Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation
Abstract
:1. Introduction
2. Actors of Phytoremediation: The Holobiont
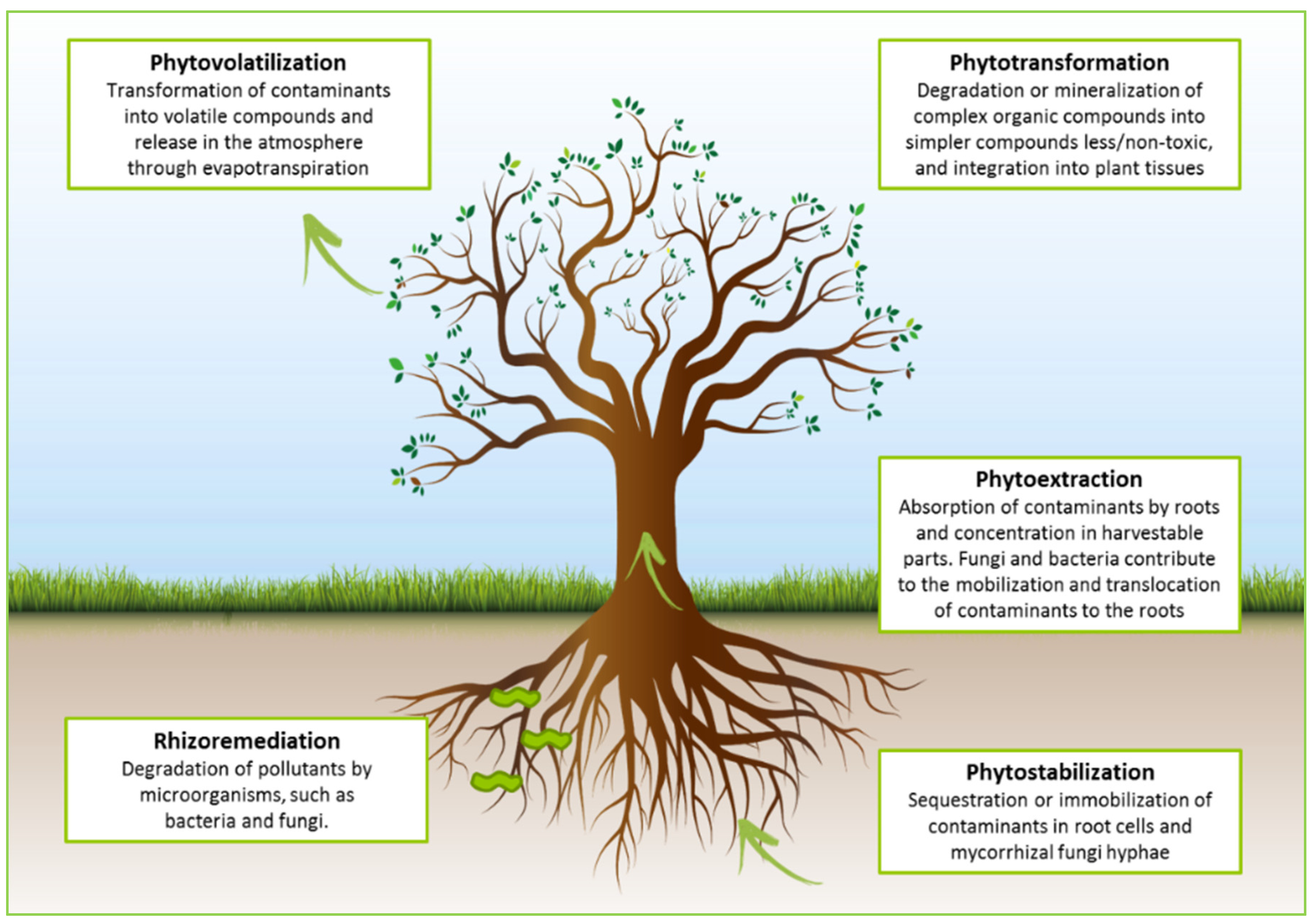
3. Hydrocarbon Rhizoremediation
4. Mechanisms of Root Exudation
5. Root Exudation, the Ecological Driver of Microbial Communities in the Rhizosphere
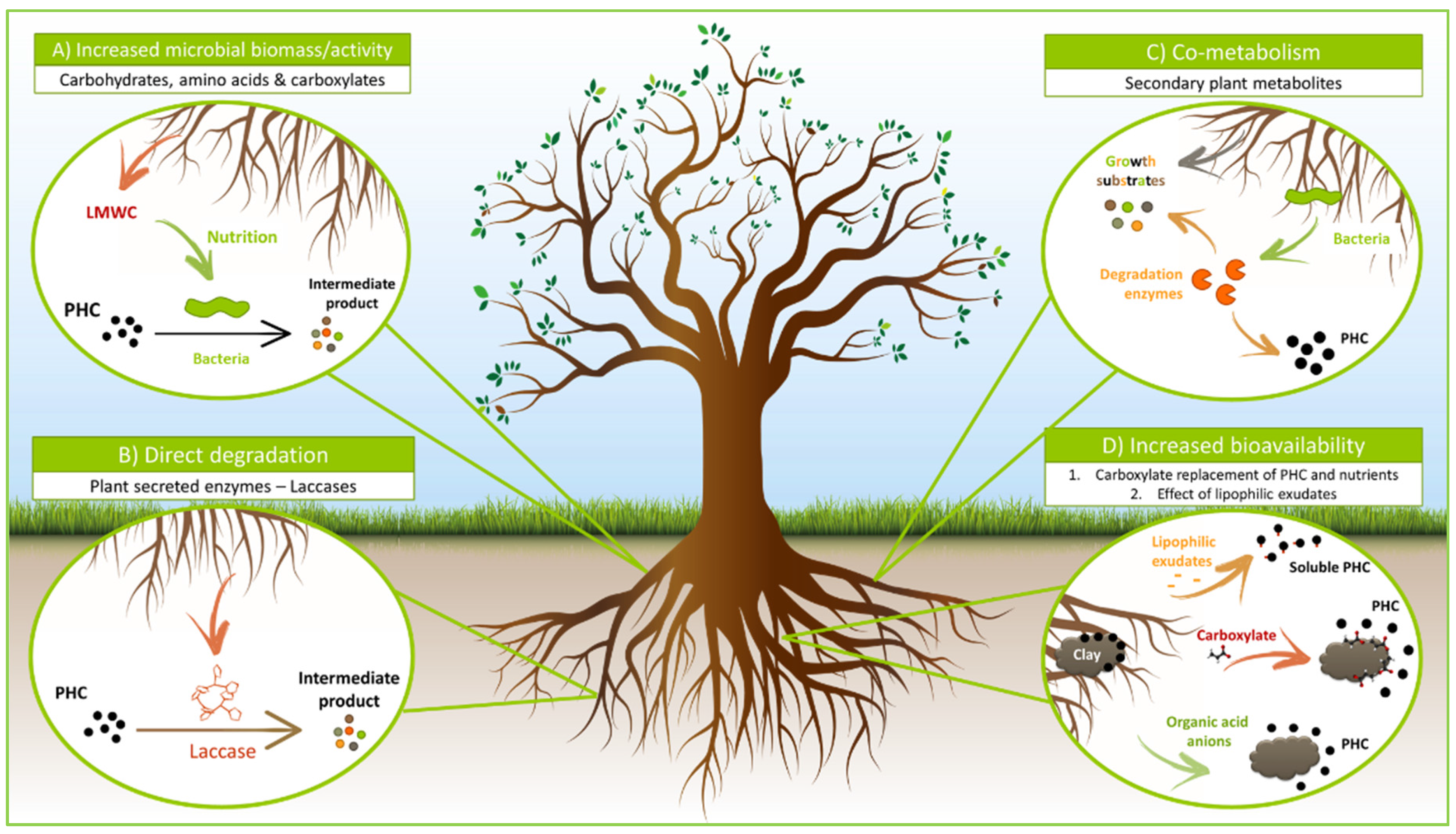
6. Impact of Root Exudates on Hydrocarbon Degradation
7. Lateral Gene Transfer in Rhizosphere and Hydrocarbon Degradation
8. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kvenvolden, K.A.; Cooper, C.K. Natural seepage of crude oil into the marine environment. Geo-Mar. Lett. 2003, 23, 140–146. [Google Scholar] [CrossRef]
- Committee on Oil in the Sea. Oil in the Sea III: Inputs, Fates and Effects; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of pahs in soil and vegetation samples from tarragona county, Spain. Environ. Pollut. 2004, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wehrer, M.; Totsche, K.U. Pah release from tar-oil contaminated soil material in response to forced environmental gradients: Implications for contaminant transport. Eur. J. Soil Sci. 2008, 59, 50–60. [Google Scholar] [CrossRef]
- An, C.J.; Huang, G.H.; Wei, J.; Yu, H. Effect of short-chain organic acids on the enhanced desorption of phenanthrene by rhamnolipid biosurfactant in soil-water environment. Water Res. 2011, 45, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Sun, R.; Gao, X.; Xu, R.; Li, H. Low-molecular-weight organic acids enhance desorption of polycyclic aromatic hydrocarbons from soil. Eur. J. Soil Sci. 2015, 66, 339–347. [Google Scholar] [CrossRef]
- De Sousa, C.A. Urban brownfields redevelopment in Canada: The role of local government. Can. Geogr. 2006, 50, 392–407. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of detroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Introduction to Phytoremediation; EPA/600/R-99/107; Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Glass, D.J. The 1998 United States Market for Phytoremediation; D. Glass Associates: Needham, MA, USA, 1998; p. 140. [Google Scholar]
- Van Epps, A. Phytoremediation of Petroleum Hydrocarbons; Environmental Protection Agency: Washington, DC, USA, 2006. [Google Scholar]
- Drake, E.N. Phytoremediation of aged petroleum hydrocarbons in soil. In Proceedings of the IBC Phytoremediation Conference, Seattle, WA, USA, 18–19 June 1997.
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; St-Arnaud, M.; Labreque, M.; Hijri, M. Phytoremediation: Biotechnological procedures involving plants and arbuscular mycorrhizal fungi. In Mycorrhizal Biotechnology; Devarajan Thangadurai, C.A.B., Hijri, M., Eds.; CRC Press: New York, NY, USA, 2010; p. 152. [Google Scholar]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Miller, R. Phytoremediation: Technology Overview Report; Ground Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1996; Volume 39, p. 80. [Google Scholar]
- Wenzel, W.W.; Adriano, D.C.; Salt, D.; Smith, R. Phytoremediation: A plant-microbe-based remediation system. Bioremediat. Contam. Soils 1999, 37, 457–508. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, L.; St-Arnaud, M.; Labrecque, M. Phytoextraction of heavy metals by two salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil 2010, 332, 55–67. [Google Scholar] [CrossRef]
- Desjardins, D.; Pitre, F.; Guidi Nissim, W.; Labrecque, M. Differential uptake of silver, copper and zinc suggests complementary species-specific phytoextraction potential. Int. J. Phytorem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guidi, W.; Kadri, H.; Labrecque, M. Establishment techniques to using willow for phytoremediation on a former oil refinery in southern Quebec: Achievements and constraints. Chem. Ecol. 2012, 28, 49–64. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Tesar, M.; Reichenauer, T.G.; Sessitsch, A. Bacterial rhizosphere populations of black poplar and herbal plants to be used for phytoremediation of diesel fuel. Soil Biol. Biochem. 2002, 34, 1883–1892. [Google Scholar] [CrossRef]
- Abrahamson, L.; Robison, D.; Volk, T.; White, E.; Neuhauser, E.; Benjamin, W.; Peterson, J. Sustainability and environmental issues associated with willow bioenergy development in New York (USA). Biomass Bioenergy 1998, 15, 17–22. [Google Scholar] [CrossRef]
- Kenney, W.; Sennerby-Forsse, L.; Layton, P. A review of biomass quality research relevant to the use of poplar and willow for energy conversion. Biomass 1990, 21, 163–188. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Quigley, M.F. Willows beyond wetlands: Uses of salix l. Species for environmental projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar] [CrossRef]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Assessment of tropical grasses and legumes for phytoremediation of petroleum-contaminated soils. Water Air Soil Pollut. 2005, 165, 195–209. [Google Scholar] [CrossRef]
- Olson, P.E.; Flechter, J.S.; Philp, P.R. Natural attenuation/phytoremediation in the vadose zone of a former industrial sludge basin. Environ. Sci. Pollut. Res. 2001, 8, 243–249. [Google Scholar] [CrossRef]
- Olson, P.E.; Fletcher, J.S. Ecological recovery of vegetation at a former industrial sludge basin and its implications to phytoremediation. Environ. Sci. Pollut. Res. 2000, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Cerniglia, C.E. Bioremediation of petroleum pollutants—Diversity and environmental aspects of hydrocarbon biodegradation. Bioscience 1995, 45, 332–338. [Google Scholar] [CrossRef]
- Karamalidis, A.K.; Evangelou, A.C.; Karabika, E.; Koukkou, A.I.; Drainas, C.; Voudrias, E.A. Laboratory scale bioremediation of petroleum-contaminated soil by indigenous microorganisms and added pseudomonas aeruginosa strain spet. Bioresour. Technol. 2010, 101, 6545–6552. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-G.; Rhee, S.-K.; Lee, S.-T. Effect of soil moisture on bioremediation of chlorophenol-contaminated soil. Biotechnol. Lett. 2000, 22, 915–919. [Google Scholar] [CrossRef]
- Bento, F.M.; Camargo, F.A.; Okeke, B.C.; Frankenberger, W.T. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour. Technol. 2005, 96, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Wolski, E.A.; Murialdo, S.E.; Gonzalez, J.F. Effect of pH and inoculum size on pentachlorophenol degradation by pseudomonas sp. Water SA 2007, 32, 93–98. [Google Scholar] [CrossRef]
- Dong, X.; Hong, Q.; He, L.; Jiang, X.; Li, S. Characterization of phenol-degrading bacterial strains isolated from natural soil. Int. Biodeterior. Biodegrad. 2008, 62, 257–262. [Google Scholar] [CrossRef]
- Cordova-Rosa, S.; Dams, R.; Cordova-Rosa, E.; Radetski, M.; Corrêa, A.; Radetski, C. Remediation of phenol-contaminated soil by a bacterial consortium and acinetobacter calcoaceticus isolated from an industrial wastewater treatment plant. J. Hazard. Mater. 2009, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Heinaru, E.; Merimaa, M.; Viggor, S.; Lehiste, M.; Leito, I.; Truu, J.; Heinaru, A. Biodegradation efficiency of functionally important populations selected for bioaugmentation in phenol-and oil-polluted area. FEMS Microbiol. Ecol. 2005, 51, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, F.M.; Rahman, R.N.Z.A.; Salleh, A.B.; Basri, M. Biodegradation of hydrocarbons in soil by microbial consortium. Int. Biodeterior. Biodegrad. 2004, 54, 61–67. [Google Scholar] [CrossRef]
- Goux, S.; Shapir, N.; El Fantroussi, S.; Lelong, S.; Agathos, S.N.; Pussemier, L. Long-term maintenance of rapid atrazine degradation in soils inoculated with atrazine degraders. Water Air Soil Pollut. Focus 2003, 3, 131–142. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.F.; Shen, Q.R.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Bowen, G.D.; Rovira, A.D. The rhizosphere and its management to improve plant growth. In Advances in Agronomy; Donald, L.S., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 66, pp. 1–102. [Google Scholar]
- Uren, N. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In The Rhizosphere; CRC Press: New York, NY, USA, 2007; pp. 1–21. [Google Scholar]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Zolla, G.; Bakker, M.G.; Manter, D.K.; Vivanco, J.M. Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytologist 2013, 198, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.F.; Shen, Q.R.; Vivanco, J.M. Application of natural blends of phytochemicals derived from the root exudates of arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013, 288, 4502. [Google Scholar] [CrossRef] [PubMed]
- Lioussanne, L.; Jolicoeur, M.; St-Arnaud, M. Mycorrhizal colonization with glomus intraradices and development stage of transformed tomato roots significantly modify the chemotactic response of zoospores of the pathogen phytophthora nicotianae. Soil Biol. Biochem. 2008, 40, 2217–2224. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Martin, B.C.; George, S.J.; Price, C.A.; Ryan, M.H.; Tibbett, M. The role of root exuded low molecular weight organic anions in facilitating petroleum hydrocarbon degradation: Current knowledge and future directions. Sci. Total Environ. 2014, 472, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Marilley, L.; Aragno, M. Phylogenetic diversity of bacterial communities differing in degree of proximity of lolium perenne and trifolium repens roots. Appl. Soil Ecol. 1999, 13, 127–136. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Buma, D.S.; de Boer, W.; Klinkhamer, P.G.; van Veen, J.A. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie van Leeuwenhoek 2002, 81, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.A.; Guthrie, E.A.; Walton, B.T. Bioremediation in the rhizosphere. Environ. Sci. Technol. 1993, 27, 2630–2636. [Google Scholar] [CrossRef]
- Cheng, W.X.; Coleman, D.C. Effect of living roots on soil organic-matter decomposition. Soil Biol. Biochem. 1990, 22, 781–787. [Google Scholar] [CrossRef]
- Nie, M.; Yang, Q.; Jiang, L.-F.; Fang, C.-M.; Chen, J.-K.; Li, B. Do plants modulate biomass allocation in response to petroleum pollution? Biol. Lett. 2010, 6, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U.; Watt, M. Rhizosphere signals for plant–microbe interactions: Implications for field-grown plants. In Progress in Botany 72; Springer: Berlin, Germany, 2010; pp. 125–161. [Google Scholar]
- Field, B.; Jordán, F.; Osbourn, A. First encounters—Deployment of defence-related natural products by plants. New Phytol. 2006, 172, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Biological activity of allelochemicals. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: Berlin, Germany, 2009; pp. 361–384. [Google Scholar]
- Ben, L. Life of microbes in the rhizosphere. In Principles of Plant-Microbe Interactions—Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Berlin, Germany, 2015; pp. 7–15. [Google Scholar]
- Heuer, H.; Smalla, K. Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol. Rev. 2012, 36, 1083–1104. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flora. In Environmental Risk Assessment of Soil Contamination; Hernandez-Soriano, M.C., Ed.; InTech: Rijeka, Croatia, 2014; pp. 485–517. [Google Scholar]
- Gao, Y.; Yang, Y.; Ling, W.; Kong, H.; Zhu, X. Gradient distribution of root exudates and polycyclic aromatic hydrocarbons in rhizosphere soil. Soil Sci. Soc. Am. J. 2011, 75, 1694–1703. [Google Scholar] [CrossRef]
- Corgié, S.; Joner, E.J.; Leyval, C. Rhizospheric degradation of phenanthrene is a function of proximity to roots. Plant Soil 2003, 257, 143–150. [Google Scholar] [CrossRef]
- Yang, Y.; Ratte, D.; Smets, B.; Pignatello, J.; Grasso, D. Mobilization of soil organic matter by complexing agents and implications for polycyclic aromatic hydrocarbon desorption. Chemosphere 2001, 43, 1013–1021. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In Pgpr: Biocontrol and Biofertilization; Siddiqui, Z., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 39–66. [Google Scholar]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Soc. Environ. 2011, 15, 327–337. [Google Scholar]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Marschner, P.; Yang, C.-H.; Lieberei, R.; Crowley, D. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol. Biochem. 2001, 33, 1437–1445. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. PNAS 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Mahaffee, W.F.; Kloepper, J.W. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (cucumis sativus l.). Microb. Ecol. 1997, 34, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversty. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Micallef, S.A.; Shiaris, M.P.; Colon-Carmona, A. Influence of arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J. Exp. Bot. 2009, 60, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Micallef, S.A.; Channer, S.; Shiaris, M.P.; Colon-Carmona, A. Plant age and genotype impact the progression of bacterial community succession in the arabidopsis rhizosphere. Plant Signal. Behav. 2009, 4, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Inceoglu, O.; Abu Al-Soud, W.; Salles, J.F.; Semenov, A.V.; van Elsas, J.D. Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS ONE 2011, 6, e23321. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Tyson, G.W.; Vivanco, J.M.; Schenk, P.M. Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE 2013, 8, e56457. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Skoneczny, D.; Weston, P.A.; Weidenhamer, J.D. Metabolic profiling: An overview—New approaches for the detection and functional analysis of biologically active secondary plant products. J. Allelochem. Interact. 2015, 1, 15–27. [Google Scholar]
- Rosenberg, E.; Zilber-Rosenberg, I. The Hologenome Concept: Human, Animal and Plant Microbiota; Springer: Berlin, Germany, 2013. [Google Scholar]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar] [PubMed]
- Kiers, E.T.; van der Heijden, M.G. Mutualistic stability in the arbuscular mycorrhizal symbiosis: Exploring hypotheses of evolutionary cooperation. Ecology 2006, 87, 1627–1636. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere bacterial signalling: A love parade beneath our feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere-microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The sinorhizobium–medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Handelsman, J.; Goodman, R.M. Genetic basis in plants for interactions with disease-suppressive bacteria. PNAS 1999, 96, 4786–4790. [Google Scholar] [CrossRef] [PubMed]
- Stougaard, J. Regulators and regulation of legume root nodule development. Plant Physiol. 2000, 124, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Kamilova, F.; Validov, S.; Gafurova, L.; Kucharova, Z.; Lugtenberg, B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in uzbekistan. Environ. Microbiol. 2008, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; He, Y.; Chen, H.-H.; Xu, J.-M.; Rengel, Z. Dissipation of polycyclic aromatic hydrocarbons (PAHS) in the rhizosphere: Synthesis through meta-analysis. Environ. Pollut. 2010, 158, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Germida, J.J.; Frick, C.M.; Farrell, R.E. Phytoremediation of oil-contaminated soils. In Soil Mineral-Organic Matter-Microorganism Interactions and Ecosystem Health, Volume 28b: Ecological Significance of the Interactions among Clay Minerals, Organic Matter and Soil Biota; Violante, A., Huang, P.M., Bollag, J.M., Gianfreda, L., Eds.; 2002; Volume 28B, pp. 169–186. [Google Scholar]
- Atlas, R.; Bragg, J. Bioremediation of marine oil spills: When and when not—The exxon valdez experience. Microb. Biotechnol. 2009, 2, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, E.; Delille, D.; Delille, B. Crude oil bioremediation in sub-antarctic intertidal sediments: Chemistry and toxicity of oiled residues. Mar. Environ. Res. 2004, 57, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Delille, D.; Coulon, F.; Pelletier, E. Effects of temperature warming during a bioremediation study of natural and nutrient-amended hydrocarbon-contaminated sub-antarctic soils. Cold Reg. Sci. Technol. 2004, 40, 61–70. [Google Scholar] [CrossRef]
- Foght, J.M.; Westlake, D.W.; Johnson, W.M.; Ridgway, H.F. Environmental gasoline-utilizing isolates and clinical isolates of pseudomonas aeruginosa are taxonomically indistinguishable by chemotaxonomic and molecular techniques. Microbiology 1996, 142, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.D.; Zhu, X. Biodegradation of crude oil contaminating marine shorelines and freshwater wetlands. Spill Sci. Technol. Bull. 2003, 8, 163–178. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Crawford, R. Effects of oxygen, nitrogen, and temperature on gasoline biodegradation in soil. Biodegradation 1995, 6, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Coulon, F.; Pelletier, E.; Gourhant, L.; Delille, D. Effects of nutrient and temperature on degradation of petroleum hydrocarbons in contaminated sub-antarctic soil. Chemosphere 2005, 58, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Chaineau, C.; Rougeux, G.; Yepremian, C.; Oudot, J. Effects of nutrient concentration on the biodegradation of crude oil and associated microbial populations in the soil. Soil Biol. Biochem. 2005, 37, 1490–1497. [Google Scholar] [CrossRef]
- Tibbett, M.; George, S.J.; Davie, A.; Barron, A.; Milton, N.; Greenwood, P.F. Just add water and salt: The optimisation of petrogenic hydrocarbon biodegradation in soils from semi-arid barrow island, western Australia. Water Air Soil Pollut. Focus 2011, 216, 513–525. [Google Scholar] [CrossRef]
- Chaillan, F.; Chaineau, C.; Point, V.; Saliot, A.; Oudot, J. Factors inhibiting bioremediation of soil contaminated with weathered oils and drill cuttings. Environ. Pollut. 2006, 144, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Oudot, J.; Merlin, F.; Pinvidic, P. Weathering rates of oil components in a bioremediation experiment in estuarine sediments. Mar. Environ. Res. 1998, 45, 113–125. [Google Scholar] [CrossRef]
- Carmichael, L.M.; Pfaender, F.K. The effect of inorganic and organic supplements on the microbial degradation of phenanthrene and pyrene in soils. Biodegradation 1997, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.; Joner, E.J. Utilising the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ. Sci. Pollut. Res. Int. 2005, 12, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Macek, T.; Mackova, M.; Kas, J. Exploitation of plants for the removal of organics in environmental remediation. Biotechnol. Adv. 2000, 18, 23–34. [Google Scholar] [CrossRef]
- Yateem, A.; Al-Sharrah, T.; Bin-Haji, A. Investigation of microbes in the rhizosphere of selected trees for the rhizoremediation of hydrocarbon-contaminated soils. Int. J. Phytoremediation 2008, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.; Vermelho, A.; Rosado, A. Petroleum-degrading enzymes: Bioremediation and new prospects. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahian, M.; Cappello, S. Crude oil biodegradation in the marine environments. In Biodegradation—Engineering and Technology; Chamy, R., Rosenkranz, F., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Sierra-Garcia, I.N.; Oliveira, V.M.D. Microbial Hydrocarbon Degradation: Efforts to Understand Biodegradation in Petroleum Reservoirs. In Biodegradation—Engineering and Technology; Chamy, R., Rosenkranz, F., Eds.; InTech: Rijeka, Croatia, 2013; pp. 47–72. [Google Scholar]
- Orphan, V.; Goffredi, S.K.; Delong, E.; Boles, J. Geochemical influence on diversity and microbial processes in high temperature oil reservoirs. Geomicrobiol. J. 2003, 20, 295–311. [Google Scholar] [CrossRef]
- Nazina, T.; Shestakova, N.; Grigor’yan, A.; Mikhailova, E.; Tourova, T.; Poltaraus, A.; Feng, C.; Ni, F.; Belyaev, S. Phylogenetic diversity and activity of anaerobic microorganisms of high-temperature horizons of the Dagang oil field (PR China). Microbiology 2006, 75, 55–65. [Google Scholar] [CrossRef]
- Garcia, J.-L.; Patel, B.K.; Ollivier, B. Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H.; Koch, M. Non-aceticlastic methanogenesis from acetate: Acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 1984, 138, 263–272. [Google Scholar] [CrossRef]
- Hattori, S.; Kamagata, Y.; Hanada, S.; Shoun, H. Thermacetogenium phaeum gen. Nov., sp. Nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Weijma, J.; Stams, A.J. Thermotoga lettingae sp. Nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int. J. Syst. Evol. Microbiol. 2002, 52, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.A.; Chater, K.F. Genetics of Bacterial Diversity; Elsevier Science: Philadelphia, PA, USA, 1988. [Google Scholar]
- Kothari, V.; Panchal, M.; Srivastava, N. Microbial Degradation of Hydrocarbons; Institute of Science: Nirma University, Ahmedabad, Gujarat, India, 2013. [Google Scholar]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Intradiol dioxygenases—The key enzymes in xenobiotics degradation. In Biodegradation of Hazardous and Special Products; Chamy, R., Rosenkranz, F., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Segura, A.; Ramos, J.L. Plant-bacteria interactions in the removal of pollutants. Curr. Opin. Biotechnol. 2013, 24, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 2003, 67, 503–549. [Google Scholar] [CrossRef] [PubMed]
- Weelink, S.B.; van Eekert, M.A.; Stams, A.M. Degradation of Btex by anaerobic bacteria: Physiology and application. Rev. Environ. Sci. Bio/Technol. 2010, 9, 359–385. [Google Scholar] [CrossRef]
- Zhang, C.; Bennett, G. Biodegradation of xenobiotics by anaerobic bacteria. Appl. Microbiol. Biotechnol. 2005, 67, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Foght, J. Anaerobic biodegradation of aromatic hydrocarbons: Pathways and prospects. J. Mol. Microbiol. Biotechnol. 2008, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, U.; Zedelius, J.; Wilkes, H.; Musat, F. Anaerobic degradation of cyclohexane by sulfate-reducing bacteria from hydrocarbon-contaminated marine sediments. Front. Microbiol. 2015, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Yan-Zheng, G.; Li-Zhong, Z. Phytoremediation for phenanthrene- and pyrene-contaminated soils. J. Environ. Sci.(China) 2005, 17, 14–18. [Google Scholar]
- Joner, E.J.; Leyval, C. Rhizosphere gradients of polycyclic aromatic hydrocarbon (PAH) dissipation in two industrial soils and the impact of arbuscular mycorrhiza. Environ. Sci. Technol. 2003, 37, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G. Root exudates and nutrient cycling. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin, Germany, 2007; Volume 10, pp. 123–157. [Google Scholar]
- Gransee, A.; Wittenmayer, L. Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J. Plant Nutr. Soil Sci. 2000, 163, 381–385. [Google Scholar] [CrossRef]
- Hutsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Leigh, M.B.; Fletcher, J.S.; Fu, X.O.; Schmitz, F.J. Root turnover: An important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environ. Sci. Technol. 2002, 36, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.P.; Sharma, S.; Singh, N.K.; Singh, V.; Tiwari, K.; Singh, A.S. Nature and role of root exudates: Efficacy in bioremediation. Afr. J. Biotechnol. 2011, 10, 9717–9724. [Google Scholar]
- Xue, K.; Wu, L.; Deng, Y.; He, Z.; Van Nostrand, J.; Robertson, P.G.; Schmidt, T.M.; Zhou, J. Functional gene differences in soil microbial communities from conventional, low-input, and organic farmlands. Appl. Environ. Microbiol. 2013, 79, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis—Model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; Ling, W.; Hu, H.; Liu, F. Effects of organic acids on copper and cadmium desorption from contaminated soils. Environ. Int. 2003, 29, 613–618. [Google Scholar] [CrossRef]
- Van Hees, P.A.; Jones, D.L.; Jentschke, G.; Godbold, D.L. Organic acid concentrations in soil solution: Effects of young coniferous trees and ectomycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 771–776. [Google Scholar] [CrossRef]
- Cooper, G. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Campbell, A.K. Intracellular Calcium; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Sanders, D.; Bethke, P. Membrane transport. In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; 2000; pp. 110–158. [Google Scholar]
- Guern, J.; Renaudin, J.P.; Brown, S.C. The compartimentation of secondary metabolites in plant cell cultures. In Cell Culture and Somatic Cell Genetics of Plants; Vasil, I.K., Ed.; Academic Press: San Diego, CA, USA, 1987; pp. 43–76. [Google Scholar]
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Et Biophys. Acta Biomembr. 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. Put the metal to the petal: Metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Hoekenga, O.A.; Maron, L.G.; Pineros, M.A.; Cancado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. Atalmt1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in arabidopsis. PNAS 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Foster, J.; Chen, J.; Voll, L.M.; Weber, A.P.M.; Tegeder, M. Aap1 transports uncharged amino acids into roots of arabidopsis. Plant J. 2007, 50, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Svennerstam, H.; Ganeteg, U.; Bellini, C.; Nasholm, T. Comprehensive screening of arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol. 2007, 143, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Cobbett, C.S. Transporters of ligands for essential metal ions in plants. New Phytol. 2007, 174, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Briat, J.F. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(iii) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Battey, N.H.; Blackbourn, H.D. The control of exocitosis in plant cells. New Phytol. 1993, 125, 307–308. [Google Scholar] [CrossRef]
- Yazaki, K. Transporters of secondary metabolites. Curr. Opin. Plant Biol. 2005, 8, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Matsuoka, H.; Shimomura, K.; Bechthold, A.; Sato, F. A novel dark-inducible protein, Ledi-2, and its involvement in root-specific secondary metabolism in lithospermum erythrorhizon. Plant Physiol. 2001, 125, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Brigham, L.A.; Woo, H.-H.; Wen, F.; Hawes, M.C. Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiol. 1998, 118, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Romheld, V. The release of root exudates as affected by the plant’s physiological status. In The Rhizosphere, Biochemistry and Organic Substances at the Soil–Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 41–93. [Google Scholar]
- Badri, D.V.; Quintana, N.; El Kassis, E.G.; Kim, H.K.; Choi, Y.H.; Sugiyama, A.; Verpoorte, R.; Martinoia, E.; Manter, D.K.; Vivanco, J.M. An Abc transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol. 2009, 151, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M.; Broeckling, C.D.; Badri, D.; Vivanco, J.M. Effect of transporters on the secretion of phytochemicals by the roots of arabidopsis thaliana. Planta 2007, 225, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Signaling from soybean roots to rhizobium: An ATP-binding cassette-type transporter mediates genistein secretion. Plant Signal. Behav. 2008, 3, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H., Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.C.; Gaxiola, R.A.; Fink, G.R. Arabidopsis Alf5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 2001, 13, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the mate and Almt families function independently to confer arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.V.; Liu, J.; Guimaraes, C.T.; Lana, U.G.; Alves, V.M.; Wang, Y.H.; Schaffert, R.E.; Hoekenga, O.A.; Pineros, M.A.; Shaff, J.E.; et al. A gene in the multidrug and toxic compound extrusion (mate) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell. Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Kakei, Y.; Shimo, H.; Bashir, K.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J. Biol. Chem. 2011, 286, 24649–24655. [Google Scholar] [CrossRef] [PubMed]
- Rentz, J.A.; Alvarez, P.J.; Schnoor, J.L. Benzo (a)pyrene co-metabolism in the presence of plant root extracts and exudates: Implications for phytoremediation. Environ. Pollut. 2005, 136, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Anderson, D.; McGrath, S. Soil microbial response during the phytoremediation of a PAH contaminated soil. Soil Biol. Biochem. 2005, 37, 2334–2336. [Google Scholar] [CrossRef]
- Yang, C.-H.; Crowley, D.E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 2000, 66, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Potts, J.M.; Millard, P. Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl. Soil Ecol. 2007, 36, 147–155. [Google Scholar] [CrossRef]
- Bonanomi, G.; Vinale, F.; Scala, F. The role of natural products in plant-microbe interactions. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: Berlin, Germany, 2009; pp. 301–320. [Google Scholar]
- Zhou, X.G.; Wu, F.Z. P-coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of fusarium oxysporum f.Sp cucumerinum owen. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Wu, F.Z. Artificially applied vanillic acid changed soil microbial communities in the rhizosphere of cucumber (cucumis sativus l.). Can. J. Soil Sci. 2013, 93, 13–21. [Google Scholar] [CrossRef]
- Gao, M.S.; Teplitski, M.; Robinson, J.B.; Bauer, W.D. Production of substances by medicago truncatula that affect bacterial quorum sensing. Mol. Plant Microbe Interact. 2003, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Robinson, J.B.; Bauer, W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant Microbe Interact. 2000, 13, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Knee, E.M.; Gong, F.C.; Gao, M.S.; Teplitski, M.; Jones, A.R.; Foxworthy, A.; Mort, A.J.; Bauer, W.D. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol. Plant Microbe Interact. 2001, 14, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.; De Vos, D.E.; Desair, J.; Raedschelders, G.; Luyten, E.; Rosemeyer, V.; Verreth, C.; Schoeters, E.; Vanderleyden, J.; Michiels, J. The cin quorum sensing locus of rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 2002, 277, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Chen, H.C.; Rajamani, S.; Gao, M.S.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Proust, H.; Hoffmann, B.; Xie, X.N.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogue, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, E.; Benizri, E.; Guckert, A. Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. Soil Biol. Biochem. 2003, 35, 1183–1192. [Google Scholar] [CrossRef]
- Benizri, E.; Dedourge, O.; Dibattista-Leboeuf, C.; Piutti, S.; Nguyen, C.; Guckert, A. Effect of maize rhizodeposits on soil microbial community structure. Appl. Soil Ecol. 2002, 21, 261–265. [Google Scholar] [CrossRef]
- Butler, J.L.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Microbial community dynamics associated with rhizosphere carbon flow. Appl. Environ. Microbiol. 2003, 69, 6793–6800. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Bakker, M.G.; Manter, D.K.; Sheflin, A.M.; Weir, T.L.; Vivanco, J.M. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil 2012, 360, 1–13. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- De Weert, S.; Vermeiren, H.; Mulders, I.H.M.; Kuiper, I.; Hendrickx, N.; Bloemberg, G.V.; Vanderleyden, J.; De Mot, R.; Lugtenberg, B.J.J. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by pseudomonas fluorescens. Mol. Plant Microbe Interact. 2002, 15, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Oku, S.; Komatsu, A.; Tajima, T.; Nakashimada, Y.; Kato, J. Identification of chemotaxis sensory proteins for amino acids in pseudomonas fluorescens Pf0–1 and their involvement in chemotaxis to tomato root exudate and root colonization. Microbes Environ. 2012, 27, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Vicre, M.; Santaella, C.; Blanchet, S.; Gateau, A.; Driouich, A. Root border-like cells of arabidopsis. Microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol. 2005, 138, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.A. Relationship between plant phenolic acids released during soil mineralization and aggregate stabilization. Soil Sci. Soc. Am. J. 2002, 66, 1857–1867. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.X.; Zhuang, Y.E.; Xu, T.C.; Li, Y.Z.; Li, Y.; Lin, W.X. Changes in rice allelopathy and rhizosphere microflora by inhibiting rice phenylalanine ammonia-lyase gene expression. J. Chem. Ecol. 2013, 39, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Michalet, S.; Rohr, J.; Warshan, D.; Bardon, C.; Rogy, J.C.; Domenach, A.M.; Czarnes, S.; Pommier, T.; Combourieu, B.; Guillaumaud, N.; et al. Phytochemical analysis of mature tree root exudates in situ and their role in shaping soil microbial communities in relation to tree N-acquisition strategy. Plant Physiol. Biochem. 2013, 72, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Blum, U.; Staman, K.L.; Flint, L.J.; Shafer, S.R. Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. J. Chem. Ecol. 2000, 26, 2059–2078. [Google Scholar] [CrossRef]
- Van Rossum, D.; Schuurmans, F.P.; Gillis, M.; Muyotcha, A.; Van Verseveld, H.W.; Stouthamer, A.H.; Boogerd, F.C. Genetic and phenetic analyses of bradyrhizobium strains nodulating peanut (arachis hypogaea l.) roots. Appl. Environ. Microbiol. 1995, 61, 1599–1609. [Google Scholar] [PubMed]
- Irisarri, P.; Milnitsky, F.; Monza, J.; Bedmar, E. Characterization of rhizobia nodulating lotus subbiflorus from Uruguayan soils. Plant Soil 1996, 180, 39–47. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M.; Das, A.; Pati, B.; Ghosh, A. Stimulation of indoleacetic acid production in a rhizobium isolate of vigna mungo by root nodule phenolic acids. Arch. Microbiol. 2009, 191, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Mathesius, U. Root exudation: The role of secondary metabolites, their localisation in roots and transport into the rhizosphere. In Root Engineering; Springer: Berlin, Germany, 2014; pp. 221–247. [Google Scholar]
- Weston, L.A.; Alsaadawi, I.S.; Baerson, S.R. Sorghum allelopathy—From ecosystem to molecule. J. Chem. Ecol. 2013, 39, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harbor Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Mandal, S.M. Fractional changes in phenolic acids composition in root nodules of arachis hypogaea l. Plant Growth Regul. 2008, 55, 159–163. [Google Scholar] [CrossRef]
- Wen, F.S.; VanEtten, H.D.; Tsaprailis, G.; Hawes, M.C. Extracellular proteins in pea root tip and border cell exudates. Plant Physiol. 2007, 143, 773–783. [Google Scholar] [CrossRef] [PubMed]
- De-La-Pena, C.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Vivanco, J.M. Root-microbe communication through protein secretion. J. Biol. Chem. 2008, 283, 25247–25255. [Google Scholar] [CrossRef] [PubMed]
- De Hoff, P.; Brill, L.; Hirsch, A. Plant lectins: The ties that bind in root symbiosis and plant defense. Mol. Genet. Genom. 2009, 282, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De-la-Pena, C.; Badri, D.V.; Lei, Z.T.; Watson, B.S.; Brandao, M.M.; Silva-Filho, M.C.; Sumner, L.W.; Vivanco, J.M. Root secretion of defense-related proteins is development-dependent and correlated with flowering time. J. Biol. Chem. 2010, 285, 30654–30665. [Google Scholar] [CrossRef] [PubMed]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Makarova, N.; Lugtenberg, B. Effects of the tomato pathogen fusarium oxysporum f. Sp radicis-lycopersici and of the biocontrol bacterium pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol. Plant Microbe Interact. 2006, 19, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Scheffknecht, S.; St-Arnaud, M.; Khaosaad, T.; Steinkellner, S.; Vierheilig, H. An altered root exudation pattern through mycorrhization affecting microconidia germination of the highly specialized tomato pathogen fusarium oxysporum f. Sp. Lycopersici (Fol) is not tomato specific but also occurs in Fol nonhost plants. Botany 2007, 85, 347–352. [Google Scholar]
- Dzantor, E.K. Phytoremediation: The state of rhizosphere 'engineering' for accelerated rhizodegradation of xenobiotic contaminants. J. Chem. Technol. Biotechnol. 2007, 82, 228–232. [Google Scholar] [CrossRef]
- Phillips, L.A.; Greer, C.W.; Farrell, R.E.; Germida, J.J. Plant root exudates impact the hydrocarbon degradation potential of a weathered-hydrocarbon contaminated soil. Appl. Soil Ecol. 2012, 52, 56–64. [Google Scholar] [CrossRef]
- Joner, E.J.; Corgie, S.C.; Amellal, N.; Leyval, C. Nutritional constraints to degradation of polycyclic aromatic hydrocarbons in a simulated rhizosphere. Soil Biol. Biochem. 2002, 34, 859–864. [Google Scholar] [CrossRef]
- Yoshitomi, K.J.; Shann, J.R. Corn (Zea mays l.) root exudates and their impact on C-14-pyrene mineralization. Soil Biol. Biochem. 2001, 33, 1769–1776. [Google Scholar] [CrossRef]
- Miya, R.K.; Firestone, M.K. Enhanced phenanthrene biodegradation in soil by slender oat root exudates and root debris. J. Environ. Qual. 2001, 30, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.L.; Kamath, R.; Alvarez, P.J. Effect of simulated rhizodeposition on the relative abundance of polynuclear aromatic hydrocarbon catabolic genes in a contaminated soil. Environ. Toxicol. Chem. 2006, 25, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Dang, H.; Liu, J. In situ gradient distribution of polycyclic aromatic hydrocarbons (PAHS) in contaminated rhizosphere soil: A field study. J. Soils Sediments 2013, 13, 677–685. [Google Scholar] [CrossRef]
- Corgie, S.C.; Beguiristain, T.; Leyval, C. Spatial distribution of bacterial communities and phenanthrene degradation in the rhizosphere of lolium perenne l. Appl. Environ. Microbiol. 2004, 70, 3552–3557. [Google Scholar] [CrossRef] [PubMed]
- Cébron, A.; Louvel, B.; Faure, P.; France-Lanord, C.; Chen, Y.; Murrell, J.C.; Leyval, C. Root exudates modify bacterial diversity of phenanthrene degraders in PAH-polluted soil but not phenanthrene degradation rates. Environ. Microbiol. 2011, 13, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.; Al-Awadhi, H.; Sorkhoh, N.; El-Nemr, I. Rhizospheric hydrocarbon-utilizing microorganisms as potential contributors to phytoremediation for the oil Kuwaiti desert. Microbiol. Res. 1998, 153, 247–251. [Google Scholar] [CrossRef]
- Seo, J.-S.; Keum, Y.-S.; Hu, Y.; Lee, S.-E.; Li, Q.X. Phenanthrene degradation in arthrobacter sp. P1–1: Initial 1, 2-, 3, 4-and 9, 10-dioxygenation, and meta-and ortho-cleavages of naphthalene-1,2-diol after its formation from naphthalene-1,2-dicarboxylic acid and hydroxyl naphthoic acids. Chemosphere 2006, 65, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Kozdrój, J.; van Elsas, J.D. Response of the bacterial community to root exudates in soil polluted with heavy metals assessed by molecular and cultural approaches. Soil Biol. Biochem. 2000, 32, 1405–1417. [Google Scholar] [CrossRef]
- Walton, B.T.; Anderson, T.A.; Guthrie, E.A. Bioremediation in the biosphere. Reply to comments. Environ. Sci. Technol. 1995, 29, 552. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J. Rhizoremediation: A beneficial plant-microbe interaction. Mol. Plant Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Van Hees, P.A.W.; Jones, D.L.; Finlay, R.; Godbold, D.L.; Lundstomd, U.S. The carbon we do not see—The impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biol. Biochem. 2005, 37, 1–13. [Google Scholar] [CrossRef]
- Darrah, P.R. Models of the rhizosphere. I. Microbial-population dynamics around a root releasing soluble and insoluble carbon. Plant Soil 1991, 133, 187–199. [Google Scholar] [CrossRef]
- Jones, D.L.; Murphy, D.V. Microbial response time to sugar and amino acid additions to soil. Soil Biol. Biochem. 2007, 39, 2178–2182. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Rengel, Z.; Marschner, P. Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 2005, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Plant-microbe interactions in the rhizosphere and nutrient cycling. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin, Germany, 2007; Volume 10, pp. 159–182. [Google Scholar]
- Singer, A. The chemical ecology of pollutant biodegradation: Bioremediation and phytoremediation from mechanistic and ecological perspectives. In Phytoremediation, Rhizoremediation; Mackova, M., Dowling, D., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 9A, pp. 5–21. [Google Scholar]
- Bais, H.P.; Broeckling, C.D.; Vivanco, J.M. Root exudates modulate plant—Microbe interactions in the rhizosphere. In Secondary metabolites in soil ecology; Karlovsky, P., Ed.; Springer: Berlin, Germany, 2008; Volume 14, pp. 241–252. [Google Scholar]
- Kanaly, R.A.; Bartha, R. Cometabolic mineralization of benzo a pyrene caused by hydrocarbon additions to soil. Environ. Toxicol. Chem. 1999, 18, 2186–2190. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Hegde, R.S. Release of phenols by perennial plant-roots and their potential importance in bioremediation. Chemosphere 1995, 31, 3009–3016. [Google Scholar] [CrossRef]
- Yergeau, E.; Sanschagrin, S.; Maynard, C.; St-Arnaud, M.; Greer, C.W. Microbial expression profiles in the rhizosphere of willows depend on soil contamination. ISME J. 2014, 8, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.D.; Berti, W.R. Remediation of contaminated soil with green plants—An overview. In Vitro Cell. Dev. Biol. Plant 1993, 29P, 207–212. [Google Scholar] [CrossRef]
- Ouvrard, S.; Lapole, D.; Morel, J. Root exudates impact on phenanthrene availability. Water Air Soil Pollut. Focus 2006, 6, 343–352. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, L.; Ling, W.; Kang, F.; Zhu, X.; Sun, B. Effects of low-molecular-weight organic acids on sorption-desorption of phenanthrene in soils. Soil Sci. Soc. Am. J. 2010, 74, 51–59. [Google Scholar] [CrossRef]
- Read, D.B.; Bengough, A.G.; Gregory, P.J.; Crawford, J.W.; Robinson, D.; Scrimgeour, C.M.; Young, I.M.; Zhang, K.; Zhang, X. Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol. 2003, 157, 315–326. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Germida, J.J.; Banks, K.; Greer, C.W. Changes in microbial community composition and function during a polyaromatic hydrocarbon phytoremediation field trial. Appl. Environ. Microbiol. 2003, 69, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.M.; Liao, M.; Yang, J.; Chai, J.J.; Fang, S.; Wang, R.H. Influence of root-exudates concentration on pyrene degradation and soil microbial characteristics in pyrene contaminated soil. Chemosphere 2012, 88, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- LeFevre, G.H.; Hozalski, R.M.; Novak, P.J. Root exudate enhanced contaminant desorption: An abiotic contribution to the rhizosphere effect. Environ. Sci. Technol. 2013, 47, 11545–11553. [Google Scholar] [CrossRef] [PubMed]
- Drever, J.I.; Stillings, L.L. The role of organic acids in mineral weathering. Colloids Surf. A 1997, 120, 167–181. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Jones, D.L.; Brassington, D.S. Sorption of organic acids in acid soils and its implications in the rhizosphere. Eur. J. Soil Sci. 1998, 49, 447–455. [Google Scholar] [CrossRef]
- White, J.C.; Mattina, M.I.; Lee, W.Y.; Eitzer, B.D.; Iannucci-Berger, W. Role of organic acids in enhancing the desorption and uptake of weathered p,p'-DDE by cucurbita pepo. Environ. Pollut. 2003, 124, 71–80. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Wang, L.-G.; Jiang, X.; Wang, F. Influence of three low-molecular-weight organic acids on the release behavior of Hchs from red soil. China Environ. Sci. 2006, 26, 324–327. [Google Scholar]
- Ling, W.; Ren, L.; Gao, Y.; Zhu, X.; Sun, B. Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil. Soil Biol. Biochem. 2009, 41, 2187–2195. [Google Scholar] [CrossRef]
- An, C.J.; Huang, G.H.; Yu, H.; Wei, J.; Chen, W.; Li, G. Effect of short-chain organic acids and pH on the behaviors of pyrene in soil-water system. Chemosphere 2010, 81, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, Q.; Zhang, J.; Li, C.; Yan, X.; Lou, X.; Xia, Y.; Hong, Q.; Li, S. Construction of a stable genetically engineered rhamnolipid-producing microorganism for remediation of pyrene-contaminated soil. World J. Microbiol. Biotechnol. 2012, 28, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.S.; Ioannidis, M.A.; Legge, R.L. Enhanced aqueous solubilization of tetrachloroethylene by a rhamnolipid biosurfactant. J. Colloid Interface Sci. 2007, 305, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fang, X.; Bai, B.; Liang, X.; Shuler, P.J.; Goddard, W.A., III; Tang, Y. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol. Bioeng. 2007, 98, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Cohan, F.M.; Koeppel, A.F. The origins of ecological diversity in prokaryotes. Curr. Biol. 2008, 18, R1024–U1017. [Google Scholar] [CrossRef] [PubMed]
- Dagan, T.; Artzy-Randrup, Y.; Martin, W. Modular networks and cumulative impact of lateral transfer in prokaryote genome evolution. PNAS 2008, 105, 10039–10044. [Google Scholar] [CrossRef] [PubMed]
- Gogarten, J.P.; Doolittle, W.F.; Lawrence, J.G. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 2002, 19, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, F.; Davies, J. Horizontal gene transfer and the origin of species: Lessons from bacteria. Trends Microbiol. 2000, 8, 128–133. [Google Scholar] [CrossRef]
- Popa, O.; Dagan, T. Trends and barriers to lateral gene transfer in prokaryotes. Curr. Opin. Microbiol. 2011, 14, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Halary, S.; Leigh, J.W.; Cheaib, B.; Lopez, P.; Bapteste, E. Network analyses structure genetic diversity in independent genetic worlds. PNAS 2010, 107, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pereira e Silva, M.C.; Chaib De Mares, M.; van Elsas, J.D. The mycosphere constitutes an arena for horizontal gene transfer with strong evolutionary implications for bacterial-fungal interactions. FEMS Microbiol. Ecol. 2014, 89, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Mela, F.; Fritsche, K.; Boersma, H.; van Elsas, J.D.; Bartels, D.; Meyer, F.; de Boer, W.; van Veen, J.A.; Leveau, J.H.J. Comparative genomics of the piPO2/pSB102 family of environmental plasmids: Sequence, evolution, and ecology of pTer331 isolated from collimonas fungivorans ter331. FEMS Microbiol. Ecol. 2008, 66, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M. Paradigms of plasmid organization. Mol. Microbiol. 2000, 37, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Top, E.M.; Wang, Y.F.; Brown, C.J.; Yao, F.; Yang, S.; Jiang, Y.; Li, H. The broad-host-range plasmid pSFA231 isolated from petroleum-contaminated sediment represents a new member of the pRoma plasmid family. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Schluter, A.; Krause, L.; Szczepanowski, R.; Goesmann, A.; Puhler, A. Genetic diversity and composition of a plasmid metagenome from a wastewater treatment plant. J. Biotechnol. 2008, 136, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, G.; Parkhill, J.; Bird, C.; Ambrose, K.; Jones, M.C.; Huys, G.; Swings, J.; Pickup, R.W. Complete nucleotide sequence of the conjugative tetracycline resistance plasmid pFBAOT6, a member of a group of IncU plasmids with global ubiquity. Appl. Environ. Microbiol. 2004, 70, 7497–7510. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Miyazaki, R.; Sota, M.; Ohtsubo, Y.; Nagata, Y.; Tsuda, M. Isolation and characterization of naphthalene-catabolic genes and plasmids from oil-contaminated soil by using two cultivation-independent approaches. Appl. Microbiol. Biotechnol. 2007, 74, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Molbak, L.; Molin, S.; Kroer, N. Root growth and exudate production define the frequency of horizontal plasmid transfer in the rhizosphere. FEMS Microbiol. Ecol. 2007, 59, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Droge, M.; Puhler, A.; Selbitschka, W. Horizontal gene transfer among bacteria in terrestrial and aquatic habitats as assessed by microcosm and field studies. Biol. Fertil. Soils 1999, 29, 221–245. [Google Scholar]
- Jussila, M.M.; Zhao, J.; Suominen, L.; Lindstrom, K. Tol plasmid transfer during bacterial conjugation in vitro and rhizoremediation of oil compounds in vivo. Environ. Pollut. 2007, 146, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Trevors, J.T.; Starodub, M.E. Bacterial conjugation between pseudomonads in the rhizosphere of wheat. FEMS Microbiol. Ecol. 1988, 53, 299–306. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Turner, S.; Bailey, M.J. Horizontal gene transfer in the phytosphere. New Phytol. 2003, 157, 525–537. [Google Scholar] [CrossRef]
- Kroer, N.; Barkay, T.; Sorensen, S.; Weber, D. Effect of root exudates and bacterial metabolic activity on conjugal gene transfer in the rhizosphere of a marsh plant. FEMS Microbiol. Ecol. 1998, 25, 375–384. [Google Scholar] [CrossRef]
- Schwaner, N.E.; Kroer, N. Effect of plant species on the kinetics of conjugal transfer in the rhizosphere and relation to bacterial metabolic activity. Microb. Ecol. 2001, 42, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, H.; Shintani, M.; Omori, T. Divergence of mobile genetic elements involved in the distribution of xenobiotic-catabolic capacity. Appl. Microbiol. Biotechnol. 2004, 64, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kita, A.; Miki, K.; Nozaki, M.; Horiike, K. Structure and reaction mechanism of catechol 2,3-dioxygenase (metapyrocatechase). Int. Congr. Ser. 2002, 1233, 213–220. [Google Scholar] [CrossRef]
- Wammer, K.H.; Peters, C.A. A molecular modeling analysis of polycyclic aromatic hydrocarbon biodegradation by naphthalene dioxygenase. Environ. Toxicol. Chem. 2006, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Lee, K.; Gibson, D.T. Diverse reactions catalyzed by naphthalene dioxygenase from pseudomonas sp strain NCIB 9816. J. Ind. Microbiol. 1996, 17, 438–457. [Google Scholar] [CrossRef]
- Parales, R.E.; Lee, K.; Resnick, S.M.; Jiang, H.Y.; Lessner, D.J.; Gibson, D.T. Substrate specificity of naphthalene dioxygenase: Effect of specific amino acids at the active site of the enzyme. J. Bacteriol. 2000, 182, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, J.D.; Wang, X.G.; Han, Y.N.; Tong, W.; Ma, L.; Liu, B.; Cai, B.L. Complete nucleotide sequence and organization of the naphthalene catabolic plasmid PND6–1 from pseudomonas sp strain ND6. Gene 2004, 336, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sentchilo, V.S.; Perebituk, A.N.; Zehnder, A.J.B.; van der Meer, J.R. Molecular diversity of plasmids bearing genes that encode toluene and xylene metabolism in pseudomonas strains isolated from different contaminated sites in belarus. Appl. Environ. Microbiol. 2000, 66, 2842–2852. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; van Elsas, J.D. Stimulatory effects of compounds present in the rhizosphere on natural transformation of acinetobacter sp BD413 in soil. Soil Biol. Biochem. 2001, 33, 345–357. [Google Scholar] [CrossRef]
- Yi, H.; Crowley, D.E. Biostimulation of PAH degradation with plants containing high concentrations of linoleic acid. Environ. Sci. Technol. 2007, 41, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Técher, D.; Laval-Gilly, P.; Henry, S.; Bennasroune, A.; Formanek, P.; Martinez-Chois, C.; D'Innocenzo, M.; Muanda, F.; Dicko, A.; Rejšek, K. Contribution of miscanthus × giganteus root exudates to the biostimulation of PAH degradation: An in vitro study. Sci. Total Environ. 2011, 409, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; Schellingen, K.; Beckers, B.; Janssen, J.; Ceulemans, R.; van der Lelie, D.; Taghavi, S.; Carleer, R.; Vangronsveld, J. Potential of willow and its genetically engineered associated bacteria to remediate mixed CD and toluene contamination. J. Soils Sediments 2013, 13, 176–188. [Google Scholar] [CrossRef]
- Filonov, A.; Akhmetov, L.; Puntus, I.; Esikova, T.; Gafarov, A.; Izmalkova, T.Y.; Sokolov, S.; Kosheleva, I.; Boronin, A. The construction and monitoring of genetically tagged, plasmid-containing, naphthalene-degrading strains in soil. Microbiology 2005, 74, 453–458. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Zhou, C.; Chen, B.; Zhao, W.; Song, J.; Fang, R.; Chen, J.; Xiao, M. In-situ remediation of contaminated farmland by horizontal transfer of degradative plasmids among rhizosphere bacteria. Soil Use Manag. 2014, 30, 303–309. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohrbacher, F.; St-Arnaud, M. Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy 2016, 6, 19. https://doi.org/10.3390/agronomy6010019
Rohrbacher F, St-Arnaud M. Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy. 2016; 6(1):19. https://doi.org/10.3390/agronomy6010019
Chicago/Turabian StyleRohrbacher, Fanny, and Marc St-Arnaud. 2016. "Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation" Agronomy 6, no. 1: 19. https://doi.org/10.3390/agronomy6010019
APA StyleRohrbacher, F., & St-Arnaud, M. (2016). Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy, 6(1), 19. https://doi.org/10.3390/agronomy6010019