Effect of Water Restriction and Supplementary Nitrogen on the Growth Dynamics of Bromus valdivianus Phil.
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Soil Nitrogen Treatment
2.2. Soil Water Restriction
2.3. Defoliation/Cutting Events and Nitrogen Treatment
2.4. Assessment of Plant Growth and Morphophysiological Variables
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Soil Water Content According to Water Restriction and Nitrogen Addition
3.2. Growth Dynamics of the Mini-Swards
3.3. Mini-Sward Nutritive Value
4. Discussion
4.1. Pasture Growth Dynamics
4.2. Nutritive Value
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boisier, J.P.; Rondanelli, R.; Garreaud, D.; Muñoz, F. Anthropogenic and natural contributions to the Southeast Pacific precipitation decline and recent megadrought in central Chile. Geophys. Res. Lett. 2016, 43, 413–421. [Google Scholar] [CrossRef]
- Villarroel, C.; Aravena, C.; Gotelli, C.; Vásquez, R.; Vilches, C. Reporte Anual de la Evolución del Clima en Chile; Dirección Meteorológica de Chile (MeteoChile), Dirección General de Aeronáutica Civil (DGAC): Santiago, Chile, 2022. [Google Scholar]
- Ordóñez, I.; López, I.F.; Kemp, P.D.; Descalzi, C.A.; Horne, R.; Zúñiga, F.; Dec, D.; Dörner, J. Effect of pasture improvement managements on physical properties and water content dynamics of a volcanic ash soil in southern Chile. Soil Till. Res. 2018, 178, 55–64. [Google Scholar] [CrossRef]
- Volaire, F.; Norton, M.R.; Lelièvre, F. Summer drought survival strategies and sustainability of perennial temperate forage grasses in Mediterranean areas. Crop Sci. 2009, 49, 2386–2392. [Google Scholar] [CrossRef]
- López, I.F.; Kemp, P.D.; Dörner, J.; Descalzi, C.A.; Balocchi, O.; García, S. Competitive strategies and growth of neighbouring Bromus valdivianus Phil. and Lolium perenne L. plants under water restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Lisar, Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; Rahman, I.M.M., Hasegawa, H., Eds.; Intech Open: Rijeka, Croatia, 2012; pp. 1–14. [Google Scholar]
- Descalzi, C.A.; López, I.F.; Kemp, P.D.; Dörner, J.; Ordóñez, I. Pasture restoration improvement methods for temperate degraded pastures and consequences of the climatic seasonality on soil–pasture complex. J. Agron. Crop Sci. 2020, 206, 130–147. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Zhang, Y.; García-Favre, J.; Hu, H.; López, I.F.; Ordóñez, I.P.; Cartmill, A.D.; Kemp, P.D. Aboveground structural attributes and morpho-anatomical response strategies of Bromus valdivianus Phil. and Lolium perenne L. to severe soil water restriction. Agronomy 2023, 13, 2964. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant. Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- López, I.F.; Balocchi, O.A.; Kemp, P.D.; Valdés, C. Phenotypic variability in Holcus lanatus L. in Southern Chile: A strategy that enhances plant survival and pasture stability. Crop Pasture Sci. 2009, 60, 768–777. [Google Scholar] [CrossRef]
- García-Favre, J.; López, I.F.; Cranston, L.M.; Donaghy, D.J.; Kemp, P.D.; Ordóñez, I.P. Functional contribution of two perennial grasses to enhance pasture production and drought resistance under a leaf regrowth stage defoliation criterion. J. Agron. Crop Sci. 2023, 209, 144–160. [Google Scholar] [CrossRef]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Dörner, J.; García-Favre, J.; Zhang, Y. A short-term effect of multi-species pastures and the plant’s physiological response on pasture growth. Eur. J. Agron. 2024, 159, 127232–127244. [Google Scholar] [CrossRef]
- Nuñez-Barrios, A.; Foster, E. Efecto del déficit hídrico sobre el crecimiento de hojas, tallos y vainas de frijol. Agric. Tec. Mex. 1996, 22, 99–109. [Google Scholar]
- García-Favre, J.; López, I.F.; Cranston, L.M.; Donaghy, D.J.; Kemp, P.D. The growth response of pasture brome (Bromus valdivianus Phil.) to defoliation frequency under two soil-water restriction levels. Agronomy 2021, 11, 300. [Google Scholar] [CrossRef]
- Muraoka, T.; Tziboy, E.A.T. Mejoramiento del uso del Agua en la Agricultura: El Papel de las Técnicas Nucleares; Universidade de São Paulo: Piracicaba, Brazil, 2001; 131p. [Google Scholar]
- Balocchi, O.; Solís, C.; Poff, J.; Keim, J.P.; López, I. Filocrono en una pradera de Lolium perenne L.: Efecto de la frecuencia de defoliación y fertilización Nitrogenada. Agro Sur 2011, 39, 165–176. [Google Scholar] [CrossRef]
- Kraiser, T.; Gras, D.E.; Gutiérrez, A.G.; González, B.; Gutiérrez, R.A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 2011, 62, 1455–1466. [Google Scholar] [CrossRef]
- Vellinga, T.V.; André, G.; Schils, R.L.M.; Oenema, O. Operational management of nitrogenous fertilizers in dairy production systems: Identification of criteria and derivation of fertilizer application rates. Grass Forage Sci. 2004, 59, 364–377. [Google Scholar] [CrossRef]
- Tisdale, S.; Nelson, W.; Beaton, J.; Havlin, J. Soil Fertility and Fertilizers, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 1993. [Google Scholar]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Kavanová, M.; Lattanzi, F.; Schnyder, H. Nitrogen deficiency inhibits leaf blade growth in Lolium perenne by increasing cell cycle duration and decreasing mitotic and post-mitotic growth rates. Plant Cell Environ. 2008, 31, 727–737. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Li, L.; Han, X. Seasonal variations in nitrogen mineralization under three land use types in a grassland landscape. Acta Oecol. 2008, 34, 322–330. [Google Scholar] [CrossRef]
- Schils, R.L. Effect of a spring application of nitrogen on the performance of perennial ryegrass-white clover swards at two sites in the Netherlands. Neth. J. Agric. Sci. 1997, 45, 263–275. [Google Scholar] [CrossRef]
- Duru, M. Effect of nitrogen fertilization rates and defoliation regimes on the vertical structure and composition (crude protein content and digestibility) of a turfgrass. J. Sci. Food Agric. 2003, 83, 1469–1479. [Google Scholar] [CrossRef]
- Blanco, J.; Balocchi, O.; López, I. Variabilidad fenotípica en accesiones de Bromus valdivianus Phil. de la provincia de Valdivia. Agro Sur 2010, 38, 68–79. [Google Scholar] [CrossRef]
- López, I.; Balocchi, O.; Lailhacar, P.; Oyarzún, C. Characterization of the growing sites of six native and naturalized species in the Dominio Húmedo of Chile. Agro Sur 1997, 25, 62–80. [Google Scholar]
- Descalzi, C.; Balocchi, O.; López, I.; Kemp, P.; Dörner, J. Different soil structure and water conditions affect the growing response of Lolium perenne L. and Bromus valdivianus Phil. growing alone or in mixture. J. Soil Sci. Plant Nutr. 2018, 18, 617–635. [Google Scholar] [CrossRef]
- Charlton, J.F.L.; Stewart, A.V. Pasture species and cultivars used in New Zealand—A list. Proc. N. Z. Grassl. Assoc. 1999, 61, 147–166. [Google Scholar] [CrossRef]
- Onstad, D.W.; Fick, G.W. Predicting crude protein, in vitro true digestibility, and leaf proportion in alfalfa herbage. Crop Sci. 1983, 23, 961–964. [Google Scholar] [CrossRef]
- Buxton, D.R.; Marter, G.C. Forage quality of plant parts of perennial grasses and relationship to phenology. Crop Sci. 1989, 29, 429–435. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Natural Resource Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2014; 372p. [Google Scholar]
- Dörner, J.; Huertas, J.; Cuevas, J.G.; Leiva, C.; Paulino, L.; Arumí, J.L. Water content dynamics in a volcanic ash soil slope in southern Chile. J. Plant Nutr. Soil Sci. 2015, 178, 693–702. [Google Scholar] [CrossRef]
- Hartge, K.; Horn, R. Die physikalische Untersuchung von Böden. J. Plant Nutr. Soil Sci. 2009, 3, 454. [Google Scholar] [CrossRef]
- Dec, D.; Bravo, S.; Clunes, J.; Granda, S.; López, I.; Ordóñez, I.; Zúñiga, F.; Dörner, J. The role of the specific properties of an Andosol as a water reservoir to improve pasture growth and prevent soil physical degradation in irrigated grazing systems. Journal of Soil Science and Plant Nutrition. J. Soil Sci. Plant Nutr. 2022, 22, 3756–3773. [Google Scholar] [CrossRef]
- Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; López, I. Water-soluble carbohydrate recovery in pastures of perennial ryegrass (Lolium perenne L.) and pasture brome (Bromus valdivianus Phil.) under two defoliation frequencies determined by thermal time. Agriculture 2020, 10, 563. [Google Scholar] [CrossRef]
- Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; López, I. Thermal time as a parameter to determine optimal defoliation frequency of perennial ryegrass (Lolium perenne L.) and pasture brome (Bromus valdivianus Phil.). Agronomy 2020, 10, 620. [Google Scholar] [CrossRef]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Zhang, Y.; Herrmann, P. Response of Bromus valdivianus (pasture brome) growth and physiology to defoliation frequency based on leaf stage development. Agronomy 2021, 11, 2058. [Google Scholar] [CrossRef]
- Okamoto, H.; Ishii, K.; An, P. Effects of soil moisture deficit and subsequent watering on the growth of four temperate grasses. Grassl. Sci. 2011, 57, 192–197. [Google Scholar] [CrossRef]
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptation strategies, functional traits, and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Blum, A. Plant water relations, plant stress and plant production. In Plant Breeding for Water-Limited Environments; Blum, A., Ed.; Springer: New York, NY, USA, 2011; pp. 11–52. [Google Scholar]
- Boyer, J.S. Cell enlargement and growth-induced water potentials. Physiol. Plant. 1988, 73, 311–316. [Google Scholar] [CrossRef]
- Matyssek, R.; Maruyama, S.; Boyer, J.S. Growth-induced water potentials may mobilize internal water for growth. Plant Cell Environ. 1991, 14, 917–923. [Google Scholar] [CrossRef]
- Wang, J.P.; Bughrara, S.S. Evaluation of drought tolerance for Atlas fescue, perennial ryegrass, and their progeny. Euphytica 2008, 164, 113–122. [Google Scholar] [CrossRef]
- Mazzanti, A.; Lemaire, G.; Gastal, F. The effect of nitrogen fertilization on forage production of tall fescue pastures under continuous grazing with sheep. 1. Forage growth dynamics. Grass Forage Sci. 1994, 49, 111–120. [Google Scholar] [CrossRef]
- Lemaire, G.; Agnusdei, M. Leaf tissue turn-over and efficiency of herbage utilization. In Grassland Ecophysiology and Grazing Ecology; Lemaire, G., Hodgson, J., de Moraes, A., Nabinger, C., Carvalho, P.C.d.F., Eds.; CABI: Oxford, UK, 2000; pp. 265–287. [Google Scholar]
- Caresani, D.; Juanicotena, M.A. Efecto de la Fertilización Nitrogenada Sobre el Crecimiento y la Utilización de Especies de un Campo Natural Bajo Pastoreo de Vacunos en el Período Otoñal. Bachelor’s Thesis, Faculty of Agronomy, Universidad de la República, Montevideo, Uruguay, 2008. [Google Scholar]
- Duru, M.; Ducrocq, H. Growth and senescence of the successive leaves on a cocksfoot tiller. Effect of nitrogen and cutting regime. Ann. Bot. 2000, 85, 645–653. [Google Scholar] [CrossRef]
- Gales, K. Effects of water supply on dry matter partitioning between roots and shoots in Lolium perenne. J. Appl. Ecol. 1979, 16, 863–877. [Google Scholar] [CrossRef]
- Brown, H.E.; Moot, D.J.; Pollock, K.M. Herbage production, persistence, nutritive characteristics and water use of perennial forages grown over 6 years on a Wakanui silt loam. N. Z. J. Agric. Res. 2005, 48, 423–439. [Google Scholar] [CrossRef]
- Black, W.N. Effects of irrigation and nitrogen on a natural pasture sward. Can. J. Plant Sci. 1978, 58, 347–356. [Google Scholar] [CrossRef]
- Mills, A.; Moot, D.J.; Jamieson, P.D. Quantifying the effect of nitrogen on productivity of cocksfoot (Dactylis glomerata L.) pastures. Eur. J. Agron. 2009, 30, 63–69. [Google Scholar] [CrossRef]
- Torres, A. Especies forrajeras mejoradas. In Praderas para Chile; Ruiz, I., Ed.; Instituto de Investigaciones Agropecuarias, Ministerio de Agricultura: Santiago, Chile, 1998; pp. 153–154. [Google Scholar]
- Norton, M.R.; Lelievre, F.; Volaire, F. Summer dormancy in Phalaris aquatica L., the influence of season of sowing and summer moisture regime on two contrasting cultivars. J. Agron. Crop Sci. 2012, 198, 1–13. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Water Relations. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; pp. 163–223. [Google Scholar]
- Weerarathne, L.V.Y.; Jahufer, Z.; Schäufele, R.; López, I.; Matthew, C. A comparative analysis of agronomic water-use efficiency and its proxy measures as derived from key morpho-physiological and supportive quantitative genetics attributes of perennial ryegrass under imposed drought. Plant-Environ. Interact. 2023, 4, 291–307. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Springer: New York, NY, USA, 2014. [Google Scholar]
- Stewart, A.V. Potential value of some Bromus species of the section Ceratochloa. N. Z. J. Agric. Res. 1996, 39, 611–618. [Google Scholar] [CrossRef]
- Chen, L.; Yi, Y.; Jun-Qin, G.; Xiao-Ya, Z.; Qian-Wei, L.; Fei-Hai, Y. Effects of soil moisture on organic and inorganic nitrogen uptake by dominant plant species in Zoigê alpine wetlands. Ecol. Indic. 2022, 141, 109087. [Google Scholar] [CrossRef]
- Navarro, F. Filocrono y Dinámica de Crecimiento de Lolium perenne L. y Bromus valdivianus Phil. con Tres Niveles de Adición de Nitrógeno en el Suelo. Master’s Thesis, Faculty of Agricultural Science, Universidad Austral de Chile, Valdivia, Chile, 2016. [Google Scholar]
- Zemenchik, R.A.; Albrecht, K.A. Nitrogen use efficiency and apparent nitrogen recovery of Kentucky bluegrass, smooth bromegrass, and orchardgrass. Agron. J. 2002, 94, 421–428. [Google Scholar] [CrossRef]
- Moyer, J.; Sweeney, D. Growth and forage quality responses of smooth Bromegrass to nitrogen placement and timing. Agron. J. 2016, 108, 2453–2461. [Google Scholar] [CrossRef]
- Lemaire, G.; Denoix, A. Summer dry matter growth of tall fescue (Festuca arundinacea Schreb.) and dactylus (Dactylis glomerata L.) stands in western France. II. Interaction between levels of water supply and nitrogenous nutrition. Agronomy 1987, 7, 381–389. [Google Scholar] [CrossRef]
- Clunes, J.; Dörner, J.; Pinochet, D. How does the functionality of the pore system affects inorganic nitrogen storage in volcanic ash soils? Soil Tillage Res. 2021, 205, 104802. [Google Scholar] [CrossRef]
- Durand, J.; Schäufele, R.; Gastal, F. Grass leaf elongation rate as a function of developmental stage and temperature: Morphological analysis and modelling. Ann. Bot. 1999, 83, 577–588. [Google Scholar] [CrossRef]
- Whitehead, D.C. Grassland Nitrogen; CABI: Wallingford, UK, 1995. [Google Scholar]
- Nelson, C.J. Shoot morphological plasticity of grasses: Leaf growth vs. tillering. In Grassland Ecophysiology and Grazing Ecology; Lemaire, G., Hodgson, J., de Moraes, A., Nabinger, C., Carvalho, P.C.d.F., Eds.; CABI: Oxford, UK, 2000; pp. 101–126. [Google Scholar]
- Wilman, D.; Wright, P.T. Some effects of applied nitrogen on the growth and chemical composition of temperate grasses. Herb. Abstr. 1983, 53, 387–393. [Google Scholar]
- Azanza, A.; Panissa, R.J.; Rodríguez, H. Evaluación de la fertilización nitrogenada de campo natural bajo pastoreo de vacunos en el período primaveral. Bachelor’s Thesis, Faculty of Agronomy, Universidad de la República, Montevideo, Uruguay, 2004. [Google Scholar]
- Bolger, T.P.; Turner, N.C. Water use efficiency and water use of Mediterranean annual grasses in South Australia. Aust. J. Agric. Res. 1999, 50, 1035–1046. [Google Scholar] [CrossRef]
- Ervin, E.H.; Koski, A.J. Drought Avoidance Aspects and Crop Coefficients of Kentucky Bluegrass and Tall Fescue Turfs in the Semiarid West. Crop Sci. 1998, 38, 788–795. [Google Scholar] [CrossRef]
- Ludlow, M.M. Strategies of response to water stress. In Structural and Functional Responses to Environmental Stresses: Water Shortage; Kreeb, K.H., Richter, H., Hinckley, T.M., Eds.; CABI: The Hague, The Netherlands, 1989; pp. 269–281. [Google Scholar]
- Lemaire, G.; Gastal, F. Nitrogen uptake and distribution in plant canopies. In Diagnosis on the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin, Germany, 1997; pp. 3–43. [Google Scholar]
- Mazzanti, A.; Lemaire, G. Effect of nitrogen fertilization on forage production of tall fescue pastures continuously grazed by sheep. 2. Forage intake and utilization efficiency. Grass Forage Sci. 1994, 49, 352–359. [Google Scholar] [CrossRef]
- Bartholomew, P.W.; Williams, R.D. Effects of the exposure to below-freezing temperatures, soil moisture content and nitrogen application on phyllochron in cool-season grasses. Grass Forage Sci. 2006, 61, 146–153. [Google Scholar] [CrossRef]
- Fulkerson, W.; Lowe, K. Grazing Management. In Encyclopedia of Dairy Sciences: Forages and Pastures; Fuquay, J.W., Ed.; Elsevier Science: Oxford, UK, 2011; pp. 594–601. [Google Scholar]
- McMaster, G.S.; Wilhelm, W.W.; Palic, D.B.; Porter, J.R.; Jamieson, P.D. Spring wheat leaf appearance and temperature: Extending the paradigm? Ann. Bot. 2003, 91, 697–705. [Google Scholar] [CrossRef]
- Longnecker, N.; Robson, A. Leaf emergence of spring wheat receiving a variable supply of nitrogen at different stages of development. Ann. Bot. 1994, 74, 1–7. [Google Scholar] [CrossRef]
- Bartholomew, P.W.; Williams, R.D. Cool-season grass development response to accumulated temperature under a range of temperature regimes. Crop Sci. 2005, 45, 529–534. [Google Scholar] [CrossRef]
- Moot, D.J.; Smith, M.C.; Mills, A. Liveweight production, dry matter yield and seasonal composition from dryland lucerne and lucerne/grass mixes over fice years. N. Z. J. Agric. Res. 2020, 63, 272–300. [Google Scholar] [CrossRef]
- Jun, S.E.; Shim, J.S.; Park, H.J. Beyond NPK: Mineral Nutrient-Mediated Modulation in Orchestrating Flowering Time. Plants 2023, 12, 3299. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.F.; Field, T.R.O. Growth characteristics of perennial ryegrass. Proc. N. Z. Grassl. Assoc. 1978, 40, 104–113. [Google Scholar] [CrossRef]
- Buxton, D.R. Characteristics related to the quality of forages influenced by the environment of the plant and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Loges, R.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Effects of grazing and precipitation on herbage production, herbage nutritive value and performance of sheep in continental steppe. Grass Forage Sci. 2012, 67, 535–545. [Google Scholar] [CrossRef]
- Tharmaraj, J.; Chapman, D.F.; Nie, Z.N.; Lane, A.P. Herbage accumulation, botanical composition, and nutritive value of five pasture types for dairy production in southern Australia. Aust. J. Agric. Res. 2008, 59, 127–138. [Google Scholar] [CrossRef]
- Lee, J.M.; Donaghy, D.J.; Roche, J.R. Effect of defoliation severity on regrowth and nutritive value of perennial ryegrass dominant swards. Agron. J. 2008, 100, 308–314. [Google Scholar] [CrossRef]
- Allen, E.; Sheaffer, C.; Martinson, K. Forage nutritive value and preference of cool-season grasses under horse grazing. Agron. J. 2013, 105, 679–684. [Google Scholar] [CrossRef]
- Davidson, A.; Da Silva, D.; Quintana, B.; Dejong, T.M. The phyllochron of Prunus persica shoots is relatively constant under controlled growth conditions but seasonally increases in the field in ways unrelated to patterns of temperature or radiation. Sci. Hortic. 2015, 184, 106–113. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Marino, M.A.; Mazzanti, A.; Assuero, S.G.; Gastal, F.; Echeverría, H.E.; Andrade, F. Nitrogen dilution curves and nitrogen use efficiency during winter-spring growth of annual ryegrass. Agron. J. 2004, 96, 601–607. [Google Scholar] [CrossRef]
- Agnusdei, M.G. Rol de la ecofisiología en el diseño de manejos especializados de pasturas. Arch. Latinoam. Prod. Anim. 2013, 21, 63–78. [Google Scholar]
- Keim, J.P.; Anrique, R. Nutritional strategies to improve nitrogen use efficiency by grazing dairy cows. Chil. J. Agric. Res. 2011, 71, 623–633. [Google Scholar] [CrossRef]
- Anrique, R.; Fuchlocher, R.; Iraira, H.; Saldaña, P. Composición Alimentos para el Ganado Bovino; Consorcio lechero, Universidad Austral de Chile: Valdivia, Chile, 2010. [Google Scholar]


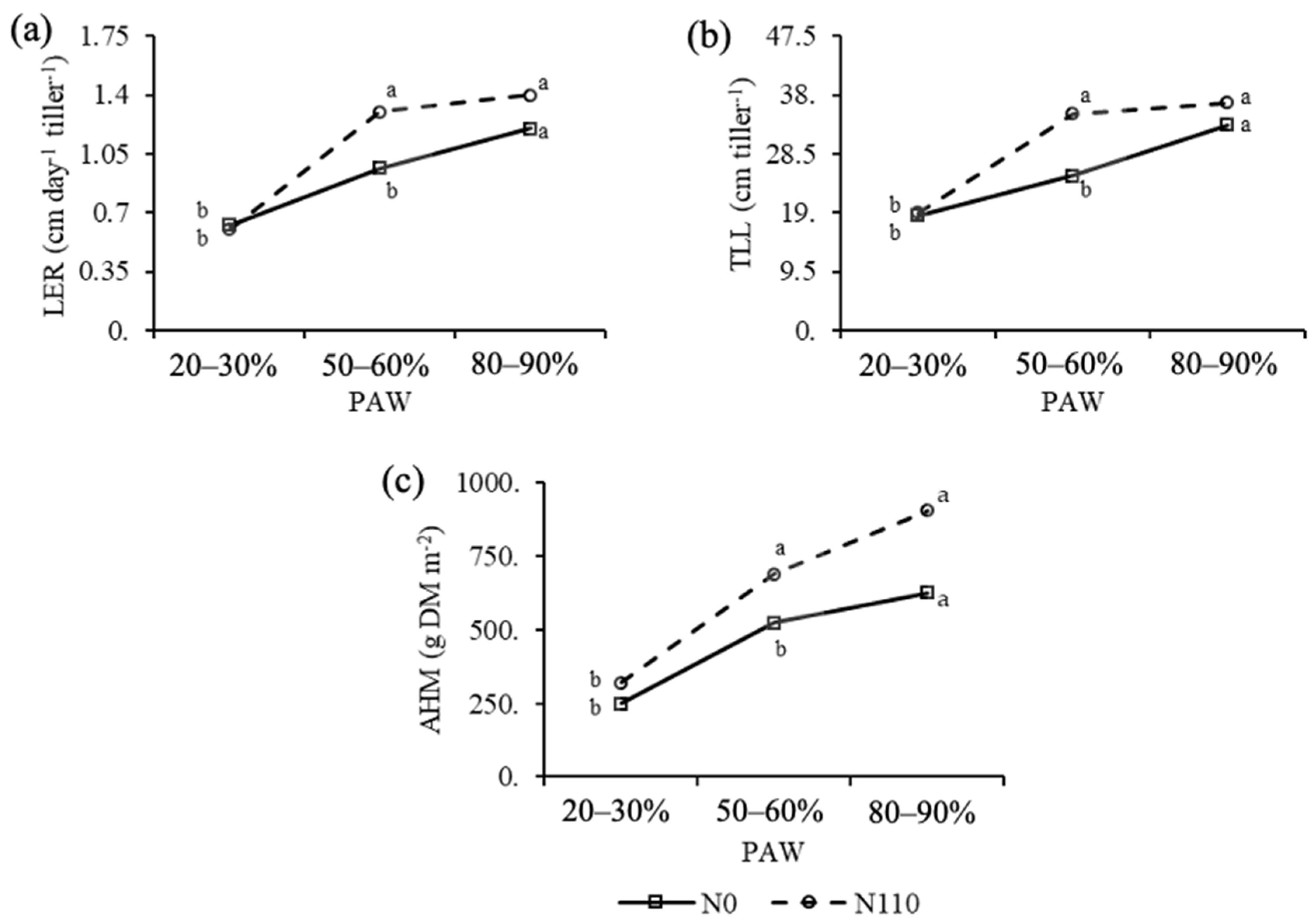
| PAW 1 (%) | LER 4 (cm day−1 tiller−1) | TLL 5 (cm tiller−1) | NLL 6 (No tiller−1) | NDL 7 (No tiller−1) | TM 8 (g tiller−1) | Phy 9 (N° days) | Phy 9 (°C day) | AHM 10 (g DM m−2) |
|---|---|---|---|---|---|---|---|---|
| 20–30 | 0.61 b | 18.76 c | 4.22 b | 0.47 | 0.04 b | 12.53 | 136.61 | 284.41 c |
| 50–60 | 1.15 a | 29.92 b | 4.56 ab | 0.75 | 0.06 b | 11.99 | 129.50 | 607.09 b |
| 80–90 | 1.22 a | 35.50 a | 4.80 a | 0.69 | 0.08 a | 11.61 | 124.41 | 723.49 a |
| Sem 2 | 0.061 | 1.525 | 0.129 | 0.089 | 0.008 | 0.586 | 6.892 | 28.267 |
| p–Value | 0.0001 | 0.0001 | 0.006 | 0.073 | 0.030 | 0.164 | 0.061 | 0.0001 |
| N 3 (kg ha−1) | ||||||||
| 0 | 0.87 b | 24.72 b | 4.38 | 0.63 | 0.05 | 12.24 | 132.61 | 437.31 b |
| 110 | 1.11 a | 30.73 a | 4.65 | 0.64 | 0.07 | 11.88 | 128.22 | 639.08 a |
| sem | 0.050 | 1.243 | 0.101 | 0.073 | 0.007 | 0.481 | 5.619 | 23.082 |
| p–Value | 0.0008 | 0.012 | 0.196 | 0.965 | 0.130 | 0.553 | 0.508 | 0.0001 |
| Interaction | p–Value | |||||||
| PAW × N | 0.042 | 0.047 | 0.170 | 0.931 | 0.914 | 0.339 | 0.259 | 0.0094 |
| PAW 1 (%) | DMC 4 (%) | CP 5 (%) | DV 6 (%) | ME 7 (Mcal kg−1 DM) | ADF 8 (%) | NDF 9 (%) | WSC 10 (g kg−1 DM) |
|---|---|---|---|---|---|---|---|
| 20–30 | 26.86 b | 22.17 a | 70.07 b | 2.56 b | 27.76 a | 56.76 a | 87.05 |
| 50–60 | 23.95 b | 19.25 b | 71.73 a | 2.61 a | 27.03 ab | 54.81 b | 87.27 |
| 80–90 | 22.64 a | 19.83 ab | 72.29 a | 2.63 a | 26.75 b | 53.20 b | 87.06 |
| Sem 2 | 0.051 | 0.596 | 0.232 | 0.009 | 0.254 | 0.281 | 3.467 |
| p–Value | 0.0002 | 0.016 | 0.0001 | 0.0003 | 0.037 | 0.0001 | 0.998 |
| N 3 (kg ha−1) | |||||||
| 0 | 25.18 | 19.92 | 71.01 | 2.59 | 26.92 | 54.90 | 88.48 |
| 110 | 23.79 | 20.9 | 71.72 | 2.60 | 27.44 | 54.89 | 88.77 |
| sem | 0.720 | 0.486 | 0.190 | 0.007 | 0.207 | 0.229 | 2.831 |
| p–Value | 0.1924 | 0.204 | 0.023 | 0.561 | 0.095 | 0.840 | 0.451 |
| Interaction | p–Value | ||||||
| PAW × N | 0.684 | 0.830 | 0.477 | 0.769 | 0.931 | 0.294 | 0.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, I.F.; Rodríguez, A.; Cartmill, A.D.; Dörner, J.; Calvache, I.; Balocchi, O. Effect of Water Restriction and Supplementary Nitrogen on the Growth Dynamics of Bromus valdivianus Phil. Agronomy 2025, 15, 2052. https://doi.org/10.3390/agronomy15092052
López IF, Rodríguez A, Cartmill AD, Dörner J, Calvache I, Balocchi O. Effect of Water Restriction and Supplementary Nitrogen on the Growth Dynamics of Bromus valdivianus Phil. Agronomy. 2025; 15(9):2052. https://doi.org/10.3390/agronomy15092052
Chicago/Turabian StyleLópez, Ignacio F., Armin Rodríguez, Andrew D. Cartmill, José Dörner, Iván Calvache, and Oscar Balocchi. 2025. "Effect of Water Restriction and Supplementary Nitrogen on the Growth Dynamics of Bromus valdivianus Phil." Agronomy 15, no. 9: 2052. https://doi.org/10.3390/agronomy15092052
APA StyleLópez, I. F., Rodríguez, A., Cartmill, A. D., Dörner, J., Calvache, I., & Balocchi, O. (2025). Effect of Water Restriction and Supplementary Nitrogen on the Growth Dynamics of Bromus valdivianus Phil. Agronomy, 15(9), 2052. https://doi.org/10.3390/agronomy15092052









