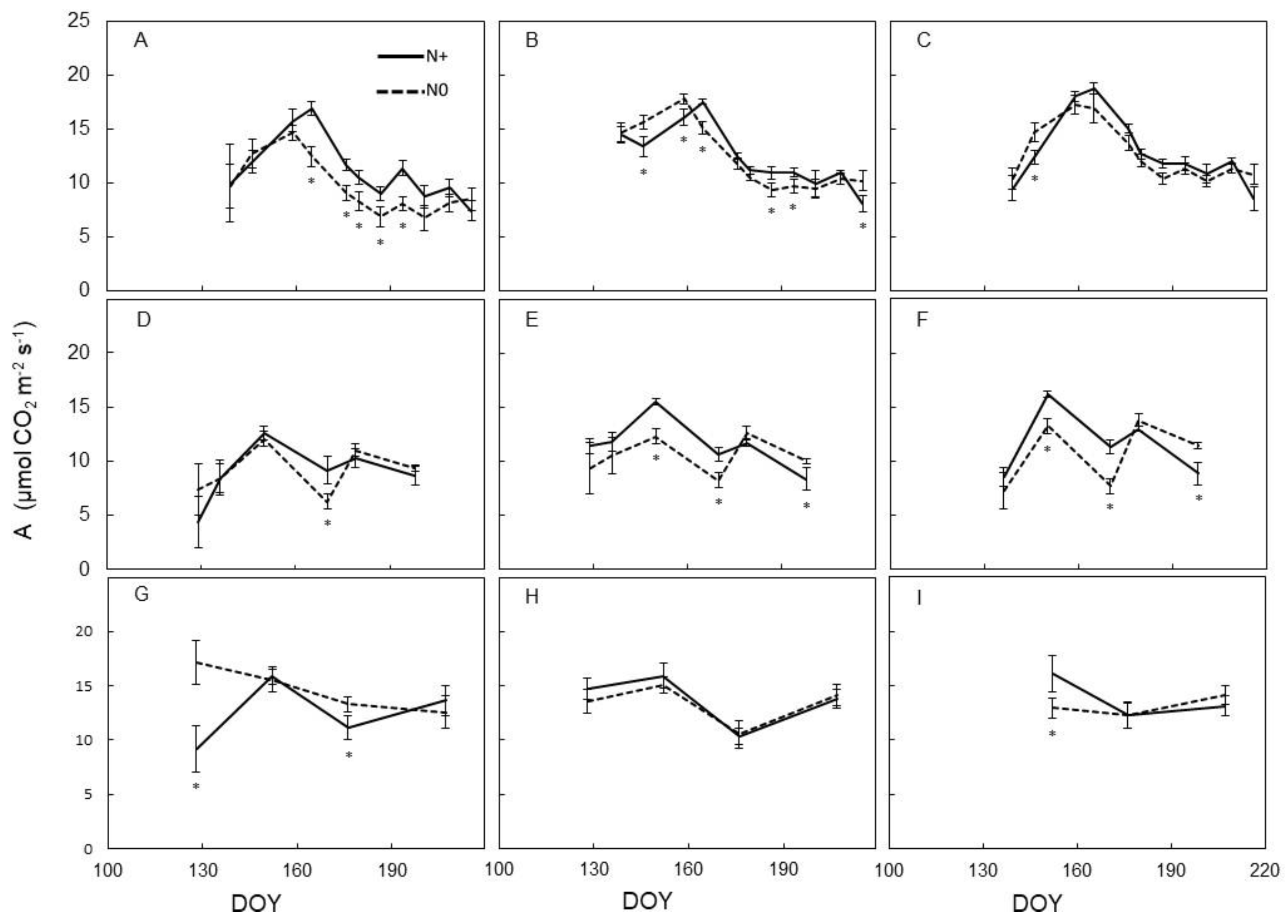
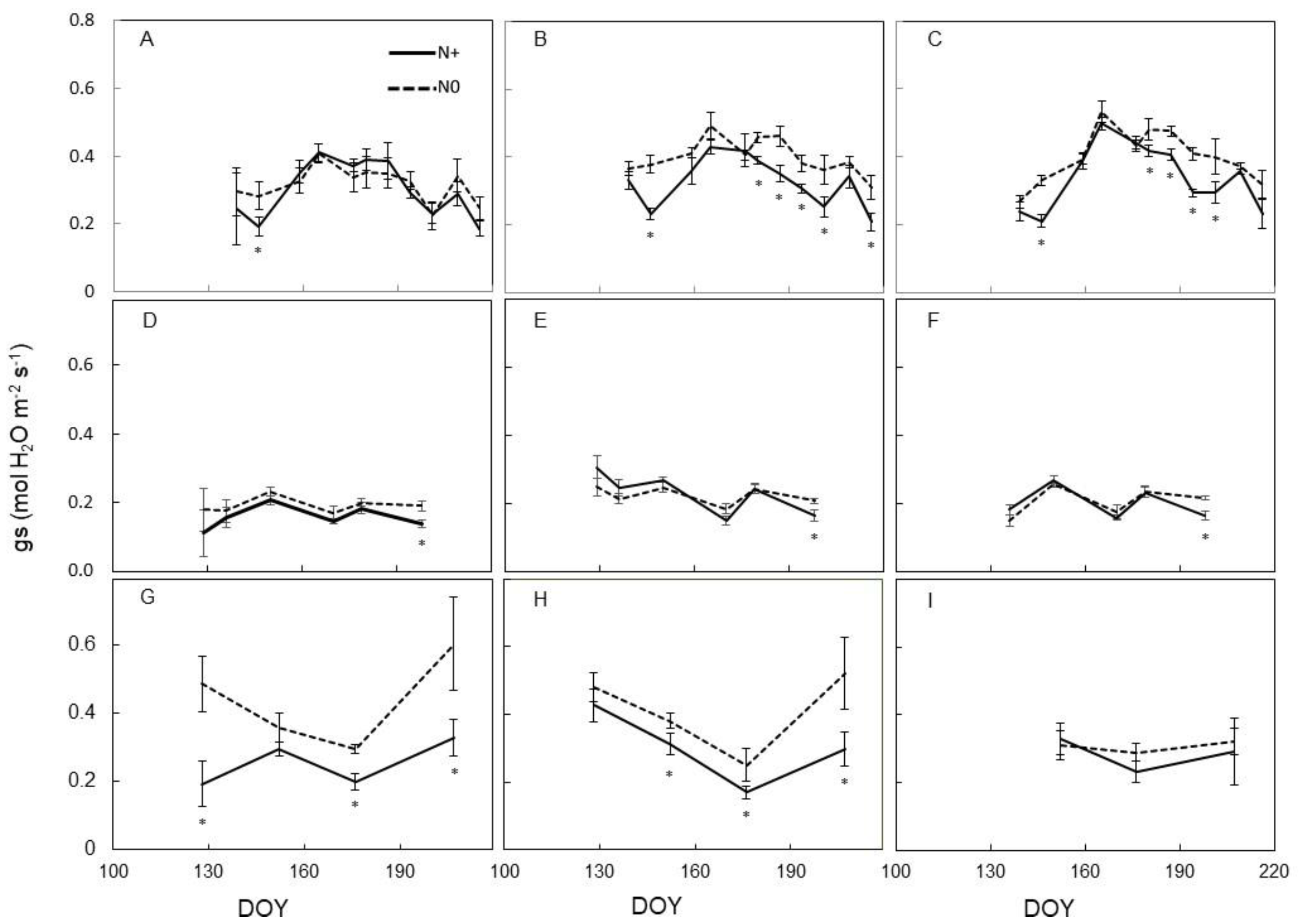
3.2. Leaf Physiology
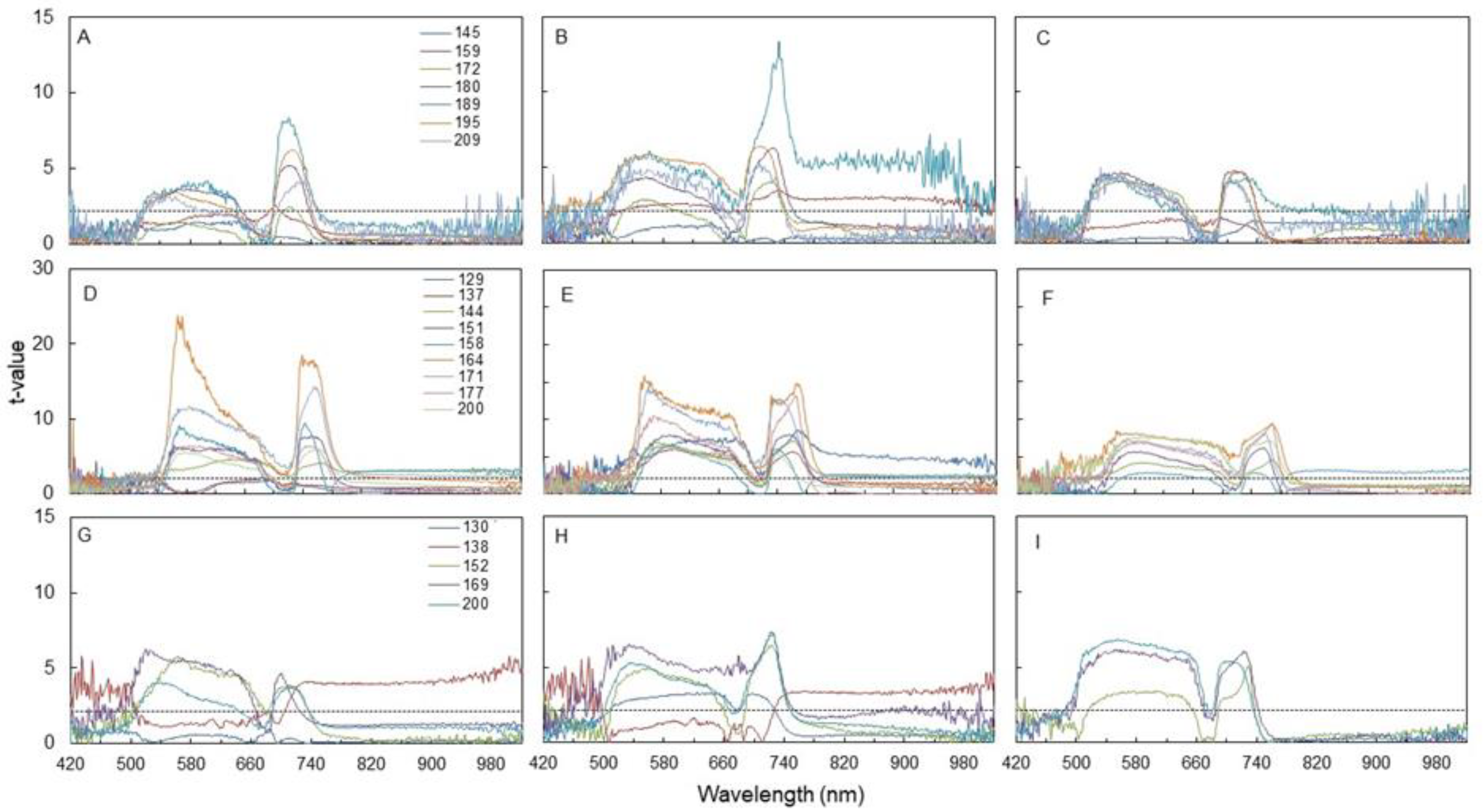
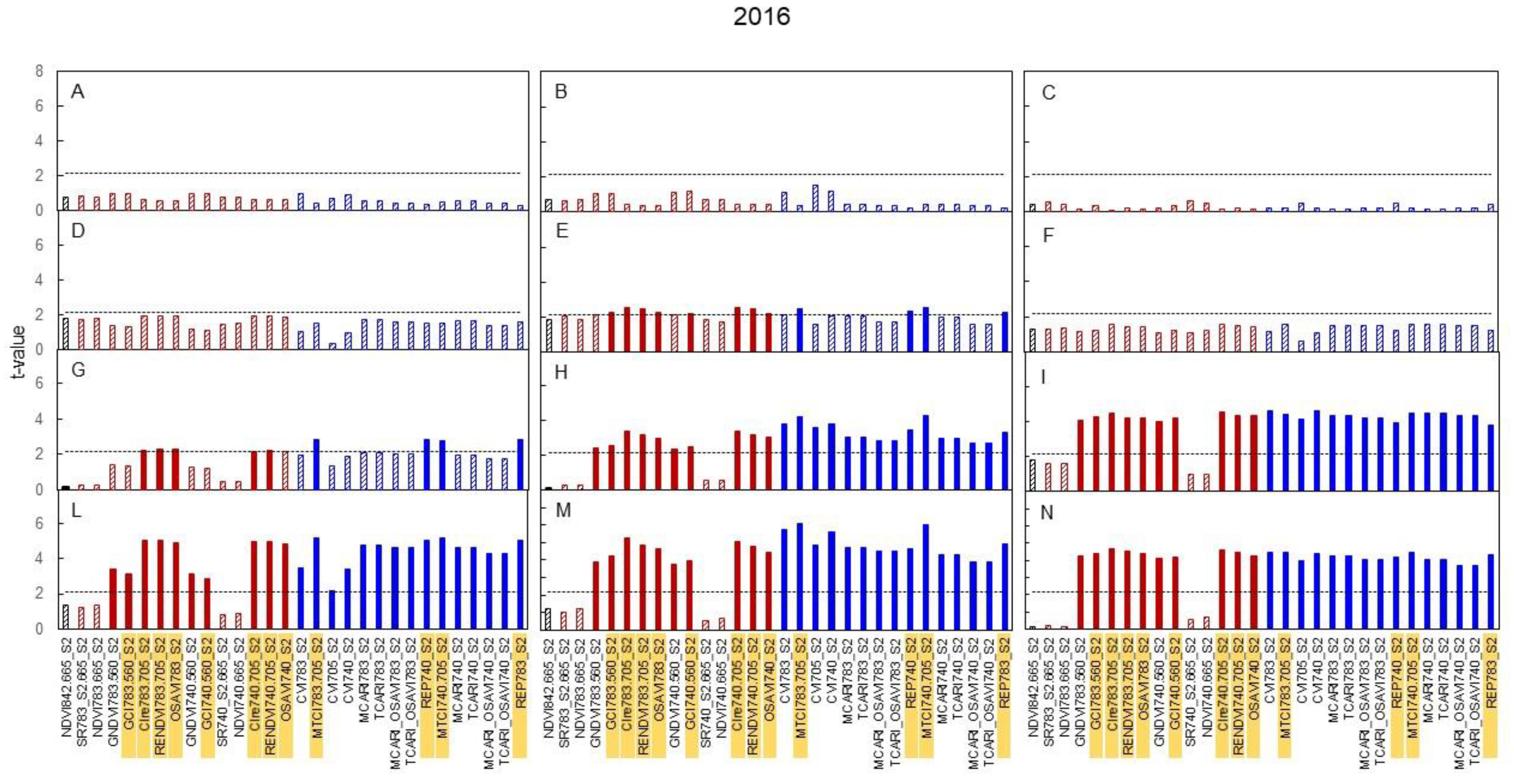
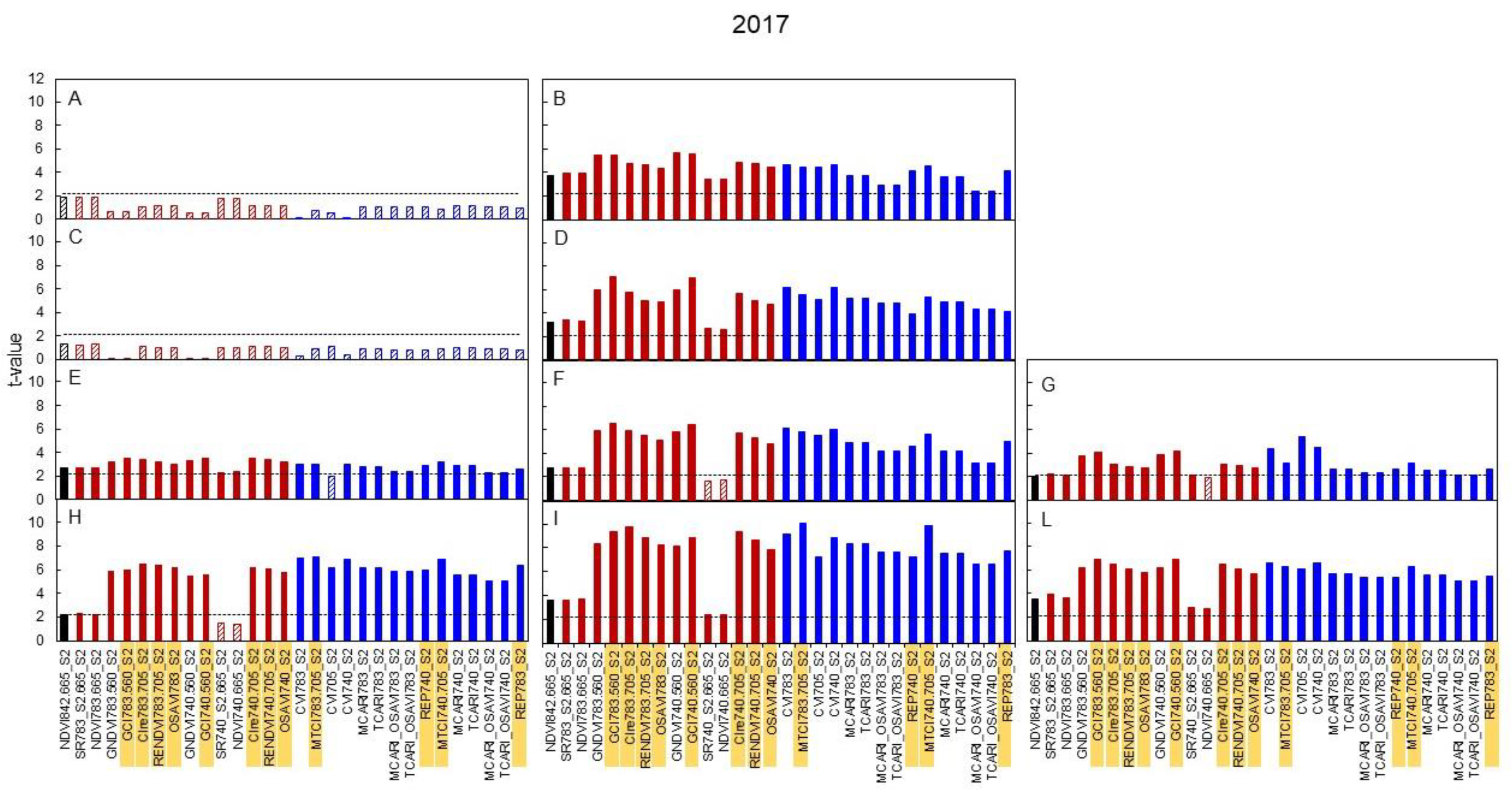
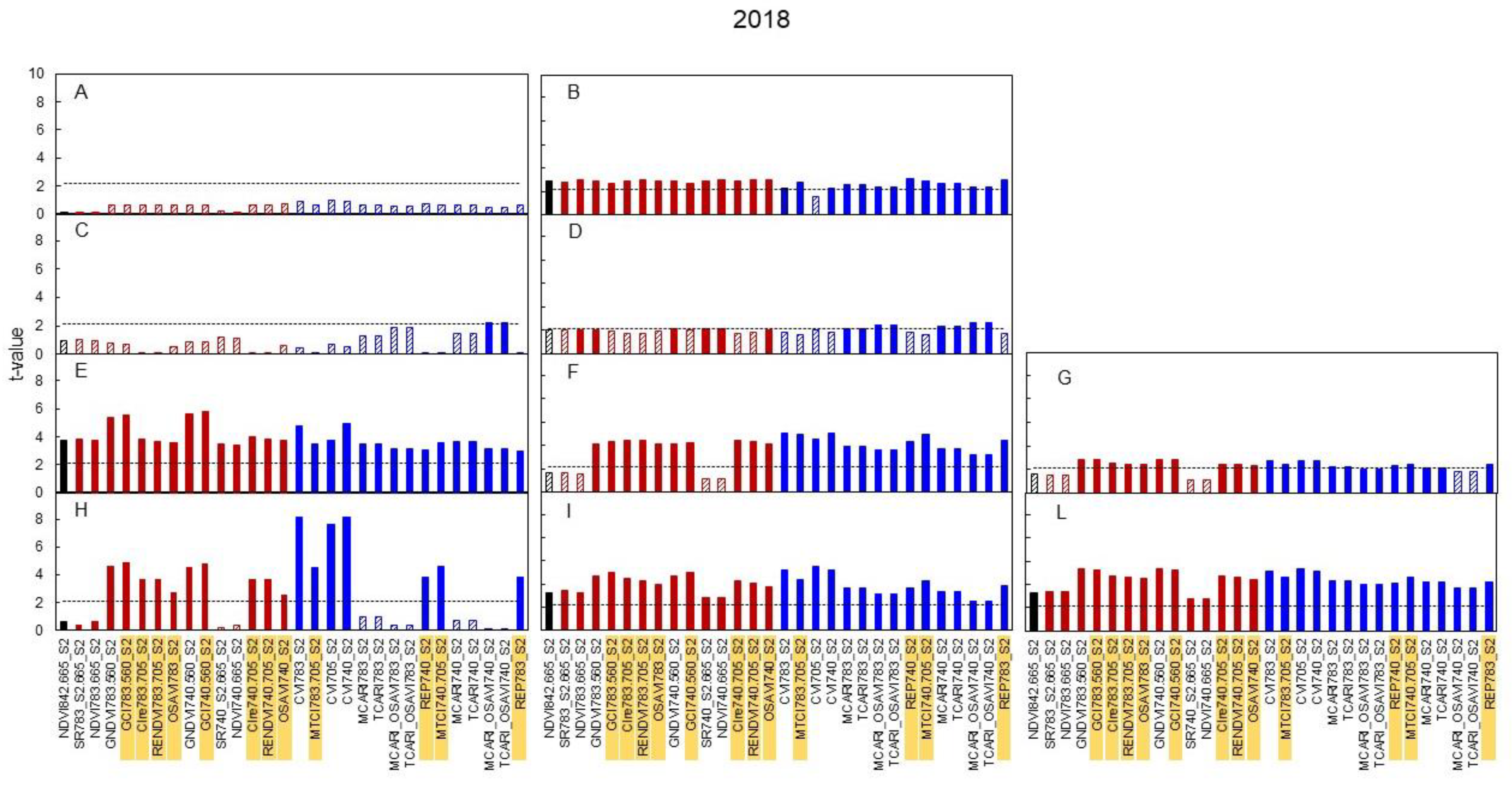
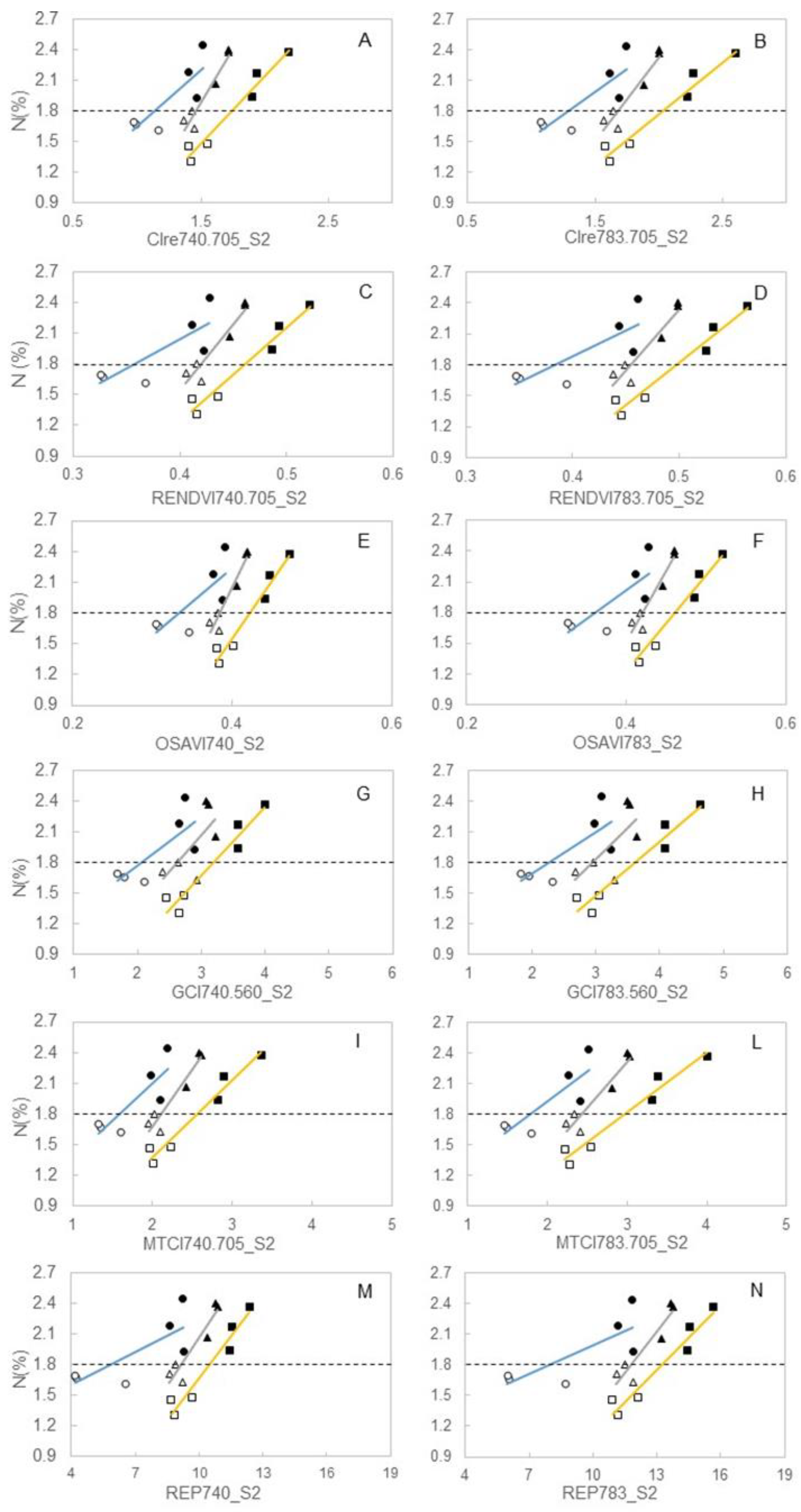
3.3. Spectral Indices and Relationships with Leaf Function
4. Discussion
4.1. Agronomic Vine Performance to N Supply
4.2. Physiological Vine Performance to N Supply
4.3. Sensitivity of Canopy Reflectance Indices versus N Leaf Status and Vine Behaviour
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keller, M. Deficit irrigation and vine mineral nutrition. Am. J. Enol. Vitic. 2005, 56, 267–283. [Google Scholar]
- Conradie, W.J. Utilization of nitrogen by the grapevine affected as affected by time of application and soil type. S. Afr. J. Enol. Vitic. 1986, 7, 76–83. [Google Scholar]
- May, P. Flowering and Fruitset in Grapevines; Phylloxera and Grape Industry Board of South Australia: Adelaide, SA, Australia, 2004.
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Waltham, MA, USA, 2012. [Google Scholar]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per cluster are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Mundy, D.C. A review on the direct and indirect effects of nitrogen on botrytis cluster rot in wine grapes. N. Z. Plant. Prot. 2008, 61, 306–310. [Google Scholar]
- Mendez-Costabel, M.P.; Wilkinson, K.L.; Bastian, S.E.P.; Jordans, C.; Mccarthy, M.; Ford, C.M.; Dokoozlian, N.K. Effect of increased irrigation and additional nitrogen fertilisation on the concentration of green aroma compounds in Vitis vinifera L. Merlot fruit and wine. Aust. J. Grape Wine Res. 2014, 20, 80–90. [Google Scholar] [CrossRef]
- Thomidis, T.; Zioziou, E.; Koundouras, S.; Karagiannidis, C.; Navrozidis, I.; Nikolaou, N. Effects of nitrogen and irrigation on the quality of grapes and the susceptibility to Botrytis cluster rot. Sci Hortic. 2016, 212, 60–68. [Google Scholar] [CrossRef]
- Palliotti, A.; Gardi, T.; Berrios, J.G.; Civardi, S.; Poni, S. Early source limitation as a tool for yield control and wine quality improvement in a high-yielding red Vitis vinifera L. cultivar. Sci Hortic. 2012, 145, 10–16. [Google Scholar] [CrossRef]
- Sabbatini, P.; Howell, G.S. Rootstock scion interaction and effects on vine vigor, phenology, and cold hardiness of interspecific hybrid grape cultivars (Vitis spp.). Int. J. Fruit Sci. 2013, 13, 466–477. [Google Scholar] [CrossRef]
- Gatti, M.; Garavani, A.; Vercesi, A.; Poni, S. Ground-truthing of remotely sensed within-field variability in a cv. Barbera plot for improving vineyard management. Aust. J. Grape Wine Res. 2017, 23, 399–408. [Google Scholar] [CrossRef]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; Van Leeuwen, C.; Delrot, S.; Gomes, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berrries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef]
- Seemann, J.R.; Sharkey, T.D.; Wang, J.; Osmond, C.B. Environmental effects on photosynthesis, nitrogen use efficiency and metabolite pools in leaves of sun and shade plants. Plant. Physiol. 1987, 84, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Intrieri, C.; Silvestroni, O. Interactions of leaf age, fruiting, and exogenous cytokinins in sangiovese grapevines under non-irrigated conditions. II. Chlorophyll and nitrogen content. Am. J. Enol. Vitic. 1994, 45, 278–284. [Google Scholar]
- Poni, S.; Intrieri, C.; Silvestroni, O. Interactions of leaf age, fruiting, and exogenous cytokinins in Sangiovese grapevines under non-irrigated conditions. I. Gas exchange. Am. J. Enol. Vitic. 1994, 45, 71–78. [Google Scholar]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Ann. Rev. Plant. Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Williams, L.E.; Dokoozlian, N.K.; Wample, R. Grape. In Handbook of Environmental Physiology of Fruit Crops Vol. I. Temperate Crops; Schaffer, B., Andersen, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; Volume I, pp. 85–133. [Google Scholar]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Ghasemi, M.; Arzani, K.; Yadollahi, K.; Ghasemi, S.; Sarikhani Khorrami, S. Estimate of leaf chlorophyll and nitrogen content in Asian pear (Pyrus serotina Rehd.) by CCM-200. Not. Sci. Biol. 2011, 3, 91–94. [Google Scholar] [CrossRef][Green Version]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for non destructive measurement of chlorophyll content in grapevine leaves. Agr. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, U.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant. Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F. Technology in precision viticulture: A state of the art review. Int. J. Wine Res. 2015, 7, 69–81. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and-3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J. In situ visible and near-infrared spectroscopy applied to vineyards as a tool for precision viticulture. Compr. Anal. Chem. 2018, 80, 252–279. [Google Scholar]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, magnesium, nitrogen and potassium deficiency symptom discrimination by reflectance spectroscopy in grapevine leaves. Sci. Hort. 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using active canopy sensors and chlorophyll meters to estimate grapevine nitrogen status and productivity. Precis. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Lopo, M.; dos santos, T.C.; Graça, A.R.; Lopes, J.A. Exploratory study on vineyards soil mapping by visible/near-infrared spectroscopy of grapevine leaves. Comp. Elec. Agric. 2016, 127, 15–25. [Google Scholar] [CrossRef]
- Caruso, G.; Tozzini, L.; Rallo, G.; Primicerio, J.; Moriondo, M.; Palai, G.; Gucci, R. Estimating biophysical and geometrical parameters of grapevine canopies (’Sangiovese’) by an unmanned aerial vehicle (UAV) and VIS-NIR cameras. Vitis 2017, 56, 63–70. [Google Scholar]
- Liu, J.; Han, J.; Chen, X.; Shi, L.; Zhang, L. Nondestructive detection of rape leaf chlorophyll level based on Vis-NIR spectroscopy. Spectrochimica Acta Part A Mol. Biomol. Spect. 2019, 222, 117202. [Google Scholar] [CrossRef]
- Soltani, I.; Fouad, Y.; Michot, D.; Pichelin, P.; Cudennec, C. Relevance of a near infrared spectral index for assessing tillage and fertilization effects on soil water retention. Soil Tillage Res. 2019, 194, 104345. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Keller, M.; Kummer, M.; Vasconcelos, M.C. Soil nitrogen utilisation for growth and gas exchange by grapevines in response to nitrogen supply and rootstock. Aust. J. Grape Wine Res. 2001, 7, 2–11. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera). Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package. R Package Vignette, Report No.: R Package Version 0.1 3. 2014. Available online: https://github.com/antoinestevens/prospectr (accessed on 4 February 2017).
- European Space Agency Home Page. Available online: https://sentinel.esa.int/web/sentinel/home (accessed on 21 October 2019).
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Rouse, J., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium. Volume 1: Technical Presentations, Section A; National Aeronautics and Space Administration: Washington, DC, USA, 1974. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. A broad-band leaf chlorophyll vegetation index at the canopy scale. Precis. Agric. 2008, 9, 303–319. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Iland, P.; Dry, P.; Proffitt, T.; Tyerman, S. The Grapevine: From the Science to the Practice of Growing Vines for Wine; Patrick Iland Wine Promotions Pty Ltd.: Campbelltown, SA, Australia, 2011. [Google Scholar]
- Keller, M. The Science of Grapevines: Anatomy and Physiology; Academic Press: Waltham, MA, USA, 2015. [Google Scholar]
- Keller, M.; Kummer, M.; Carmo Vasconcelos, M. Reproductive growth of grapevines in response to nitrogen supply and rootstocks. Aust. J. Grape Wine Res. 2001, 7, 12–18. [Google Scholar] [CrossRef]
- Schreiner, P.; Lee, J.; Skinkis, P.A. N, P and K supply to pinot noir grapevines: Impact on vine nutrient status, growth, physiology and yield. Am. J. Enol. Vitic. 2013, 64, 26–38. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front. Plant. Sci. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
| N (%) | P (%) | K (%) | Ca (%) | Mg (%) | S (%) | Na (ppm) | Fe (ppm) | Mn (ppm) | B (ppm) | Zn (ppm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||||||
| N+ | 2.21a | 0.34b | 0.64 | 2.89 | 0.66 | 0.21 | 73 | 102 | 59b | 25 | 17b |
| N0 | 1.60b | 0.59a | 0.66 | 3.12 | 0.63 | 0.19 | 85 | 94 | 76a | 27 | 20a |
| Position (P) | |||||||||||
| Basal | 1.75c | 0.42b | 0.69 | 2.69b | 0.53b | 0.18b | 83 | 107 | 79a | 25 | 22a |
| Median | 1.94b | 0.49a | 0.64 | 3.19a | 0.68a | 0.19b | 80 | 100 | 67ab | 27 | 17b |
| Apical | 2.02a | 0.49a | 0.62 | 3.12a | 0.73a | 0.22a | 74 | 88 | 56b | 26 | 17b |
| Year (Y) | |||||||||||
| 2016 | 2.00a | 0.43b | 0.71a | 2.60b | 0.55b | 0.24a | 58b | 126a | 78a | 29a | 19 |
| 2017 | 1.80c | 0.47ab | 0.54b | 3.29a | 0.69a | 0.19b | 66b | 72c | 68ab | 29a | 18 |
| 2018 | 1.92b | 0.49a | 0.70a | 3.12a | 0.69a | 0.18b | 112a | 97b | 57b | 20b | 18 |
| T | ** | ** | ns | ns | ns | ns | ns | ns | * | Ns | * |
| P | ** | * | ns | * | * | * | ns | ns | * | Ns | * |
| Y | ** | * | ** | ** | * | ** | ** | ** | * | ** | ns |
| T × P | ** | ns | ns | ns | ns | ns | ns | ns | ns | Ns | ns |
| T × Y | * | ** | ns | ns | ns | ns | ns | ns | ns | Ns | ns |
| P × Y | Ns | ns | ns | ns | ns | ns | ns | ns | ns | Ns | ns |
| Main Pruning Weight (g/vine) | Lateral Pruning Weight (g/vine) | Total Pruning Weight (g/vine) | Yield (g/vite) | Cluster Weight (g) | Berry Weight (g) | Cluster Compactness (g/cm) | Shot Berries (%) | Live Green Ovaries (%) | Ravaz Index (kg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | ||||||||||
| N+ | 200.4a | 66.2a | 266.67a | 651a | 155a | 2.08a | 19. 10 a | 8.44b | 0.99a | 2.80a |
| N0 | 169.7b | 26.8b | 196.50b | 291b | 72b | 1.64b | 9.76b | 22.72a | 0.27b | 1.55b |
| Year (Y) | ||||||||||
| 2016 | 187.1ab | 53.7a | 240.87a | 529b | 137a | 2.13a | 17.59a | 26.22a | 1.24a | 2.19b |
| 2017 | 166.2b | 39.7b | 206.00b | 706a | 139a | 1.99a | 17.96a | 4.58c | 0.48b | 3.54a |
| 2018 | 201.8a | 46.1ab | 247.87a | 178c | 65b | 1.46b | 7.73b | 15.94b | 0.15b | 0.80c |
| T | ** | ** | ** | ** | ** | ** | ** | ** | * | ** |
| Y | * | * | * | ** | ** | ** | ** | ** | ** | ** |
| T × Y | * | ** | ** | ** | * | ns | Ns | ns | Ns | ** |
| Skin Weight (g/berry) | Flesh Weight (g/berry) | Total Seed Weight (g/berry) | Mean Seed Weight (mg) | Seed Number (n/berry) | Skin-to-berry Ratio (%) | Seed-to-berry Ratio (%) | Flesh-to-berry Ratio (%) | Skin-to-flesh Ratio (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||||
| N+ | 0.152 | 1.960a | 0.092a | 36.79a | 2.49a | 7.39b | 4.22a | 88.39a | 8.48b |
| N0 | 0.141 | 1.515b | 0.066b | 32.77b | 2.00b | 8.97a | 3.83b | 87.28b | 10.42a |
| Year (Y) | |||||||||
| 2016 | 0.138b | 2.012a | 0.080b | 32.52b | 2.54a | 6.38c | 3.52b | 90.09a | 7.12b |
| 2017 | 0.156a | 1.703b | 0.090a | 38.18a | 2.29ab | 8.48b | 4.31a | 87.22b | 9.85a |
| 2018 | 0.143b | 1.375c | 0.066b | 33.64b | 1.92b | 9.68a | 4.25a | 86.19b | 11.39a |
| T | ns | ** | ** | ** | ** | ** | * | * | ** |
| Y | * | ** | ** | ** | ** | ** | ** | ** | ** |
| T × Y | ** | ** | ** | Ns | ** | ns | ns | ns | ns |
| Total Soluble Solids (Brix) | pH | Titratable Acidity (g/L) | Tartrate (g/L) | Malate (g/L) | Total Anthocyanins (mg/g) | Total Phenols (mg/g) | |
|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||
| N+ | 23.6b | 3.42 | 11.82a | 7.57b | 5.89a | 0.584b | 1.865b |
| N0 | 25.8a | 3.39 | 7.70b | 8.58a | 2.65b | 1.047a | 2.939a |
| Year (Y) | |||||||
| 2016 | 25.1 | 3.36 | 8.15c | 8.57a | 3.65c | 1.083a | 2.750a |
| 2017 | 23.7 | 3.43 | 9.55b | 9.27a | 4.35b | 0.667b | 1.703b |
| 2018 | 25.2 | 3.42 | 11.58a | 6.40b | 4.81a | 0.697b | 2.754a |
| T | ** | ns | ** | * | ** | ** | ** |
| Y | * | ns | ** | ** | ** | ** | ** |
| T × Y | ** | ns | Ns | ** | ** | ** | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squeri, C.; Gatti, M.; Garavani, A.; Vercesi, A.; Buzzi, M.; Croci, M.; Calegari, F.; Vincini, M.; Poni, S. Ground Truthing and Physiological Validation of Vis-NIR Spectral Indices for Early Diagnosis of Nitrogen Deficiency in cv. Barbera (Vitis vinifera L.) Grapevines. Agronomy 2019, 9, 864. https://doi.org/10.3390/agronomy9120864
Squeri C, Gatti M, Garavani A, Vercesi A, Buzzi M, Croci M, Calegari F, Vincini M, Poni S. Ground Truthing and Physiological Validation of Vis-NIR Spectral Indices for Early Diagnosis of Nitrogen Deficiency in cv. Barbera (Vitis vinifera L.) Grapevines. Agronomy. 2019; 9(12):864. https://doi.org/10.3390/agronomy9120864
Chicago/Turabian StyleSqueri, Cecilia, Matteo Gatti, Alessandra Garavani, Alberto Vercesi, Marta Buzzi, Michele Croci, Ferdinando Calegari, Massimo Vincini, and Stefano Poni. 2019. "Ground Truthing and Physiological Validation of Vis-NIR Spectral Indices for Early Diagnosis of Nitrogen Deficiency in cv. Barbera (Vitis vinifera L.) Grapevines" Agronomy 9, no. 12: 864. https://doi.org/10.3390/agronomy9120864
APA StyleSqueri, C., Gatti, M., Garavani, A., Vercesi, A., Buzzi, M., Croci, M., Calegari, F., Vincini, M., & Poni, S. (2019). Ground Truthing and Physiological Validation of Vis-NIR Spectral Indices for Early Diagnosis of Nitrogen Deficiency in cv. Barbera (Vitis vinifera L.) Grapevines. Agronomy, 9(12), 864. https://doi.org/10.3390/agronomy9120864








