Abstract
Soybean mosaic virus (SMV) is a major viral pathogen that causes significant yield losses and a reduction in seed quality in susceptible soybean cultivars. Resistance breeding is the most effective, economical, and eco-friendly strategy for prevention of SMV-induced damage. Accurate and convenient assessment of SMV resistance is an essential prerequisite for resistance breeding. In this study, we constructed a green fluorescent protein (GFP)-tagged SMV recombinant virus (SMV-GFP) by yeast homologous recombination technology. It was proved that the recombinant virus can not only be used to track the viral infection process in Nicotiana benthamiana and soybean, but also to quantify the viral load based on relative fluorescence area (RFA) value. Using this recombinant virus, the resistance of 286 soybean germplasms from Northeast China to SMV was evaluated. A genome-wide association study (GWAS) was conducted using the RFA values of the 286 soybean accessions to find possible SMV-resistance genes. The results revealed 72 single nucleotide polymorphism (SNP) loci on chromosome 13 closely associated with SMV resistance, and a total of 40 genes were discovered within the candidate regions. By integrating the results of gene functional annotation and haplotype analysis, Glyma.13g176600 encoding a membrane attack complex/perforin (MACPF) domain-containing protein and Glyma.13g177000 encoding a DUF761-containing protein were identified as the most probable candidate genes associated with SMV resistance. Overall, the GFP-based rapid evaluation system developed in this study will facilitate breeding for resistance to SMV in soybean.
1. Introduction
Soybean (Glycine max (L.) Merr.) is a globally significant agricultural commodity, serving as a vital source of edible oil, plant-based protein, and biofuel feedstock while supporting both human nutrition and livestock production systems [1]. The cultivation of soybean is challenged by a range of biotic and abiotic stresses that compromise both yield and quality. Among these threats, viral diseases pose particular concern, with infections by plant viruses like soybean mosaic virus (SMV), bean pod mottle virus (BPMV), cucumber mosaic virus (CMV), tobacco ringspot virus (TRSV), and alfalfa mosaic virus (AMV) emerging as major constraints to soybean production [2,3]. SMV is one of the most common and devastating viruses infecting soybean, causing significant losses in soybean production all over the world [4].
SMV is a member of the genus Potyvirus in the family Potyviridae. It has a single-stranded positive-sense RNA genome of about 10 kb inside filamentous viral particles of about 750 nm in length. The genome contains a large open reading frame (ORF) that is translated and cleaved into 10 mature proteins (P1, HC-Pro, P3, 6K1, CI, 6K2, VPg, NIa-Pro, NIb, and CP), and a small ORF which expresses a fusion protein (P3N-PIPO) by a frameshift in the P3 cistron [5]. SMV can be transmitted through seeds, aphids, and mechanical inoculation, causing various symptoms in soybean such as leaf mosaic, distortion, vein necrosis, and seedcoat mottling [6,7,8]. The pathogenicity of SMV is co-determined by both the viral genotype and the host genotype [2]. Consequently, SMV isolates were grouped into various strains based on differences in their pathogenicity across various soybean varieties in the major of global soybean-producing regions; for example, the G1–G7 strains in USA and SC1–SC21 strains in China [9,10].
Breeding and planting soybean cultivars with resistance is the most effective, economical, and eco-friendly strategy to reduce the SMV damage [11]. To date, several independent loci for SMV resistance have been identified. Four dominant loci including Rsv1, Rsv3, Rsv4, and Rsv5 were identified to confer resistance to G1–G7 strains in America [12,13,14,15,16]. A series of Rsc loci for resistance to SCs strains in China including Rsc3, Rsc4, Rsc5, Rsc6, Rsc7, Rsc8, Rsc10, Rsc11, Rsc12, Rsc13, Rsc14, Rsc15, Rsc17, Rsc18, Rsc20, and Rsc21 were also identified [6,17,18,19,20,21,22,23,24]. By positional cloning, Ishibashi et al. demonstrated that Rsv4 encodes an RNase H family protein with dsRNA-degrading activity, which can target and degrade viral dsRNA [14]. However, the precise resistance genes have yet to be cloned from other Rsv or Rsc loci to date, although multiple candidate genes, including NLR protein encoding genes, have been identified in numerous loci [25].
Accurate disease resistance phenotyping is fundamental to the study of plant disease resistance. The evaluation of symptom severity, such as calculating disease index (DI), is the most prevalent method for assessing viral disease resistance, although it is susceptible to environmental changes and subjective judgments of investigators [26]. Measuring viral load in inoculated plants through laboratory techniques such as quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) is also widely used for evaluating viral disease resistance [27,28]. However, these laboratory techniques entail substantial time and financial commitments while requiring specialized personnel, rendering them poorly suited for large-scale resistance.
In this study, we constructed a green fluorescent protein (GFP)-tagged SMV recombinant virus (SMV-GFP) based on the full-length cDNA infectious clone vector, which served as an indicator of viral load in soybean leaves. Using this system, a total of 286 soybean germplasm resources were inoculated by SMV-GFP, and the relative fluorescence areas in the systemic leaves were used for genome-wide association study (GWAS) to identify genomic regions associated with SMV-resistance.
2. Materials and Methods
2.1. Plant Materials
A total of 286 soybean germplasm resources, including 122 cultivars, 88 landraces and 76 wild soybeans, for which the whole genome had been resequenced by our research team previously [29], were used in this work (Supplementary Table S1). All the soybean accessions were sown in plastic pots 15 cm in diameter and 20 cm in height. Three plants were grown in each pot, and three pots per soybean accession were used for subsequent virus inoculation. They were grown in an insect-proof net greenhouse under a 14 h light and 10 h dark photoperiod at a temperature of 23 ± 3 °C.
2.2. Construction of the GFP-Tagged SMV Recombinant Virus
The SMV-SC7 strain used in this work was kindly provided by Prof. Haijian Zhi from the Nanjing Agricultural University. To construct an SMV-SC7 infectious clone vector harboring a GFP gene insertion between P1 and HC-Pro cistrons, the SMV genome was divided into two fragments for PCR amplification, fragment-N (5′UTR+P1) and fragment-C (HC-Pro+P3+6K1+CI+ 6K2+NIa+NIb+CP+3′UTR). The DNA fragment of GFP was amplified using the primer pair GFP-F/GFP-R (Supplementary Table S2), with coding sequences for the P1 cleavage site (DIQHY/S) introduced at the 5′ end and the NIa cleavage site (ESVSLQ/SQNPE) at the 3′ end. The vector backbone of pCB301-2μ-HDV was linearized by PCR amplification with primers pCB-F/pCB-R (Supplementary Table S2). All of the above fragments were co-transformed into Y2H Gold yeast cells for homologous recombination and positive yeast colonies were screened on tryptophan-lacking media [30]. The resulting vector, designated pSMV-GFP, was isolated from yeast cells using E.Z.N.A Yeast Plasmid Mini Kit (Omega Bio-tek, Norcross, GA, USA) and transformed into Agrobacterium tumefaciens strain GV3101 via electrotransformation for agroinfiltration.
The GV3101 isolate containing pSMV-GFP was grown overnight in LB media, adjusted to a 0.6 optical density at 600 nm (OD600) in infiltration buffer (1M MgCl2, 1M MES, 200 μM acetosyringone) and induced at room temperature for 2 h before infiltration. The inoculum was infiltrated into the abaxial side of 3-week-old Nicotiana benthamiana plants using a 1 mL syringe without a needle [31]. The fluorescence of GFP was monitored using a LUYOR-3410 handheld 365 nm UV lamp. The systemic leaves of N. benthamiana plants with GFP fluorescence that RT-PCR showed were positive for SMV were collected and used as the viral source of SMV-GFP.
2.3. Resistance Assessment Based on Fluorescent Area
In order to evaluate the resistance of soybean germplasm resources to SMV, all the 286 soybean accessions were rub-inoculated with SMV-GFP. In brief, the SMV-GFP-infected N. benthamiana leaves were homogenized in 0.01 M phosphate-buffered saline (PBS) solution (w/v 1:10) to prepare the inoculum. The inoculum was mixed with a portion of 600-mesh carborundum power, and rub-inoculated onto fully expanded primary leaves of two-week-old soybean plants using a paintbrush, followed by rinsing with tap water after 5 min. At 14 days post-inoculation (dpi), the uninoculated top young leaves of inoculated soybean plants were observed and photographed under a UV lamp for further analysis. The ratio of fluorescent area to the whole leaf area, designed as relative fluorescence area (RFA) in this work, was quantified through grayscale-based image analysis using ImageJ v1.8 software (National Institutes of Health, Rockville, MD, USA) by applying an automated thresholding algorithm that discriminates fluorescent signals from background based on pixel intensity distributions.
2.4. Genome-Wide Association Study
The whole genome of all the 286 soybean germplasm resources had been re-sequenced using the BGI platform [29]. After filtering out sites with a minor allele frequency of less than 0.05 and a missing rate of more than 20%, a total of 7,470,624 high-quality single nucleotide polymorphism sites (SNPs) screened out from the genome sequences were used for GWAS. Based on the RFA values, GWAS was conducted using the R package rmvp (V 1.0.8) (parameter: nPC.MLM = 20 and vc.method = “EMMA”) with mixed linear models (MLMs) [32,33]. The effective number of sites in the soybean resource population was calculated using GEC software (v0.2) [34], and the significant threshold for the genome-wide association analysis was set as 1/N, where N represents the effective number of sites [35]. The GWAS results of quantile–quantile plot (Q–Q plot) and Manhattan plot analysis were generated using the R package CMplot (V 4.4.1).
2.5. Linkage Disequilibrium (LD) Decay Distance Analysis
LD decay distance analysis was performed on the soybean germplasm resource population consisting of 286 accessions using the PopLDdecay software (v3.41) (parameters: MaxDist 800). The LD decay distance for the population was determined as the distance at which the r2 value dropped to half of its maximum.
2.6. Candidate Gene Analysis
Based on the reference genome Glycine max Wm82.a2.v1 (https://phytozome-next.jgi.doe.gov (accessed on 1 May 2025), genes within the regions flanking each significant SNP by the population-averaged LD decay distance (7.6 kb) were selected as candidate genes. The function of candidate genes was annotated utilizing online databases QuickGO (https://www.ebi.ac.uk/QuickGO/ (accessed on 5 May 2025) and Soybase (https://www.soybase.org/ (accessed on 5 May 2025). Genes with annotations related to disease resistance were retained for further analysis. The haplotype analysis was conducted using the R package vcfR (V 1.15.0), and haplotypes containing more than 5% of the total population were considered as predominant haplotypes. The statistical analysis of RFA values among different predominant haplotypes was conducted using the GraphPad Prism 5.0 (GraphPad Prism Software Inc., San Diego, CA, USA).
2.7. Double Antibody Sandwich ELISA (DAS-ELISA)
To determine the extent of viral particle accumulation in SMV-infected soybean leaves, an entire sampled leaf was flash-frozen in liquid nitrogen and ground into fine powder. A subsample of the powdered tissue was homogenized with 0.01 M phosphate-buffered saline (PBS) solution (w/v 1:10) to prepare analytical specimens. Viral quantification was analyzed by the SMV DAS-ELISA kit (Agdia Inc., Elkhart, IN, USA) following the manufacturer’s protocol. The OD405 was measured using a microplate reader (Thermo Multiskan MK3, Shanghai, China). Each sample was tested in triplicate.
2.8. qRT-PCR
Total RNA was extracted and used to synthesize the first-strand cDNA using the TRIzol reagents (Invitrogen, Carlsbad, CA, USA) and the PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China), respectively, according to the manufacturer’s instructions. qPCR was performed with specific primers (Supplementary Table S2) using the ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China). GmUKN1 was selected as the reference gene, and the expression level was quantified using the 2−∆∆Ct method.
3. Results
3.1. Construction of the GFP-Tagged SMV Recombinant Virus
Using yeast homologous recombination technology, the ORF sequence of GFP was inserted in-frame between the P1 and HC-Pro genes of SMV-SC7, and the recombinant SMV-GFP cDNA was integrated into the yeast-agrobacterium shuttle vector pCB301-2μ-HDV under the control of the CaMV 35S promoter (Figure 1). The recombinant plasmid pSMV-GFP was delivered into 4-week-old N. benthamiana plants via agroinfiltration. At 4 dpi, scattered green fluorescent spots were first observed in the inoculated leaves under a handheld 352 nm UV light. The green fluorescent spots gradually increased in number and size, until they covered the entire infiltrated areas (Figure 2A). At 10 dpi, sporadic green fluorescent spots began to appear on the non-inoculated systemic leaves, and became more obvious in the vein areas (Figure 2A). The infection of SMV-GFP on systemic leaves was also confirmed by RT-PCR. Meanwhile, the SMV-GFP-inoculated N. benthamiana plants did not exhibit any disease symptoms (Figure 2A).
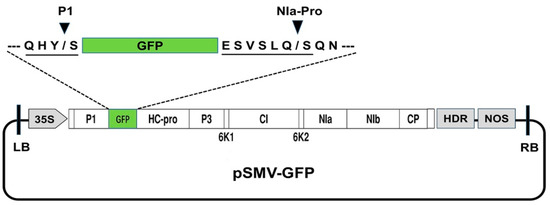
Figure 1.
Schematic representation of the infectious clone of GFP-tagged SMV. Between the T-DNA left border (LB) and right border (RB), the infectious clone vector contains a cauliflower mosaic virus 35S promoter (35S), full-length cDNA of the SMV-SC7 with GFP gene inserted between P1 and HC-Pro cistrons, a cis-cleaving ribozyme sequence from hepatitis delta virus (HDR), and a NOS terminator (NOS). The P1 cleavage site and NIa-Pro cleavage site were added to the N-terminal and C-terminal of GFP, respectively.
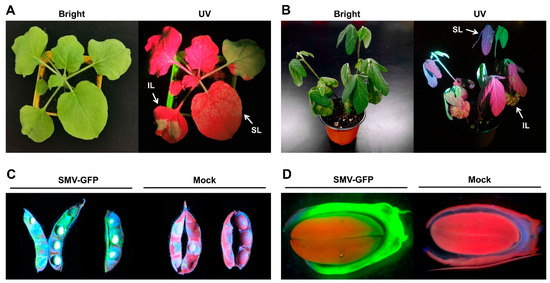
Figure 2.
GFP expression in SMV-GFP-inoculated (A) Nicotiana benthamiana at 10 days post-inoculation (dpi), (B) susceptible soybean cultivar SN14 at 10 dpi, (C) pod, and (D) seed in maturation stage. IL: inoculated leaf; SL: non-inoculated systemic leaf.
3.2. Infectivity of SMV-GFP on Soybean
The SMV-GFP was rub-inoculated onto the leaves of SMV-susceptible soybean cultivar SN14. Starting from 3 dpi, scattered green fluorescent spots were observed on the inoculated leaves under UV lamp, although no disease symptoms were visible under white light. Brown necrotic spots and partial vein necrosis began to appear on the inoculated leaves at 5 dpi. At 7–10 dpi, the non-inoculated systemic leaves from all of the inoculated soybean plants began showing symptoms of mosaic and distortion with green fluorescence (Figure 2B). By rub-inoculation, the progeny SMV-GFP from diseased systemic leaves was able to continuously infect healthy soybean plants, confirming that the pathogenicity of this recombinant virus was stable. Like the wild-type SMV-SC7 strain, the SMV-GFP recombinant virus was also incapable of being transmitted to offspring plants. It was consistent with this phenomenon that the green fluorescence could be observed in pod and seedcoat, while not in seed embryo (Figure 2C,D). Taken together, these results confirmed that the constructed SMV-GFP recombinant virus not only can effectively infect soybean, but also showed similar pathogenicity to the wild SMV strain.
3.3. SMV-GFP Can Be Quantified by Green Fluorescence
In order to determine whether green fluorescence can be used to quantify SMV-GFP content in soybean leaves, we proposed the concept of RFA that is defined as the ratio of fluorescent area to the total leaf area and can be calculated using ImageJ software through pixelated analysis. A total of 15 soybean leaves with varying RFA values were randomly selected (Figure 3A), and the relative levels of genomic RNA and viral protein of SMV-GFP in these samples were measured by qRT-PCR and DAS-ELISA, respectively. The correlation analysis results showed that the RFA values exhibited a significantly positive correlation (p < 0.0001) with both the viral genomic content (R2 = 0.9603) (Figure 3B) and viral protein content (R2 = 0.9069) (Figure 3C). It was indicated that the RFA value can be used to characterize the viral load in soybean leaf samples.
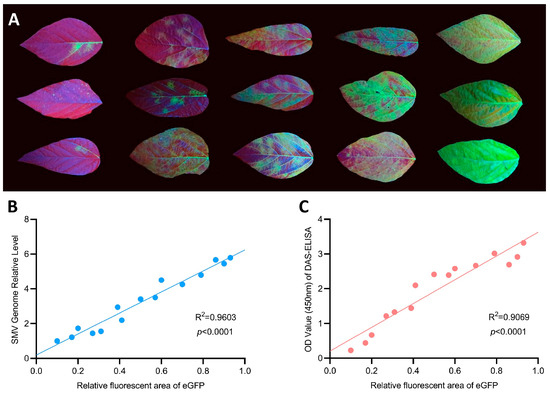
Figure 3.
Correlation analysis between the relative fluorescence area (RFA) value and viral load in soybean leaves. (A) Samples of soybean systemic leaves exhibiting varying levels of RFA values following inoculation of SMV-GFP. (B) Scatter plot of RFA value and viral genomic RNA content determined by qRT-PCR. (C) Scatter plot of RFA value and viral protein content determined by DAS-ELISA.
3.4. Phenotypic Analysis of RFA in Soybean Germplasms from Northeast China
In order to apply the RFA-based virus content detection for mining SMV-resistant genes of soybean, a total of 286 soybean germplasm resources from Northeast China were mechanically inoculated by SMV-GFP. The results showed that the RFA values of the uninoculated top young leaves of the 286 soybean accessions ranged from 0% to 100%, at 14 dpi, with average value and median value of 43.83% and 37.14%, respectively (Supplementary Table S1). Among the 286 evaluated accessions, the RFA values of 114 accessions were from 0% to 20%, 35 were from 20% to 40%, 32 were from 40% to 60%, 25 were from 60% to 80%, and 80 were from 80% to 100% (Figure 4A). A total of 85 accessions, including 63 cultivars, 15 landraces, and 7 wild soybeans, showed no green fluorescence on the uninoculated top young leaves, demonstrating their high resistance to SMV (Supplementary Table S1).
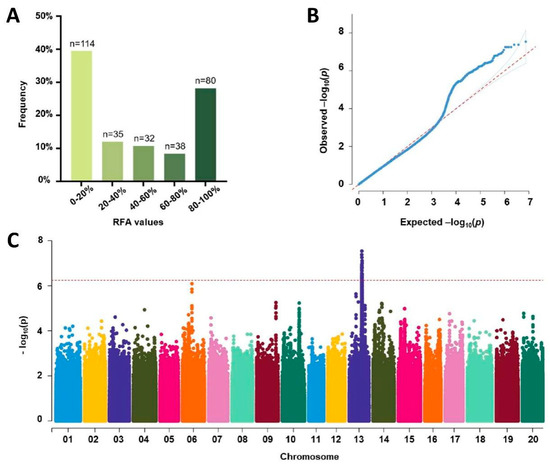
Figure 4.
Genome-wide association study (GWAS) for soybean mosaic virus (SMV) resistance. (A) Frequency distribution of the relative fluorescence area (RFA) values of 286 soybean germplasm resources. (B) Quantile–quantile plot of GWAS result. (C) Manhattan plot of GWAS result. The horizontal red line indicates the significance threshold (−log10P = 6.24).
3.5. GWAS for SMV Resistance
Whole genome resequencing (WRGS) was performed on all of the 286 soybean germplasm resources, and a total of 7,470,624 high-quality SNPs were screened out from the genome sequences previously, which were used for GWAS in this study [29]. Quantile–quantile plots (Q–Q plots) of the GWAS result showed significant upwards deviation beginning from the expected p value as the −log10P increased to approximately 3.5, indicating a significant association between phenotype and genotype (Figure 4B). A total of 72 SNPs exceeding the significant threshold with Bonferroni correction (−log10P ≥ 6.24), located between the physical positions 29060125 and 30171041 on chromosome 13, were identified to be significantly associated with RFA values (Figure 4C and Supplementary Table S3).
3.6. Candidate Gene Prediction
A linkage disequilibrium (LD) analysis was performed to determine the suitable chromosomal region for candidate gene prediction. The results showed that the physical decay distance of LD, equal to half the maximum value of r2, was about 7.6 kb. Based on the GWAS results, a total of 40 candidate genes were found in the 7.6 kb interval upstream and downstream of the significant SNPs, according to the reference genome Glycine max Wm82.a2.v1, and the gene functions were predicted by GO annotations. Five GO terms including defense response (GO:0006952), pathogenesis (GO:0009405), plant-type hypersensitive response (GO:0009626), programmed cell death (GO:0012501), and regulation of salicylic acid-mediated signaling pathway (GO:2000031) were found to be associated with disease resistance, and five candidate genes (Glyma.13g176600, Glyma.13g177000, Glyma.13g184800, Glyma.13g184900, and Glyma.13g187900) contained in these GO terms were selected for further analysis (Supplementary Table S4). Glyma.13g176600 encodes a membrane attack complex/perforin (MACPF) domain-containing protein that plays vital roles in immune response [36,37]. Glyma.13g177000 encodes a protein containing a DUF761 domain, which has been found to be involved in plant defense responses [38]. Glyma.13g184800, Glyma.13g184900, and Glyma.13g187900 encode proteins containing a leucine-rich repeat (LRR) domain that all share the GO term of defense response.
Haplotype polymorphism analyses showed that Glyma.13g176600 had four predominant haplotypes, Hap1, Hap5, Hap2, and Hap7, accounting for 52.10%, 15.73%, 13.64%, and 13.29% of all the 286 soybean accessions, respectively. The RFA values of soybean accessions containing Hap2 of Glyma.13g176600 were significantly lower than those containing other three predominant haplotypes (Figure 5). Glyma.13g177000 had five predominant haplotypes, Hap1, Hap4, Hap2, Hap3, and Hap10, which accounted for 47.55%, 26.57%, 11.19%, 7.34%, and 5.59% of the 286 accessions, respectively, and Hap2 showed a significantly lower RFA value than other predominant haplotypes (Figure 5). It was worth noting that all of the 32 soybean accessions carrying Hap2 of Glyma.13g177000 were included in the 39 accessions carrying Hap2 of Glyma.13g176600, indicating potential tight linkage between the two candidate genes. Glyma.13g184800 and Glyma.13g184900 had two predominant haplotypes, and Glyma.13g187900 had three predominant haplotypes. However, the RFA values showed no significant differences among the soybean accessions carrying these predominant haplotypes for these three genes (Figure 5). Finally, integrating the findings from the GWAS, gene function annotation, and haplotype analysis, Glyma.13g176600 and Glyma.13g177000 were identified as strong candidate genes associated with SMV resistance.
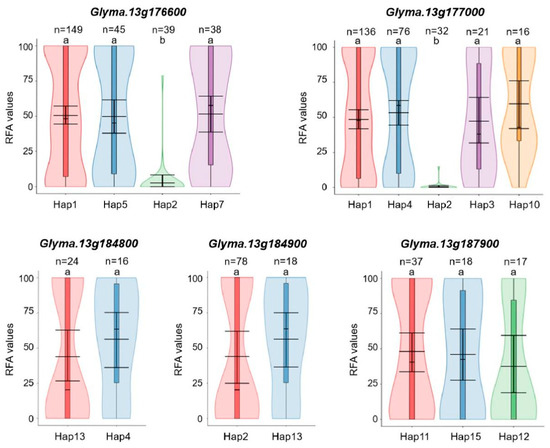
Figure 5.
Differences in the relative fluorescence area (RFA) values among accessions carrying different predominant haplotypes of Glyma.13g176600, Glyma.13g177000, Glyma.13g184800, Glyma.13g184900, and Glyma.13g187900. Different letters indicate significant differences (p < 0.05) among groups.
4. Discussion
Full-length cDNA infectious clones of RNA viruses are powerful genetics tools for virus studies, such as those on viral gene function, genome expression, replication, and interaction mechanisms with the host [39]. The successful construction of fluorescent protein-tagged recombinant viruses using infectious clones has been achieved for a variety of plant viruses, like tobacco mosaic virus (TMV) [40], cucumber mosaic virus (CMV) [41], and tomato chlorosis virus (ToCV) [42]. El-Gebaly et al. constructed a GFP-tagged tobacco rattle virus (TRV-GFP) and developed a rapid screening system to identify formulations that enhance plant resistance to viral infection [43]. However, most of the fluorescent protein-tagged recombinant viruses were used for tracking viral infection on specific host plants, while their application in large-scale evaluation of disease resistance across diverse varieties has rarely been documented. In this study, we constructed a GFP-tagged SMV recombinant virus, and developed a rapid evaluation system for SMV resistance that can be used to identify possible SMV-resistance genes via GWAS. This work would make a contribution to the development of disease-resistant breeding of soybean.
Potyvirus is the largest genus of plant viruses causing serious losses in a wide range of crops across the globe [44]. However, the construction of infectious clones for potyviruses is technically challenging due to the relatively large genome size of about 10 kb and the toxic effect of certain viral components on Escherichia coli growth [45]. Sun et al. [30] developed a yeast-Agrobacterium shuttle vector pCB301-2μ-HDV that enables highly efficient homologous recombination (HR) in yeast for vector assembly, and the extracted yeast plasmids can be transformed directly into Agrobacterium, obviating the toxic effect on E. coli in the process of cloning. In this study, using this yeast HR system, we inserted the coding sequence of GFP in-frame between the P1 and HC-Pro genes and constructed a GFP-tagged SMV recombinant virus (SMV-GFP). In potyviruses, their ten mature viral proteins are derived from the proteolytic cleavage of a large polyprotein encoded on the genomic RNA. In SMV-GFP-infected cells, the expression level of GFP should theoretically be consistent with that of other viral proteins and correlate with the viral genomic RNA content. Therefore, we proposed the concept of relative fluorescence area (RFA) and proved that it can be applied for quantifying viral content in soybean leaves.
Using this RFA-based virus content detection method, in this study, the resistance to SMV of 286 soybean germplasm resources from Northeast China was evaluated, and the obtained RFA values ranging from 0 to 100% were used for GWAS to screen the SMV resistance-associated SNPs. A total of 72 significantly SNPs were identified on chromosome 13 (physical position: 29.06–30.17 Mb). Chromosome 13 is internationally recognized as a critical genetic region for SMV-resistance in soybean, on which multiple SMV-resistance loci, like Rsv1 [46], Rsc3 [18], Rsc3Q [47], Rsc12 [48], Rsc14 [19], Rsc18 [49], and Rsc20 [50], have been identified to date. The interval on chromosome 13 identified in this study overlapped partially with the previously reported Rsv1 and Rsc3Q loci, and three candidate genes identified in this work encoding LRR kinase, including Glyma.13g184800, Glyma.13g184900, and Glyma.13g187900, were also among the candidate gene lists of Rsv1 and Rsc3Q [46,47]. However, no significant differences were found among the RFA values of soybean accessions carrying the predominant haplotypes for these three genes (Figure 5).
Glyma.13g176600 encodes a MACPF domain-containing protein. MACPF domain proteins were reported to play vital roles in innate and adaptive immunity in vertebrates [37]. This domain is conserved across mammals and plants [37]. Some studies have shown that this type of protein plays a significant role in the plant immune response. For example, a MACPF domain-containing protein in Arabidopsis, NSL1, was indicated to be associated with programmed cell death in microbe-associated molecular pattern (MAMP)-triggered immunity [51]. Wang et al. [37] indicated that a MACPF domain protein of rice, OsCAD1, negatively regulates salicylic acid levels to modulate plant growth and immunity against Xanthomonas oryzae pv. oryzae. By GWAS analysis, Qin et al. [36] identified Glyma.13g176600 as a candidate gene of resistance to Phytophthora sojae. Meanwhile, MACPF domain proteins also play roles in abiotic stress response. Chen et al. [52] found that silencing GhMACPF26 could increase the cold tolerance of cotton. With regard to another SMV resistance candidate gene identified in this study, Glyma.13g177000 encodes a protein containing a DUF761 domain. The proteins containing the domain of unknown function (DUF) have been proved to play various roles in plant development and stress responses [53]. Zhang et al. [38] indicated that overexpression of DUF761 constitutively activated defense responses and enhanced resistance to Pseudomonas syringae in Arabidopsis thaliana. The function of DUF761 in A. thaliana was also associated with plant architecture, including altered leaf and root morphology, inhibition of inflorescence stem elongation, and defects in flower development [38].
Taken together, based on the GWAS results and gene functional annotations, by further integrating the haplotype analysis findings, it was indicated that Glyma.13g176600 and Glyma.13g177000 were associated with resistance to SMV infection. However, confirmation through experimental support, such as gene knockout and gene supplementation, is required for further investigation.
5. Conclusions
In conclusion, this study constructed a GFP-tagged SMV recombinant virus (SMV-GFP) and developed a rapid evaluation system for viral load in soybean leaves based on the RFA value caused by SMV-GFP. Using this system, the resistance of 286 soybean germplasms from Northeast China to SMV was evaluated. A total of 72 SMV resistance-associated SNPs and 40 candidate genes on chromosome 13 were identified by GWAS based on RFA values. Glyma.13g176600 encoding a MACPF domain-containing protein and Glyma.13g177000 encoding a DUF761-containing protein were identified as the most probable candidate genes associated with SMV resistance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15081960/s1, Table S1: Relative fluorescence area (RFA) values of 286 evaluated soybean accessions; Table S2: Primers used for construction of SMV-GFP infectious clone and qRT-PCR of SMV genomic RNA; Table S3: Single nucleotide polymorphism sites (SNPs) identified to be significantly associated with relative fluorescence area (RFA) values; Table S4: GO annotation of the 40 genes within the candidate regions determined by GWAS.
Author Contributions
Conceptualization, J.Z. and S.S.; methodology, J.Z., H.S., and Y.G.; software, H.T. and Y.H. (Yun Hao); validation, Y.H. (Yuxi Hu) and M.Z.; formal analysis, J.Z., H.S. and H.T.; investigation, Y. Hu, Y.H. (Yun Hao); resources, Y.H. (Yun Hao) and Q.C.; data curation, H.T.; writing—original draft preparation, J.Z. and H.S.; writing—review and editing, D.X. and S.S.; visualization, H.T.; supervision, Q.C.; project administration, Q.C. and D.X.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Heilongjiang Provincial Natural Science Foundation of China (YQ2024C008).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to the Collaborative Innovation Center of Soybean Biotechnology and Nutrition Efficiency, Henan Province.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Coradi, P.C.; Müller, A.; Souza, G.A.C.; Steinhaus, J.I.; Wagner, R. Quality of soybean cultivars in the drying and storage processes in real scale and experimental. J. Food Process. Eng. 2020, 43, e13418. [Google Scholar] [CrossRef]
- Widyasari, K.; Alazem, M.; Kim, K. Soybean resistance to Soybean mosaic virus. Plants 2020, 9, 219. [Google Scholar] [CrossRef]
- Hill, J.H.; Whitham, S.A. Control of virus disease in soybean. Adv. Virus Res. 2014, 90, 355–390. [Google Scholar]
- Ross, J.P. Effect of aphid-transmitted Soybean mosaic virus on yields of closely related resistant and susceptible soybean lines. Crop Sci. 1997, 17, 869–872. [Google Scholar] [CrossRef]
- Hajimorad, M.R.; Domier, L.L.; Tolin, S.A.; Whitham, S.A.; Saghai Maroof, M.A. Soybean mosaic virus: A successful potyvirus with a wide distribution but restricted natural host range. Mol. Plant Pathol. 2018, 19, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Liu, S.; Karthikeyan, A.; Wang, T.; Niu, H.; Yin, J.; Yang, Y.; Wang, L.; Yang, Q.; Zhi, H.; et al. Fine-mapping and identification of a novel locus Rsc15 underlying soybean resistance to Soybean mosaic virus. Theor. Appl. Genet. 2017, 130, 2395–2410. [Google Scholar] [CrossRef]
- Koning, G.; TeKrony, D.M.; Ghabrial, S.A. Soybean seedcoat mottling: Association with Soybean mosaic virus and Phomopsis spp. Seed Infection. Plant Dis. 2003, 87, 413–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Senda, M. Patterning of virus-infected Glycine max seedcoat is associated with suppression of endogenous silencing of chalcone synthase genes. Plant Cell 2004, 16, 807–818. [Google Scholar] [CrossRef]
- Li, K.; Yang, Q.H.; Zhi, H.J.; Gai, J.Y. Identification and distribution of Soybean mosaic virus strains in Southern China. Plant Dis. 2010, 94, 351–357. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; An, J.; Huang, W.; Liu, X.; Xue, Y.; Luan, X.; Lin, F.; Sun, L. Pathogenicity and genome-wide sequence analysis reveals relationships between Soybean mosaic virus strains. Arch. Virol. 2022, 167, 517–529. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Li, K.; Jiang, H.; Ren, R.; Li, C.; Zhi, H.; Chen, S.; Gai, J. Inheritance, fine-mapping, and candidate gene analyses of resistance to Soybean mosaic virus strain SC5 in soybean. Mol. Genet. Genom. 2017, 292, 811–822. [Google Scholar] [CrossRef]
- Hayes, A.J.; Ma, G.R.; Buss, G.R.; Maroof, M.A.S. Molecular marker mapping of RSV4, a gene conferring resistance to all known strains of Soybean mosaic virus. Crop Sci. 2000, 40, 1434–1437. [Google Scholar] [CrossRef]
- Hayes, A.J.; Maroof, M.A.S. Targeted resistance gene mapping in soybean using modified AFLPs. Theor. Appl. Genet. 2000, 100, 1279–1283. [Google Scholar] [CrossRef]
- Ishibashi, K.; Saruta, M.; Shimizu, T.; Shu, M.; Anai, T.; Komatsu, K.; Yamada, N.; Katayose, Y.; Ishikawa, M.; Ishimoto, M.; et al. Soybean antiviral immunity conferred by dsRNase targets the viral replication complex. Nat. Commun. 2019, 10, 4033. [Google Scholar] [CrossRef]
- Suh, S.J.; Bowman, B.C.; Jeong, N.; Yang, K.; Kastl, C.; Tolin, S.A.; Maroof, M.A.S.; Jeong, S.C. The Rsv3 locus conferring resistance to Soybean mosaic virus is associated with a cluster of coiled-coil nucleotide-binding leucine-rich repeat genes. Plant Genome. 2011, 4, 55–64. [Google Scholar] [CrossRef]
- Klepadlo, M.; Chen, P.Y.; Shi, A.N.; Mason, R.E.; Korth, K.L.; Srivastava, V.; Wu, C.J. Two tightly linked genes for Soybean mosaic virus resistance in soybean. Crop Sci. 2017, 57, 1844–1853. [Google Scholar] [CrossRef]
- Fu, S.; Zhan, Y.; Zhi, H.; Gai, J.; Yu, D. Mapping of SMV resistance gene Rsc-7 by SSR markers in soybean. Genetica 2006, 128, 63–69. [Google Scholar] [CrossRef]
- Li, C.; Adhimoolam, K.; Yuan, Y.; Yin, J.L.; Ren, R.; Yang, Y.Q.; Zhi, H.J. Identification of candidate genes for resistance to Soybean mosaic virus strain SC3 by using fine mapping and transcriptome analyses. Crop Pasture Sci. 2017, 68, 156–166. [Google Scholar] [CrossRef]
- Li, H.C.; Zhi, H.J.; Gai, J.Y.; Guo, D.Q.; Wang, Y.W.; Li, K.; Bai, L.; Yang, H. Inheritance and gene mapping of resistance to Soybean mosaic virus strain SC14 in soybean. J. Integr. Plant Biol. 2006, 48, 1466–1472. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Liu, N.; Yang, Z.; Zheng, G.; Zhi, H. Fine mapping and identification of the soybean RSC4 resistance candidate gene to Soybean mosaic virus. Plant Breed. 2011, 130, 653–659. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Yang, Y.; Liu, N.; Li, C.; Song, Y.; Zhi, H. Fine mapping and analyses of R (SC8) resistance candidate genes to Soybean mosaic virus in soybean. Theor. Appl. Genet. 2011, 122, 555–565. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, D.G.; Li, H.C.; Zheng, G.J.; Yang, Y.Q.; Li, H.W.; Zhi, H.J. Fine mapping of the R-SC14Q locus for resistance to Soybean mosaic virus in soybean. Euphytica 2011, 181, 127–135. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, D.; Zhang, H.; Shen, Y.; Yang, Y.; Li, K.; Wang, L.; Yang, Y.; Zhi, H.; Morris, B. Fine mapping of the RSC8 locus and expression analysis of candidate SMV resistance genes in soybean. Plant Breed. 2016, 135, 701–706. [Google Scholar] [CrossRef]
- Jiang, H.; Jia, H.; Hao, X.; Li, K.; Gai, J. Mapping locus RSC11K and predicting candidate gene resistant to Soybean mosaic virus SC11 through linkage analysis combined with genome resequencing of the parents in soybean. Genomics 2022, 114, 110387. [Google Scholar] [CrossRef]
- Usovsky, M.; Chen, P.; Li, D.; Wang, A.; Shi, A.; Zheng, C.; Shakiba, E.; Lee, D.; Vieira, C.C.; Lee, Y.C.; et al. Decades of genetic research on Soybean mosaic virus resistance in soybean. Viruses 2022, 14, 1122. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Ren, R.; Wang, T.; Gao, L.; Song, P.; Yang, Y.; Zhi, H.; Li, K. Development of comprehensive serological techniques for sensitive, quantitative and rapid detection of Soybean mosaic virus. Int. J. Mol. Sci. 2022, 23, 9457. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ding, X.; Li, K.; Liao, W.; Zhong, Y.; Ren, R.; Liu, Z.; Karthikeyan, A.; Zhi, H. Characterization of Soybean mosaic virus resistance derived from inverted repeat-SMV-HC-Pro genes in multiple soybean cultivars. Theor. Appl. Genet. 2015, 128, 1489–1505. [Google Scholar] [CrossRef]
- Jin, S.; Tian, H.; Ti, M.; Song, J.; Hu, Z.; Zhang, Z.; Xin, D.; Chen, Q.; Zhu, R. Genetic analysis of soybean flower size phenotypes based on computer vision and genome-wide association studies. Int. J. Mol. Sci. 2024, 25, 7622. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, D.; Liu, Y.; Huang, C.; Zhang, W.; Li, Z. Rapid construction of complex plant RNA virus infectious cDNA clones for Agroinfection using a yeast-E. coli-Agrobacterium shuttle vector. Viruses 2017, 9, 332. [Google Scholar] [CrossRef]
- Zaulda, F.A.; Yang, S.H.; Han, J.P.; Mlotshwa, S.; Dorrance, A.; Qu, F. A cowpea severe mosaic virus-based vector simplifies virus induced gene silencing and foreign protein expression in soybean. Plant Methods 2022, 18, 116. [Google Scholar] [CrossRef]
- Yin, L.L.; Zhang, H.H.; Tang, Z.S.; Xu, J.Y.; Yin, D.; Zhang, Z.W.; Yuan, X.H.; Zhu, M.J.; Zhao, S.H.; Li, X.Y.; et al. rMVP: A memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Ersoz, E.; Lai, C.Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.M.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef]
- Li, M.X.; Yeung, J.M.; Cherny, S.S.; Sham, P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef]
- Li, N.; He, Q.; Wang, J.; Wang, B.; Zhao, J.T.; Huang, S.Y.; Yang, T.; Tang, Y.P.; Yang, S.B.; Aisimutuola, P.; et al. Super-pangenome analyses highlight genomic diversity and structural variation across wild and cultivated tomato species. Nat. Genet. 2023, 55, 852–860. [Google Scholar] [CrossRef]
- Qin, J.; Song, Q.; Shi, A.; Li, S.; Zhang, M.; Zhang, B. Genome-wide association mapping of resistance to Phytophthora sojae in a soybean [Glycine max (L.) Merr.] germplasm panel from maturity groups IV and V. PLoS ONE 2017, 12, e0184613. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, T.; Ruan, Z.; Tu, R.; He, Y.; Liu, Q.; Cheng, S.; He, G.; Shen, X. A MACPF protein OsCAD1 balances plant growth and immunity through regulating salicylic acid homeostasis in rice. Plant Cell Environ. 2025, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Huang, X. Characterization of an Arabidopsis thaliana DUF761-contaning protein with a potential role in development and defense responses. Theor. Exp. Plant Phys. 2019, 31, 303–316. [Google Scholar] [CrossRef]
- Seo, J.; Lee, H.; Kim, K. Systemic gene delivery into soybean by simple rub-inoculation with plasmid DNA of a Soybean mosaic virus-based vector. Arch. Virol. 2009, 154, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Baek, E.; Yoon, J.; Palukaitis, P. The use of a Tobacco mosaic virus-based expression vector system in chrysanthemum. Plant Pathol. J. 2017, 33, 429–433. [Google Scholar] [CrossRef]
- Kawakubo, A.; Gallois, J.; Nakahara, K.S. Monitoring systemic infection by cucumber mosaic virus using a small fluorescent protein iLOV in plants. J. Gen. Plant Pathol. 2023, 89, 47–52. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, Y.; Cho, Y.; Byun, H.; Seo, J. Engineering of stable infectious cDNA constructs of a fluorescently tagged tomato chlorosis virus. Virology 2024, 593, 110010. [Google Scholar] [CrossRef]
- El-Gebaly, F.E.; Ckurshumova, W.; Liu, J.; Fefer, M.; Krysmanski, E.C.; Cameron, R.K. Development of a rapid screen to identify formulations that enhance plant resistance to viral infection. Physiol. Mol. Plant Pathol. 2025, 139, 102783. [Google Scholar] [CrossRef]
- Revers, F.; García, J.A. Molecular biology of potyviruses. Adv. Virus Res. 2015, 92, 101–199. [Google Scholar]
- Bao, W.; Yan, T.; Deng, X.; Wuriyanghan, H. Synthesis of full-length cDNA infectious clones of Soybean mosaic virus and functional identification of a key amino acid in the silencing suppressor HC-Pro. Viruses 2020, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Y.-N.; Zhang, C.; Liu, X.-T.; Liu, C.-C.; Guo, R.; Niu, K.-X.; Zhu, A.-Q.; Yang, J.-Y.; Chen, J.-Q.; et al. Molecular mapping of the gene(s) conferring resistance to Soybean mosaic virus and bean common mosaic virus in the soybean cultivar raiden. Theor. Appl. Genet. 2019, 132, 3101–3114. [Google Scholar] [CrossRef]
- Zheng, G.J.; Yang, Y.Q.; Ying, M.A.; Yang, X.F.; Chen, S.Y.; Rui, R.E.; Wang, D.G.; Yang, Z.L.; Zhi, H.J. Fine mapping and candidate gene analysis of resistance gene RSC3Q to Soybean mosaic virus in Qihuang 1. J. Integr. Agric. 2014, 13, 2608–2615. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Wang, D.; Liu, N.; Zhi, H. Molecular mapping and marker assisted selection of Soybean mosaic virus resistance gene RSC12 in soybean. Legume Genomes Genet. 2010, 8, 41–46. [Google Scholar]
- Liu, S.; Cheng, Y.; Ma, Q.; Li, M.; Jiang, Z.; Xia, Q.; Nian, H. Fine mapping and genetic analysis of resistance genes, Rsc18, against Soybean mosaic virus. J. Integr. Agric. 2022, 21, 644–653. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Li, K.; Li, C.; Yin, J.; Li, N.; Yang, Y.; Song, Y.; Ren, R.; Zhi, H.; Gai, J. Fine-mapping and identifying candidate genes conferring resistance to Soybean mosaic virus SC20 in soybean. Theor. Appl. Genet. 2018, 131, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Sogame, M.; Hata, M.; Singkaravanit-Ogawa, S.; Piślewska-Bednarek, M.; Onozawa-Komori, M.; Nishiuchi, T.; Hiruma, K.; Saitoh, H.; Terauchi, R.; et al. Dysfunction of Arabidopsis MACPF domain protein activates programmed cell death via tryptophan metabolism in MAMP-triggered immunity. Plant J. 2017, 89, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Jian, H.; Wei, F.; Gu, L.; Hu, T.; Lv, X.; Guo, X.; Lu, J.; Ma, L.; Wang, H.; et al. Phylogenetic analysis of the membrane attack complex/perforin domain-containing proteins in Gossypium and the role of GhMACPF26 in cotton under cold stress. Front. Plant Sci. 2021, 12, 68227. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, T.D.; Thamm, A.M.; de Crecy-Lagard, V.; Hanson, A.D. Proteins of unknown biochemical function: A persistent problem and a roadmap to help overcome it. Plant Physiol. 2015, 169, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).