1. Introduction
Soil-dwelling parasitic nematodes represent a diverse group of organisms with profound ecological and agricultural importance. They play crucial roles in belowground food webs, influencing nutrient cycling, plant health, and pest population dynamics. Among them, entomopathogenic nematodes (EPNs) have been extensively studied and successfully applied as biological control agents against insect pests, demonstrating their effectiveness and environmental safety compared with chemical pesticides [
1,
2]. In contrast, slug-parasitic nematodes (SPNs) remain relatively underexplored despite their potential as sustainable alternatives to molluscicides, which are increasingly restricted due to their non-target impacts and environmental concerns [
3,
4,
5,
6].
Several SPN species, such as
Phasmarhabditis hermaphrodita (Schneider),
Phasmarhabditis papillosa (Schneider),
Oscheius myriophilus Poinar, and
Oscheius onirici Torrini et al., have shown the ability to parasitize and suppress pestiferous slugs, including
Deroceras reticulatum (Müller) and
Arion vulgaris (Moquin-Tandon) [
3,
4,
7]. These mollusks cause substantial economic damage in agriculture, particularly during early crop establishment, where they feed on seedlings and reduce plant vigor [
6]. The development of reliable biological control methods against slugs is therefore an urgent priority for integrated pest management (IPM) strategies.
Host location is a critical factor determining the success of parasitic nematodes as biocontrol agents. Like EPNs, SPNs rely on chemical cues to navigate complex soil environments and locate their hosts [
8,
9]. Previous studies on EPNs have demonstrated that plant-emitted volatile organic compounds (VOCs) serve as important attractants, guiding nematodes toward insect herbivores or their damage sites [
10,
11,
12,
13,
14]. However, comparable studies on SPNs are limited. Recent evidence suggests that SPNs can respond to plant- and host-associated volatiles, including aldehydes, ketones, and hydrocarbons released from damaged potato tubers and
Brassica roots [
11,
15]. These findings highlight the importance of VOC-mediated interactions in nematode foraging ecology but also underscore the lack of systematic research into how SPNs interact with cereal crops.
Cereal systems, and barley (
Hordeum vulgare L.) in particular, warrant specific attention. Barley ranks among the world’s most widely cultivated cereals and is highly susceptible to slug damage during its early growth stages [
6,
16]. Its roots release a diverse array of VOCs, including dimethyl sulfide (DMS), hexanal (H), 2-pentylfuran (2PF), and (E)-non-2-enal (N2E) [
17,
18,
19]. These compounds are known to mediate interactions with soil microbes and invertebrates [
18,
19,
20], raising the possibility that they also influence nematode behavior. Yet, despite the ecological and economic relevance, no studies have investigated whether SPNs exploit barley root VOCs for host location, nor how environmental conditions such as temperature modulate these responses.
Addressing this gap is essential for advancing both fundamental and applied perspectives. From an ecological standpoint, understanding SPN responses to cereal root volatiles contributes to the broader knowledge of belowground multitrophic interactions. From a practical perspective, such insights can inform the development of SPN-based biocontrol strategies tailored to cereal production systems, where slug damage remains a persistent challenge.
The present study therefore examines the chemotactic responses of three SPN species—P. papillosa, O. myriophilus, and O. onirici—to individual barley VOCs and their synthetic blends under contrasting temperature regimes. Specifically, we aimed to:
- (i)
assess species-specific responses of nematodes to individual barley VOCs (DMS, H, 2PF, and N2E);
- (ii)
determine whether synthetic blends of these compounds enhance nematode attraction;
- (iii)
evaluate how environmental factors, particularly temperature, influence chemotactic behavior.
By elucidating how SPNs perceive and respond to barley root volatiles, this research advances our understanding of the chemical ecology of nematode–plant–slug interactions and provides a foundation for developing innovative, environmentally sustainable slug management practices in cereal cropping systems.
2. Materials and Methods
2.1. Collection, Isolation, and Storage Preparation of Nematodes
For this study, native populations of
P. papillosa (GenBank accession number MT800511.1),
O. myriophilus (GenBank accession number OP684306.1), and
O. onirici (GenBank accession number PQ876382) were used, as their presence has recently been confirmed in Slovenia [
7,
15].
The nematodes were cultured in vivo using freeze-killed Spanish slugs (
A. vulgaris) as a substrate [
11]. After 10 days of incubation at 20 ± 1 °C, IJs were extracted from slug cadavers using a modified White trap method, followed by centrifugation in a 5% sodium hypochlorite (NaOCl) solution to separate nematodes from organic debris. Two subsequent rinses in sterile distilled water ensured the removal of residual bleach and slug tissue [
15].
The cleaned IJs were suspended in M9 buffer—an isotonic physiological solution containing KH
2PO
4, Na
2HPO
4, NaCl, and NH
4Cl—commonly used for maintaining nematode viability and osmotic balance [
15]. Suspensions were stored at 4 °C and used within 14 days of harvesting. Prior to each experiment, a 100-IJ subsample was assessed for viability using a stereomicroscope (25× magnification). Nematodes were considered viable if they exhibited active movement, including sinusoidal locomotion or twitching. Batches with a viability rate of ≥95% were used in bioassays to ensure consistent and reliable behavioral responses [
11].
2.2. Tested Volatile Compounds
The selection of VOCs for this study was guided by the findings of Gfeller et al. [
17], who analyzed VOCs emissions from 21-day-old barley (
H. vulgare) roots using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC–MS). Their study identified a complex mixture of 29 VOCs, several of which are known to mediate belowground interactions with soil organisms.
Based on their relative abundance and ecological relevance in the barley rhizosphere, four VOCs were selected for chemotaxis assays: dimethyl sulfide (DS), hexanal (H), 2-pentylfuran (2PF), and (E)-non-2-enal (N2E). Synthetic standards of these compounds were purchased from Sigma-Aldrich (Merck, St. Louis, MO, USA) with the following purities: dimethyl sulfide (≥99%), hexanal (≥98%), 2-pentylfuran (≥99%), and (E)-non-2-enal (≥98%).
All compounds were tested at a working concentration of 0.03 ppm, which corresponds to average soil-level concentrations measured approximately 10 cm from the root zone of barley plants [
17]. This standardized concentration was selected to allow comparability across treatments and with previous studies. Each VOC was individually diluted in 96% ethanol by precisely measuring and mixing the appropriate volume of pure compound with solvent. The resulting solutions were vortexed for uniformity and freshly prepared prior to each assay to ensure chemical stability and consistency. We recognize that VOC concentrations in the field may fluctuate depending on soil characteristics, microbial interactions, and plant physiological state; this limitation is acknowledged in the Discussion.
Both individual VOCs and their binary or multi-component blends (mixed at a 1:1 v/v ratio) were tested. Blends were prepared by combining equal volumes of 0.03 ppm stock solutions of each compound. All solutions were freshly prepared immediately prior to testing to preserve chemical stability and minimize potential degradation.
Ethanol was chosen as the solvent due to its widespread application in agar-based nematode chemotaxis assays, its miscibility with water, and chemical inertness at low volumes. While solvent effects can potentially bias behavioral results, our design included ethanol-only controls (10 μL applied to both treated and control area, see
Figure 1). These controls consistently yielded chemotaxis index (CI) values near zero across all nematode species, confirming that ethanol at the applied concentration did not influence nematode behavior. Alternative solvents such as n-hexane were considered but excluded due to high volatility, limited agar compatibility, and greater safety risks.
All dilutions were prepared using calibrated micropipettes and homogenized by vortexing to ensure consistent concentrations. Bioassays were carried out in a climate-controlled chamber at 18 °C and 22 °C, under 75% relative humidity and complete darkness, conditions chosen to minimize VOC degradation and maintain experimental consistency. The two temperature regimes were selected in line with previous related studies [
11,
15], allowing for direct comparison across experiments. Moreover, they represent biologically relevant conditions that approximate suboptimal versus more favorable thermal environments for slug-parasitic nematodes, thereby enabling a controlled yet ecologically meaningful assessment of chemotactic responses to root-emitted VOCs.
2.3. Chemotaxis Assay
Chemotactic responses were assessed using a modified version of the standard protocol first described by O’Halloran and Burnell [
21], with adaptations from Laznik et al. [
11,
15] to fit the experimental system (see
Figure 1). All assays were conducted in sterile 9 cm polystyrene Petri dishes (Greiner Bio-One), each filled with 25 mL of 1.6% technical agar (Biolife, Milan, Italy). The agar medium was prepared using a chemotaxis-supporting buffer composed of 5 mM potassium phosphate (pH 6.0), 1 mM calcium chloride, and 1 mM magnesium sulfate to support optimal nematode movement and directional sensing.
Each plate was divided into three areas: a treated area on left side, a control area on the right side, and a central area where nematodes were introduced (see
Figure 1). For each test, 10 µL of a VOCs solution or blends was applied to the treated area, and 10 µL of 96% ethanol (solvent control) was applied to the opposite side. A 10 µL droplet containing approximately 100 IJs, suspended in M9 buffer, was pipetted at the center of the dish to ensure uniform starting conditions.
The M9 buffer was prepared by dissolving 6.0 g of Na
2HPO
4 (42.5 mM), 3.0 g of KH
2PO
4 (22.0 mM), and 0.5 g of NaCl (8.5 mM) in 1 L of distilled water. Following autoclaving, 1.0 mL of sterile 1 M MgSO
4·7H
2O was added to achieve a final concentration of 1.0 mM. The buffer was stored at 4 °C and used freshly to maintain nematode viability and physiological stability during assays [
11].
To avoid cross-exposure between volatile treatments, only one compound was tested per assay series. Dishes were sealed with Parafilm™ immediately after application to limit VOC diffusion and maintain localized concentrations within the agar [
15].
Each treatment was replicated ten times, and the entire assay was repeated in three independent runs for statistical robustness, resulting in a total sample size of n = 30 per treatment. All trials were conducted under controlled environmental conditions using a programmable climate chamber (RK-900 CH, Kambič Laboratory Equipment, Semič, Slovenia). Assays were incubated at two test temperatures—18 °C and 22 °C—with 75% relative humidity and complete darkness to simulate natural soil conditions and eliminate light-mediated behavioral interference.
After a 24 h incubation period, the Petri dishes were briefly frozen at −20 °C for 3 min to halt nematode movement, preserving their final locations. Nematode distribution across treatment zones was analyzed using a Nikon SMZ800N stereomicroscope (Nikon Corporation, Tokyo, Japan) equipped with a 4K UHD HDMI camera (XCAM4K8MPB) at 25× magnification.
Chemotactic behavior was quantified by calculating the Chemotaxis Index (CI) using the following formula:
The CI offers a standardized approach to quantify nematode behavioral responses, effectively measuring the extent of attraction or repulsion triggered by each volatile compound tested.
CI values span a theoretical range from +1.0 (indicating maximum attraction) to −1.0 (indicating maximum repulsion). Based on these values, volatile compounds were classified into five behavioral categories: strong attractants (CI ≥ 0.2), mild attractants (0.2 > CI ≥ 0.1), neutral (−0.1 ≤ CI < 0.1), mild repellents (−0.2 ≤ CI < −0.1), and strong repellents (CI ≤ −0.2) [
11,
15].
2.4. Statistical Analysis
Directional movement of nematodes from the central zone toward either the treated or control areas of the Petri dish was interpreted as a chemotactic response and assessed using a paired Student’s t-test. Statistical significance was established at p < 0.05.
To assess behavioral differences among treatments, the proportion of IJs that migrated to the peripheral zones or remained in the central area of the assay dish was calculated for each replicate. These data were analyzed using a two-way analysis of variance (ANOVA), with statistical significance set at p < 0.05. The model accounted for the main effects of VOCs identity (including both single compounds and their binary or complex blends), nematode species, and temperature, as well as all possible interactions.
A separate two-way ANOVA was also conducted on the chemotaxis index (CI) values to compare overall nematode responsiveness to individual VOCs and their binary or complex blends mixtures. Where significant differences were identified, post hoc comparisons were performed using Duncan’s multiple range test (p < 0.05) to distinguish among treatment groups.
All data are reported as mean ± standard error (SE). Statistical analyses were carried out using Statgraphics Plus for Windows, Version 4.0 (Statistical Graphics Corp., Manugistics, Inc., Rockville, MD, USA), and graphical outputs were prepared using Microsoft Excel 2010.
3. Results
3.1. Nematode Motility
Motility, measured as the proportion of IJs migrating from the central zone of the Petri dish toward either treated or control areas (
Figure 1), was strongly influenced by nematode species, temperature, and VOC identity (
Table 1). Significant two-way and three-way interactions further highlighted the complex interplay between biotic and abiotic factors shaping nematode behavior. Replication effects (temporal and spatial) were not significant, confirming the repeatability and robustness of the experimental design. Overall, nematode motility was highly responsive to chemical and thermal cues, with species-specific behavioral patterns strongly influenced by environmental context.
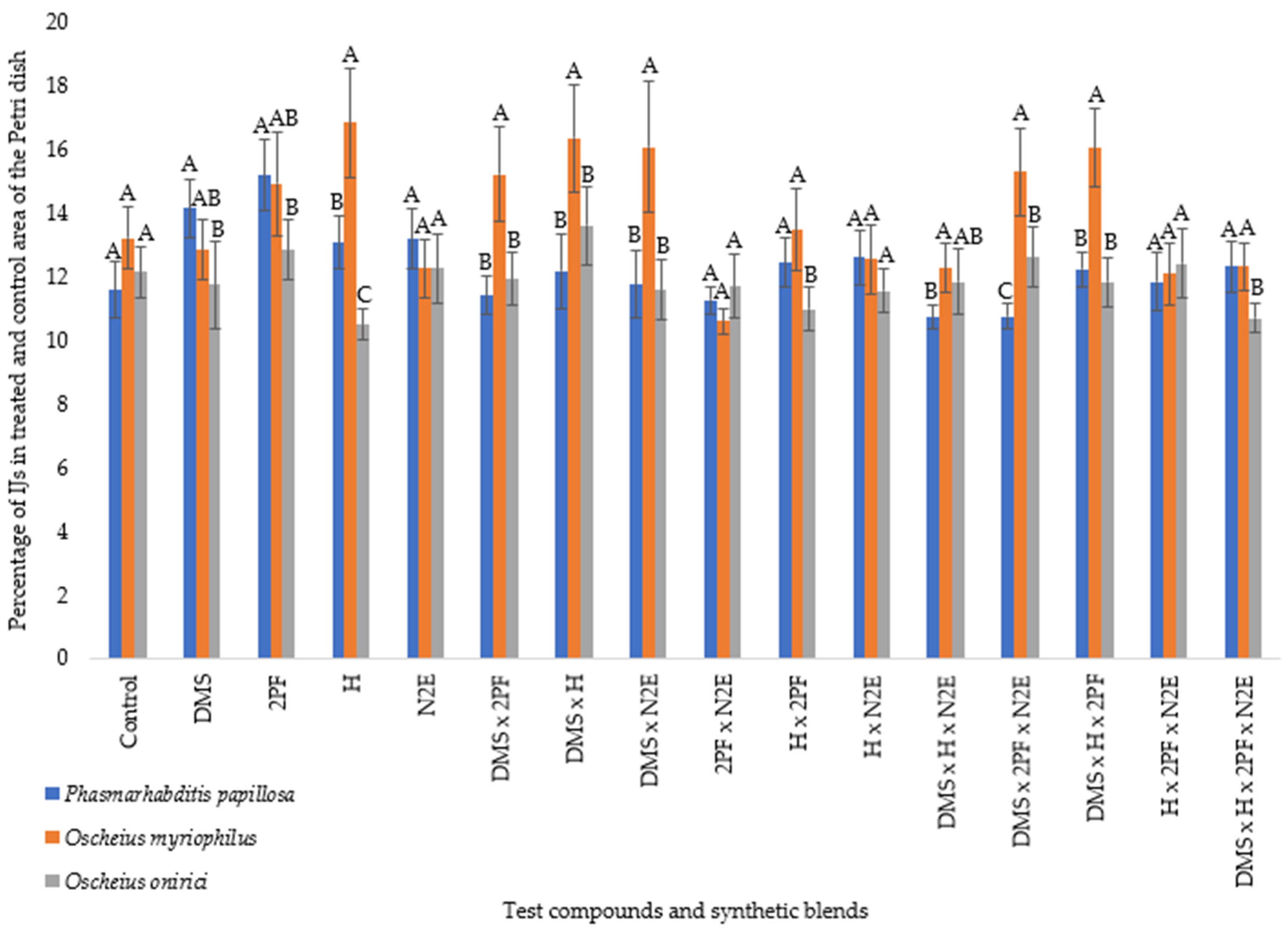
At 18 °C, clear differences in motility were observed among the three nematode species (
P. papillosa,
O. myriophilus, and
O. onirici) in response to individual VOCs and their blends (
Figure 2). Among the single compounds, 2-pentylfuran (2PF) elicited the highest levels of IJ movement, particularly in
P. papillosa and
O. myriophilus, indicating that 2PF acts as a strong locomotor stimulant. Hexanal (H) also promoted elevated motility in
P. papillosa and
O. myriophilus, though
O. onirici exhibited the lowest response to H, suggesting species-specific differences in sensitivity. Dimethyl sulfide (DMS) produced moderate stimulation across species, while (E)-non-2-enal (N2E) consistently induced lower activity, often comparable to or below control values.
Binary blends generally enhanced motility relative to single compounds, though responses varied by species. For example, H × 2PF elicited strong motility in P. papillosa, while O. myriophilus responded more strongly to DMS × 2PF. Conversely, H × N2E produced neutral to low motility across species.
Ternary and quaternary blends tended to reduce motility relative to individual VOCs, suggesting possible masking or inhibitory effects. For instance, DMS × H × N2E and DMS × 2PF × N2E generally led to reduced IJ dispersal in P. papillosa and O. onirici, although O. myriophilus remained comparatively more active. The strongest reduction in motility was observed in P. papillosa when exposed to the four-component blend (DMS × H × 2PF × N2E). Notably, while previous text suggested this effect was uniform across all species, revisions clarify that O. myriophilus maintained higher motility under several ternary blends, indicating species-specific tolerance.
The ethanol control yielded some of the lowest motility values, though for O. onirici several binary and ternary blends produced similar or lower values, confirming that solvent alone did not bias nematode responses.
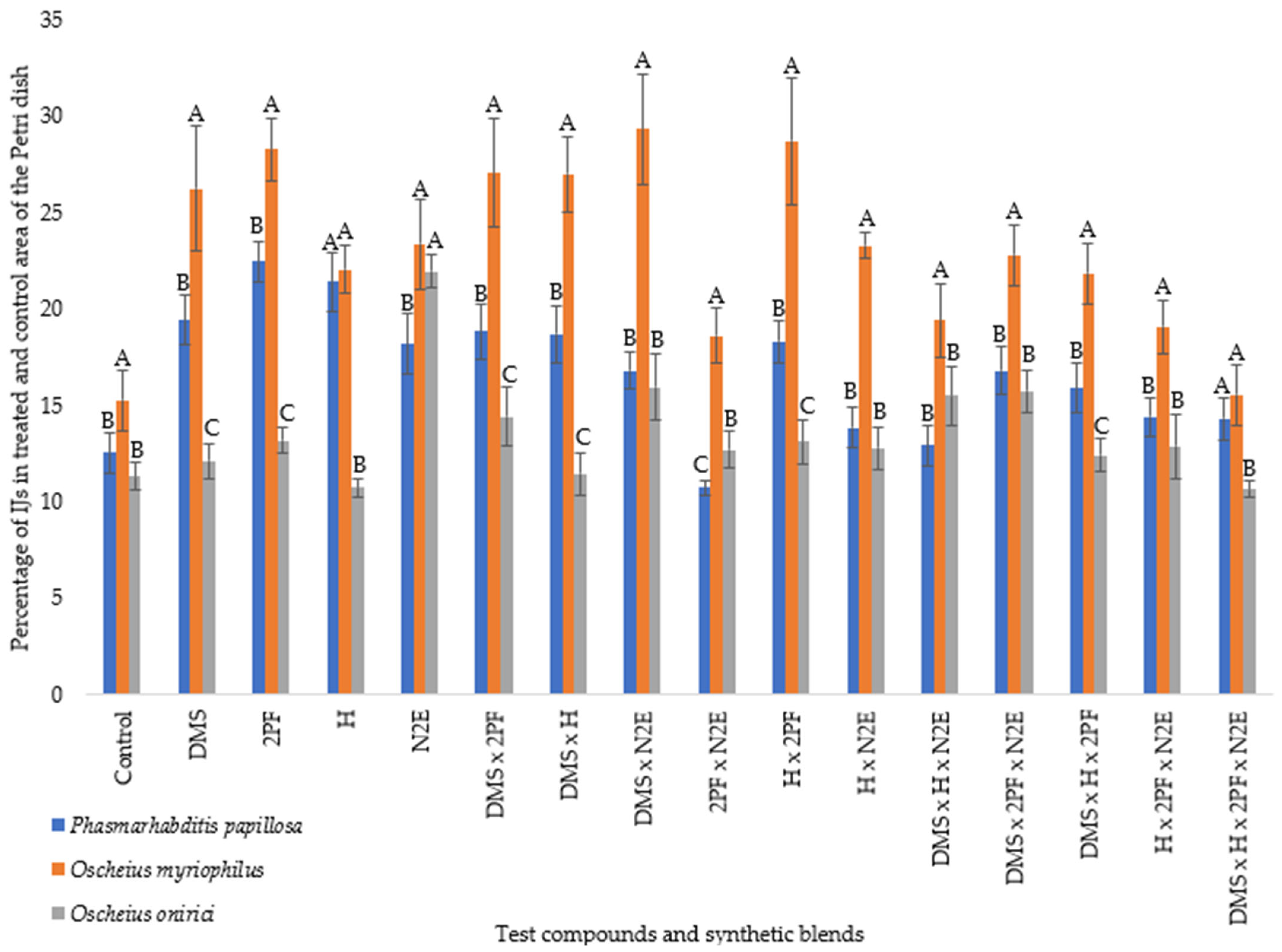
At 22 °C, motility increased overall across all species (
Figure 3).
O. myriophilus consistently exhibited the highest levels of IJ movement across most treatments, significantly exceeding
P. papillosa and
O. onirici in many cases. Among individual compounds, 2PF and H elicited particularly strong responses in
O. myriophilus, while
O. onirici showed more modest activity. Binary blends such as DMS × 2PF supported elevated motility in both
O. myriophilus and
O. onirici. In contrast, blends containing N2E (e.g., DMS × H × N2E) reduced dispersal, particularly in
O. onirici. The four-component blend (DMS × H × 2PF × N2E) elicited neutral or repellent responses across species, though the H × N2E treatment produced an even more pronounced reduction. These observations underscore the role of compound interactions in shaping nematode behavioral outcomes.
3.2. Chemotaxis Index
Chemotactic responses, quantified using the CI, varied significantly with nematode species, VOC identity, and temperature (
Table 2). Interaction effects were also significant, indicating that responsiveness to VOCs differed among species and was further modulated by thermal conditions. Replication effects (temporal and spatial) were not significant, confirming the robustness of the experimental design. Together, these results highlight the context-dependent nature of nematode chemotaxis, shaped by both chemical and environmental cues.
At 18 °C (
Table 3), 2PF emerged as a mild attractant for
P. papillosa and
O. onirici (CI = 0.11 ± 0.01 each), but a strong attractant for
O. myriophilus (CI = 0.29 ± 0.01). DMS also stimulated strong attraction in
P. papillosa (CI = 0.20 ± 0.01) and a milder response in
O. myriophilus. In contrast, H and N2E acted as neutral to weak repellents across species, with
P. papillosa and
O. myriophilus showing the strongest negative responses to H.
Binary blends produced variable effects. DMS × 2PF acted as a strong attractant for O. myriophilus (CI = 0.27 ± 0.07), whereas H × N2E induced weak repellence in P. papillosa and O. myriophilus.
Complex blends often reduced chemotaxis, with the four-component blend (DMS × H × 2PF × N2E) acting as a mild repellent for P. papillosa (CI = −0.18 ± 0.06). However, contrary to the earlier generalization, O. onirici showed a more positive response to DMS × 2PF × N2E (CI = 0.19 ± 0.01).
At 22 °C (
Table 4), species-specific variation was more pronounced. DMS elicited the strongest attraction across all species, particularly
P. papillosa (CI = 0.26 ± 0.02). H also produced strong attraction in
P. papillosa and
O. myriophilus (CIs = 0.23–0.24), though
O. onirici responded more moderately. Binary and ternary blends displayed contrasting effects, with DMS × H × 2PF attractive to both
O. myriophilus and
O. onirici, while H × N2E induced a strong repellent effect in
P. papillosa (CI = −0.33 ± 0.06). Importantly, the DMS × 2PF × N2E blend was found to be strongly attractive to both
O. myriophilus and
O. onirici (CIs > 0.20).
Overall, O. myriophilus displayed the highest sensitivity to VOCs across both temperatures, whereas P. papillosa and O. onirici showed more variable responses depending on compound identity and blend complexity. These findings reinforce the importance of evaluating both individual compounds and blends, as well as considering temperature effects, when characterizing nematode chemotaxis.
4. Discussion
This study advances understanding of the chemical ecology of SPNs by demonstrating that their chemotactic responses to barley-derived volatile organic compounds (VOCs) are strongly shaped by species identity, compound identity, and temperature. The findings emphasize the complexity of belowground signaling and highlight both opportunities and challenges for exploiting VOCs in biological control.
Species- and compound-specific responses were evident throughout.
O. myriophilus consistently exhibited the strongest attraction, particularly to 2PF and DMS, suggesting high chemosensory acuity and flexibility. These volatiles are well-known products of microbial metabolism and plant root exudation [
12,
19,
22,
23] and may therefore act as indirect indicators of gastropod presence or decaying organic substrates.
P. papillosa also responded positively to DMS and 2PF but with more variable responses across other compounds, while
O. onirici was less responsive overall, except for certain blends such as DMS × 2PF × N2E at 22 °C. This species-level variation supports earlier suggestions that slug-parasitic nematodes differ in host-searching strategies and ecological niches [
24,
25,
26].
In contrast, aldehydes such as hexanal (H) and (E)-non-2-enal (N2E) elicited neutral or negative responses in most cases. For
O. onirici, hexanal consistently ranked among the least attractive compounds, while
P. papillosa and
O. myriophilus displayed weak to moderate attraction only under specific conditions. Aldehydes are often associated with plant stress, senescence, or toxicity [
11,
22,
23], which may explain their limited attractiveness to SPNs. These results suggest that SPNs may actively avoid cues linked to unfavorable or degraded habitats.
Blend effects further highlight the integrative nature of nematode chemosensory behavior. Several binary blends (e.g., DMS × 2PF) enhanced attraction compared with individual compounds, while more complex blends, such as DMS × H × 2PF × N2E, often suppressed responsiveness. Importantly, antagonistic or masking interactions were not uniform across species: for example,
O. onirici responded positively to DMS × 2PF × N2E at 22 °C, whereas
P. papillosa showed repulsion to the four-component blend. These outcomes mirror findings in other nematode systems, where blends can override or amplify responses to individual components [
11,
18]. Such complexity reinforces that nematodes likely process odor cues hierarchically, prioritizing some volatiles while ignoring or suppressing others.
Temperature emerged as a major modulator of nematode responsiveness. Across species, stronger motility and chemotaxis were generally observed at 22 °C compared with 18 °C. This pattern is consistent with studies showing enhanced nematode mobility, sensory acuity, and infection rates at warmer temperatures [
9,
11,
24,
27]. However, higher temperatures did not uniformly increase attraction: some blends remained neutral or repellent, and species-specific differences persisted. These results indicate that field applications of VOC-based lures must consider seasonal and microclimatic variability, as nematode performance will depend heavily on temperature regimes.
The three-way interaction among species, VOC identity, and temperature underscores the multifactorial nature of nematode chemical ecology. For practical applications, this means that semiochemical-based control approaches (e.g., lure-and-infect strategies [
28,
29]) cannot rely on single attractants but must account for the species of nematode deployed and the environmental conditions in which they operate. A “one-size-fits-all” attractant is unlikely; instead, tailored blends may be required to optimize foraging and infection in target habitats.
Finally, while our standardized dose of 0.03 ppm was chosen to reflect average reported levels near barley roots [
17], actual field concentrations are dynamic, influenced by soil type, microbial activity, and plant growth stage. Future work should therefore test identified attractants under more realistic soil systems and across a range of concentrations. Evaluating persistence, diffusion, and microbial interactions of these volatiles will be crucial for determining their field relevance and biocontrol potential.