Effect of Grassland Vegetation Units on Soil Biochemical Properties and the Abundance of Selected Microorganisms in the Obra River Valley
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Phytosociological Survey
2.3. Soil Survey and Sampling
2.4. Chemical Analyses
2.5. Abundance of Microorganisms In Vitro
2.6. Enzymatic Activity of Soils
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Selected Grasslands
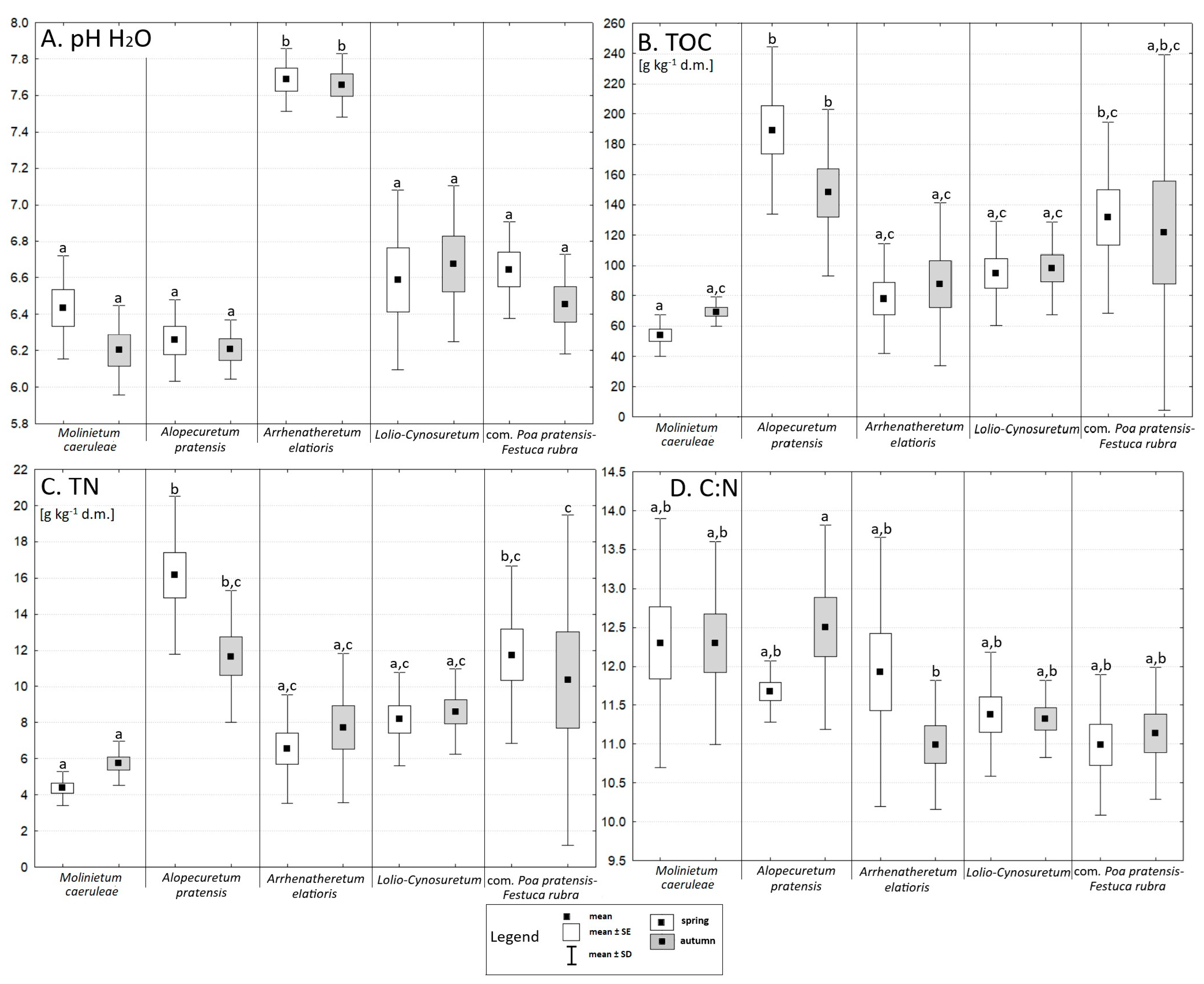
3.2. Chemical Soil Properties
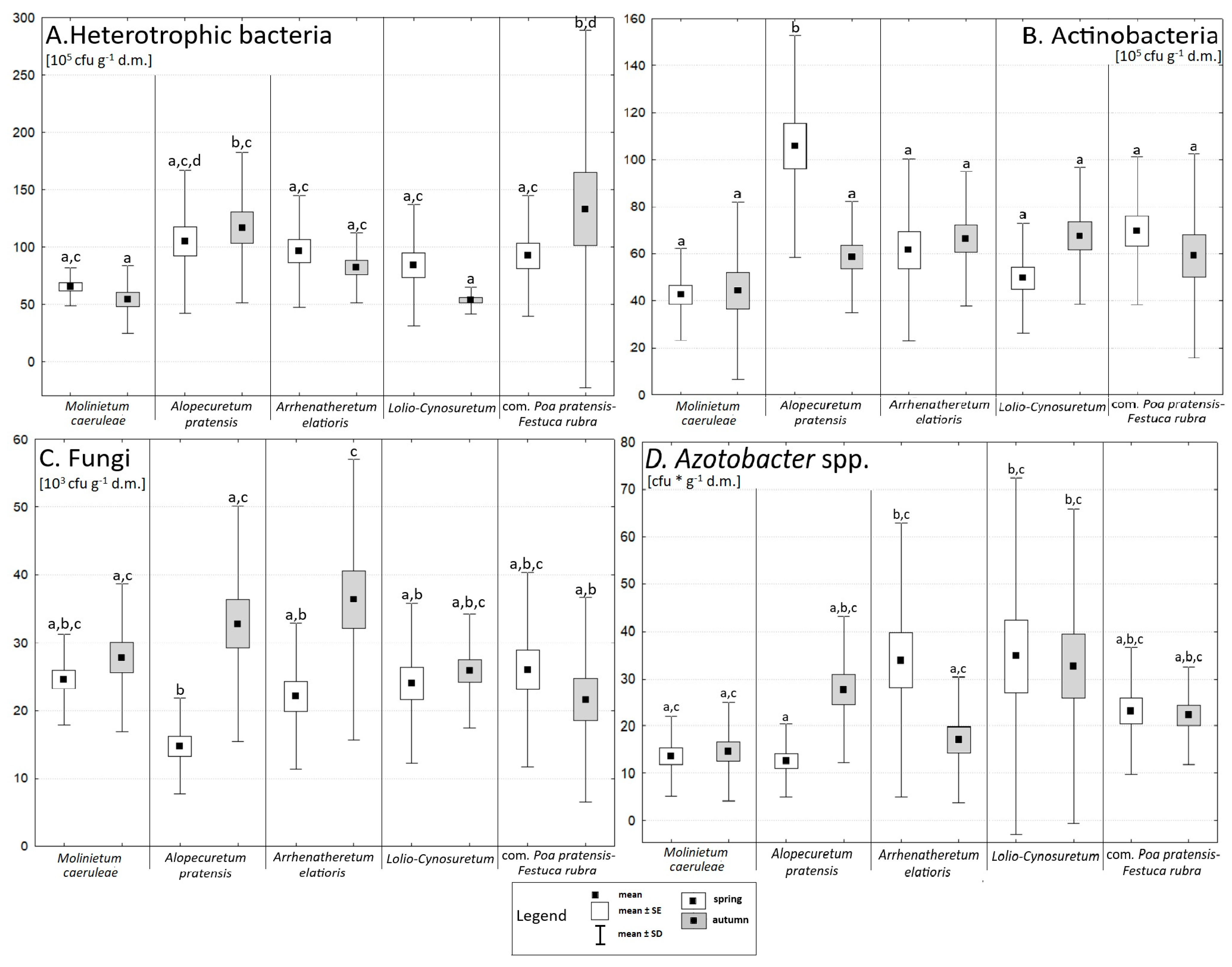
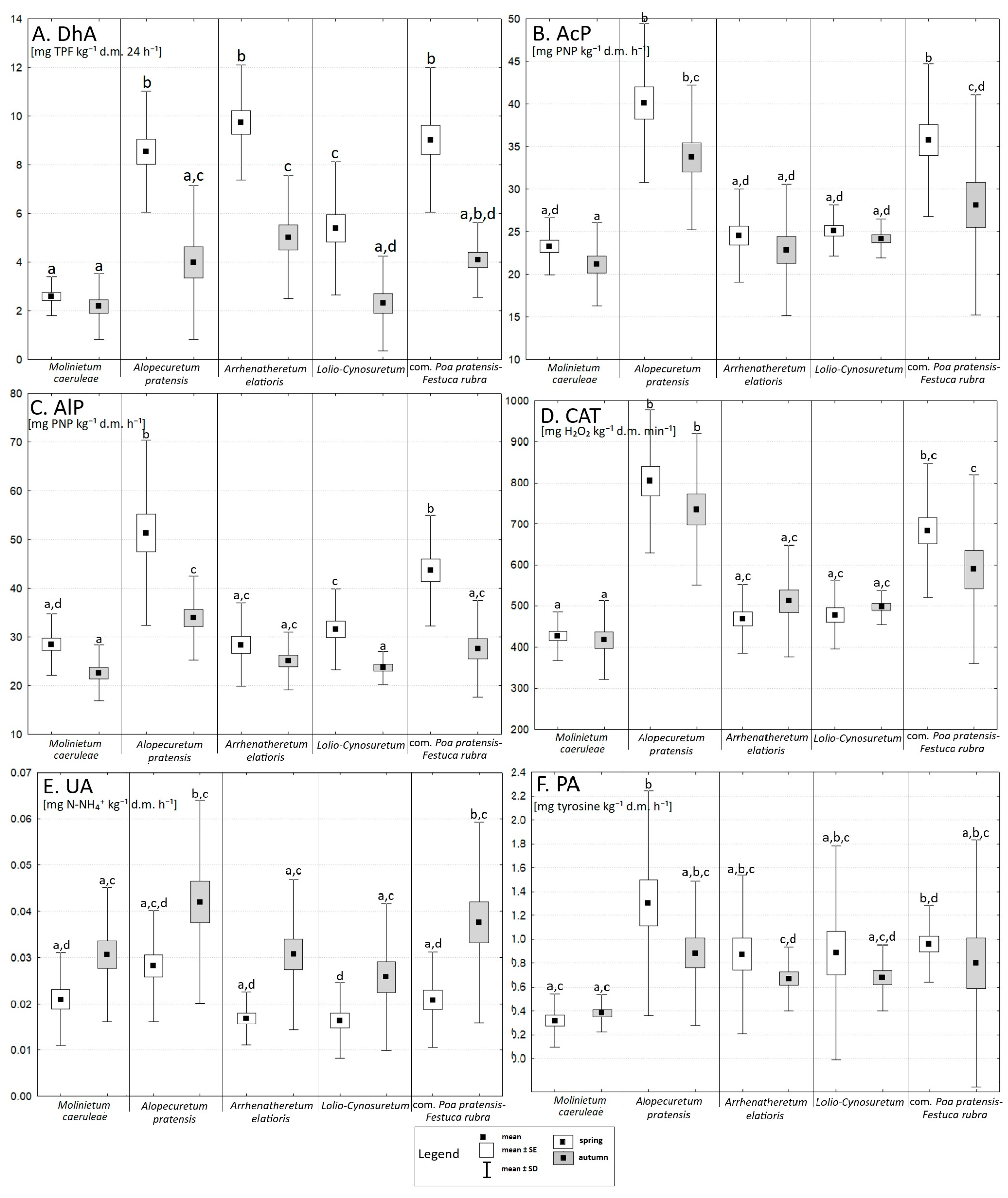
3.3. Microbial Abundance and Enzymatic Activity
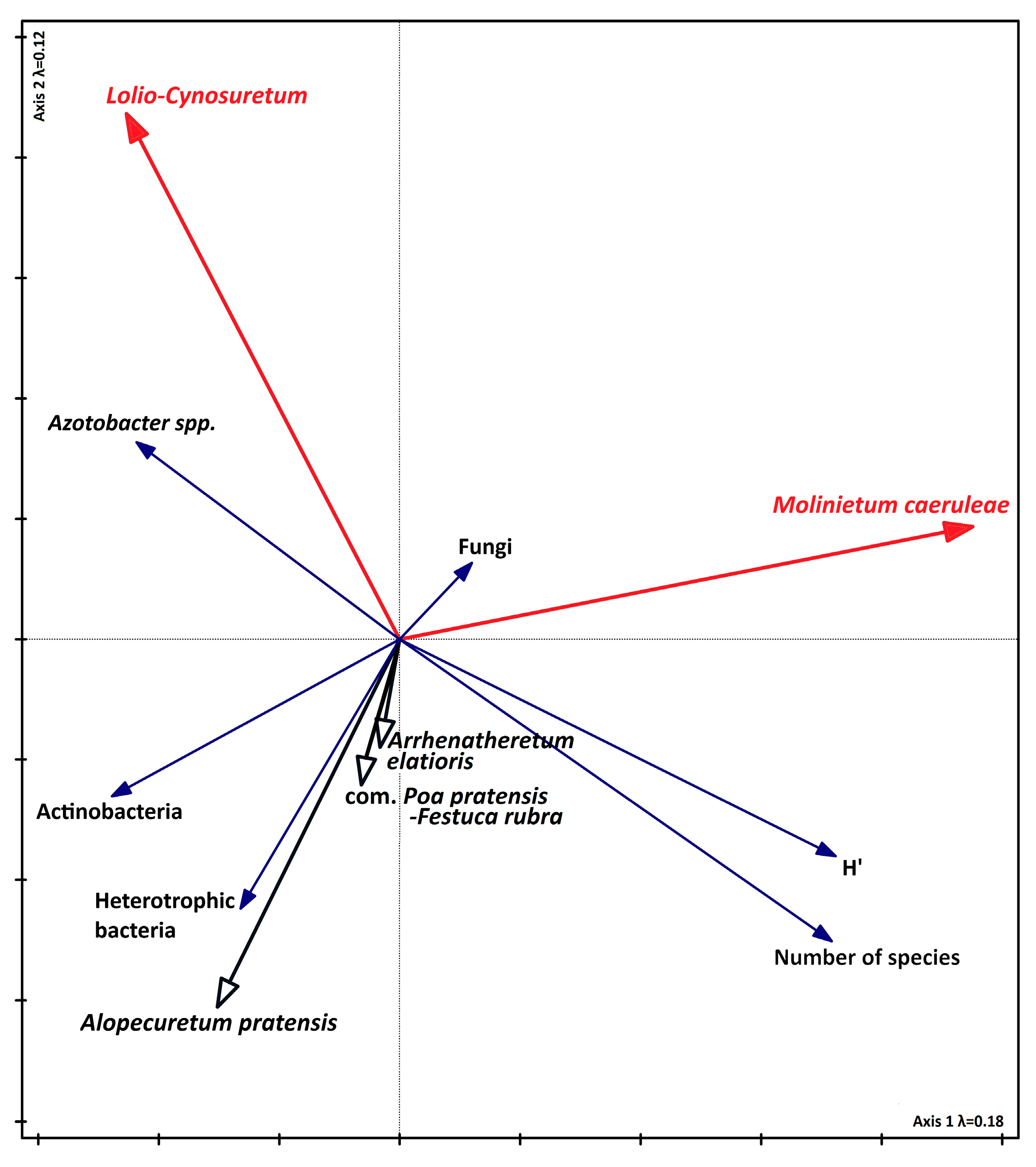
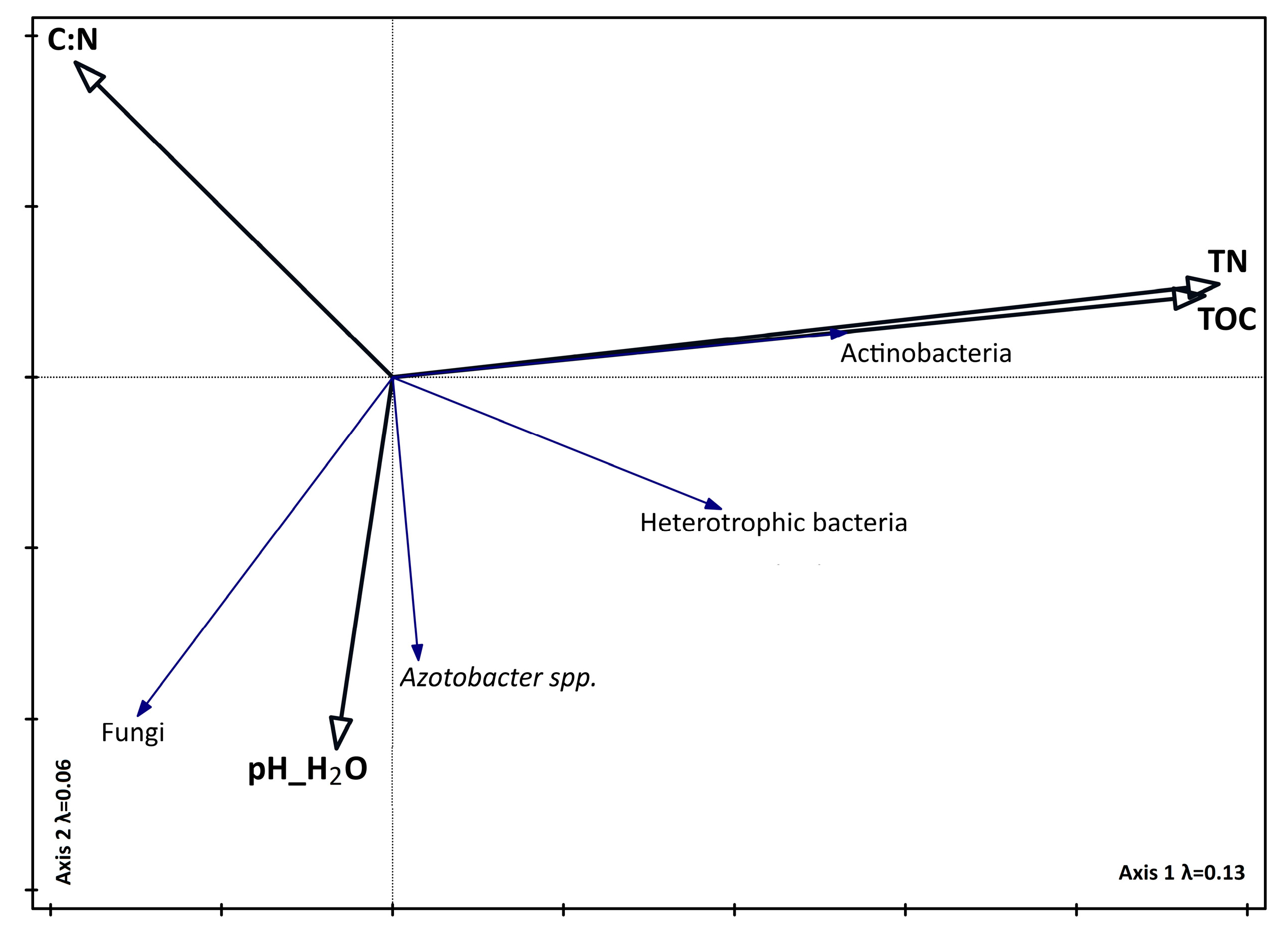
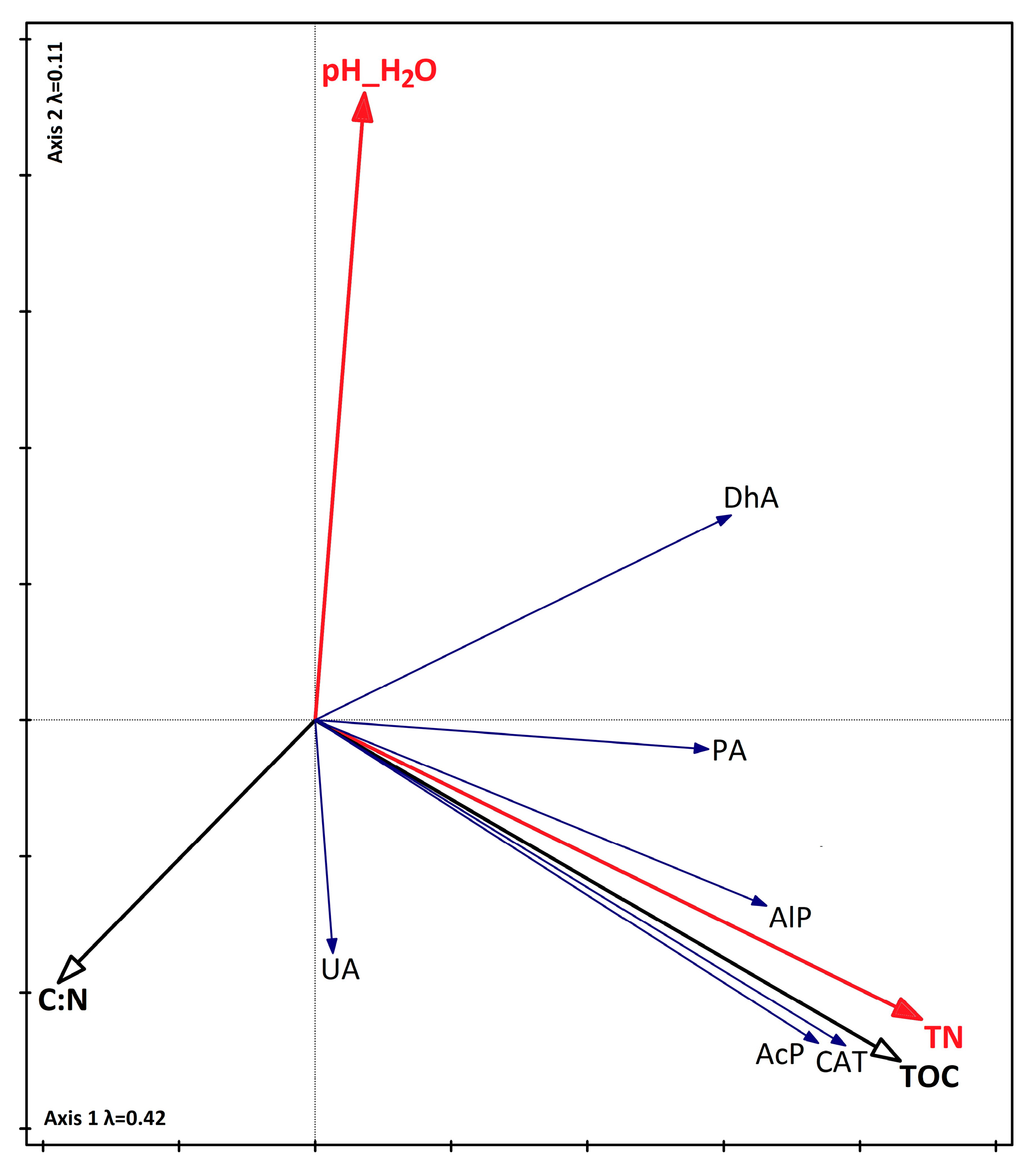
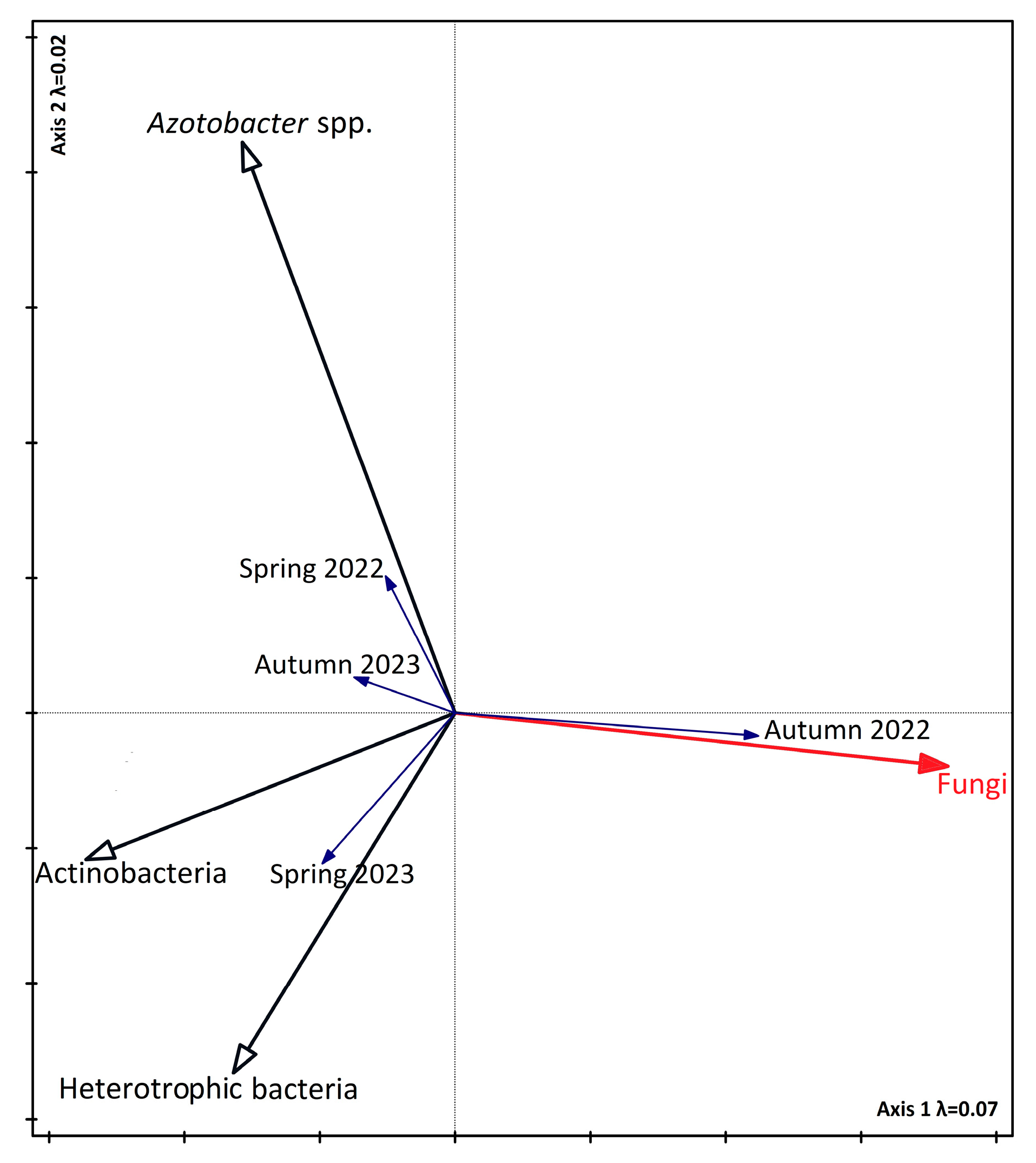
3.4. Relationship Between Biodiversity, Soil Parameters, and Seasons
4. Discussion
4.1. The Impact of Seasonality on Vegetation, the Abundance of Selected Microorganisms, and Enzyme Activity
4.2. The Influence of Chemical Properties on Vegetation, the Abundance of Selected Microorganisms, and Enzyme Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcP | acid phosphatase |
| AlP | alkaline phosphatase |
| AMF | arbuscular mycorrhizae fungi |
| CAT | catalase |
| CFU | colony-forming units |
| com. | community |
| DhA | dehydrogenases |
| PA | proteases |
| PGPR | plant growth-promoting rhizobacteria |
| RDA | redundancy analysis |
| SOC | soil organic carbon |
| SOM | soil organic matter |
| TN | total nitrogen |
| TOC | total organic carbon |
| UA | urease |
References
- Van Oijen, M.; Bellocchi, G.; Höglind, M. Effects of Climate Change on Grassland Biodiversity and Productivity: The Need for a Diversity of Models. Agronomy 2018, 8, 14. [Google Scholar] [CrossRef]
- Gaujour, E.; Amiaud, B.; Mignolet, C.; Plantureux, S. Factors and Processes Affecting Plant Biodiversity in Permanent Grasslands. A Review. Agron. Sustain. Dev. 2012, 32, 133–160. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Erdős, S. Grassland versus Non-Grassland Bird Abundance and Diversity in Managed Grasslands: Local, Landscape and Regional Scale Effects. Biodivers. Conserv. 2007, 16, 871–881. [Google Scholar] [CrossRef]
- Sienkiewicz-Paderewska, D.; Paderewski, J.; Klarzyńska, A.; Wolański, P.; Rogut, K. Floristic Diversity versus Utilization Value of Selected Semi-Natural Central-European Grassland Communities: A Study from Poland. Ecol. Indic. 2021, 132, 108316. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Guide to the Identification of Plant Communities of Poland; PWN: Warsaw, Poland, 2023. [Google Scholar]
- Bonari, G.; Fajmon, K.; Malenovský, I.; Zelený, D.; Holuša, J.; Jongepierová, I.; Kočárek, P.; Konvička, O.; Uřičář, J.; Chytrý, M. Management of Semi-Natural Grasslands Benefiting Both Plant and Insect Diversity: The Importance of Heterogeneity and Tradition. Agric. Ecosyst. Environ. 2017, 246, 243–252. [Google Scholar] [CrossRef]
- Telo Da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Qiao, B.; Jia, K.; Li, C.; Zhao, C. Advances in Plant–Soil Feedback Driven by Root Exudates in Forest Ecosystems. Forests 2024, 15, 515. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, M.; Xiao, R.; Cui, Y.; Yu, F. Changes in Soil Microbial Biomass and Community Composition in Coastal Wetlands Affected by Restoration Projects in a Chinese Delta. Geoderma 2017, 289, 124–134. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Bielinska, E.J.; Futa, B.; Mocek-Płóciniak, A. Soil Enzymes as Bioindicators of Soil Quality and Health; Libropolis Scientific 550 Publishers Society: Lublin, Poland, 2014; ISBN 978-83-63761-25-7. [Google Scholar]
- Ibáñez De Aldecoa, A.L.; Zafra, O.; González-Pastor, J.E. Mechanisms and Regulation of Extracellular DNA Release and Its Biological Roles in Microbial Communities. Front. Microbiol. 2017, 8, 1390. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.; Song, S. Important Soil Microbiota’s Effects on Plants and Soils: A Comprehensive 30-Year Systematic Literature Review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Iqbal Khan, M.S.; Shahid, N.; Aaliya, K. Bottlenecks in Commercialisation and Future Prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using Plant Growth-Promoting Rhizobacteria (PGPR) to Improve Plant Growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A Potential Bio-Fertilizer for Soil and Plant Health Management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef]
- Al-Baldawy, M.S.M.; Matloob, A.A.A.H.; Almammory, M.K.N. The Importance of Nitrogen-Fixing Bacteria Azotobacter Chroococcum in Biological Control to Root Rot Pathogens (Review). IOP Conf. Ser. Earth Environ. Sci. 2023, 1259, 012110. [Google Scholar] [CrossRef]
- Prasad, R.; Bhola, D.; Akdi, K.; Cruz, C.; Kvss, S.; Tuteja, N.; Varma, A. Introduction to Mycorrhiza: Historical Development. In Mycorrhiza-Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–7. ISBN 978-3-319-53063-5. [Google Scholar]
- Verbruggen, E.; Van Der Heijden, M.G.A.; Weedon, J.T.; Kowalchuk, G.A.; Röling, W.F.M. Community Assembly, Species Richness and Nestedness of Arbuscular Mycorrhizal Fungi in Agricultural Soils. Mol. Ecol. 2012, 21, 2341–2353. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of Arbuscular Mycorrhizal Fungi on Soil Fertility: Contribution in the Improvement of Physical, Chemical, and Biological Properties of the Soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, M.; Ramasamy, M. Role of Fungi in Agriculture. In Biostimulants in Plant Science; IntechOpen: London, UK, 2020; p. 12. [Google Scholar]
- Koorem, K.; Sepp, S.; Bueno, C.G.; Davison, J.; Liu, S.; Meng, Y.; Semchenko, M.; Vasar, M.; Zobel, M.; Moora, M. Plant Mycorrhizal Status Indicates Partner Selectivity in Arbuscular Mycorrhizal Interaction Networks. Funct. Ecol. 2024, 39, 1358–1368. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal Networks: Mechanisms, Ecology and Modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kuhar, S.; Sharma, K.K.; Shrivastava, B. Microorganisms and Enzymes Involved in Lignin Degradation Vis-à-Vis Production of Nutritionally Rich Animal Feed: An Overview. In Biotechnology for Environmental Management and Resource Recovery; Kuhad, R.C., Singh, A., Eds.; Springer: New Delhi, India, 2013; pp. 3–44. ISBN 978-81-322-0875-4. [Google Scholar] [CrossRef]
- Wang, L.; Hamel, C.; Lu, P.; Wang, J.; Sun, D.; Wang, Y.; Lee, S.-J.; Gan, G.Y. Using Enzyme Activities as an Indicator of Soil Fertility in Grassland—An Academic Dilemma. Front. Plant Sci. 2023, 14, 1175946. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A. Enzymatic Activity as a Popular Parameter Used to Determine the Quality of the Soil Environment. Pol. J. Agron. 2019, 37, 22–30. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil Microbial Communities and Activities under Intensive Organic and Conventional Vegetable Farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Impact of Various Grass Species on Soil Bacteriobiome. Diversity 2020, 12, 212. [Google Scholar] [CrossRef]
- Mencel, J.; Mocek-Płóciniak, A.; Kryszak, A. Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review. Agronomy 2022, 12, 1136. [Google Scholar] [CrossRef]
- Marcos, M.S.; Carrera, A.L.; Bertiller, M.B.; Olivera, N.L. Grazing Enhanced Spatial Heterogeneity of Soil Dehydrogenase Activity in Arid Shrublands of Patagonia, Argentina. J. Soils Sediments 2020, 20, 883–888. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Q.; Cao, Q.; Hu, J. Effects of Ecological Restoration Measures on Vegetation and Soil Properties in Semi-Humid Sandy Land on the Southeast Qinghai-Tibetan Plateau, China. Glob. Ecol. Conserv. 2022, 33, e02000. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Chen, J. Assessment of Grassland Ecosystem Services and Analysis on Its Driving Factors: A Case Study in Hulunbuir Grassland. Front. Ecol. Evol. 2022, 10, 841943. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, W.; Zhou, L.; Liang, J.; Jiang, T. Interactive Effect of Dissolved Organic Matter and Phenanthrene on Soil Enzymatic Activities. J. Environ. Sci. 2010, 22, 607–614. [Google Scholar] [CrossRef]
- Wang, H.; Ma, S.; Shao, G.; Dittert, K. Use of Urease and Nitrification Inhibitors to Decrease Yield-Scaled N2O Emissions from Winter Wheat and Oilseed Rape Fields: A Two-Year Field Experiment. Agric. Ecosyst. Environ. 2021, 319, 107552. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Coulter, J.A.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Gan, Y. Slow-Release Fertilizer Improves the Growth, Quality, and Nutrient Utilization of Wintering Chinese Chives (Allium tuberosum Rottler Ex Spreng.). Agronomy 2020, 10, 381. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Wyszkowski, M. Activity of Soil Dehydrogenases, Urease, and Acid and Alkaline Phosphatases in Soil Polluted with Petroleum. J. Toxicol. Environ. Health 2010, 73, 1202–1210. [Google Scholar] [CrossRef]
- Liao, P.; Ros, M.B.H.; Van Gestel, N.; Sun, Y.; Zhang, J.; Huang, S.; Zeng, Y.; Wu, Z.; Van Groenigen, K.J. Liming Reduces Soil Phosphorus Availability but Promotes Yield and P Uptake in a Double Rice Cropping System. J. Integr. Agric. 2020, 19, 2807–2814. [Google Scholar] [CrossRef]
- Wahdan, S.F.M.; Heintz-Buschart, A.; Sansupa, C.; Tanunchai, B.; Wu, Y.-T.; Schädler, M.; Noll, M.; Purahong, W.; Buscot, F. Targeting the Active Rhizosphere Microbiome of Trifolium Pratense in Grassland Evidences a Stronger-Than-Expected Belowground Biodiversity-Ecosystem Functioning Link. Front. Microbiol. 2021, 12, 629169. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Z.; Cheng, C. Effect of Conservation Tillage Practices on Soil Phosphorus Nutrition in an Apple Orchard. Hortic. Plant J. 2016, 2, 331–337. [Google Scholar] [CrossRef]
- Moreno, J.L.; García, C.; Hernández, T. Toxic Effect of Cadmium and Nickel on Soil Enzymes and the Influence of Adding Sewage Sludge. Eur. J. Soil Sci. 2003, 54, 377–386. [Google Scholar] [CrossRef]
- Naga Raju, M.; Golla, N.; Vengatampalli, R. Soil Protease. In Soil Enzymes; SpringerBriefs in Environmental Science; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–24. ISBN 978-3-319-42654-9. [Google Scholar]
- Lepinay, C.; Větrovský, T.; Chytrý, M.; Dřevojan, P.; Fajmon, K.; Cajthaml, T.; Kohout, P.; Baldrian, P. Effect of Plant Communities on Bacterial and Fungal Communities in a Central European Grassland. Environ. Microbiome 2024, 19, 42. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Hilal, M.G.; Guo, L.; Zhang, Y.; Ji, Y.; Jiang, X.; Hao, L.; Lin, K. Effects of Grassland Degradation on Soil Ecological Stoichiometry and Soil Microbial Community on the South of the Greater Khingan Mountains. Front. Microbiol. 2024, 15, 1438787. [Google Scholar] [CrossRef]
- Roux, X.L.; Recous, S.; Attard, E. Soil Microbial Diversity in Grasslands and Its Importance for Grassland Functioning and Services. In Grassland Productivity and Ecosystem Services; Lemaire, G., Hodgson, J., Chabbi, A., Eds.; CABI: Wallingford, UK, 2011; pp. 158–165. ISBN 978-1-84593-809-3. [Google Scholar]
- Gao, X.; Zheng, Z.; Diao, Z.; Zhang, Y.; Wang, Y.; Ma, L. The Effects of Litter Input and Increased Precipitation on Soil Microbial Communities in a Temperate Grassland. Front. Microbiol. 2024, 15, 1347016. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Siebert, J.; Sünnemann, M.; Hautier, Y.; Risch, A.C.; Bakker, J.D.; Biederman, L.; Blumenthal, D.M.; Borer, E.T.; Bugalho, M.N.; Broadbent, A.A.D.; et al. Drivers of Soil Microbial and Detritivore Activity across Global Grasslands. Commun. Biol. 2023, 6, 1220. [Google Scholar] [CrossRef]
- Breulmann, M.; Schulz, E.; Weißhuhn, K.; Buscot, F. Impact of the Plant Community Composition on Labile Soil Organic Carbon, Soil Microbial Activity and Community Structure in Semi-Natural Grassland Ecosystems of Different Productivity. Plant Soil 2012, 352, 253–265. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Grego, S.; Kandeler, E. Soil Organic Carbon Distribution Drives Microbial Activity and Functional Diversity in Particle and Aggregate-Size Fractions. Pedobiologia 2012, 55, 101–110. [Google Scholar] [CrossRef]
- Wordell-Dietrich, P.; Don, A.; Helfrich, M. Controlling Factors for the Stability of Subsoil Carbon in a Dystric Cambisol. Geoderma 2017, 304, 40–48. [Google Scholar] [CrossRef]
- Hagh-Doust, N.; Mikryukov, V.; Anslan, S.; Bahram, M.; Puusepp, R.; Dulya, O.; Tedersoo, L. Effects of Nitrogen Deposition on Carbon and Nutrient Cycling along a Natural Soil Acidity Gradient as Revealed by Metagenomics. New Phytol. 2023, 238, 2607–2620. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- IMGW PIB. Meteorological Yearbook 2022; IMGW PIB: Warszawa, Poland, 2022. [Google Scholar]
- IMGW PIB. Meteorological Yearbook 2023; IMGW PIB: Warszawa, Poland, 2023. [Google Scholar]
- Szałajdewicz, J. Szczegółowa Mapa Geologiczna Polski (Detailed Geological Map of Poland), 1:50,000. Rakoniewice (541); Państwowy Instytut Geologiczny: Warsaw, Poland, 2004; ISBN 978-83-7372-649-9. [Google Scholar]
- Jodłowski, J. Szczegółowa Mapa Geologiczna Polski (Detailed Geological Map of Poland), 1:50,000. Wolsztyn (540); Państwowy Instytut Geologiczny: Warsaw, Poland, 2003; ISBN 978-83-7372-626-0. [Google Scholar]
- Krzysztofka, M. Szczegółowa Mapa Geologiczna Polski (Detailed Geological Map of Poland), 1:50,000. Kościan (542); Państwowy Instytut Geologiczny: Warsaw, Poland, 1993. [Google Scholar]
- Mencel, J.; Klarzyńska, A.; Piernik, A.; Mocek-Płóciniak, A. Differentiation of Grassland Vegetation in Relation to the Physicochemical Properties of Peat Soils in the Obra River Valley, Western Poland. Soil Sci. Annu. 2024, 75, 190113. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge Der Vegetationskunde; Springer: Vienna, Austria, 1964. [Google Scholar]
- Hennekens, S.M.; Schaminée, J.H.J. Turboveg, a Comprehensive Data Base Management System for Vegetation Data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L.; Holt, J.; Nejezchlebová, M. JUICE Program for Management, Analysis and Classification of Ecological Data, 2nd ed.; Vegetation Science Group: Masaryk University Brno, Czech Republic, 2011. [Google Scholar]
- Shannon, C.E.; Weaver, W. A Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Martin, J.P. Use of Acid, Rose Bengal and Streptomycin in the Plate Method for Estimating Soil Fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Grabińska-Łoniewska, A. Laboratory Exercises in General Microbiology; Warsaw Technical University: Warsaw, Poland, 1999. [Google Scholar]
- Fenglerowa, W. Simple Method for Counting Azotobacter in Soil Samples. Acta Microbiol. Pol. 1970, 14, 203–206. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik Der Bestimmung Der Dehydrogenaseaktivität Im Boden Mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsh 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wyczółkowski, A.J.; Dabek-Szreniawska, M. Enzymes Taking Part in Organic Nitrogen Mineralization. Acta Agrophysica. Monogr. 2005, 3, 37–61. [Google Scholar]
- Hoffmann, G.; Teichert, K. Ein Kolorimetrisches Verfahren Zur Bestimmung Der Ureaseaktivität in Böden. Z. Für Pflanzenernährung Düngung Bodenkd. 1961, 91, 55–63. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide: Software Ordination (Version 5.0); Biometrics: Wageningen, České Budějowice, 2012. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 9.0. 2009. Available online: http://www.statsoft.com (accessed on 19 March 2025).
- Garbowski, M.; Boughton, E.; Ebeling, A.; Fay, P.; Hautier, Y.; Holz, H.; Jentsch, A.; Jurburg, S.; Ladouceur, E.; Martina, J.; et al. Temperature Seasonality and Nutrient Enrichment Drive Intra-Annual Community Turnover in Global Grasslands. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pan, J.J.; Ammerman, D.; Mitchell, R.J. Nutrient Amendments in a Temperate Grassland Have Greater Negative Impacts on Early Season and Exotic Plant Species. Plant Ecol. 2011, 212, 853–864. [Google Scholar] [CrossRef]
- Doležal, J.; Lanta, V.; Mudrák, O.; Lepš, J. Seasonality Promotes Grassland Diversity: Interactions with Mowing, Fertilization and Removal of Dominant Species. J. Ecol. 2019, 107, 203–215. [Google Scholar] [CrossRef]
- Fischer, F.M.; Chytrý, K.; Chytrá, H.; Chytrý, M.; Těšitel, J. Seasonal Beta-Diversity of Dry Grassland Vegetation: Divergent Peaks of above-Ground Biomass and Species Richness. J. Veg. Sci. 2023, 34, e13182. [Google Scholar] [CrossRef]
- Yan, P.; Lu, X.; Li, W.; Zhang, J.; Li, P.; Li, Y.; Wang, K.; Ding, S. Seasonal Variations in Plant Species Diversity and Phylogenetic Diversity in Abandoned Farmland of China’s Huang–Huai Plain. Diversity 2023, 15, 922. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.; Montgomery, R.; Townsend, P.; Zygielbaum, A.; Bitan, K.; Tilman, D.; Cavender-Bares, J. Seasonal Variation in the NDVI–Species Richness Relationship in a Prairie Grassland Experiment (Cedar Creek). Remote Sens. 2016, 8, 128. [Google Scholar] [CrossRef]
- Qiao, Y.; Cheng, H.; Zhu, H.; Zhu, Y.; Jia, Q.; Yang, Y.; Zhong, H.; Zohner, C.; Liu, J. Species Diversity Advances Autumn Senescence in Grasslands. Authorea 2024, preprint. [Google Scholar] [CrossRef]
- Fóti, S.; Bartha, S.; Balogh, J.; Pintér, K.; Koncz, P.; Biró, M.; Süle, G.; Petrás, D.; Luca, G.D.; Ladányi, M.; et al. Fluctuations and Trends in Spatio-Temporal Patterns of Plant Species and Diversity in a Sandy Pasture. J. Veg. Sci. 2023, 34, e13190. [Google Scholar] [CrossRef]
- Hsin, K.-T.; Lee, H.; Lin, Y.-C.J.; Chen, P.-Y. Lignocellulose Degradation in Bacteria and Fungi for Biomass Conversion. Front. Microbiol. 2024, 16, 1583746. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal Dynamics of Soil Fungal and Bacterial Communities in Cool-Temperate Montane Forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef]
- Schnecker, J.; Baldaszti, L.; Gündler, P.; Pleitner, M.; Sandén, T.; Simon, E.; Spiegel, F.; Spiegel, H.; Urbina Malo, C.; Zechmeister-Boltenstern, S.; et al. Seasonal Dynamics of Soil Microbial Growth, Respiration, Biomass, and Carbon Use Efficiency in Temperate Soils. Geoderma 2023, 440, 116693. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Wen, Y.; Ye, C.; Wang, P.; Bai, T.; Gu, X.; Guo, L.; Qiu, Y.; Zhang, Y.; et al. Temporal Dynamics of Climate Sensitivity of Litter Decomposition in a Semi-Arid Grassland. Geoderma 2025, 453, 117157. [Google Scholar] [CrossRef]
- Cereghetti, E.; Peller, T.; Kaeser, S.; Gounand, I.; Altermatt, F. Seasonal Dynamics of Detritus Flows and Decomposition across Ecosystem Boundaries. Curr. Biol. 2025, 35, p2139–p2145.e3. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Qu, A.; Feng, E.; Chen, R.; Yang, X.; Lai, Y. Seasonal Dynamics of Soil Enzymatic Activity under Different Land-Use Types in Rocky Mountainous Region of North China. Forests 2023, 14, 536. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Wilczewski, E. Assessment of Soil Nitrogen and Related Enzymes as Influenced by the Incorporation Time of Field Pea Cultivated as a Catch Crop in Alfisol. Environ. Monit. Assess. 2014, 186, 8425–8441. [Google Scholar] [CrossRef]
- Alizadeh, H.; Kandula, D.R.W.; Hampton, J.G.; Stewart, A.; Leung, D.W.M.; Edwards, Y.; Smith, C. Urease Producing Microorganisms under Dairy Pasture Management in Soils across New Zealand. Geoderma Reg. 2017, 11, 78–85. [Google Scholar] [CrossRef]
- Song, W.; Han, F.; Bao, Z.; Chai, Y.; Wang, L.; Huang, C.; Cheng, H.; Chang, L. Mulching Practices Improve Soil Moisture and Enzyme Activity in Drylands, Increasing Potato Yield. Agronomy 2024, 14, 1077. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Abdul Sukor, A.S.; Adibah, A.M. Urea Application in Soil: Processes, Losses, and Alternatives—A Review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of Soil Microbial and Enzyme Activity in the Rhizosphere Zone under Different Land Use/Cover of a Semiarid Region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Puissant, J.; Jassey, V.E.J.; Mills, R.T.E.; Robroek, B.J.M.; Gavazov, K.; De Danieli, S.; Spiegelberger, T.; Griffiths, R.; Buttler, A.; Brun, J.-J.; et al. Seasonality Alters Drivers of Soil Enzyme Activity in Subalpine Grassland Soil Undergoing Climate Change. Soil Biol. Biochem. 2018, 124, 266–274. [Google Scholar] [CrossRef]
- Chmolowska, D.; Nobis, M.; Rożej-Pabijan, E.; Grześ, I.M.; Radzikowski, P.; Okrutniak, M.; Celary, W.; Sternalski, J.; Shrubovych, J.; Wasak-Sęk, K. Matching the Puzzle Piece to a New Jigsaw: The Effect of Surrounding Environments on Plants and Invertebrates in the Translocated Wet Meadow. Sci. Total Environ. 2023, 904, 166637. [Google Scholar] [CrossRef]
- Wójcik, T.; Kostrakiewicz-Gierałt, K.; Makuch-Pietraś, I. The Effect of Accidental Burning on Habitat Conditions and Species Composition of Molinion Caeruleae Meadows. J. Nat. Conserv. 2022, 70, 126294. [Google Scholar] [CrossRef]
- Marciniuk, P.; Marciniuk, J.; Sychut-Czapla, E.; Oklejewicz, K. Meadows of the Molinietalia order as a refugium of rare plant species in the Nadhużański Landscape Park (F. Poland). Fragm. Florist. Geobot. Pol. 2016, 23, 73–81. [Google Scholar]
- Zelnik, I.; Čarni, A. Wet Meadows of the Alliance Molinion and Their Environmental Gradients in Slovenia. Biologia 2008, 63, 187–196. [Google Scholar] [CrossRef]
- Kozłowski, S.; Zielewicz, W.; Swędrzyński, A.; Olejarnik, Ł. Chemical properties of forest grasses. Grassl. Sci. Pol. 2012, 15, 109–118. [Google Scholar]
- Swacha, G.; Botta-Dukát, Z.; Kącki, Z.; Pruchniewicz, D.; Żołnierz, L. The Effect of Abandonment on Vegetation Composition and Soil Properties in Molinion Meadows (SW Poland). PLoS ONE 2018, 13, e0197363. [Google Scholar] [CrossRef]
- Suder, A. Vegetation of wet meadows (order Molinietalia caeruleae W. Koch 1926) in the eastern part of Silesia Upland. Grassl. Sci. Pol. 2007, 10, 159–172. [Google Scholar]
- Han, X.; Doménech-Pascual, A.; Pere Casas-Ruiz, J.; Donhauser, J.; Jordaan, K.; Ramond, J.-B.; Priemé, A.; Romaní, A.M.; Frossard, A. Soil Organic Matter Properties Drive Microbial Enzyme Activities and Greenhouse Gas Fluxes along an Elevational Gradient. Geoderma 2024, 449, 116993. [Google Scholar] [CrossRef]
- Xu, Q.; Li, L.; Guo, J.; Guo, H.; Liu, M.; Guo, S.; Kuzyakov, Y.; Ling, N.; Shen, Q. Active Microbial Population Dynamics and Life Strategies Drive the Enhanced Carbon Use Efficiency in High-Organic Matter Soils. mBio 2024, 15, e00177-24. [Google Scholar] [CrossRef]
- Jnawali, A.D.; Ojha, R.B.; Marahatta, S. Role of Azotobacter in Soil Fertility and Sustainability—A Review. Adv. Plants Agric. Res. 2015, 2, 250–253. [Google Scholar] [CrossRef]
- Martyniuk, S.; Martyniuk, M. Occurrence of Azotobacter Spp. in Some Polish Soils. Pol. J. Environ. Stud. 2002, 12, 371–374. [Google Scholar]
- Lenart, A. Occurrence, Characteristics, and Genetic Diversity of Azotobacter Chroococcum in Various Soils of Southern Poland. Pol. J. Environ. Stud. 2012, 21, 415–424. [Google Scholar]
- Mazinani, Z.; Asgharzadeh, A. Genetic Diversity of Azotobacter Strains Isolated from Soils by Amplified Ribosomal DNA Restriction Analysis. Cytol. Genet. 2014, 48, 293–301. [Google Scholar] [CrossRef]
- Kozieł, M.; Gałązka, A.; Martyniuk, S. Free-Living Atmospheric Nitrogen-Fixing Bacteria of the Azotobacter Genus—534 Occurrence, Abundance, and Significance. Stud. Rep. IUNG-PIB 2018, 56, 57–70. [Google Scholar] [CrossRef]
- Tejera, N.; Lluch, C.; Martìnez-Toledo, M.V.; Gonzàlez-López, J. Isolation and Characterization of Azotobacter and Azospirillum Strains from the Sugarcane Rhizosphere. Plant Soil 2005, 270, 223–232. [Google Scholar] [CrossRef]
- Kizilkaya, R. Nitrogen Fixation Capacity of Azotobacter Spp. Strains Isolated from Soils in Different Ecosystems and Relationship Between Them and the Microbiological Properties of Soils. J. Environ. Biol. 2009, 30, 73–82. [Google Scholar] [PubMed]
- Li, Z.; Liu, X.; Zhang, M.; Xing, F. Plant Diversity and Fungal Richness Regulate the Changes in Soil Multifunctionality in a Semi-Arid Grassland. Biology 2022, 11, 870. [Google Scholar] [CrossRef]
- Meliani, A.; Bensoltane, A.; Mederbel, K. Microbial Diversity and Abundance in Soil: Related to Plant and Soil Type. Am. J. Plant Nutr. Fertil. Technol. 2012, 2, 10–18. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Huang, X.; Lang, X.; Tang, R.; Zhang, R.; Li, S.; Su, J. Effects of Plant Diversity, Soil Microbial Diversity, and Network Complexity on Ecosystem Multifunctionality in a Tropical Rainforest. Front. Plant Sci. 2023, 14, 1238056. [Google Scholar] [CrossRef] [PubMed]
- Jayaramaiah, R.H.; Martins, C.S.C.; Egidi, E.; Macdonald, C.A.; Wang, J.-T.; Liu, H.; Reich, P.B.; Delgado-Baquerizo, M.; Singh, B.K. Soil Function-Microbial Diversity Relationship Is Impacted by Plant Functional Groups under Climate Change. Soil Biol. Biochem. 2025, 200, 109623. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil Fungal Diversity in Natural Grasslands of the Tibetan Plateau: Associations with Plant Diversity and Productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Li, T.; Guo, M.; Guo, S.; Wang, R. Responses of Bacterial and Fungal Community to Long-Term Nitrogen Application in Loess Plateau. J. Plant Nutr. Fertil. 2024, 30, 232–241. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, L.C. Organic Carbon and Nitrogen Availability Determine Bacterial Community Composition in Paddy Fields of the Indo-Gangetic Plain. 3 Biotech 2017, 7, 199. [Google Scholar] [CrossRef]
- Steinauer, K.; Tilman, D.; Wragg, P.D.; Cesarz, S.; Cowles, J.M.; Pritsch, K.; Reich, P.B.; Weisser, W.W.; Eisenhauer, N. Plant Diversity Effects on Soil Microbial Functions and Enzymes Are Stronger than Warming in a Grassland Experiment. Ecology 2015, 96, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, X.; Schmid, B.; Poly, F.; Barnard, R.L.; Niklaus, P.A.; Guillaumaud, N.; Habekost, M.; Oelmann, Y.; Philippot, L.; Salles, J.F.; et al. Soil Environmental Conditions and Microbial Build-Up Mediate the Effect of Plant Diversity on Soil Nitrifying and Denitrifying Enzyme Activities in Temperate Grasslands. PLoS ONE 2013, 8, e61069. [Google Scholar] [CrossRef]
- Xie, L.; Gong, L.; Zhu, M. Soil Enzyme Activities and Their Correlation with Physicochemical Factors in the Oasis of Southern Margin of Tarim Basin. Res. Environ. Sci. 2014, 27, 1306–1313. [Google Scholar]
- Liu, Q.; Wu, F.; Liu, H.; Zhang, Q.-F.; Zhang, J.; Li, H. Study on the Relationships Between Soil Enzyme Activities and Soil Fertility in Zhifanggou Watershed. J. Plant Nutr. Fertil. 2009, 15, 1100–1106. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil Enzyme Activity in Response to Long-Term Organic Matter Manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Xu, R.; Li, J.; Li, X.; Zhang, J.; Song, W. Effect of Coal Mining Subsidence on Soil Enzyme Activity in Mining Areas with High Underground Water Levels. Water 2024, 16, 1704. [Google Scholar] [CrossRef]
- Ochmian, I.; Kozos, K.; Jaroszewska, A.; Malinowski, R. Chemical and Enzymatic Changes of Different Soils during Their Acidification to Adapt Them to the Cultivation of Highbush Blueberry. Agronomy 2020, 11, 44. [Google Scholar] [CrossRef]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R.; et al. The pH Optimum of Soil Exoenzymes Adapt to Long Term Changes in Soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]

| Sampling Sites | Grassland Units | Coordinates WGS 84 (N/E) |
|---|---|---|
| 1. | Molinietum caeruleae | 52°05′42″ N 16°31′30″ E |
| 2. | Molinietum caeruleae | 52°05′42″ N 16°31′33″ E |
| 3. | Molinietum caeruleae | 52°05′43″ N 16°31′37″ E |
| 4. | Molinietum caeruleae | 52°05′43″ N 16°31′39″ E |
| 5. | com. Poa pratensis–Festuca rubra | 52°06′07″ N 16°22′12″ E |
| 6. | com. Poa pratensis–Festuca rubra | 52°06′07″ N 16°22′08″ E |
| 7. | com. Poa pratensis–Festuca rubra | 52°06′04″ N 16°22′03″ E |
| 8. | com. Poa pratensis–Festuca rubra | 52°01′27″ N 16°16′23″ E |
| 9. | Arrhenatheretum elatioris | 52°06′03″ N 16°22′07′′ E |
| 10. | Arrhenatheretum elatioris | 52°06′00″ N 16°21′56′′ E |
| 11. | Arrhenatheretum elatioris | 52°00′49″ N 16°16′52″ E |
| 12. | Arrhenatheretum elatioris | 52°01′01″ N 16°16′43″ E |
| 13. | Lolio–Cynosuretum | 52°06′02″ N 16°22′04″ E |
| 14. | Lolio–Cynosuretum | 52°05′59″ N 16°22′00″ E |
| 15. | Lolio–Cynosuretum | 52°06′00″ N 16°22′01″ E |
| 16. | Lolio–Cynosuretum | 52°04′23″ N 16°14′11″ E |
| 17. | Alopecuretum pratensis | 52°05′58″ N 16°21′57″ E |
| 18. | Alopecuretum pratensis | 52°00′47″ N 16°16′54″ E |
| 19. | Alopecuretum pratensis | 52°00′47″ N 16°16′57″ E |
| 20. | Alopecuretum pratensis | 52°01′02″ N 16°16′44″ E |
| Grassland Units | Total Number of Species | Number of Species in the Reléve in Spring (Range and Mean) | Number of Species in the Reléve in Autumn (Range and Mean) | H′ Spring | H′ Autumn | ||
|---|---|---|---|---|---|---|---|
| Molinietum caeruleae | 57 | 17–20 | 19 | 22–29 | 26 | 2.25 | 2.45 |
| Alopecuretum pratensis | 44 | 10–22 | 16 | 12–22 | 17 | 1.72 | 1.79 |
| Arrhenatheretum elatioris | 54 | 16–20 | 18 | 14–24 | 19 | 2.00 | 2.06 |
| Lolio–Cynosuretum | 36 | 8–12 | 11 | 9–16 | 13 | 1.49 | 1.48 |
| com. Poa pratensis–Festuca rubra | 50 | 12–22 | 19 | 15–24 | 19 | 2.17 | 2.19 |
| Grassland Units | Heterotrophic Bacteria | Actinobacteria | Fungi | Azotobacter spp. |
|---|---|---|---|---|
| Molinietum caeruleae | ns | ns | ns | ns |
| Alopecuretum pratensis | ns | ns | ns | ns |
| Arrhenatheretum elatioris | −0.53 * | ns | ns | ns |
| Lolio–Cynosuretum | ns | ns | ns | ns |
| com. Poa pratensis–Festuca rubra | ns | ns | −0.63 ** | ns |
| Grassland Units | DhA | AcP | AlP | CAT | UA | PA |
|---|---|---|---|---|---|---|
| Molinietum caeruleae | ns | ns | ns | ns | ns | ns |
| Alopecuretum pratensis | ns | −0.33 * | ns | −0.33 ** | ns | ns |
| Arrhenatheretum elatioris | ns | ns | ns | ns | ns | ns |
| Lolio–Cynosuretum | ns | ns | ns | ns | −0.65 ** | ns |
| com. Poa pratensis–Festuca rubra | ns | −0.63 ** | −0.51 * | −0.62 ** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencel, J.; Wojciechowska, A.; Mocek-Płóciniak, A. Effect of Grassland Vegetation Units on Soil Biochemical Properties and the Abundance of Selected Microorganisms in the Obra River Valley. Agronomy 2025, 15, 1573. https://doi.org/10.3390/agronomy15071573
Mencel J, Wojciechowska A, Mocek-Płóciniak A. Effect of Grassland Vegetation Units on Soil Biochemical Properties and the Abundance of Selected Microorganisms in the Obra River Valley. Agronomy. 2025; 15(7):1573. https://doi.org/10.3390/agronomy15071573
Chicago/Turabian StyleMencel, Justyna, Anna Wojciechowska, and Agnieszka Mocek-Płóciniak. 2025. "Effect of Grassland Vegetation Units on Soil Biochemical Properties and the Abundance of Selected Microorganisms in the Obra River Valley" Agronomy 15, no. 7: 1573. https://doi.org/10.3390/agronomy15071573
APA StyleMencel, J., Wojciechowska, A., & Mocek-Płóciniak, A. (2025). Effect of Grassland Vegetation Units on Soil Biochemical Properties and the Abundance of Selected Microorganisms in the Obra River Valley. Agronomy, 15(7), 1573. https://doi.org/10.3390/agronomy15071573






