Impact of Organic and Conventional Agricultural Management on Subsurface Soil Microbiota in Mediterranean Vineyards
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Study
2.2. Soil Sample Collection
2.3. Physicochemical Assessment of Soil Samples
2.4. Microbial Community Assessment: Microbial Population Abundance
2.5. Microbial Community Assessment: 16S rRNA-Metabarcoding and Bioinformatics
2.6. Statistical Analysis
3. Results and Discussion
3.1. Impact of Crop Management on the Subsurface Soil Physicochemical Soil Parameters
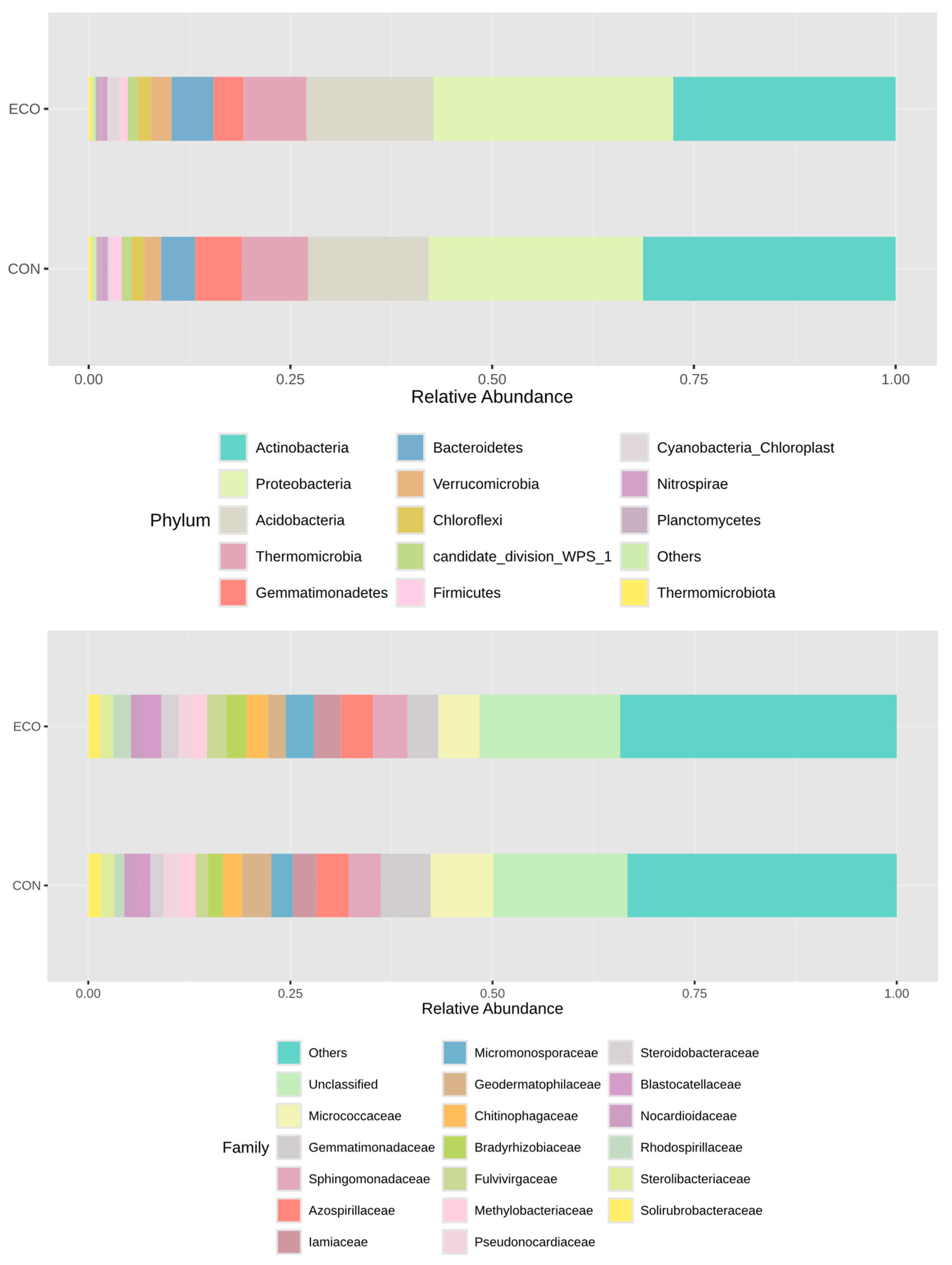
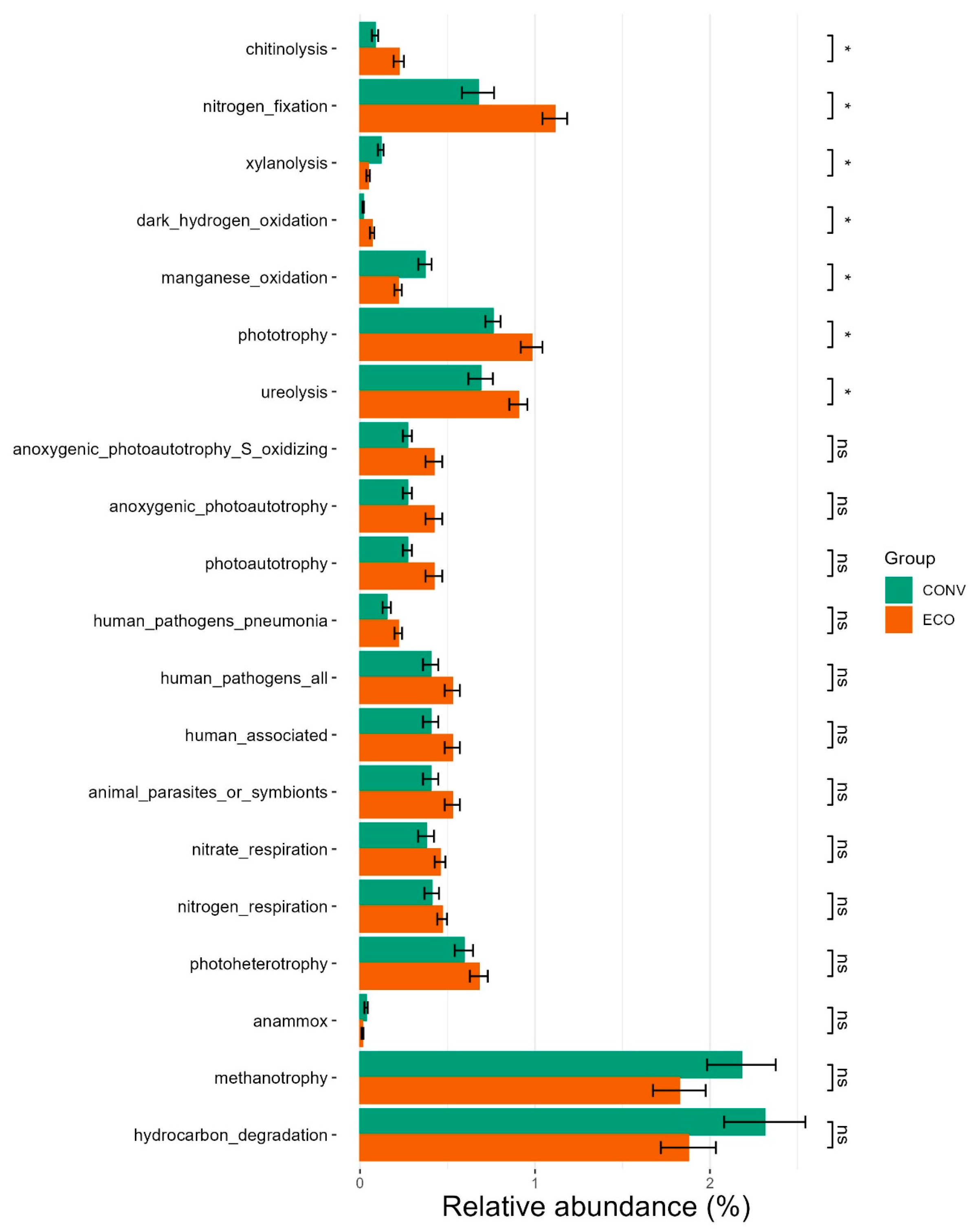
3.2. Impact of Crop Management on the Subsurface Soil Microbiota
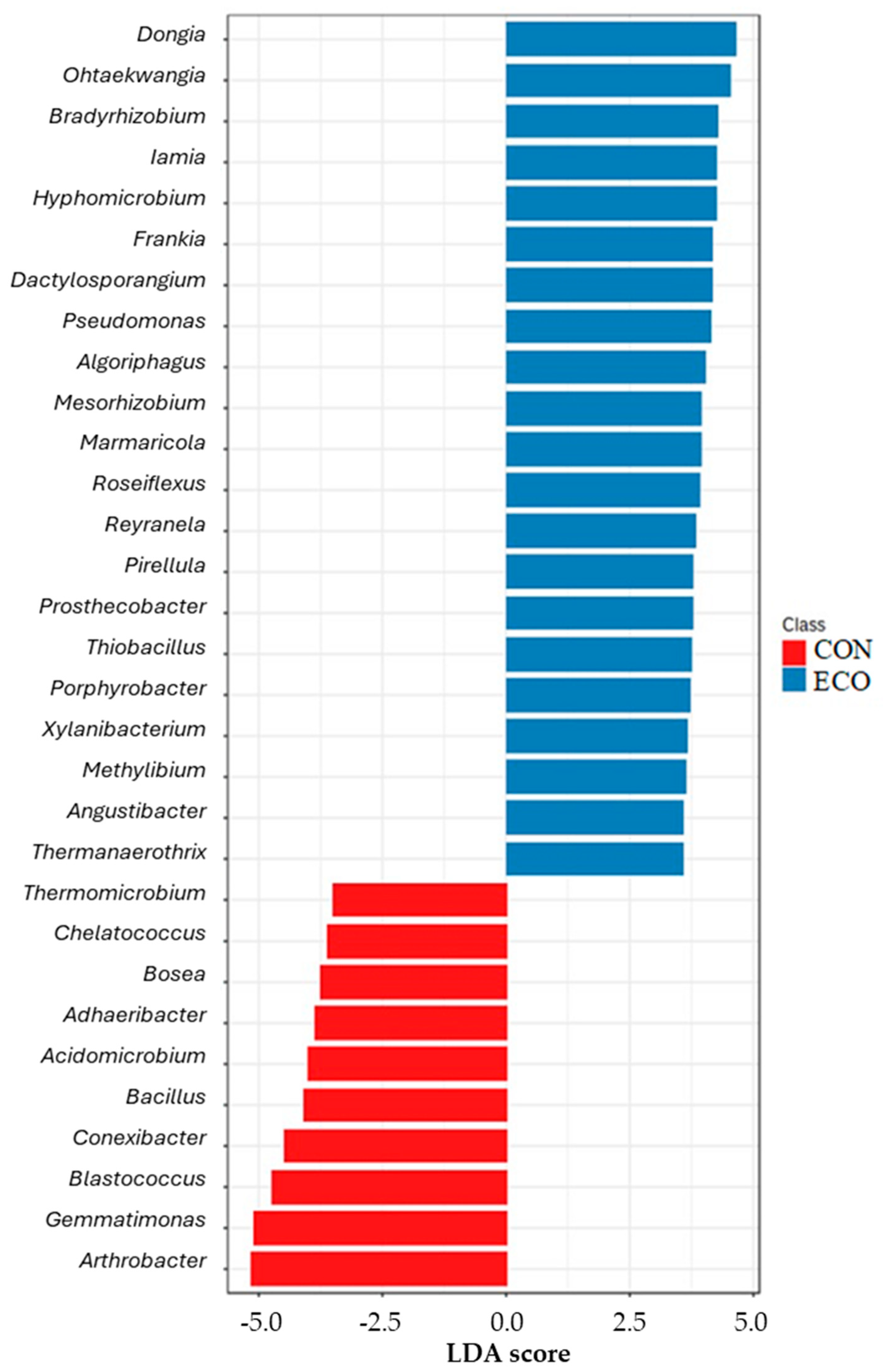
3.3. Main Drivers That Impact Subsurface Soil Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M.A.; Domingo, N.G.G.; Colgan, K.; Thakrar, S.K.; Tilman, D.; Lynch, J.; Azevedo, I.L.; Hill, J.D. Global food system emissions could preclude achieving the 1.5° and 2 °C climate change targets. Science 2020, 370, 705–708. [Google Scholar] [CrossRef]
- FAO. The 10 Elements of Agroecology; Guiding the Transition to Sustainable Food and Agricultural Systems; FAO: Rome, Italy, 2018. [Google Scholar]
- Novara, A.; Stallone, G.; Cerdà, A.; Gristina, L. The Effect of Shallow Tillage on Soil Erosion in a Semi-Arid Vineyard. Agronomy 2019, 9, 257. [Google Scholar] [CrossRef]
- Mrabet, R.; Savé, R.; Toreti, A.; Caiola, N.; Chentouf, M.; Llasat, M.C.; Mohamed, A.A.A.; Santeramo, F.G.; Sanz-Cobena, A.; Tsikliras, A. First Mediterranean Assessment Report—Chapter 3.2: Resources–Food. In Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future; MedECC: Marseille, France, 2020; pp. 237–264. [Google Scholar] [CrossRef]
- OIV. General Assembly of the International Organisation of Vine and Wine; Resolution OIV/VITI 333/2010; OIV: Paris, France, 2010; Available online: https://www.oiv.int/public/medias/379/viti-2010-1-en.pdf (accessed on 1 March 2025).
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Vaudour, E.; Costantini, E.; Jones, G.; Mocali, S. An overview of the recent approaches to terroir functional modelling, footprinting and zoning. Soil 2015, 1, 287–312. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Roby, J.P.; De Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Gil, J.; Alter, E.; La Rota, M.J.; Tello, E.; Galletto, V.; Padró, R.; Martínez, T.; Darnay, S.; Marull, J. Towards an agroecological transition in the Mediterranean: A bioeconomic assessment of viticulture farming. J. Clean. Prod. 2022, 380, 134999. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- OIV. Resolution OIV-VITI 655-2021. OIV Recommendations About Valuation and Importance of Microbial Biodiversity in a Sustainable Vitiviniculture Context. 2021. Available online: https://www.oiv.int/standards/oiv-recommendations-about-valuation-and-importance-of-microbial-biodiversity-in-a-sustainable-vitiviniculture-context (accessed on 1 March 2025).
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Sánchez, R.; Castañeda, L.E.; Godoy, K.; Barbosa, O. Is microbial terroir related to geographic distance between vineyards? Environ. Microbiol. Rep. 2017, 9, 742–749. [Google Scholar] [CrossRef]
- Burns, K.N.; Bokulich, N.A.; Cantu, D.; Greenhut, R.F.; Kluepfel, D.A.; O’Geen, A.T.; Strauss, S.L.; Steenwerth, K.L. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by vineyard management. Soil Biol. Biochem. 2016, 103, 337–348. [Google Scholar] [CrossRef]
- Longa, C.M.O.; Nicola, L.; Antonielli, L.; Mescalchin, E.; Zanzotti, R.; Turco, E.; Pertot, I. Soil microbiota respond to green manure in organic vineyards. J. Appl. Microbiol. 2017, 123, 1547–1560. [Google Scholar] [CrossRef]
- Unc, A.; Eshel, G.; Unc, G.A.; Doniger, T.; Sherman, C.; Leikin, M.; Steinberger, Y. Vineyard soil microbial community under conventional, sustainable and organic management practices in a Mediterranean climate. Soil Res. 2020, 59, 253–265. [Google Scholar] [CrossRef]
- Salome, C.; Nunan, N.; Pouteau, V.; Lerch, T.Z.; Chenu, C. Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob. Change Biol. 2010, 16, 416–426. [Google Scholar] [CrossRef]
- Hobley, E.; Baldock, J.; Hua, Q.; Wilson, B. Land-use contrasts reveal instability of subsoil organic carbon. Glob. Change Biol. 2017, 23, 955–965. [Google Scholar] [CrossRef]
- Funes, I.; Savé, R.; Rovira, P.; Molowny-Horas, R.; Alcañiz, J.M.; Ascaso, E.; Herms, I.; Herrero, C.; Boixadera, J.; Vayreda, J. Agricultural soil organic carbon stocks in the north-eastern Iberian Peninsula: Drivers and spatial variability. Sci. Total Environ. 2019, 668, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Steenwerth, K.; Drenovsky, R.; Lambert, J.-J.; Kluepfel, D.; Scow, K.; Smart, D. Soil morphology, depth and grapevine root frequency influence microbial communities in a Pinot noir vineyard. Soil Biol. Biochem. 2008, 40, 1330–1340. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Liu, J.; Liu, J.; Han, L.; Wang, X.; Liu, H.; Xu, M.; Yang, G.; Ren, C. The contribution of microbial necromass carbon to soil organic carbon in soil aggregates. Appl. Soil Ecol. 2023, 190, 104985. [Google Scholar] [CrossRef]
- Rotchés-Ribalta, R.; Marull, J.; Pino, J. Organic farming increases functional diversity and ecosystem service provision of spontaneous vegetation in Mediterranean vineyards. Ecol. Indic. 2023, 147, 110023. [Google Scholar] [CrossRef]
- ISO 14235:1998; Soil Quality—Determination of Organic Carbon in Soil by Sulfochromic Oxidation. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 11261:1995; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO/TS 14256-1:2003; Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field-Moist Soils by Extraction with Potassium Chloride Solution—Part 1: Manual Method. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 11263:1994; Soil Quality—Determination of Phosphorus Soluble in Sodium Hydrogen Carbonate Solution. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. International Organization for Standardization: Geneva, Switzerland, 1994.
- Prenafeta-Boldú, F.X.; Guivernau, M.; Gallastegui, G.; Viñas, M.; de Hoog, G.S.; Elías, A. Fungal/bacterial interactions during the biodegradation of TEX hydrocarbons (toluene, ethylbenzene and p-xylene) in gas biofilters operated under xerophilic conditions. FEMS Microbiol. Ecol. 2012, 80, 722–734. [Google Scholar] [CrossRef]
- Pelissari, C.; Guivernau, M.; Viñas, M.; García, J.; Velasco-Galilea, M.; Souza, S.S.; Sezerino, P.H.; Ávila, C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018, 141, 185–195. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Kern, J.; Johnson, M. Conservation tillage impacts on national soil and atmospheric carbon levels. Soil Sci. Soc. Am. J. 1993, 57, 200–210. [Google Scholar] [CrossRef]
- Preece, C.; Peñuelas, J. A Return to the Wild: Root Exudates and Food Security. Trends Plant Sci. 2020, 25, 14–21. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root exudates induce soil macroaggregation facilitated by fungi in subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Fleishman, S.M.; Bock, H.W.; Eissenstat, D.M.; Centinari, M. Undervine groundcover substantially increases shallow but not deep soil carbon in a temperate vineyard. Agric. Ecosyst. Environ 2021, 313, 107362. [Google Scholar] [CrossRef]
- Mayer, M.; Leifeld, J.; Szidat, S.; Mäder, P.; Krause, H.-M.; Steffens, M. Dynamic stability of mineral-associated organic matter: Enhanced stability and turnover through organic fertilization in a temperate agricultural topsoil. Soil Biol. Biochem. 2023, 184, 109095. [Google Scholar] [CrossRef]
- Preece, C.; Farré-Armengol, G.; Peñuelas, J. Drought is a stronger driver of soil respiration and microbial communities than nitrogen or phosphorus addition in two Mediterranean tree species. Sci. Total. Environ. 2020, 735, 139554. [Google Scholar] [CrossRef]
- Paula, F.S.; Tatti, E.; Thorn, C.; Abram, F.; Wilson, J.; O’Flaherty, V. Soil prokaryotic community resilience, fungal colonisation and increased cross-domain co-occurrence in response to a plant-growth enhancing organic amendment. Soil Biol. Biochem. 2020, 149, 107937. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A global microbiome survey of vineyard soils highlights the microbial dimension of viticultural terroirs. Commun. Biol. 2022, 5, 241. [Google Scholar] [CrossRef]
- Wuchter, C.; Abbas, B.; Coolen, M.J.; Herfort, L.; Van Bleijswijk, J.; Timmers, P.; Strous, M.; Teira, E.; Herndl, G.J.; Middelburg, J.J. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. USA 2006, 103, 12317–12322. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 2017, 113, 161–172. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nicol, G.W.; Neufeld, J.D. Differential responses of soil ammonia-oxidizing archaea and bacteria to temperature and depth under two different land uses. Soil Biol. Biochem. 2018, 120, 272–282. [Google Scholar] [CrossRef]
- Sainju, U.M.; Schomberg, H.H.; Singh, B.P.; Whitehead, W.F.; Tillman, P.G.; Lachnicht-Weyers, S.L. Cover crop effect on soil carbon fractions under conservation tillage cotton. Soil Tillage Res. 2007, 96, 205–218. [Google Scholar] [CrossRef]
- Darriaut, R.; Antonielli, L.; Martins, G.; Ballestra, P.; Vivin, P.; Marguerit, E.; Mitter, B.; Masneuf-Pomarède, I.; Compant, S.; Ollat, N. Soil composition and rootstock genotype drive the root associated microbial communities in young grapevines. Front. Microbiol. 2022, 13, 1031064. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.R.; Männistö, M.K.; Bromberg, Y.; Häggblom, M.M. Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol. Ecol. 2012, 82, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Mohanty, S.; Guru, P.K.; Swain, C.K.; Tripathi, R.; Shahid, M.; Kumar, U.; Kumar, A.; Bhattacharyya, P.; Gautam, P.; et al. Comparative assessment of urea briquette applicators on greenhouse gas emission, nitrogen loss and soil enzymatic activities in tropical lowland rice. Agric. Ecosyst. Environ. 2018, 252, 178–190. [Google Scholar] [CrossRef]
- van Wyk, D.A.B.; Adeleke, R.; Rhode, O.H.J.; Bezuidenhout, C.C.; Mienie, C. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in North West Province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [CrossRef]
- Michas, A.; Pastore, G.; Chiba, A.; Grafe, M.; Clausing, S.; Polle, A.; Schloter, M.; Spohn, M.; Schulz, S. Phosphorus Availability Alters the Effect of Tree Girdling on the Diversity of Phosphorus Solubilizing Soil Bacterial Communities in Temperate Beech Forests. Front. For. Glob. Change 2021, 4, 696983. [Google Scholar] [CrossRef]
- Fu, L.; Wu, X.; Dini-Andreote, F.; Wang, B.; Tao, C.; Ruan, Y.; Shen, Z.; Li, R.; Shen, Q. Changes in bulk soil affect the disease-suppressive rhizosphere microbiome against Fusarium wilt disease. Front. Agric. Sci. Eng. 2020, 7, 307–316. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Wentzien, N.M.; Zorrilla-Fontanesi, Y.; Valverde-Corredor, A.; Fernández-González, A.J.; Fernández-López, M.; Mercado-Blanco, J. Impacts of the Biocontrol Strain Pseudomonas simiae PICF7 on the Banana Holobiont: Alteration of Root Microbial Co-occurrence Networks and Effect on Host Defense Responses. Front. Microbiol. 2022, 13, 809126. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Minamisawa, K.; Uchiumi, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Iriguchi, M.; Kawashima, K.; et al. Complete Genomic Sequence of Nitrogen-fixing Symbiotic Bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002, 9, 189–197. [Google Scholar] [CrossRef]
- Liu, C.; Yan, J.; Huang, Q.; Liu, H.; Qiao, C.; Li, R.; Shen, B.; Shen, Q. The addition of sawdust reduced the emission of nitrous oxide in pig manure composting by altering the bacterial community structure and functions. Environ. Sci. Pollut. Res. 2022, 29, 3733–3742. [Google Scholar] [CrossRef]
- Hol, W.H.G.; Bezemer, T.M.; Biere, A. Getting the ecology into interactions between plants and the plant growth-promoting bacterium Pseudomonas fluorescens. Front. Plant Sci. 2013, 4, 81. [Google Scholar] [CrossRef]
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef]
- Benítez, M.-S.; Tustas, F.B.; Rotenberg, D.; Kleinhenz, M.D.; Cardina, J.; Stinner, D.; Miller, S.A.; McSpadden Gardener, B.B. Multiple statistical approaches of community fingerprint data reveal bacterial populations associated with general disease suppression arising from the application of different organic field management strategies. Soil Biol. Biochem. 2007, 39, 2289–2301. [Google Scholar] [CrossRef]
- Arias-Giraldo, L.F.; Guzmán, G.; Montes-Borrego, M.; Gramaje, D.; Gómez, J.A.; Landa, B.B. Going Beyond Soil Conservation with the Use of Cover Crops in Mediterranean Sloping Olive Orchards. Agronomy 2021, 11, 1387. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Martínez-Hidalgo, P.; Cobo-Díaz, J.F.; Villadas, P.J.; Martínez-Molina, E.; Toro, N.; Tringe, S.G.; Fernández-López, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 6008. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Roudet, J.; Aveline, N.; Davidou, L.; Dupin, S.; Fermaud, M. Microbial Antagonism Toward Botrytis Bunch Rot of Grapes in Multiple Field Tests Using One Bacillus ginsengihumi Strain and Formulated Biological Control Products. Front. Plant Sci. 2019, 10, 105. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Baril, X.; Constant, P. Carbon amendments in soil microcosms induce uneven response on H2 oxidation activity and microbial community composition. FEMS Microbiol. Ecol. 2023, 99, fiad159. [Google Scholar] [CrossRef]
- Liu, B.; Hou, L.; Zheng, Y.; Zhang, Z.; Tang, X.; Mao, T.; Du, J.; Bi, Q.; Dong, H.; Yin, G.; et al. Dark carbon fixation in intertidal sediments: Controlling factors and driving microorganisms. Water Res. 2022, 216, 118381. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.F.; Nouioui, I.; Sangal, V.; Trujillo, M.E.; Montero-Calasanz Md, C.; Rahmani, T.; Bull, A.T.; Asenjo, J.A.; Andrews, B.A.; Goodfellow, M. Geodermatophilus chilensis sp. nov., from soil of the Yungay core-region of the Atacama Desert, Chile. Syst. Appl. Microbiol. 2018, 41, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cheng, S.; Fang, H.; Xu, M.; Yang, Y.; Li, Y.; Zhang, J.; Müller, C. Organic nitrogen addition causes decoupling of microbial nitrogen cycles by stimulating gross nitrogen transformation in a temperate forest soil. Geoderma 2021, 385, 114886. [Google Scholar] [CrossRef]
- Matschullat, J.; Reimann, C.; Birke, M.; dos Santos Carvalho, D.; Albanese, S.; Anderson, M.; Baritz, R.; Batista, M.J.; Bel-Ian, A.; Cicchella, D.; et al. GEMAS: CNS concentrations and C/N ratios in European agricultural soil. Sci. Total Environ. 2018, 627, 975–984. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Bååth, E. Interaction between pH and Cu toxicity on fungal and bacterial performance in soil. Soil Biol. Biochem. 2016, 96, 20–29. [Google Scholar] [CrossRef]
- Signorini, M.; Borruso, L.; Randall, K.C.; Dumbrell, A.J.; Pii, Y.; Mimmo, T.; Cesco, S. Soil heterogeneity within a vineyard impacts the beta but not the alpha microbial agro-diversity. Appl. Soil Ecol. 2021, 166, 104088. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Shinfuku, M.; Junier, P.; Poirier, S.; Verrecchia, E.; Sebag, D.; DeAngelis, K.M. Direct evidence for the role of microbial community composition in the formation of soil organic matter composition and persistence. ISME Commun. 2021, 1, 64. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.L.; Jastrow, J.D.; Grimley, D.A.; Gonzalez-Meler, M.A. Edaphic controls on soil organic carbon stocks in restored grasslands. Geoderma 2015, 251–252, 117–123. [Google Scholar] [CrossRef]
- Seaton, F.M.; George, P.B.L.; Lebron, I.; Jones, D.L.; Creer, S.; Robinson, D.A. Soil textural heterogeneity impacts bacterial but not fungal diversity. Soil Biol. Biochem. 2020, 144, 107766. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]



| Characteristics and Practices | Plots | |||||
|---|---|---|---|---|---|---|
| CON 1 | CON 2 | CON 3 | ECO 3 | ECO 8 | ECO 12 | |
| Surface (ha) | 2.5 | 0.99 | 2.12 | 1.8 | 1.14 | 0.67 |
| Age (years) | 25 | 25 | 11 | 14 | 26 | 33 |
| Variety | Xarel·lo | Macabeu | Xarel·lo | Xarel·lo | Macabeu | Xarel·lo |
| Rootstock | 110R | 110R | SO4 | 110R | 110R | 161-49C |
| Vine spacing (m) | 2.8 × 1.2 | 3 × 1.4 | 2.8 × 1.2 | 2.6 × 1.20 | 3 × 1.2 | 2.6 × 1.2 |
| Training system | Doble cordon | Doble cordon | Doble cordon | Single cordon | Doble cordon | Gobelet |
| Continued management (years) | 25 | 25 | 11 | 10 | 16 | 10 |
| Yield (kg·ha−1) | 6548 | 8768 | 8697 | 8716 | 8079 | 9194 |
| Tillage | Always | Always | Always | Combined with mowing or grazing | Combined with mowing or grazing | Combined with mowing or grazing |
| Cover crop | No | No | No | Temporary (spontaneous) [31] | Temporary (spontaneous) [31] | Temporary (spontaneous) [31] |
| Fertilizer 1 | Organo-mineral Fertilizer (4-6-10) 2 13 kg N ha−1 year−1 8.5 kg P ha−1 year−1 27 kg K ha−1 year−1 | Organo-mineral Fertilizer (4-6-10) 2 13 kg N ha−1 year−1 8.5 kg P ha−1 year−1 27 kg K ha−1 year−1. | Organo-mineral Fertilizer (4-6-10) 2 13 kg N ha−1 year−1 8.5 kg P ha−1 year−1 27 kg K ha−1 year−1 | Composted cow manure and biodynamic amendments 10 kg N ha−1 year−1 10 kg P ha−1 year−1 20 kg K ha−1 year−1 | Composted cow manure and biodynamic amendments 10 kg N ha−1 year−1 10 kg P ha−1 year−1 20 kg K ha−1 year−1 | Composted cow manure and biodynamic amendments 10 kg N ha−1 year−1 10 kg P ha−1 year−1 20 kg K ha−1 year−1 |
| Plot | Soil Texture | ||||
|---|---|---|---|---|---|
| Soil Texture (USDA) | Clay (%) | Silt (%) | Sand (%) | ||
| Conventional | CON_1 | Loam | 17.82 ± 1.42 | 41.66 ± 4.63 | 40.52 ± 5.70 |
| CON_2 | Loam | 20.76 ± 1.40 | 38.94 ± 3.44 | 40.30 ± 4.57 | |
| CON_3 | Sandy Loam | 13.98 ± 2.29 | 29.00 ± 4.90 | 57.02 ± 7.10 | |
| Organic | ECO_3 | Loam | 18.96 ± 2.45 | 36.88 ± 1.79 | 44.16 ± 4.22 |
| ECO_8 | Silty Clay Loam | 28.32 ± 1.64 | 49.44 ± 4.01 | 22.24 ± 5.21 | |
| ECO 12 | Clay Loam | 25.10 ± 2.74 | 39.38 ± 3.87 | 35.52 ± 5.87 | |
| Parameters | CON (n = 15) Estimate ± SE | ECO (n = 15) Estimate ± SE | F-Statistic | p-Value | Mantel Test Statistic | Mantel Test (p-Values) |
|---|---|---|---|---|---|---|
| Clay (%) | 17.50 ± 1.42 a | 24.10 ± 2.01 b | 10.770 | 0.003 | 0.405 | 0.000 |
| Ca2+/Mg2+ | 46.44 ± 3.59 a | 26.42 ± 5.07 b | 15.570 | 0.000 | 0.371 | 0.000 |
| EC (dS/m) | 0.15 ± 0.00 a | 0.16 ± 0.00 b | 5.271 | 0.029 | 0.368 | 0.001 |
| Sand (%) | 45.90 ± 3.72 a | 34.00 ± 5.26 b | 5.190 | 0.031 | 0.353 | 0.000 |
| K+ (mg/kg) | 153.53 ± 21.48 a | 381.00 ± 30.38 b | 5.890 | 0.022 | 0.264 | 0.005 |
| Mg2+ (mg/kg) | 149.53 ± 35.53 a | 342.00 ± 50.25 b | 14.680 | 0.001 | 0.239 | 0.020 |
| SOC Ratio (5–20)/(20–40) | 0.99 ± 0.09 a | 1.61 ± 0.13 b | 22.200 | 0.000 | 0.216 | 0.001 |
| Ca2+/K+ | 57.1 ± 6.82 a | 33.00 ± 9.64 b | 6.246 | 0.019 | 0.209 | 0.002 |
| OOM Ratio (5–20)/(20–40) | 1.07 ± 0.08 a | 1.64 ± 0.12 b | 23.760 | 0.000 | 0.201 | 0.007 |
| C/N | 8.33 ± 0.42 a | 5.59 ± 0.59 b | 21.850 | 0.000 | 0.181 | 0.008 |
| OOM 20–40 cm (%) | 1.04 ± 0.11 a | 0.73 ± 0.11 b | 4.574 | 0.041 | 0.149 | 0.045 |
| Diff SOC (%) (top-sub) | −0.04 ± 0.04 a | 0.24 ± 0.05 b | 31.090 | 0.000 | 0.141 | 0.051 |
| Diff OOM (%) (top-sub) | −0.02 ± 0.06 a | 0.44 ± 0.07 b | 31.100 | 0.000 | 0.141 | 0.039 |
| Na (mg/kg) | 16.30 ± 1.66 a | 23.10 ± 2.35 b | 8.378 | 0.007 | 0.140 | 0.111 |
| Fe (mg/kg) | 74.50 ± 5.24 a | 35.10 ± 7.41 b | 28.150 | 0.000 | 0.121 | 0.114 |
| SOC 20–40 cm (%) | 0.61 ± 0.09 a | 0.42 ± 0.06 b | 4.625 | 0.040 | 0.118 | 0.099 |
| NO3− (mg/kg) | 3.67 ± 0.46 a | 2.29 ± 0.57 b | 5.474 | 0.027 | 0.096 | 0.194 |
| Cu (mg/kg) | 19.40 ± 2.07 a | 12.50 ± 2.93 b | 5.612 | 0.025 | 0.092 | 0.134 |
| Zn (mg/kg) | 3.47 ± 0.30 a | 2.47 ± 0.39 b | 6.275 | 0.018 | 0.045 | 0.270 |
| NH4+ (mg/kg) | 4.24 ± 0.30 a | 5.90 ± 0.42 b | 15.710 | 0.000 | −0.019 | 0.565 |
| S (mg/kg) | 352.45 ± 58.33 a | 186.00 ± 82.49 a | 4.054 | 0.054 | 0.140 | 0.117 |
| SO4 (mg/kg) | 22.13 ± 5.43 a | 37.20 ± 7.69 a | 3.843 | 0.060 | 0.204 | 0.038 |
| Silt (%) | 36.50 ± 2.54 a | 41.90 ± 3.60 a | 2.225 | 0.147 | 0.405 | 0.000 |
| Mo (mg/kg) | 0.10 ± 0.00 a | 0.11 ± 0.01 a | 1.312 | 0.262 | 0.157 | 0.051 |
| Mg2+/K+ | 1.26 ± 0.21 a | 1.55 ± 0.29 a | 1.021 | 0.321 | 0.297 | 0.002 |
| Ca2+ (mg/kg) | 6591.00 ± 105.92 a | 6451.00 ± 149.80 a | 0.868 | 0.360 | 0.232 | 0.002 |
| P Olsen (mg/kg) | 13.95 ± 1.62 a | 14.97 ± 2.29 a | 0.198 | 0.660 | 0.186 | 0.009 |
| Mn (mg/kg) | 83.70 ± 10.10 a | 85.40 ± 14.28 a | 0.015 | 0.904 | 0.128 | 0.070 |
| pH | 8.43 ± 0.02 a | 8.43 ± 0.03 a | 0.000 | 1.000 | 0.264 | 0.118 |
| N-Kjeldahl (%) | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.256 | 0.617 | 0.067 | 0.2291 |
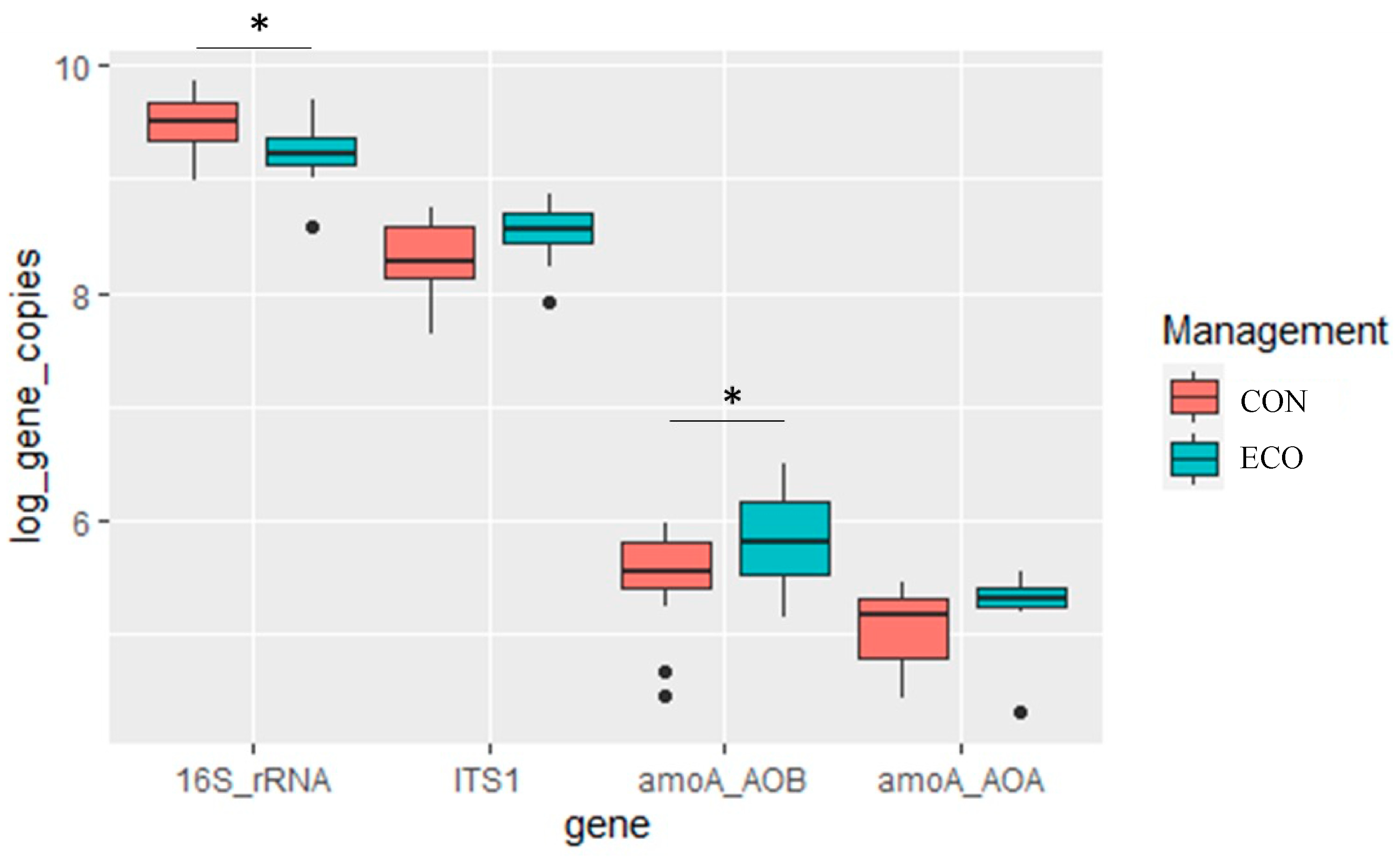
| EUB16S rRNA (log10 gene copies/g) | 9.48 ± 0.07 a | 9.24 ± 0.08 b | 5.6863 | 0.024 | 0.136 | 0.080 |
| FUNG ITS (log10 gene copies/g) | 8.33 ± 0.08 a | 8.54 ± 0.07 a | 3.9513 | 0.057 | 0.198 | 0.007 |
| ITS/16S rRNA | 0.09 ± 0.02 a | 0.21 ± 0.02 b | 11.582 | <0.001 | 0.085 | 0.214 |
| AOB (log amoA gene copies/g) | 5.47 ± 0.14 a | 5.84 ± 0.09 b | 5.0963 | 0.041 | 0.182 | 0.033 |
| AOA (log amoA gene copies/g) | 5.06 ± 0.10 a | 5.27 ± 0.08 a | 3.0582 | 0.080 | 0.149 | 0.046 |
| AOB/AOA | 3.03 ± 0.49 a | 4.72 ± 0.95 a | 1.3642 | 0.243 | 0.113 | 0.145 |
| Indexes | Mean ECO (n = 14) | Mean CON (n = 15) | Mann–Whitney (ECO vs. CON p-Value) | Statistic (U) |
|---|---|---|---|---|
| Reads (contigs) | 15,693 ± 3051 | 15,059 ± 6125 | 0.918 | 102 |
| Coverage | 0.899 ± 0.008 | 0.898 ± 0.003 | 0.913 | 102 |
| Sobs (Richness) | 1810 ± 132 | 1789 ± 65 | 0.285 | 80 |
| Chao1 (Richness) | 1845 ± 119 | 1790 ± 103 | 0.1225 | 94 |
| Shannon (H) (Diversity) | 6.80 ± 0.27 a | 6.76 ± 0.06 b | * 0.029 | 62 |
| Inverse Simpson (Diversity) | 489.2 ± 88.1 a | 390.3 ± 167.9 b | * 0.012 | 50 |
| Parameter | R | p-Value |
|---|---|---|
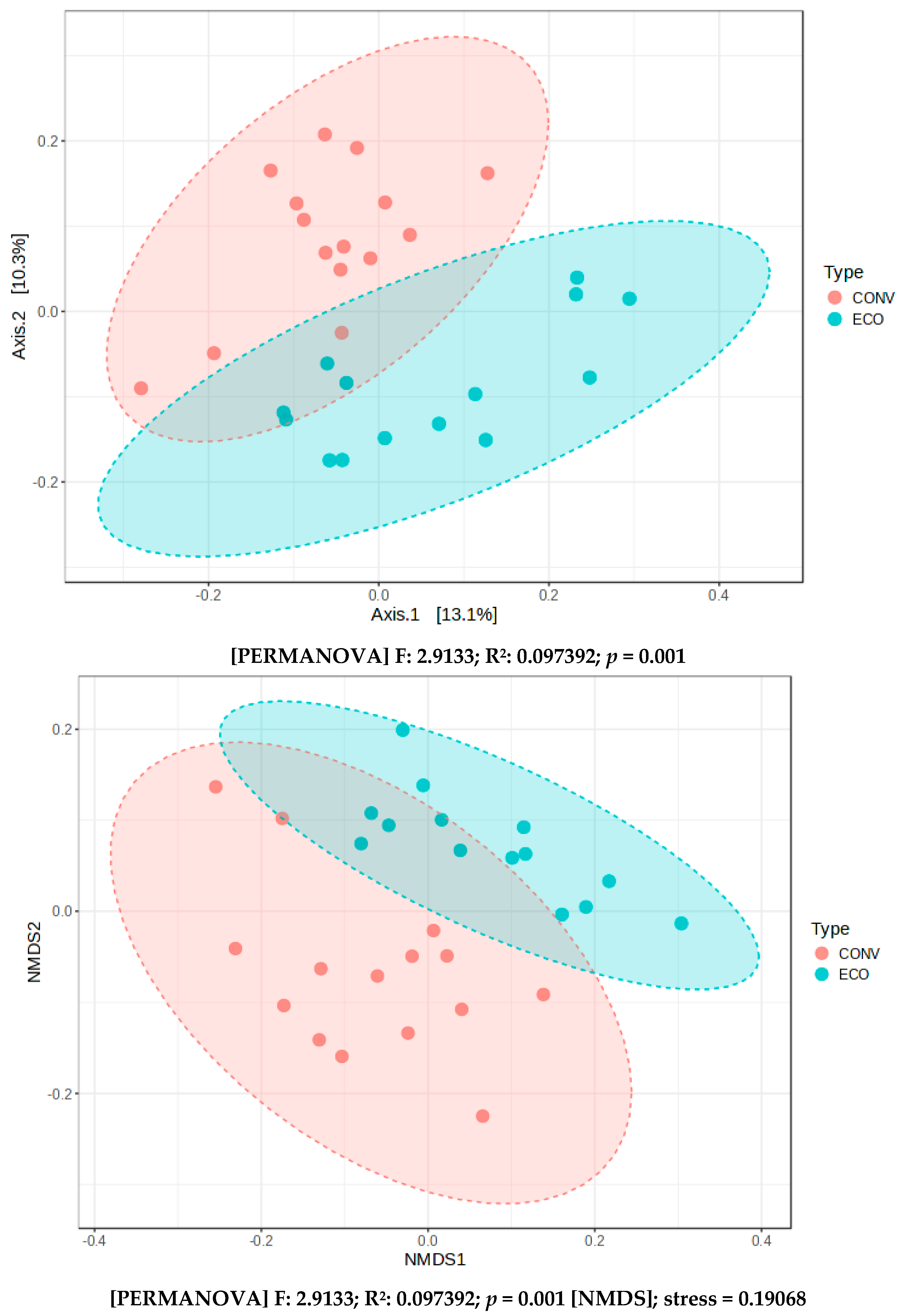
| Management (CON vs. ECO) | 0.3603 | 0.0001 |
| Plot (all) | 0.5483 | 0.0001 |
| Plot in CON | 0.448 | 0.0001 |
| Plot in ECO | 0.4651 | 0.0006 |
| Variety (all) | −0.0748 | 0.8216 |
| Variety in ECO | −0.04541 | 0.5687 |
| Variety in CON | 0.1047 | 0.2114 |
| Soil Texture | 0.3419 | 0.0022 |
| Rootstock | 0.3022 | 0.0061 |
| Training system | 0.2316 | 0.0356 |
| CON (n = 15) | ECO (n = 14) | |
|---|---|---|
| Average path length | 1.93 | 2.01 |
| Network diameter | 5 | 5 |
| Global clustering coefficient | 0.712 | 0.535 |
| Average local clustering coefficient | 0.725 | 0.553 |
| Modularity (Louvain) | 0.172 | 0.331 |
| Parameter | Statistic | log10 16S rRNA gene Copies (n = 30) | log10 ITS Copies (n = 30) | Ratio ITS/16S rRNA (n = 30) | Sobs Richness (n = 29) | Chao1 Richness (n = 29) | Shannon (n = 29) | Inv. Simpson (n = 29) |
|---|---|---|---|---|---|---|---|---|
| Clay (%) | p-adjusted (FDR) | 0.338 | 0.662 | 0.156 | 0.866 | 0.831 | 0.527 | 0.409 |
| [rs] | −0.257 | 0.132 | 0.349 | 0.058 | −0.070 | 0.178 | 0.224 | |
| Ca2+/Mg2+ | p-adjusted (FDR) | 0.108 | 0.647 | 0.071 | 0.859 | 0.893 | 0.701 | 0.376 |
| [rs] | 0.389 | −0.138 | −0.426 | −0.061 | 0.047 | −0.116 | −0.240 | |
| EC (dS/m) | p-adjusted (FDR) | 0.670 | 0.845 | 0.732 | 0.739 | 0.351 | 0.968 | 0.856 |
| [rs] | −0.129 | −0.066 | 0.104 | −0.100 | −0.251 | −0.016 | 0.062 | |
| Sand (%) | p-adjusted (FDR) | 0.341 | 0.986 | 0.422 | 0.963 | 0.733 | 0.808 | 0.701 |
| [rs] | 0.255 | −0.008 | −0.217 | −0.018 | 0.102 | −0.078 | −0.116 | |
| K+ (mg/kg) | p-adjusted (FDR) | 0.739 | 0.358 | 0.408 | 0.378 | 0.806 | 0.110 | 0.129 |
| [rs] | −0.100 | 0.248 | 0.225 | 0.239 | 0.079 | 0.387 | 0.371 | |
| Mg2+ (mg/kg) | p-adjusted (FDR) | 0.128 | 0.682 | 0.090 | 0.902 | 0.824 | 0.732 | 0.389 |
| [rs] | −0.372 | 0.125 | 0.405 | 0.041 | −0.073 | 0.105 | 0.235 | |
| Ca2+/K+ | p-adjusted (FDR) | 0.603 | 0.410 | 0.339 | 0.365 | 0.814 | 0.099 | 0.114 |
| [rs] | 0.150 | −0.223 | −0.256 | −0.245 | −0.076 | −0.397 | −0.384 | |
| C/N | p-adjusted (FDR) | 0.041 | 0.667 | 0.013 | 0.701 | 0.491 | 1.000 | 0.318 |
| [rs] | 0.466 | −0.130 | −0.541 | 0.116 | 0.190 | −0.001 | −0.267 | |
| OOM 20–40 cm (%) | p-adjusted (FDR) | 0.129 | 0.893 | 0.220 | 0.144 | 0.121 | 0.162 | 0.794 |
| [rs] | 0.370 | 0.047 | −0.312 | 0.357 | 0.378 | 0.344 | 0.083 | |
| Na (mg/kg) | p-adjusted (FDR) | 0.230 | 0.435 | 0.050 | 0.597 | 0.351 | 0.900 | 0.875 |
| [rs] | −0.307 | 0.210 | 0.453 | −0.153 | −0.252 | −0.042 | 0.055 | |
| Fe (mg/kg) | p-adjusted (FDR) | 0.161 | 0.314 | 0.019 | 0.585 | 0.582 | 0.394 | 0.133 |
| [rs] | 0.346 | −0.269 | −0.516 | −0.157 | −0.158 | −0.232 | −0.367 | |
| SOC 20–40 cm (%) | p-adjusted (FDR) | 0.107 | 0.893 | 0.200 | 0.180 | 0.150 | 0.191 | 0.844 |
| [rs] | 0.389 | 0.046 | −0.323 | 0.334 | 0.353 | 0.328 | 0.066 | |
| NO3− (mg/kg) | p-adjusted (FDR) | 0.252 | 0.325 | 0.051 | 0.816 | 0.835 | 0.890 | 0.183 |
| [rs] | 0.298 | −0.264 | −0.450 | 0.076 | 0.068 | −0.050 | −0.332 | |
| Cu (mg/kg) | p-adjusted (FDR) | 0.097 | 0.893 | 0.137 | 0.207 | 0.094 | 0.410 | 0.996 |
| [rs] | 0.398 | 0.049 | −0.364 | 0.319 | 0.402 | 0.223 | −0.003 | |
| Zn (mg/kg) | p-adjusted (FDR) | 0.351 | 0.960 | 0.323 | 0.465 | 0.338 | 0.701 | 0.916 |
| [rs] | 0.251 | 0.019 | −0.264 | 0.199 | 0.257 | 0.116 | −0.035 | |
| NH4+ (mg/kg) | p-adjusted (FDR) | 0.733 | 0.021 | 0.056 | 0.422 | 0.362 | 0.190 | 0.136 |
| [rs] | −0.103 | 0.508 | 0.444 | 0.216 | 0.246 | 0.328 | 0.365 | |
| S (mg/kg) | p-adjusted (FDR) | 0.418 | 0.409 | 0.159 | 0.603 | 0.900 | 0.547 | 0.977 |
| [rs] | 0.219 | −0.224 | −0.347 | 0.150 | 0.042 | 0.171 | 0.013 | |
| SO4 (mg/kg) | p-adjusted (FDR) | 0.036 | 0.794 | 0.241 | 0.935 | 1.000 | 0.893 | 0.742 |
| [rs] | −0.476 | −0.083 | 0.303 | 0.028 | 0.000 | −0.047 | 0.099 | |
| Silt (%) | p-adjusted (FDR) | 0.269 | 0.900 | 0.462 | 0.875 | 0.805 | 0.693 | 0.404 |
| [rs] | −0.288 | −0.041 | 0.200 | 0.055 | −0.080 | 0.121 | 0.227 | |
| Mo (mg/kg) | p-adjusted (FDR) | 0.816 | 0.407 | 0.435 | 0.598 | 0.659 | 0.659 | 0.667 |
| [rs] | −0.075 | 0.226 | 0.210 | 0.152 | 0.133 | 0.133 | 0.130 | |
| Mg2+/K+ | p-adjusted (FDR) | 0.180 | 0.875 | 0.285 | 0.045 | 0.065 | 0.039 | 0.544 |
| [rs] | −0.334 | −0.055 | 0.281 | −0.461 | −0.432 | −0.471 | −0.172 | |
| Ca2+ (mg/kg) | p-adjusted (FDR) | 0.15 | 0.598 | 0.521 | 0.378 | 0.362 | 0.370 | 0.893 |
| [rs] | 0.353 | 0.153 | −0.180 | 0.239 | 0.246 | 0.243 | 0.047 | |
| P Olsen (mg/kg) | p-adjusted (FDR) | 0.778 | 0.955 | 0.893 | 0.406 | 0.581 | 0.255 | 0.475 |
| [rs] | −0.087 | 0.021 | 0.046 | 0.226 | 0.158 | 0.296 | 0.196 | |
| Mn (mg/kg) | p-adjusted (FDR) | 0.567 | 0.982 | 0.732 | 0.732 | 0.491 | 0.732 | 0.733 |
| [rs] | −0.163 | −0.011 | 0.104 | −0.105 | −0.190 | −0.105 | −0.103 | |
| pH | p-adjusted (FDR) | 0.763 | 0.567 | 0.856 | 0.017 | 0.009 | 0.049 | 0.410 |
| [rs] | −0.092 | −0.163 | 0.063 | −0.525 | −0.560 | −0.454 | −0.223 | |
| N-Kjeldahl (%) | p-adjusted (FDR) | 0.542 | 0.358 | 0.926 | 0.140 | 0.230 | 0.034 | 0.138 |
| [rs] | 0.173 | 0.248 | 0.031 | 0.361 | 0.307 | 0.480 | 0.363 | |
| OOM 5–20 cm (%) | p-adjusted (FDR) | 0.021 | 0.701 | 0.129 | 0.250 | 0.263 | 0.139 | 0.269 |
| [rs] | 0.509 | 0.116 | −0.370 | 0.299 | 0.291 | 0.362 | 0.288 | |
| SOC 5–20 cm (%) | p-adjusted (FDR) | 0.019 | 0.673 | 0.133 | 0.263 | 0.275 | 0.148 | 0.282 |
| rs | 0.518 | 0.127 | −0.367 | 0.291 | 0.286 | 0.355 | 0.282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viñas, M.; Marull, J.; Guivernau, M.; Tello, E.; Lucas, Y.; Carreras-Sempere, M.; Giol-Casanova, X.; Funes, I.; Sánchez-Costa, E.; Savé, R.; et al. Impact of Organic and Conventional Agricultural Management on Subsurface Soil Microbiota in Mediterranean Vineyards. Agronomy 2025, 15, 2001. https://doi.org/10.3390/agronomy15082001
Viñas M, Marull J, Guivernau M, Tello E, Lucas Y, Carreras-Sempere M, Giol-Casanova X, Funes I, Sánchez-Costa E, Savé R, et al. Impact of Organic and Conventional Agricultural Management on Subsurface Soil Microbiota in Mediterranean Vineyards. Agronomy. 2025; 15(8):2001. https://doi.org/10.3390/agronomy15082001
Chicago/Turabian StyleViñas, Marc, Joan Marull, Miriam Guivernau, Enric Tello, Yolanda Lucas, Mar Carreras-Sempere, Xavier Giol-Casanova, Immaculada Funes, Elisenda Sánchez-Costa, Robert Savé, and et al. 2025. "Impact of Organic and Conventional Agricultural Management on Subsurface Soil Microbiota in Mediterranean Vineyards" Agronomy 15, no. 8: 2001. https://doi.org/10.3390/agronomy15082001
APA StyleViñas, M., Marull, J., Guivernau, M., Tello, E., Lucas, Y., Carreras-Sempere, M., Giol-Casanova, X., Funes, I., Sánchez-Costa, E., Savé, R., & de Herralde, F. (2025). Impact of Organic and Conventional Agricultural Management on Subsurface Soil Microbiota in Mediterranean Vineyards. Agronomy, 15(8), 2001. https://doi.org/10.3390/agronomy15082001






