Abstract
Soil organic matter (SOM) decomposition is a critical biogeochemical process that regulates the carbon cycle, nutrient availability, and agricultural sustainability of cropland systems. Recent progress in multi-omics and microbial network analyses has provided us with a better understanding of the decomposition process at different spatial and temporal scales. Climate factors, such as temperature and seasonal variations in moisture, play a critical role in microbial activity and enzyme kinetics, and their impacts are mediated by soil physical and chemical properties. Soil mineralogy, texture, and structure create different soil microenvironments, affecting the connectivity of microbial habitats, substrate availability, and protective mechanisms of organic matter. Moreover, different microbial groups (bacteria, fungi, and archaea) contribute differently to the decomposition of plant residues and SOM. Recent findings suggest the paramount importance of living microbial communities as well as necromass in forming soil organic carbon pools. Microbial functional traits such as carbon use efficiency, dormancy, and stress tolerance are essential drivers of decomposition in the soil. Furthermore, the role of microbial necromass, alongside live microbial communities, in the formation and stabilization of persistent SOM fractions is increasingly recognized. Based on this microbial perspective, feedback between local microbial processes and landscape-scale carbon dynamics illustrates the cross-scale interactions that drive agricultural productivity and regulate soil climate. Understanding these dynamics also highlights the potential for incorporating microbial functioning into sustainable agricultural management, which offers promising avenues for increasing carbon sequestration without jeopardizing soil nutrient cycling. This review explores current developments in intricate relationships between climate, soil characteristics, and microbial communities determining SOM decomposition, serving as a promising resource in organic fertilization and regenerative agriculture. Specifically, we examine how nutrient availability, pH, and oxygen levels critically influence these microbial contributions to SOM stability and turnover.
1. Introduction
Soil organic matter (SOM) decomposition in agricultural landscapes is a fundamental biogeochemical process governing terrestrial carbon cycling, nutrient availability, and ecosystem functioning. SOM is mainly produced by incomplete decomposition and transformation of crop residues by microbes [1]. Croplands, which occupy approximately 12% of the Earth’s ice-free land surface, serve as a dynamic interface where plant-derived organic materials undergo continuous transformation through complex interactions between climatic drivers, soil properties, and microbial communities [2].
The structural and chemical compositions of crop residues vary significantly, greatly affecting their decomposition rates and stabilization potential [3]. For instance, if recalcitrants such as lignin, tannins, and phenols are high in crop residues, they hinder residue decomposition [4]. Likewise, aboveground crop residues, including plant stems and leaves, demonstrate better organic matter (OM) quality than belowground residues, such as roots [5]. Similarly, substrate availability and quality directly influence soil microbial turnover [6]. Therefore, if substrate availability is interrupted, microbes may undergo dormancy or their biomass reduces following cell death, and a decrease in microbial biomass leads to the accumulation of microbial necromass, which is considered an important source of stable SOM. Because microbial necromasses are heterogeneous in nature, they are used as labile substrates by living microorganisms [7].
New paradigm changes have highlighted the importance of microbial necromass as a central driver of stable SOC pools and have shifted the focus away from traditional views of decomposition as a loss process [8,9]. This recognition has significant implications for understanding how agricultural practices affect long-term soil carbon pools and fertility. Additionally, modeling techniques and frameworks at the “meso-scale” to “micro-scale” have been developed that provide a more mechanistic characterization of decomposition (not just simple temperature and moisture functions), such as trait-based decomposition models that mechanistically represent microbial community dynamics.
This review integrates the current knowledge of climate–soil–microbe interactions in croplands and explores how they determine the cycling of organic matter at multiple scales, ranging from molecular to landscape. In general, the organic matter decomposition process varies across a hierarchy of scales. For instance, different chemical bonds are broken down by microbial enzymes at the molecular scale [10,11]. Conversely, at the microaggregate scale, organic matter is typically present in the bound form in soil aggregates, which physically protect its decomposition by enzymatic activity [12,13]. At the field scale, differences exist in soil texture, topology, and farming practices (like tillage and fertilization), resulting in variations in decomposition rates [11,14]. Finally, at the landscape scale, regional climate patterns, vegetation types, and land use are key determinants of large-scale carbon cycling dynamics [15,16]. The article also assesses the impact of climate variability on microbial decomposition processes, explores the impact of soil environmental factors as context-dependent modifiers, and evaluates how microbial functional ecology mediates decomposition outcomes. The critical feedback between decomposition processes and broader agroecosystem functioning, current modeling capabilities, and priority areas for future research is also highlighted.
2. Organic Matter and the Soil Microbial Engine
2.1. Composition and Fate of SOM in Croplands
The chemical composition of crop residues is a primary driver of decomposition. The initial chemical composition of different crops varies significantly in carbon, nitrogen, carbon–nitrogen (C–N) ratio, and major organic fractions, including the soluble fraction, hemicellulose, cellulose, and lignin. These variations, especially in the C–N ratio and lignin content, affect the rate and nature of microbial decomposition and the eventual SOM formation. For instance, a lower C–N ratio indicates a more easily decomposable material, whereas a higher lignin content implies higher recalcitrance [17].
The SOM in croplands consists of diverse carbon pools with varying degrees of stability and turnover rates [18,19]. Particulate organic matter represents a more labile fraction, primarily derived from plant residues, and is easily accessible for microbial decomposition [20,21]. Conversely, mineral-associated organic matter forms through organo–mineral interactions and represents the most persistent soil carbon pool [19,22]. Carbon transformation and stabilization within these fractions are strongly influenced by climate, soil texture, and microbial traits [23,24,25]. For example, higher temperature and optimum moisture content trigger particulate organic carbon (POC) degradation by stimulating microbial activity and enzyme kinetics, thereby reducing turnover times [23,25,26]. However, simultaneously, these climatic conditions can indirectly promote the formation of mineral-associated organic carbon (MAOC) by stimulating the degradation of complex organic matter into simpler, microbial-derived molecules, which can easily adsorb on mineral surfaces [23,27,28]. Additionally, fine-textured soils (such as, clays) provide greater surface area and pore spaces for organo–mineral associations and physical protection; hence, promoting MAOC formation and stability compared to sandy soils [29,30,31]. This is further mediated by microbial traits; certain microbial communities (for example, K-strategists) are more efficient in producing necromass and metabolic byproducts that preferentially form MAOC, even under nutrient limitation or during drying–rewetting cycles that induce cell lysis and subsequent adsorption [32,33,34,35]. Therefore, the interaction between climate-mediated microbial activity, soil mineralogy, and microbial functional diversity is the key determinant controlling carbon allocation between labile POC and stable MAOC pools [24,27,31]. Recent studies have demonstrated that cropland soils typically contain lower SOM concentrations than natural ecosystems owing to frequent disturbances and reduced carbon inputs [36,37].
The fate of SOM in agricultural systems is largely determined by the balance between organic matter inputs from crop residues, root exudates, and external amendments and outputs through microbial decomposition and soil respiration [38,39]. Crop residues contribute to varying amounts of carbon with different chemical compositions, affecting the decomposition rates and stabilization potential [40]. The conversion of forest or grassland to cropland typically results in substantial SOC loss, often exceeding 50% of the original stock within decades of cultivation [36]. However, improved management practices can partially restore these losses by enhancing carbon inputs and reducing decomposition rates [38].
2.2. Key Microbial Groups and Their Decomposition Roles
Soil microbial communities in croplands are dominated by bacteria and fungi, which exhibit distinct functional roles in organic matter decomposition [41,42]. Bacterial communities, particularly Proteobacteria and Bacteroidetes, are typically enriched in agricultural soils and respond rapidly to changes in substrate availability and environmental conditions [41,43]. These copiotrophic organisms excel at decomposing readily available organic compounds and are particularly abundant in the rhizosphere, where root exudates provide labile carbon sources [40,42]. Fungal communities, dominated by ascomycetes and basidiomycetes, play crucial roles in decomposing more recalcitrant plant materials through their extensive hyphal networks and specialized enzyme systems [41,44].
Different extracellular enzymes released by these microbial groups can degrade organic compounds into their bioavailable forms. For instance, β-1,4-glucosidase (BG) and cellobiohydrolase (CBH) are essential for decomposing cellulose [45], whereas polyphenol oxidase and peroxidase are involved in decomposing lignin [46]. Other enzymes, such as β-1,4-N-acetylglucosaminidase (NAG), leucine aminopeptidase (LAP), and alkaline phosphatase (AP), can assist in nutrient mineralization [47]. The comparative activities of BG:AP, BG:NAG, and NAG:AP have been proposed as indicators of nutrient acquisition strategies (carbon versus phosphorus, carbon versus nitrogen, and nitrogen versus phosphorus) [10,48]. Table 1 lists the major groups microorganisms responsible for decomposition of organic matter in croplands, emphasizing their distinct ecological strategies and management responses [49,50].
Besides enzyme profiles, microbial functional traits are also largely responsible for decomposition [51]. For instance, strategies of microbial dormancy and resistance are primarily important; many soil microbes may become dormant (spore formation or reduced metabolic activity) under unfavorable conditions (such as drought and nutrient starvation), allowing them to survive and rapidly reactivate when conditions become favorable [52]. This accelerated resuscitation leads to episodic release of carbon and nutrients [53]. Additionally, microbial growth strategies (mostly identified as r- and K-strategists) influence their resource management and participation in the decomposition process [54]. For example, r-strategists (such as, many Proteobacteria and Bacteroidetes) are often copiotroph, with high growth rates and encoded enzymes for degradation of labile carbon, resulting in a dominance under high substrate availability, but with a relatively smaller contribution to stable SOM [55]. In contrast, K-strategists (such as, many fungi and Acidobacteria) are oligotrophs, with lower growth rates, increased CUE activity, and stronger allocation to decomposing recalcitrant compounds (for example, ligninolytic enzymes). They also contribute to the stable microbial necromass and long-term carbon sequestration, particularly in nutrient-poor or stable environments [56,57]. Understanding these characteristics provides a mechanistic link between microbial functional ecology (from their enzyme repertoires, survival and growth strategies) and decomposition outcomes across diverse soil conditions [56].

Table 1.
Key microbial groups involved in decomposition processes, their ecological strategies, and responses to climate and soil type and agricultural management practices.
Table 1.
Key microbial groups involved in decomposition processes, their ecological strategies, and responses to climate and soil type and agricultural management practices.
| Microbial Group | Primary Function | Habitat Preference | Response to Management | Influence of Climate and Soil Type | References |
|---|---|---|---|---|---|
| Proteobacteria | Rapid decomposition of labile organic compounds | Rhizosphere, nutrient-rich environments | Increases with fertilization and organic inputs | Favored by warmer temperatures and higher moisture in fertile, loamy soils. | [58,59,60,61] |
| Bacteroidetes | Decomposition of readily available substrates | Agricultural soils, organic-rich zones | Enhanced by agricultural practices | Prevalent in moist, neutral pH soils; sensitive to drought stress. | [62,63,64,65] |
| Ascomycetes | Decomposition of recalcitrant plant materials | Diverse soil environments | Sensitive to soil disturbance | Exhibit broad environmental tolerance; active across diverse soil types and moisture regimes, including dry conditions. | [66,67] |
| Basidiomycetes | Decomposition of complex organic compounds | Forest soils, organic matter-rich zones | Decreases with intensive cultivation | Prefer cooler, stable environments and higher moisture; dominant in forest soils with abundant lignin. | [68,69,70,71] |
| Actinobacteria | Decomposition of complex organic matter | Diverse soil conditions | Variable response to management | Resilient to desiccation and high pH; prominent in arid and alkaline soils. | [72,73,74] |
| Acidobacteria | Slow decomposition of stable organic compounds | Nutrient-poor, stable environments | Stable under low-input systems | Dominant in acidic, oligotrophic soils and stable, undisturbed environments; sensitive to rapid environmental shifts. | [60,75,76,77] |
Microbial community structure and diversity are important indicators of soil health [78]. Organic matter decomposition is a highly specialized process that depends on various microbial guilds, implying that management practices that influence microbial diversity, such as monoculture versus diverse crop rotations or the application of particular chemical fertilizers, will directly influence the decomposition rate and efficiency. For instance, the prevalence and activity of ligninolytic fungi are essential for the breakdown of recalcitrant molecules, with a significant impact on the rate of stable SOM formation [79]. The relative abundance and activity of bacterial and fungal communities significantly impact decomposition patterns and soil carbon cycling [43,80]. Therefore, planned rotation of crops increases microbial diversity and network complexity, which subsequently improves nutrient cycling and ecosystem functioning [40].
2.3. Microbial Necromass and Its Contribution to Stable SOM
Microbial necromass—the dead cells and cell-wall fragments of soil microbes—provides a critical link between fresh crop inputs and long-lived soil organic matter [32]. As microbes grow on crop residues, carbon is assimilated into biomass and ultimately released as necromass when cells die (via turnover, predation, or stress) [9,34]. This microbial residue carbon (bacterial and fungal necromass) is now recognized as a major SOM component [9,81]. Amino-sugar biomarker studies show necromass often makes up on around 30–60% of total SOC across ecosystems (higher in grasslands and croplands than in forests) [82,83]. For instance, cropland soils typically derive about half of their organic carbon from microbial necromass [84,85], reflecting the fact that large portions of crop-residue carbon are first cycled through the soil microbial communities [34].
Unlike fresh plant litter, microbial necromass is enriched in N-bearing compounds (amino sugars, proteins, lipids) and has strong affinity for mineral surfaces [86,87]. After cell death, these necromass molecules bind tightly to clay and silt particles or become physically trapped within soil microaggregates [88,89]. Through such organo–mineral associations and aggregate entombment, microbial necromass is effectively stabilized, forming MAOM that resists rapid decomposition [86,90]. This “microbial carbon pump” (MCP) pathway—where microbes transform plant carbon into cell biomass that is later preserved as necromass in protected soil pools—has been highlighted as a fundamental mechanism of soil carbon sequestration [32,34].
Field and modeling studies confirm the quantitative importance of necromass in croplands [85,91]. For instance, global surveys report that soil microbial necromass contributes roughly 50–51% of SOC in cultivated soils [82,84]. By comparison, contributions are ~33% in forests and ~60% in some grasslands [92]. Importantly, management practices strongly influence these fractions [93]. Long-term intensive tillage or monocropping tends to deplete microbial biomass and reduce necromass accumulation, whereas practices that support microbial activity (organic amendments, reduced disturbance, crop rotations) tend to boost necromass-derived carbon [85,94]. A recent meta-analysis found that “climate-smart” cropping systems (conservation tillage, nutrient management, organic inputs) increased soil microbial necromass by ~18% relative to conventional systems [93]. Thus, practices that enhance microbial growth and turnover (for example, adding compost or cover crops, improving soil fertility, minimizing soil disturbance) generally raise the share of SOC coming from stable microbial residues [95].
In summary, necromass provides a mechanistic bridge between crop residue inputs and stable soil carbon pools [32,96]. Crop residues supply the substrate, microbes convert a portion of that carbon into cell biomass, and after microbial death, the remnants are rapidly protected by mineral and aggregate processes [86]. This microbial pathway often sequesters carbon more effectively than direct plant-residue humification [96,97]. A future understanding of residue decomposition must therefore account not only for plant litter chemistry, but also for microbial growth, turnover, and necromass stabilization [9,81]. By integrating necromass dynamics with aggregate and mineral protection, it is possible to obtain a coherent framework linking substrate inputs to the long-term formation of stable SOM in croplands [88,98].
3. Climate Influences Decomposition Dynamics
3.1. Temperature Sensitivity of Microbial Processes and Enzyme Kinetics
Temperature significantly controls microbial decomposition processes by impacting enzyme kinetics and microbial metabolism [99,100]. The temperature sensitivity of SOM decomposition, commonly expressed as Q10 values, varies significantly among different SOM fractions and microbial processes, with particulate organic matter typically exhibiting higher temperature sensitivity than mineral-associated organic matter due to differences in substrate accessibility and protection mechanisms [20]. To clarify these complex interactions, Figure 1a,b illustrate generalized enzyme Q10 ranges and microbial CUE responses across varying temperature and moisture gradients. Enzyme Q10 typically peaks within an optimal temperature range, while microbial CUE can show more complex responses, potentially increasing or decreasing with warming depending on factors like substrate availability and microbial community shifts [101].
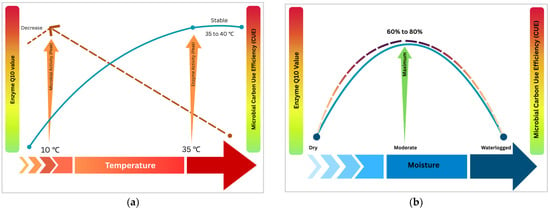
Figure 1.
(a) A schematic diagram indicating the effects of soil temperature on the enzyme Q10 and microbial CUE. Generally, enzyme Q10 values elevate with increase in temperature at lower ranges, but decline as temperature rises above 40 °C, consistent with reduced temperature sensitivity. Microbial activity usually plateaus at moderate temperatures, whereas the microbial CUE decreases with temperature rise due to higher respiratory losses. Hence, these separate thermal optima for microbial and enzymatic processes structure soil carbon dynamics. The figure represents a general trend of enzyme Q10 and microbial CUE under varying temperatures, and response under multiple factors prevailing in natural conditions may be complex and context-dependent, necessitating consideration of integrative approaches. (b) A schematic diagram indicating the effects of soil moisture on the enzyme Q10 and microbial CUE. This figure illustrates the non-linear response of microbial CUE and enzyme Q10 to changes in soil moisture. Both microbial CUE and enzyme Q10 increase with rise in moisture from dry to moderate conditions (60–80%), which provide optimal substrate diffusion, microbial activity, and enzymatic function. However, further increase in moisture leads to waterlogged conditions, which inhibit oxygen diffusion and result in reduced aerobic metabolism, lower CUE, and diminished enzyme efficiency. The figure represents a general trend of enzyme Q10 and microbial CUE under varying moisture, and response under multiple factors prevailing in natural conditions may be complex and context-dependent, necessitating consideration of integrative approaches. Blue lines indicate enzyme Q10 values and red lines depict CUE. Figures created with Canva (https://www.canva.com).
Temperature sensitivity patterns reveal intricate relationships between intracellular and extracellular processes that differ depending on the temperature range examined [101]. For instance, between 5 and 15 °C, both intra- and extracellular metabolic processes show similar temperature sensitivities. However, as temperature increases (15–37 °C), intracellular metabolic processes demonstrate higher temperature sensitivity. Conversely, at higher temperatures (26–37 °C), extracellular enzyme activity becomes more temperature sensitive, indicating that depolymerization of complex carbon compounds becomes increasingly sensitive to temperature changes [101]. Additionally, different enzymes exhibit varying Q10 values (1.78 to 3.28) [102], which reveal their unique catalytic efficiencies and thermal stabilities. For instance, cellulose-degrading enzymes (cellobiohydrolase) show higher temperature sensitivity, while protein-degrading enzymes (leucine aminopeptidase) demonstrate lower Q10 values but are highly sensitive to drought [103]. These enzyme-specific responses are crucial for predicting climate change impacts on decomposition processes, as the relative importance of different enzymatic pathways may shift under warming scenarios [104].
Beyond enzymatic processes, temperature and seasonal variations in moisture content directly impact overall microbial activity and enzyme kinetics through enzyme conformation, substrate diffusion, and microbial physiological state [105,106]. Microorganisms and their cellular metabolites are extremely sensitive to these environmental conditions [107,108]. Enzymes require specific temperature and moisture conditions for optimal three-dimensional structure and catalytic function, and any deviations can lead to enzyme denaturation or reduced activity [109,110]. For instance, drying–rewetting cycles alter substrate accessibility and enzyme-substrate diffusion rates, and microbial activity by causing osmotic stress, microbial dormancy, or reactivation [110,111]. During dry periods, low substrate diffusion along with desiccation stress limits the microbial activity and enzyme production, whereas rewetting exerts a “Birch effect” and results in reactivation of dormant microbes and the release of enzymes from lysed cells, hence, rapidly increasing microbial metabolic activity and SOM decomposition [103,112,113]. This dynamic interplay of temperature and moisture dictates the availability of water, microbial community structure, and the expression of enzymes, which are essential for both microbial metabolism and the movement of substrates to enzymes, thereby directly affecting decomposition rates [114].
Changes in soil temperature specifically influence microbial community composition by affecting metabolic processes, aeration, nutrient availability, microstructure connectivity, and microbial motility. These conditions exhibit different effects on bacteria and fungi, possibly because of their distinct composition and biology [115]. Moreover, microbial respiration increases under high thermal conditions (until certain limits), which affects both the quality and quantity of the organic matter [116,117]. Therefore, elevated temperatures within a suitable range positively affect soil nitrate content, likely due to fast decomposition of plant litter and organic matter, resulting in the accumulation of mineral nitrogen and the release of carbon dioxide [118]. Such temperature regimes are also dominated by fast-growing bacteria (such as Proteobacteria) that can decompose organic matter. Contrarily, fungi (such as saprotrophic fungi) tend to dominate low-temperature environments because of their capacity to decompose complex organic compounds, for instance, lignin [107].
Enzyme catalytic efficiency serves as the key determinant of temperature sensitivity [99,119] in soil carbon cycling, with important implications for predicting climate change impacts on decomposition processes [120]. This sensitivity is further regulated by nutrient availability, such as nitrogen and phosphorus fertilization, highlighting complex interactions within soil ecosystems [121]. Microbial carbon use efficiency (CUE) also demonstrates complex temperature dependencies that influence soil carbon cycling [122,123]. Warming can either increase or decrease CUE, depending on substrate quality, nutrient availability, and microbial community composition [123]. Recent studies have indicated that CUE is at least four times more important than other factors in determining global soil organic carbon storage [122]. The effects of temperature on decomposition are further modulated by soil moisture, nutrient availability, and substrate quality [99,100]. Long-term warming experiments have revealed that temperature sensitivity may decline over time owing to substrate depletion and microbial acclimation [100].
3.2. Moisture Effects on Soil Aeration, Redox, and Microbial Activity
Moisture conditions in the soil have a considerable influence on organic matter decomposition. Many biochemical reactions occurring in the soil are strongly affected by soil moisture conditions. They even affect organic matter content, mobility, gaseous exchange, microbial growth, activity, and community composition, and ultimately, decomposition of organic matter [124]. Moisture regulates decomposition by influencing oxygen availability, substrate diffusion, and microbial habitat connectivity [125]. Optimal moisture conditions for decomposition typically occur at intermediate levels that balance adequate substrate mobility with sufficient aeration for aerobic processes [126]. Soils that are rich in moisture, well-ventilated, and have neutral pH are believed to be ideal for organic matter decomposition [127,128]. Moreover, SOM turnover is regulated by precipitation, which is strongly associated with soil moisture [129]. Changes in precipitation trends also cause changes in soil moisture, thereby affecting substrate availability and organic matter decomposition [130].
Excessive moisture can create anaerobic conditions that shift microbial metabolism toward fermentation and methanogenesis, reduce decomposition rates, and alter carbon cycling pathways [100], leading to increased emission of methane in waterlogged croplands and a slower, less efficient breakdown of complex carbon compounds [131,132]. Regarding oxygen availability, aerobic microorganisms can effectively decompose organic matter, whereas mutualistic consortia are involved in anaerobic decomposition because single bacteria cannot perform complete decomposition. Similarly, the rate of aerobic decomposition is five to ten times higher than anaerobic decomposition [133]. Hence, oxygen availability serves as a critical regulator of decomposition pathways, with aerobic processes generally proceeding more rapidly than anaerobic processes [134]. Similarly, an oxygen-limited environment encourages the growth and activity of methanogenic archaea and facultative anaerobes compared with aerobic conditions, favoring the growth of Actinobacteria and fungi [133]. A recent study has demonstrated that oxygen availability regulates the quality of soil-dissolved organic matter by mediating microbial metabolism and iron oxidation, with implications for carbon cycling in agricultural systems [121]. In contrast, drought stress can limit microbial activity by reducing substrate diffusion and increasing osmotic stress [100,135], often resulting in microbial dormancy, reduced biomass, and the accumulation of less labile organic matter due to suppressed enzymatic activity [136,137]. Soil texture and structure significantly mediate the effects of moisture on decomposition by controlling pore size distribution and water retention characteristics [125,138]. Fine-textured soils with high clay content tend to retain more moisture and provide better protection for organic matter through physical occlusion in micropores [138].
Aggregation promotes decomposition through improved microbial habitat and substrate accessibility while simultaneously protecting organic matter within aggregate cores [139,140]. Rabbi et al. [125] found that microbial decomposition of organic matter and wetting–drying cycles promote aggregation, highlighting the complex feedback between moisture fluctuations, microbial activity, and soil structure development [25,140]. Although all types of microorganisms (actinomycetes, bacteria, and fungi) are involved in aerobic and anaerobic degradation, fungi are more active in decomposing highly recalcitrant constituents owing to their ability to produce diverse extracellular enzymes [141]. These dynamics have important implications for understanding the decomposition responses to changing precipitation patterns under future changing scenarios [140,142].
The relationship between moisture, oxygen, and decomposition creates complex threshold behaviors in many soils, with slight changes in water content potentially triggering substantial shifts in decomposition rates and pathways when critical thresholds are crossed [143]. Moisture fluctuations, particularly wetting–drying cycles, can significantly influence decomposition through physical disruption of aggregates, osmotic stress on microbial communities, and substrate redistribution [139]. These dynamic processes often result in decomposition patterns that cannot be predicted from average moisture conditions alone, highlighting the importance of considering temporal variability in moisture regimes when assessing decomposition dynamics [140].
3.3. Climate Variability, Land Use Change, and Long-Term Shifts in OM Decomposition
Climate variability and extreme weather events have increasingly influenced SOM dynamics in agricultural systems [100]. Changes in precipitation patterns affect soil carbon decomposition through altered moisture regimes and increased frequency of drought–flood cycles [135]. Land use conversion from natural ecosystems to croplands represents one of the most significant drivers of soil carbon loss, with effects persisting for decades after initial conversion [36,144].
Sanderman et al. [145] developed a global soil organic carbon loss map induced by past land use changes, specifically transformation from natural to cultivated ecosystems, and estimated the SOC loss in the top two m of soil. Their analysis indicated loss of carbon over time, an extensive and pronounced influence of anthropogenic activities on terrestrial carbon pools, and the long-term persistence of these effects. The “soil carbon debt” associated with historical land use change highlights the need for widespread sustainable land management. This stresses that agricultural systems have been and remain a major source of CO2 to the atmosphere, and the future development of such systems must focus on carbon sequestration to reverse this trend.
Land use change affects different soil environmental factors, biological interactions, and nutrient conditions, thereby shaping microbial communities [146]. For instance, changes from woodland to farmland or pasture decrease microbial abundance and diversity, possibly due to nutrient cycling performed by soil microbes [147]. Microorganisms largely facilitate changes in soil ecosystems, including degraded land. The biomass carbon, enzyme activity, and basal respiration of microbes are essential for determining their adaptation and response to degraded land [148,149]. Land use changes in croplands negatively impact all these indicators due to retarded microbial activity [150]. Similarly, a decrease in plant growth promoting rhizobacteria and unbalanced carbon and nitrogen cycling were observed in severely degraded subalpine meadows [151].
The magnitude of these effects varies with climate, soil type, and management intensity [19,37]. Long-term impacts of climate change on decomposition exhibit complex temporal dynamics with lag effects that may extend for several years. A four-year lag in soil organic carbon responses to elevated temperatures has been observed, highlighting the importance of considering temporal scales in climate impact assessments. Extreme precipitation events can trigger substantial nutrient leaching and alter the soil carbon cycling trajectories [100]. The interaction between climate change and land management creates nonlinear responses that challenge predictive modeling efforts [135,152]. A previous study found that elevated temperatures and abnormal precipitation significantly impact soil carbon and nitrogen dynamics, with important implications for agricultural sustainability under changing climatic conditions [25].
Climate–soil–management interactions create complex patterns of carbon sequestration potential across agricultural landscapes [153]. Emerging research has emphasized the importance of considering local soil conditions, climate projections, and management options when developing strategies to enhance soil carbon storage in croplands [120,154].
4. Soil Properties as Contextual Modifiers
4.1. Soil Aggregation, Texture, and Porosity: Regulators of Microbial Habitat and OM Protection
Soil aggregation provides fundamental control for organic matter protection and microbial habitat organization [155]. Macroaggregates (>250 μm) serve as dynamic structures that facilitate rapid carbon cycling, while microaggregates (<250 μm) provide long-term protection for organic matter through physical occlusion [125]. The microbial processing of organic matter drives aggregate formation and stability through the production of binding agents and extracellular polymeric substances [125]. The spatial distribution of bacteria between macro- and microaggregates creates distinct microenvironments with varying oxygen concentrations, nutrient availability, and microbial interactions [155]. The relationship between aggregation and organic matter protection is context-dependent and varies with the soil type, management history, and environmental conditions [125]. The mechanism by which soil mineralogy affects SOM protection and microbial habitat is depicted in Figure 2.
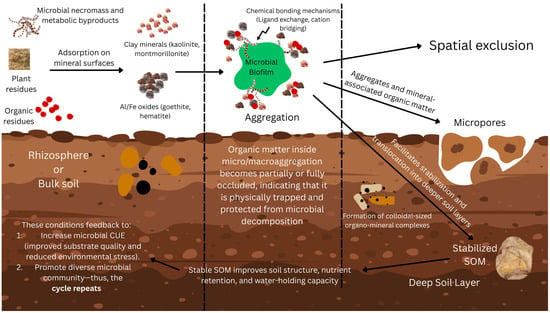
Figure 2.
Schematic representation of how soil mineralogy influences microbial habitats and SOM stabilization. The figure highlights how different types of minerals influence microbial accessibility, carbon protection mechanisms, and SOM stabilization through interactions between microbial products and mineral surfaces. Plant and microbial residues adhere to the surface of different clay minerals (for example, kaolinite and montmorillonite) and the iron/aluminum oxides (for example, goethite and hematite), resulting in the generation of mineral-bonded organic matter. Microbial bio-films promote aggregation, thus forming micro- and macroaggregates that physically exclude microbes and enclose organic matter to protect it from decomposition. These aggregates then move through micropores into deeper soil layers, resulting in long-term stabilization of SOM. This feedback loop increases microbial CUE and promotes the growth of diverse microbial species. Stabilized SOM in turn enhances soil structure, nutrient retention, and water-holding capacity of soil. Figure created with Canva (https://www.canva.com).
Soil texture exerts a fundamental control on organic matter decomposition through its influence on pore size distribution and microbial accessibility. Clay-rich soils typically exhibit slower decomposition rates because of enhanced organo–mineral interactions and reduced pore connectivity. Fine-textured soils, which are rich in clay particles, serve as microhabitats for the growth of slow decomposers, such as Actinobacteria and mycorrhizal fungi, whereas sandy soils have larger poor sizes that allow for better aeration and water retention, thus favoring the growth of copiotrophs, such as Proteobacteria [156]. Soil texture is the second most important indicator after pH for determining the soil microbial community. Table 2 presents the impact of soil texture on microbial community and organic matter stabilization. It has greater impact on alpha diversity of fungi than that of bacteria, as fungal species richness is favored in coarse-textured soils. Conversely, clay and silt soils facilitate the growth of filamentous bacteria and some fungi. Similarly, finer-textured soils have higher inherent potential for organic carbon degradation [157].

Table 2.
Influence of soil texture on microbial community and organic matter stabilization.
The relationship between texture and decomposition is mediated by the pore neck size, which determines whether microorganisms can access soil microsites [138]. Porosity influences decomposition by affecting water movement, gas diffusion, and microbial colonization patterns [125]. The total porosity and pore size distribution also determine substrate accessibility and microbial habitat connectivity. Sandy soils with larger pore sizes typically exhibit higher decomposition rates owing to improved aeration and reduced physical protection [138]. The interaction between texture and moisture creates complex feedback effects on decomposition, with clayey soils showing greater sensitivity to moisture variations [126].
4.2. Soil pH
Soil pH is one of the most important determinants of microbial community composition, enzyme activity, and organic matter stabilization [41,161]. Rhizosphere processes often reduce soil pH, particularly in neutral to alkaline soils, creating favorable conditions for particular microbial groups [42]. pH influences microbial community composition, diversity, biomass, and enzymatic activity but also the microbial turnover of organic matter and microbial-mediated mineralization of carbon and nitrogen [162]. The microbial decomposition of organic matter is also affected by soil pH. Near-neutral pH (approximately 7) is generally considered ideal for soil microbial enzymatic activity and organic matter decomposition, as it optimizes enzyme conformation and enhances the bioavailability of key nutrients directly required for microbial metabolic and enzymatic processes [10,163]. Conversely, extreme pH conditions can denature important intra- and extra-cellular enzymes, and select for specialized, often less diverse and less efficient, microbial guilds adapted to stress [164].
The acidic pH inhibits the growth of microorganisms, thereby negatively affecting the decomposition of organic matter. For instance, acidic conditions usually promote the growth of fungal communities over bacterial populations, thereby influencing decomposition pathways [165,166]. Moreover, toxic metals such as aluminum are abundant in acidic soils. These metals inhibit microbial enzymatic activity and form a strong bond with organic matter, thereby reducing its decomposition. Finally, alkaline pH also affects microbial activity [167], often suppressing key enzyme activities vital for decomposition and favoring distinct, alkali-tolerant bacterial groups such as Actinobacteria [168,169].
4.3. Nutrient Stoichiometry and Microbial CUE
Nutrient availability, particularly nitrogen and phosphorus, fundamentally alters decomposition dynamics by affecting microbial growth and enzyme production [21,119]. In particular, soil nitrogen and phosphorus affect the primary productivity and community composition of organic matter decomposers [170]. Studies have suggested that high nitrogen and phosphorus levels stimulate microbial decomposition of holocellulose and soluble compounds, which are preferentially lost during the early stages of decomposition [171]. However, during the secondary stages (when lignin dominates the substrate), nitrogen addition reduces the decomposition rate due to lignin binding with complex organic compounds, changes in the fungal community (that are lignocellulolytic), promotion of bacterial growth (which prefers carbohydrates and aromatic compounds) [172], and reduction in the production of lignolytic enzymes through gene regulation [173]. Similarly, nutrient-rich soils are dominated by copiotrophs (Actinobacteria and Proteobacteria), whereas nutrient-poor soils encourage the growth of oligotrophic bacteria (such as Firmicutes) and fungi that feed on recalcitrant organic matter [174,175]. This nutrient-driven microbial adaptation, including shifts in resource allocation, directly impacts microbial CUE and thus the rate and extent of SOM stabilization [175,176]. Under nutrient-limited conditions (for example, high C–N or C–P), microbes allocate more energy to produce nutrient-acquiring enzymes, causing excess carbon to be respired and reducing CUE; conversely, abundant nutrients (low C–N/P) tend to increase CUE [177,178].
Besides nitrogen and phosphorus, the carbon-to-nitrogen (C–N) ratio is also an important indicator of organic matter decomposition. Organic matter with a low C–N ratio contains more nitrogen that is readily available for microbes, leading to faster decomposition. Conversely, under a high C–N ratio, less nitrogen is readily available to microbes. Therefore, the decomposition process is slow, leading to nitrogen immobilization [179]. At the microbial level, changes in C–N ratios affect the expression of enzymes and nutrient-acquisition strategies of bacteria. For instance, a high C–N ratio (>25:1) indicates a nitrogen limitation to microbes, resulting in an upregulation of enzymes to acquire nitrogen (for example, N-acetylglucosaminidase and leucine aminopeptidase) and a downregulation of carbon-acquiring enzymes [180]. In contrast, low C–N ratios (<20:1) favor nitrogen mineralization because of an excessive release of nitrogen from microbial biomass [181]. However, thresholds are very important: a C–N ratio of about 20–25:1 is considered a critical threshold where neither strong net immobilization nor mineralization occurs, and microbial demand balances with the substrate supply. Nutrient ratios well above this threshold enhance nitrogen immobilization (thereby slowing carbon decomposition), while low ratios promote carbon decomposition and nitrogen release [182]. Microbial biomass C–N is typically low (averaging around 7:1) [183], so substrates with much higher C–N force microbes to immobilize N for their own stoichiometric needs, slowing decomposition [184].
5. Cross-Scale Interactions and Emergent Feedback
5.1. Feedback Between Microbial Activity and Soil Microenvironment
Microbial activity creates dynamic feedback that modifies soil microenvironmental conditions and subsequently influences decomposition processes [152]. Microbial respiration alters local pH and redox conditions, affecting nutrient availability and mineral–organic matter interactions [161]. Extracellular enzyme production by soil microorganisms modifies substrate accessibility and chemical composition of SOM pools [185]. These enzyme-catalyzed reactions may enhance or retard subsequent decomposition depending on the nature of the products formed and their reactivity [99].
The production of microbial necromass is a major feedback mechanism for the long-term storage of soil carbon [81]. Microbial–mineral interactions work as feedback processes [25]. Microbial activity can modify the surface properties of minerals by producing organic acids and siderophores, affecting their ability to stabilize organic matter [134]. Mineral surfaces, on the other hand, affect microbial community composition by changing substrate availability, water films, and predation protection [153]. The chemical composition of necromass is different from that of living biomass and is characterized by modified C–N ratios as well as increased resistance to decomposition. Mechanisms of microbial death influence necromass composition and their ultimate fate in soil [22]. In particular, the stabilization of microbial necromass is controlled by the adsorption of recalcitrant components, like chitin and peptidoglycans, on mineral surfaces, which generate stable organo–mineral complexes [32,186]. This phenomenon is especially promoted by iron and aluminum oxides, which strongly adsorb microbial residues [187,188]. Microorganisms also produce extracellular polymeric substances (EPSs), which adhere to minerals and provide physical protection to organic matter within biofilms [189]. Through variations in moisture and temperature, climate directly impacts microbial turnover and CUE; for instance, drying–rewetting cycles induce microbial mortality and necromass pulses [190], whereas optimal conditions increase CUE, thus channeling more carbon into biomass and potentially stable necromass [85,122].
The new paradigm of the “soil–microbe complex” underscores the mutualistic and intimate relationship between soil environment and microbial communities [153,154]. This perspective acknowledges that microorganisms are both responsive and can actively alter their habitat, which can generate feedback that influences decomposition dynamics over various timescales [120]. The MCP is an important component of this complex. It facilitates the sequestration of SOC by converting labile carbon into the more recalcitrant forms, such as microbial necromass and metabolic byproducts [191]. Metabolic byproducts are ultimately stabilized by adsorption of carbon to mineral surfaces or its physical protection into soil aggregates [192]. The MCP’s efficiency is largely dependent on the availability of nutrients and climate-induced changes in microbial community composition, which enhance microbial growth and necromass production and preferentially promote characteristics that enhance stable carbon allocation [34,193]. This process depicts the role of microbes in controlling the fate of carbon in the soil and, ultimately, the global carbon cycle [194].
5.2. Landscape-Level Implications of Local Decomposition Processes
Local decomposition processes collectively influence carbon cycling at the landscape scale via spatial heterogeneity and connectivity effects [152,195]. Soil formation processes and weathering patterns result in spatial variations in decomposition rates across landscapes [142,152]. Topographic gradients influence moisture distribution, temperature regimes, and erosion-deposition patterns that affect organic matter dynamics [153]. The interaction between local soil properties and regional climate creates complex spatial mosaics of decomposition [154,195].
Landscape-scale soil carbon storage depends on the spatial distribution of different soil types and their decomposition characteristics [120]. Wetland and lowland positions typically store more soil organic carbon than upland areas because of reduced decomposition rates under anaerobic conditions [195]. Agricultural land use patterns at the landscape scale influence regional carbon balances through edge effects and spatial connectivity [44]. Understanding these cross-scale interactions is essential for scaling local measurements to regional and global carbon cycle assessments [139].
The spatial configuration of agricultural landscapes creates important constraints on decomposition processes through effects on the microclimate, water movement, and organism dispersal [134,142]. Landscape features such as hedgerows, riparian buffers, and field margins can significantly influence decomposition patterns by altering temperature, moisture, and litter input quality. These edge effects create transition zones with distinct decomposition characteristics that differ from those of adjacent fields or natural areas [153]. The impact of various temporal and spatial scales on SOM decomposition in cropland systems is summarized in Table 3. In particular, mesoscale consequences of decomposition at the landscape level arise from integrated molecular-level interactions. For example, the temperature dependance of any specific enzyme-substrate reaction at the molecular scale [196] is scaled up across millions of microbial cells through a soil pore network (micro-scale) to control the local respiration rates. Such localized biochemical processes, which are often spatially heterogeneous because of soil texture, moisture, and nutrient hot-spots across a field, then translate to field-scale differentiated carbon fluxes [197]. At the broader landscape scale, the integrated outcome of these field-scale differences, as modulated by factors such as regional climatic gradients, land use type (for example, forest patches versus cropland), and hydrologic connectivity, gives rise to the regional net ecosystem carbon balance [101,198]. Regional carbon dynamics can be assessed and predicted using spatial modeling frameworks and geospatial tools, such as Geographic Information Systems (GISs) and remote sensing data, which integrate diverse environmental datasets to map and monitor carbon fluxes and stocks across complex landscapes [199]. For instance, satellite-derived vegetation indices (e.g., NDVI) can be integrated with climate models and soil maps within GIS platforms to simulate regional decomposition rates and predict long-term soil organic carbon (SOC) sequestration [200]. This allows researchers to assess the landscape-scale impact of land use changes, such as reforestation or agricultural management practices, on carbon balances and to identify hotspots of carbon loss or gain [201]. Accordingly, the sub-micron scale chemical interactions between soil minerals and individual organic compounds dictate the mass balance of the whole SOC pool in a watershed [202].

Table 3.
Impact of temporal and spatial scales on SOM decomposition in cropland systems.
6. Decomposition, Carbon Sequestration, and Cropland Sustainability
6.1. Balancing Decomposition and Carbon Stabilization
Regenerative agricultural systems must balance the beneficial aspects of organic matter decomposition for nutrient cycling with the need for long-term carbon sequestration [39]. Decomposition provides essential nutrients for crop growth but simultaneously releases CO2 into the atmosphere [38]. The challenge lies in optimizing management practices to maintain adequate nutrient mineralization while maximizing carbon stabilization in persistent soil pools [21]. Recent research has demonstrated that targeting mineral-associated organic matter formation can enhance carbon sequestration without compromising nutrient availability [19].
Thresholds of soil organic carbon are important drivers of effective management interventions. For instance, soils with low carbon concentrations show a different response to nitrogen fertilization compared to soils rich in carbon, which might have implications for carbon sequestration strategies. Partitioning between particulate and mineral-associated organic matter pools may differ depending on the initial amount of soil organic carbon and management history [20,21]. Knowledge of these threshold effects is important for target-specific implementation of carbon sequestration strategies in different agricultural systems [140]. Recent studies also indicate that short-term warming promotes carbon stabilization in mineral-associated fractions in abandoned croplands, implying a strong interaction between climate change and carbon stabilization [25,140]. Microbial CUE is critical in connecting the formation of POC/MAOC to the carbon sequestration strategies [122,203]. For instance, higher CUE indicates that more carbon is assimilated into microbial biomass and then stable necromass, directly enhancing MAOC formation rather than CO2 efflux [122]. Soil physical properties, such as texture (for example, clay content) and aggregate stability, strongly affect the dynamics of POC and MAOC by controlling physical protection and establishing a microenvironment for microbial activity [204,205]. Fine-textured soils are rich in reactive mineral surfaces that favor the formation of MAOC by stronger organo-mineral associations [204,206]. Under different climate regimes, the balance shifts; in warm and wet conditions, rapid POC decomposition is often linked to increased MAOC formation through microbial turnover and concomitant necromass production [207]. In contrast, severe drought can decrease these processes by inhibiting microbial activity and the disruption of physical protection [207,208]. Therefore, effective SOC sequestration strategies must incorporate land management practices that increase microbial CUE and effects on soil aggregation and consider site-specific climatic conditions to ensure maximum transformation of labile POC to stable MAOC [205,208].
The concept of carbon saturation has been established to interpret the diminishing potential of soil carbon sequestration in agricultural systems [142]. This perspective acknowledges that soil has a limited capacity to stabilize organic matter, with potential consequences for the long-term efficacy of carbon sequestration strategies [134]. Management practices that affect unsaturated carbon pools are more likely to enhance soil carbon storage than those that target saturated fractions [153]. Practices that enhance microbial CUE and promote the stabilization of microbial products may be adopted as ecological strategies to synchronize improvements in nutrient cycling and carbon sequestration [120].
6.2. Role of Microbial Communities in SOC Formation and Loss
Microbial communities contribute to both the loss of soil carbon by decomposition and its formation via necromass production and SOM stabilization [32,209]. This trade-off is modulated by the microbial CUE, community structure, and environmental conditions [210]. The ultimate fate of soil organic carbon is affected by microbial properties such as enzyme production profiles and stress tolerance [211]. Recent research has highlighted that microbial functional diversity is more important than taxonomic diversity in predicting soil carbon outcomes [212]. Unlike taxonomic richness, functional diversity reflects differences in microbial strategies for carbon acquisition, transformation, and stabilization [213,214,215]. Communities with diverse functional traits—such as complementary enzyme systems and metabolic pathways—are better equipped to process a broader range of substrates and direct more carbon into necromass and stable SOM components [9,12,81,216].
Farming methods that promote beneficial microbial communities can simultaneously improve soil carbon sequestration and crop productivity. Cover crops and diverse rotations increase the diversity of microorganisms and the quality of carbon input. Low tillage practices help preserve soil structure and habitat for microbes and promote increased carbon retention [217,218,219]. The combinatorial effect of these practices leads to synergistic changes exceeding the sum of individual management impacts [220,221]. Lu et al. [140] described that catalytic efficiency of soil enzymes can predict temperature sensitivity of soil carbon cycling, underscoring the role of microbially mediated processes in carbon kinetics.
The emerging concept of the MCP emphasizes the central role of microorganisms in transforming plant inputs into stable SOM [25]. This perspective highlights the importance of considering not only the quantity of carbon inputs but also their processing efficiency within the soil microbial community [134,142]. Management practices that enhance microbial CUE while promoting the formation of stable microbial products offer promising pathways for simultaneously improving nutrient cycling and carbon sequestration in cropland systems [153].
Mechanistically, beyond necromass formation, MCP’s efficiency in enhancing SOC sequestration also depends on several other microbial processes, including direct synthesis of recalcitrant microbial metabolites and EPS that act as glues for soil aggregate formation, physically protecting organic matter from decomposition [222]. Additionally, microbial resource acquisition strategies modulate the MCP. For instance, competitive interactions among microbial groups can drive a higher proportion of assimilated carbon toward stable biomass and byproducts rather than respiration [223]. Specific microbial taxa, particularly those with slower growth rates (K-strategists) and high investment in complex biopolymer synthesis, are disproportionately important in forming stable carbon forms via the MCP [224]. Furthermore, the enzymatic breakdown of complex plant litter into simpler compounds by microbes often results in the formation of novel, chemically altered organic molecules that are more prone to adsorption onto mineral surfaces or occlusion within aggregates, representing another facet of the MCP [192]. This highlights a broader feedback loop where microbial activity not only processes carbon inputs but actively transforms them into persistent forms, driven by both intrinsic microbial traits and extrinsic environmental conditions. The interplay between climate (shifts in temperature and moisture regimes) and soil properties (mineral composition and aggregate stability) directly dictates the optimal conditions for these MCP-related mechanisms, influencing microbial community composition toward taxa and metabolic pathways that favor stable SOC formation [122]. The role of different microbial taxa in decomposition processes under different environments is illustrated in Figure 3.
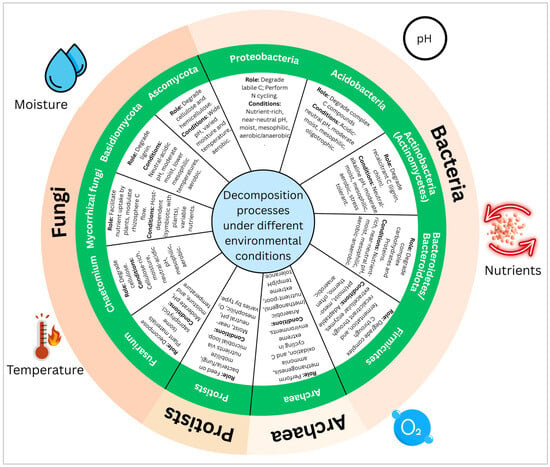
Figure 3.
A functional wheel representing how key microbial taxa contribute to decomposition processes under varying environmental conditions, including moisture, temperature, pH, oxygen availability, and nutrient levels. The inner ring describes microbial functional roles (for example, lignin decomposition, nitrogen cycling, and carbon mineralization). Bacterial taxa such as Proteobacteria, Acidobacteria, Actinobacteria, Bacteroidota, and Firmicutes exhibit distinct decomposition capabilities, which are linked to specific environmental niches. Fungal groups, including Ascomycota, Basidiomycota, and mycorrhizal fungi, specialize in degrading complex organic compounds such as cellulose and lignin. Protists, archaea, and some specific fungal genera (including Fusarium and Chaetomium) play critical roles in nutrient cycling. Although these microbial taxa perform under diverse ecological environments, their activity impacts soil conditions (for example, substrate quality, pH, moisture content, and oxygen), resulting in shifts in microbial communities—demonstrating a feedback loop between environmental contexts and microbial functions. Figure created with Canva (https://www.canva.com).
6.3. Effects of Agricultural Practices on Decomposition Dynamics
Different agricultural management practices exert varying influences on decomposition processes and soil carbon cycling. These practices collectively influence microbial traits such as CUE and enzyme activities, which critically determine the fate of SOM [225,226]. Regenerative practices, including cover cropping, reduced tillage, diverse rotations, and agroforestry, generally enhance SOC accumulation [38,227]. Carbon sequestration rates for woody perennial and arable land uses indicate the contribution of different regenerative practices to achieving sequestration in arable cropland systems. Studies indicate that agroforestry and double cover crops have the higher mean sequestration rates, while other practices have a lesser but positive effect on below-ground carbon sequestration [228,229]. These diverse practices increase microbial diversity, CUE, and enzyme activities, thereby promoting greater SOM stabilization [230]. No-till systems combined with cover crops maximize the potential for SOM accumulation [231]. Crop rotation and diversity enhance microbial community complexity and functional redundancy, leading to more stable decomposition [227].
Tillage practices also significantly influence decomposition through their effects on soil structure, aggregate stability, and microbial habitat. Conventional tillage disrupts stable mineral-associated organic matter, resulting in a net loss of SOM [232]. High-intensity tillage and disturbance reduce microbial biomass and efficiency, undermining CUE and SOM protection [233]. Conversely, reduced or no-till systems help maintain soil aggregates and microbial habitats, often leading to higher microbial CUE and enhanced SOC retention [234]. Although the ratio of Gram-positive to Gram-negative bacteria increases with increasing land degradation, the ratio of bacteria to fungi decreases. Generally, fresh organic inputs are preferred by Gram-negative bacteria, whereas Gram-positive bacteria favor recalcitrant or low-quality organic matter for decomposition [235]. These results indicate changes in the quality of SOM and a decrease in microbial ecosystem stability, which may be attributed to the high carbon content that transits microbial communities from commensals to oligotrophic groups and an increase in the soil pH of degraded lands, generating physiological pressure on soil microbes and affecting their competition and reproduction. Conversely, fungi are less sensitive to soil pH than bacteria and are generally more resistant to intense land degradation [236].
Conventional agricultural practices can also lead to environmental disturbances such as water and soil pollution and reduced soil health [237]. These practices often involve intensive use of synthetic fertilizers, pesticides, and other chemical inputs. Excessive use of these chemicals can suppress microbial CUE and enzyme activities, undermining SOM formation and soil health [238]. If applied in excessive concentrations, chemical fertilizers and pesticides can result in the loss of organic matter, a reduction in cellulose-decomposing bacteria, and an increase in soil salinity, thereby affecting the soil structure, nutrients, and microbial diversity and abundance. Hence, conventional farming significantly decreases soil earthworm community, food webs, and microbial diversity [170,239,240]. Soil has the specific ability to filter, adsorb, and precipitate substances. Therefore, soil exposure to biologically active chemicals, such as heavy metals, pesticides, polychlorinated biphenyls and furans, polycyclic carbohydrates, petroleum products, and dioxins, significantly influences soil quality, microbial biocenosis, and ultimately human health [241]. Soil fungi and bacteria play essential roles in pollutant migration, morphological transformation, and decomposition [241,242]. Gram-positive bacteria prefer utilizing complex pollutants more than Gram-negative bacteria [243]. Although soil microbes can actively degrade pollutants, these pollutants may significantly affect microbial ecological succession and kill various microbes, resulting in the loss of resistance in the soil ecosystem.
Persistent organic pollutants further illustrate these impacts. For example, dichlorodiphenyltrichloroethane affects soil respiration and reduces microbial growth and activity. Specifically, it decreases the bacterial and actinomycete counts and inhibits the growth of Trichoderma viride, Botryotrichum species, Alternaria humicola, Sepedonium species, Helmintosporium sativum, Botrytis, Rhizoctonia solani, Mucor species, Azotobacter, and nodulation. Similarly, other pollutants, including aldrin and polychlorinated biphenyls, negatively affect soil fertility, inhibit the growth of nematodes and the nitrifying bacterium Nitrobacter agilis, and decrease the nitrite-oxidizing bacterial count [244,245]. Furthermore, heavy metals may accumulate in high concentrations in the soil. They are persistent and cannot be degraded through metabolic processes, negatively affecting microbial diversity, activity, and biomass [246]. Overall, chemical pollutants tend to lower microbial enzyme activities and CUE, weakening decomposition and SOM protection [247].
Site-specific and precision management strategies (for example, variable-rate fertilizer applications and targeted cover crop use) further influence soil microbial processes. By tailoring interventions to local soil and climate conditions, these approaches can maximize microbial efficiency and carbon retention [248]. The timing and intensity of management interventions create promising effects that persist over multiple growing seasons [40]. Recent research has highlighted the potential of precision management approaches for optimizing decomposition processes based on site-specific soil and climate conditions [153]. These approaches recognize that optimal management strategies vary considerably across different landscape positions and soil types, necessitating spatially explicit recommendations [120]. The development of decision support tools that integrate decomposition science with precision agriculture technologies offers promising opportunities for optimizing both agricultural productivity and environmental outcomes [121].
7. Future Research Directions
Although there has been considerable progress, some key knowledge gaps are still present in the SOM decomposition in croplands. First, there is a lack of comprehensive understanding of the contributions of soil fauna (arthropods, nematodes, and earthworms) to OM decomposition; especially their trophic relationships with microbial communities and impact on microbial CUE and enzyme expression. Therefore, fauna-mediated turnover should be incorporated into trait-based decomposition frameworks in future studies. Second, the soil–microbiome–plant interaction is not only complex but poorly characterized at field scales. Trait-based experimental designs in relation to root exudation, microbial growth kinetics, and necromass formation are required among diverse crop species to determine crop-specific SOM stabilization. Third, the climate change impact on soil microbiomes under different moisture/warming is required to be extensively investigated in the climatic zones. Long-term field trials are essential to predict how precipitation changes, temperature extremes, and drought interact to influence microbial traits (dormancy, enzyme allocation, and necromass production) and thereby MAOM formation. Fourth, there is a pressing need for the standardization of microbial trait measurements such as CUE, growth efficiency, and enzyme stoichiometry across agricultural systems. This allows for cross-study comparisons and model calibration, especially for trait-explicit biogeochemical models, such as MIMICS and CORPSE. Fifth, combining the multi-omics data (metagenomics, transcriptomics, and metabolomics) with the soil physico-chemical models is a major frontier. This is important in the context of quantifying microbial contributions to SOM persistence and predicting community shifts; for example, relationships between the abundance of ligninolytic enzyme genes and soil aggregation models to infer the decomposition of recalcitrant carbon. Sixth, it is necessary to scale up the laboratory results to more realistic field and landscape conditions. Although much of our current understanding originates from these lab microcosms, decomposition performed under natural conditions is spatially heterogeneous. Furthermore, connecting field mesocosms with remote sensing and geospatial modeling (soil moisture sensors, thermal infrared imaging, and NDVI) may efficiently scale microbial-driven decomposition. Lastly, inter-disciplinary efforts need to focus on applicable technologies. With the integration of sensor networks, in situ probes, and HTS of DNA/RNA sequencing, in situ monitoring of soil respiration and redox dynamics can be developed to follow a microbial response. It appears that AI/ML, using multi-site, multi-omics data, can generate adaptive predictive decomposition models for a specific soil–climate–crop combination that would allow for greater accuracy in these predictions.
8. Conclusions
This review emphasizes the critical importance of climate–soil–microbe interactions on SOM decomposition, which is influential in sustaining crop land, carbon sequestration, and nutrient availability. The advancement of multi-omics technologies and microbial network approaches has provided unprecedented understanding of these dynamics at different spatial and temporal scales, highlighting the crucial roles of climate and soil in shaping such dynamics. Microbes are the central players in decomposition, with different groups like bacteria that act on labile carbon, and the fungi that release specialized enzymes to break down recalcitrant materials play distinct complementary roles. Importantly, microbial functional traits, including CUE, dormancy, and growth strategy, are key to decomposition responses and to the fate of carbon in the soil. The formation of microbial necromass, dead microbial cells stabilized by mineral association, is a major component of stable SOM and contributes as a key mechanism in long-term carbon sequestration through the MCP. An enhanced knowledge of these microbial processes directly supports climate-smart agriculture. This suggests that harnessing microbial functioning in sustainable management would provide opportunities for improving carbon sequestration and nutrient cycling. Regenerative farming systems, including cover crops, reduced tillage, agroforestry, and diverse rotations are effective strategies to increase soil microbial diversity, CUE, and enzyme activities, which can favor greater SOM stabilization and agriculture productivity. This highlights the importance of regionally specific recommendations that consider soil, climate, and economic status for the successful and widespread adoption of sustainable organic carbon management. This paper therefore links theoretical developments with their application. In principle, it integrates a mechanistic, multi-scale approach to SOM dynamics, moving beyond simplistic conceptualizations by emphasizing the MCP and the importance of microbial functional diversity. Operationally, it leads to decision-support strategies for developing climate-resilient agricultural systems. By highlighting microbial functions and responses to management, this work guides farmers and policy makers toward successful regenerative strategies that improve both soil health and global food security.
Author Contributions
Conceptualization, M.T.K., S.S. and J.A.; data curation, M.T.K.; writing—original draft preparation, M.T.K. and S.S.; writing—review and editing, S.S., R.Ž. and J.A.; supervision, J.A.; funding acquisition, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This review article was funded by the Ministry of Agriculture of the Republic of Lithuania, Project MTE-24-8, entitled “The Possibilities for Soil Humus Preservation and Increase under Agricultural Practices.”
Data Availability Statement
All data is available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cotrufo, M.F.; Lavallee, J.M. Chapter One-Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 172, pp. 1–66. [Google Scholar]
- Zhao, X.; Liu, B.-Y.; Liu, S.-L.; Qi, J.-Y.; Wang, X.; Pu, C.; Li, S.-S.; Zhang, X.-Z.; Yang, X.-G.; Lal, R.; et al. Sustaining Crop Production in China’s Cropland by Crop Residue Retention: A Meta-Analysis. Land Degrad. Dev. 2020, 31, 694–709. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Liang, A.; Duan, Y.; Chen, H.; Yu, Z.; Fan, R.; Liu, H.; Pan, H. Chemical Composition of Plant Residues Regulates Soil Organic Carbon Turnover in Typical Soils with Contrasting Textures in Northeast China Plain. Agronomy 2022, 12, 747. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, H.; Zhang, Q.; Said Mgelwa, A.; Zhu, B.; Hu, Y. Negative Priming Effect from Tree Leaf and Root Residues with Contrasting Chemical Composition. Geoderma 2022, 427, 116118. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Pei, J.; Li, M.; Shan, T.; Zhang, W.; Wang, J. Below Ground Residues Were More Conducive to Soil Organic Carbon Accumulation than above Ground Ones. Appl. Soil Ecol. 2020, 148, 103509. [Google Scholar] [CrossRef]
- Luu, A.T.; Hoang, N.T.; Dinh, V.M.; Bui, M.H.; Grandy, S.; Hoang, D.T.T. Effects of Carbon Input Quality and Timing on Soil Microbe Mediated Processes. Geoderma 2022, 409, 115605. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial Decomposition of Soil Organic Matter Is Mediated by Quality and Quantity of Crop Residues: Mechanisms and Thresholds. Biol. Fertil. Soils. 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Miltner, A.; Zheng, T.; Liang, C.; Kästner, M. Microbial Necromass as a Source for Soil Organic Matter Formation-Implications for Soil Processes. In Proceedings of the Copernicus Meetings, Online, 4–8 May 2020. [Google Scholar]
- Yang, Y.; Gunina, A.; Cheng, H.; Liu, L.; Wang, B.; Dou, Y.; Wang, Y.; Liang, C.; An, S.; Chang, S.X. Unlocking Mechanisms for Soil Organic Matter Accumulation: Carbon Use Efficiency and Microbial Necromass as the Keys. Glob. Change Biol. 2025, 31, e70033. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef] [PubMed]
- Whalen, E.D.; Grandy, A.S.; Geyer, K.M.; Morrison, E.W.; Frey, S.D. Microbial Trait Multifunctionality Drives Soil Organic Matter Formation Potential. Nat. Commun. 2024, 15, 10209. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.; Błońska, E.; Piaszczyk, W. State of Soil Enzymatic Activity in Relationship to Some Chemical Properties of Brunic Arenosols. Soil Sci. Annu. 2021, 72, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in Soil Carbon Fractions and Enzyme Activities under Different Vegetation Types of the Northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Li, Z.; Zhang, Y.; Lu, L. Microbial Community and Soil Enzyme Activities Driving Microbial Metabolic Efficiency Patterns in Riparian Soils of the Three Gorges Reservoir. Front. Microbiol. 2023, 14, 1108025. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Jiang, W.; Zou, S.; Kang, D.; Yan, X. Microbial Carbohydrate-Active Enzymes Influence Soil Carbon by Regulating the of Plant- and Fungal-Derived Biomass Decomposition in Plateau Peat Wetlands under Differing Water Conditions. Front. Microbiol. 2023, 14, 1266016. [Google Scholar] [CrossRef] [PubMed]
- Weiler, D.A.; Bastos, L.M.; Schirmann, J.; Aita, C.; Giacomini, S.J. Changes in Chemical Composition of Cover Crops Residue during Decomposition. Cienc. Rural. 2021, 52, e20210357. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Q.; Wang, X.; Wang, Y.-P.; Ciais, P.; Zhang, H.; Goll, D.S.; Zhu, L.; Zhao, Z.; Guo, Z.; et al. Modeling Biochar Effects on Soil Organic Carbon on Croplands in a Microbial Decomposition Model (MIMICS-BC_v1.0). Geosci. Model Dev. 2024, 17, 4871–4890. [Google Scholar] [CrossRef]
- Hondroudakis, L.; Kopittke, P.M.; Dalal, R.C.; Barnard, M.; Weng, Z.H. The Influence of Land Use and Management on the Behaviour and Persistence of Soil Organic Carbon in a Subtropical Ferralsol. SOIL 2024, 10, 451–465. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, H.; Gao, X.; Huang, S.; Niu, S.; Lugato, E.; Xia, X. Short-Term Warming Supports Mineral-Associated Carbon Accrual in Abandoned Croplands. Nat. Commun. 2025, 16, 344. [Google Scholar] [CrossRef]
- Ling, J.; Dungait, J.A.J.; Delgado-Baquerizo, M.; Cui, Z.; Zhou, R.; Zhang, W.; Gao, Q.; Chen, Y.; Yue, S.; Kuzyakov, Y.; et al. Soil Organic Carbon Thresholds Control Fertilizer Effects on Carbon Accrual in Croplands Worldwide. Nat. Commun. 2025, 16, 3009. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Y.; Lou, M.; Cheng, Z.; Ma, R.; Guo, H.; Zhou, J.; Jia, H.; Fan, L.; Wang, T. Microbial Transformation Mechanisms of Particulate Organic Carbon to Mineral-Associated Organic Carbon at the Chemical Molecular Level: Highlighting the Effects of Ambient Temperature and Soil Moisture. Soil Biol. Biochem. 2024, 195, 109454. [Google Scholar] [CrossRef]
- Hansen, P.M.; Even, R.; King, A.E.; Lavallee, J.; Schipanski, M.; Cotrufo, M.F. Distinct, Direct and Climate-Mediated Environmental Controls on Global Particulate and Mineral-Associated Organic Carbon Storage. Glob. Change Biol. 2024, 30, e17080. [Google Scholar] [CrossRef]
- Liang, Y.; Leifheit, E.F.; Lehmann, A.; Rillig, M.C. Soil Organic Carbon Stabilization Is Influenced by Microbial Diversity and Temperature. Sci. Rep. 2025, 15, 13990. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Influence of Water Availability and Temperature on Estimates of Microbial Extracellular Enzyme Activity. PeerJ 2021, 9, e10994. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Cotrufo, M.F. Mechanisms of Soil Organic Carbon and Nitrogen Stabilization in Mineral-Associated Organic Matter–Insights from Modeling in Phase Space. Biogeosciences 2024, 21, 4077–4098. [Google Scholar] [CrossRef]
- Dohnalkova, A.C.; Tfaily, M.M.; Chu, R.K.; Smith, A.P.; Brislawn, C.J.; Varga, T.; Crump, A.R.; Kovarik, L.; Thomashow, L.S.; Harsh, J.B.; et al. Effects of Microbial-Mineral Interactions on Organic Carbon Stabilization in a Ponderosa Pine Root Zone: A Micro-Scale Approach. Front. Earth Sci. 2022, 10, 799694. [Google Scholar] [CrossRef]
- Matus, F.J. Fine Silt and Clay Content Is the Main Factor Defining Maximal C and N Accumulations in Soils: A Meta-Analysis. Sci. Rep. 2021, 11, 6438. [Google Scholar] [CrossRef]
- Calero, J.; García-Ruiz, R.; Torrús-Castillo, M.; Vicente-Vicente, J.L.; Martín-García, J.M. Role of Clay Mineralogy in the Stabilization of Soil Organic Carbon in Olive Groves under Contrasted Soil Management. Minerals 2023, 13, 60. [Google Scholar] [CrossRef]
- Poeplau, C.; Dechow, R.; Begill, N.; Don, A. Towards an Ecosystem Capacity to Stabilise Organic Carbon in Soils. Glob. Change Biol. 2024, 30, e17453. [Google Scholar] [CrossRef] [PubMed]
- Kästner, M.; Miltner, A.; Thiele-Bruhn, S.; Liang, C. Microbial Necromass in Soils—Linking Microbes to Soil Processes and Carbon Turnover. Front. Environ. Sci. 2021, 9, 756378. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, D.; Wei, B.; Yang, Y. Dual Roles of Microbes in Mediating Soil Carbon Dynamics in Response to Warming. Nat. Commun. 2024, 15, 6439. [Google Scholar] [CrossRef]
- Kou, X.; Morriën, E.; Tian, Y.; Zhang, X.; Lu, C.; Xie, H.; Liang, W.; Li, Q.; Liang, C. Exogenous Carbon Turnover within the Soil Food Web Strengthens Soil Carbon Sequestration through Microbial Necromass Accumulation. Glob. Change Biol. 2023, 29, 4069–4080. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, J.; Wang, P.; Zhu, B. The Direct and Legacy Effects of Drying-Rewetting Cycles on Active and Relatively Resistant Soil Carbon Decomposition. Land Degrad. Dev. 2023, 34, 2124–2135. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A Global Meta-Analysis of Soil Organic Carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Zhu, G.; Lu, S.; Qiu, D.; Jiao, Y.; Meng, G.; Chen, L.; Li, R.; Zhang, W.; et al. Agricultural Activities Increased Soil Organic Carbon in Shiyang River Basin, a Typical Inland River Basin in China. Sci. Rep. 2025, 15, 11727. [Google Scholar] [CrossRef] [PubMed]
- Villat, J.; Nicholas, K.A. Quantifying Soil Carbon Sequestration from Regenerative Agricultural Practices in Crops and Vineyards. Front. Sustain. Food Syst. 2024, 7, 1234108. [Google Scholar] [CrossRef]
- Abrar, M.M.; Waqas, M.A.; Mehmood, K.; Fan, R.; Memon, M.S.; Khan, M.A.; Siddique, N.; Xu, M.; Du, J. Organic Carbon Sequestration in Global Croplands: Evidenced through a Bibliometric Approach. Front. Environ. Sci. 2025, 13, 1495991. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Narsing Rao, M.P.; Shi, Y.; Lian, Z.-H.; Jin, P.-J.; Wang, W.; Li, Y.-M.; Wang, K.-K.; Banerjee, A.; et al. The Shift of Soil Microbial Community Induced by Cropping Sequence Affect Soil Properties and Crop Yield. Front. Microbiol. 2023, 14, 1095688. [Google Scholar] [CrossRef]
- Wei, X.; Fu, T.; He, G.; Zhong, Z.; Yang, M.; Lou, F.; He, T. Characteristics of Rhizosphere and Bulk Soil Microbial Community of Chinese Cabbage (Brassica Campestris) Grown in Karst Area. Front. Microbiol. 2023, 14, 1241436. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Fu, Q.; Qiu, Y.; Zhao, J.; Li, J.; Wu, X.; Yang, Y.; Liu, H.; Yang, X.; et al. Critical Transition of Soil Microbial Diversity and Composition Triggered by Plant Rhizosphere Effects. Front. Plant Sci. 2023, 14, 1252821. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, X.; Elsgaard, L.; Du, T.; Siddique, K.H.M.; Kang, S.; Butterbach-Bahl, K. Diversified Crop Rotations Improve Soil Microbial Communities and Functions in a Six-Year Field Experiment. J. Environ. Manag. 2024, 370, 122604. [Google Scholar] [CrossRef] [PubMed]
- Speckert, T.C.; Wiesenberg, G.L.B. Source or Decomposition of Soil Organic Matter: What Is More Important with Increasing Forest Age in a Subalpine Setting? Front. For. Glob. Change 2023, 6, 1290922. [Google Scholar] [CrossRef]
- Chaudhari, Y.B.; Várnai, A.; Sørlie, M.; Horn, S.J.; Eijsink, V.G.H. Engineering Cellulases for Conversion of Lignocellulosic Biomass. Protein Eng. Des. Sel. 2023, 36, gzad002. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Paice, M.; Zhang, X. Enzymatic Oxidation of Lignin: Challenges and Barriers Toward Practical Applications. ChemCatChem 2020, 12, 401–425. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial Extracellular Enzymes in Biogeochemical Cycling of Ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Cheng, J.; Jin, H.; Zhang, J.; Xu, Z.; Yang, X.; Liu, H.; Xu, X.; Min, D.; Lu, D.; Qin, B. Effects of Allelochemicals, Soil Enzyme Activities, and Environmental Factors on Rhizosphere Soil Microbial Community of Stellera Chamaejasme L. along a Growth-Coverage Gradient. Microorganisms 2022, 10, 158. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, T.; Wang, L.; Liu, E. Drip Irrigation Affects Soil Bacteria Primarily through Available Nitrogen and Soil Fungi Mainly via Available Nutrients. Front. Microbiol. 2024, 15, 1453054. [Google Scholar] [CrossRef]
- Ahmed, N.; Li, J.; Li, Y.; Deng, L.; Deng, L.; Chachar, M.; Chachar, Z.; Chachar, S.; Hayat, F.; Raza, A.; et al. Symbiotic Synergy: How Arbuscular Mycorrhizal Fungi Enhance Nutrient Uptake, Stress Tolerance, and Soil Health through Molecular Mechanisms and Hormonal Regulation. IMA Fungus 2025, 16, e144989. [Google Scholar] [CrossRef]
- Bernier, L.S.; Estoppey, A.; Bindschedler, S.; Stan, G.-B.; Junier, P.; Stanley, C.E. Microfluidic Platform for Microbial Spore Germination Studies in Multiple Growth Conditions. BMC Methods 2024, 1, 12. [Google Scholar] [CrossRef]
- Hwang, Y.; Rahlff, J.; Schulze-Makuch, D.; Schloter, M.; Probst, A.J. Diverse Viruses Carrying Genes for Microbial Extremotolerance in the Atacama Desert Hyperarid Soil. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, J.A.W.W.; Seneviratne, G.; Wijepala, P.C. Application of Microbial Biofilms to Reinstate Lost Soil Microbial Diversity under Global Warming: A Case Study. In Proceedings of International Forestry and Environment Symposium; University of Sri Jayewardenepura: Nugegoda, Sri Lanka, 2022; Volume 26. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q.; et al. Environmental Behaviors of Bacillus Thuringiensis (Bt) Insecticidal Proteins and Their Effects on Microbial Ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Stefanova, V.V.; Petrov, P.G. Soil Development and Properties of Microbial Biomass Succession in Reclaimed Sites in Bulgaria. In CBU International Conference Proceedings; CBU Research Institute: Prague, Czech Republic, 2019; Volume 7, pp. 1008–1014. [Google Scholar] [CrossRef]
- Furiosi, M.; Hasanaliyeva, G.; Caffi, T.; Rossi, V. Soil Covering and Biofumigant Effect of Armoracia Rusticana against Spore Dispersal and Viability of Downy Mildew Inoculum in Viticultural Systems-BIOVINE. BIO Web Conf. 2022, 50, 03004. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, J.; Ongena, M.; Liu, W.; Wei, D.; Zhao, B.; Guan, D.; Jiang, X.; Li, J. Effect of Long-Term Fertilization Strategies on Bacterial Community Composition in a 35-Year Field Experiment of Chinese Mollisols. AMB Express 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, X.; Xiang, Q.; Penttinen, P.; Gu, Y. Response of Soil Microbial Community Structure and Function to Different Altitudes in Arid Valley in Panzhihua, China. BMC Microbiol. 2022, 22, 86. [Google Scholar] [CrossRef]
- Wang, X.; Huang, T.; Li, Y.; Zhao, G.; Zhao, J. Elevational Characteristics of Soil Bacterial Community and Their Responses to Soil Translocation at a Mountainside in Northwest Sichuan, China. Sci. Rep. 2023, 13, 17906. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Yang, Y.; Wang, S.; Chen, K.; Zhang, N. The Response Mechanism of the cbbM Carbon Sequestration Microbial Community in the Alpine Wetlands of Qinghai Lake to Changes in Precipitation. Biology 2024, 13, 1090. [Google Scholar] [CrossRef]
- Cui, J.; Yang, B.; Zhang, M.; Song, D.; Xu, X.; Ai, C.; Liang, G.; Zhou, W. Investigating the Effects of Organic Amendments on Soil Microbial Composition and Its Linkage to Soil Organic Carbon: A Global Meta-Analysis. Sci. Total Environ. 2023, 894, 164899. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef]
- Roley, S.S. Diazotrophic Nitrogen Fixation in the Rhizosphere and Endosphere. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Singapore, 2021; pp. 93–108. ISBN 978-981-15-6125-2. [Google Scholar]
- Zhang, N.; Chen, K.; Wang, S.; Qi, D.; Zhou, Z.; Xie, C.; Liu, X. Dynamic Response of the cbbL Carbon Sequestration Microbial Community to Wetland Type in Qinghai Lake. Biology 2023, 12, 1503. [Google Scholar] [CrossRef]
- Shi, L.; Dossa, G.G.O.; Paudel, E.; Zang, H.; Xu, J.; Harrison, R.D. Changes in Fungal Communities across a Forest Disturbance Gradient. Appl. Environ. Microbiol. 2019, 85, e00080-19. [Google Scholar] [CrossRef]
- Das, S.K. Biochar Application Method and Amount Both Changed the Dynamics of Soil Temperature-Moisture-Metals in an Acidic Inceptisols. Water Air Soil Pollut. 2024, 235, 303. [Google Scholar] [CrossRef]
- Muneer, M.A.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.Z.; Wu, L.; Zheng, C. Response of Fungal Diversity, Community Composition, and Functions to Nutrients Management in Red Soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef]
- Vicentini, M.E.; Pinotti, C.R.; Hirai, W.Y.; de Moraes, M.L.T.; Montanari, R.; Filho, M.C.M.T.; Milori, D.M.B.P.; Júnior, N.L.S.; Panosso, A.R. CO2 Emission and Its Relation to Soil Temperature, Moisture, and O2 Absorption in the Reforested Areas of Cerrado Biome, Central Brazil. Plant Soil 2019, 444, 193–211. [Google Scholar] [CrossRef]
- Gallo, A.C.; Holub, S.M.; Littke, K.; Lajtha, K.; Maguire, D.; Hatten, J.A. Short-Term Effects of Organic Matter and Compaction Manipulations on Soil Temperature, Moisture, and Soil Respiration for 2 Years in the Oregon Cascades. Soil Sci. Soc. Am. J. 2023, 87, 156–171. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The Sensitivity of Soil Respiration to Soil Temperature, Moisture, and Carbon Supply at the Global Scale. Glob. Change Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Cui, J. Microbial Community Structure and Carbon Transformation Characteristics of Different Aggregates in Black Soil. PeerJ 2024, 12, e17269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Tai, X.; Sun, L.; Wu, M.; Zhang, W.; Chen, X.; Zhang, G.; Chen, T.; Liu, G.; et al. Variation in Actinobacterial Community Composition and Potential Function in Different Soil Ecosystems Belonging to the Arid Heihe River Basin of Northwest China. Front. Microbiol. 2019, 10, 2209. [Google Scholar] [CrossRef]
- Fang, J.; Wei, S.; Shi, G.; Cheng, Y.; Zhang, X.; Zhang, F.; Lu, Z.; Zhao, X. Potential Effects of Temperature Levels on Soil Bacterial Community Structure. E3S Web Conf. 2021, 292, 01008. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, K.; Schneider, R.L.; Morreale, S.J.; Xie, Y. Comparison of Microbial Community Structures in Soils with Woody Organic Amendments and Soils with Traditional Local Organic Amendments in Ningxia of Northern China. PeerJ 2019, 7, e6854. [Google Scholar] [CrossRef]
- Li, M.; Dai, G.; Mu, L. Composition and Diversity of Soil Bacterial Communities under Identical Vegetation along an Elevational Gradient in Changbai Mountains, China. Front. Microbiol. 2022, 13, 1065412. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Rowińska, P.; Gutarowska, B.; Janas, R.; Szulc, J. Biopreparations for the Decomposition of Crop Residues. Microb. Biotechnol. 2024, 17, e14534. [Google Scholar] [CrossRef]
- Glassman, S.I.; Weihe, C.; Li, J.; Albright, M.B.N.; Looby, C.I.; Martiny, A.C.; Treseder, K.K.; Allison, S.D.; Martiny, J.B.H. Decomposition Responses to Climate Depend on Microbial Community Composition. Proc. Natl. Acad. Sci. USA 2018, 115, 11994–11999. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Du, M.; Chen, J.; Tie, L.; Zhou, S.; Buckeridge, K.M.; Cornelissen, J.H.C.; Huang, C.; Kuzyakov, Y. Microbial Necromass under Global Change and Implications for Soil Organic Matter. Glob. Change Biol. 2023, 29, 3503–3515. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; He, N.; Tiemann, L. Global Microbial Necromass Contribution to Soil Organic Matter. In Proceedings of the AGU Fall Meeting 2021, New Orleans, LA, USA, 13–17 December 2021. [Google Scholar]
- Jikai, S.; Yimei, H.; Qian, H.; Fengjing, X.U. Accumulation of Microbial Necromass Carbon and Their Contribution to Soil Organic Carbon in Different Vegetation Types on the Loess Plateau, Northwest China. Chin. J. Appl. Ecol./Yingyong Shengtai Xuebao 2024, 35, 124–132. [Google Scholar]
- Li, S.-J.; Sheng, M.-J.; Li, G.; Wang, R.; Li, J.; Zhang, G.-L.; Xiu, W.-M. Impacts of Land Use Intensification Level on Fluvo-aquic Cropland Soil Microbial Community Abundance and Necromass Accumulation in North China. Huan Jing Ke Xue 2023, 44, 4611–4622. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.; Tiemann, L.K.; Zhang, F.; Kuzyakov, Y.; Tian, J. Microbial Necromass in Cropland Soils: A Global Meta-Analysis of Management Effects. Glob. Change Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef] [PubMed]
- Beidler, K.V.; Huenupi, E.; DeLancey, L.C.; Maillard, F.; Zhang, B.; Persson, P.; Kennedy, P.G.; Phillips, R. Melanization Interacts with Soil Mineral and Microbial Properties to Determine Fungal Carbon and Nitrogen Persistence in Soils. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Cotrufo, M.F.; Sun, L.; Jiang, P.; Liu, Z.; Bai, E. Elevated Temperature Increases the Accumulation of Microbial Necromass Nitrogen in Soil via Increasing Microbial Turnover. Glob. Change Biol. 2020, 26, 5277–5289. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.; Xiao, J.; Duan, P.; Tang, T.; et al. Lithological Controls on Soil Aggregates and Minerals Regulate Microbial Carbon Use Efficiency and Necromass Stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef] [PubMed]
- Gies, H.; Hagedorn, F.; Lupker, M.; Montluçon, D.; Haghipour, N.; van der Voort, T.S.; Eglinton, T.I. Millennial-Age Glycerol Dialkyl Glycerol Tetraethers (GDGTs) in Forested Mineral Soils: 14C-Based Evidence for Stabilization of Microbial Necromass. Biogeosciences 2021, 18, 189–205. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Fan, X.; Sun, L.; Sang, C.; Wang, X.; Jiang, P.; Fang, Y.; Bai, E. Mineral Composition Controls the Stabilization of Microbially Derived Carbon and Nitrogen in Soils: Insights from an Isotope Tracing Model. Glob. Change Biol. 2024, 30, e17156. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Lynch, L.; Zheng, T.; Chen, F.; Liang, C. Factors Driving Microbial Biomass and Necromass Relationships Display Ecosystem-Dependent Responses. Eur. J. Soil Sci. 2024, 75, e13555. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, G.; Zhai, G.; Yi, W.; Ma, L.; Huang, Z.; Ye, X.; Ma, W.; Wang, Y.; Zhang, P.; et al. Root-Borne Microbial Necromass—An Overlooked Source of Grassland Soil Organic Carbon. Geophys. Res. Lett. 2024, 51, e2024GL110908. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Yang, Y.; Ren, L.; Wang, Z.; Liao, Y.; Yong, T. Global Synthesis on the Response of Soil Microbial Necromass Carbon to Climate-Smart Agriculture. Glob. Change Biol. 2024, 30, e17302. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Zhang, X.; Li, Y.; Virk, A.L.; Li, F.-M.; Yang, H.; Liu, S.; Kan, Z.-R. No-till Reduced Subsoil Organic Carbon Due to Decreased Microbial Necromass in Micro-Aggregates. Land Degrad. Dev. 2024, 35, 1792–1803. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, T.; Thomas, B.W.; Chen, J.; Xie, J.; Hu, Y.; Kong, F.; Yang, Y.; Chen, X.; Zhang, Y.; et al. Legume Cover Crops Enhance Soil Organic Carbon via Microbial Necromass in Orchard Alleyways. Soil Tillage Res. 2023, 234, 105858. [Google Scholar] [CrossRef]
- Si, Q.; Chen, K.; Wei, B.; Zhang, Y.; Sun, X.; Liang, J. Dissolved Carbon Flow to Particulate Organic Carbon Enhances Soil Carbon Sequestration. SOIL 2024, 10, 441–450. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart Grandy, A.; Liang, C.; Phillips, R.P. Fast-Decaying Plant Litter Enhances Soil Carbon in Temperate Forests but Not through Microbial Physiological Traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Abs, E.; Allison, S.D.; Tao, F.; Huang, Y.; Manzoni, S.; Abramoff, R.; Bruni, E.; Bowring, S.P.K.; Chakrawal, A.; et al. Emerging Multiscale Insights on Microbial Carbon Use Efficiency in the Land Carbon Cycle. Nat. Commun. 2024, 15, 8010. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, H.; Gu, X.; Li, N.; Zhao, X.; Lei, M.; Alharbi, H.; Megharaj, M.; He, W.; Kuzyakov, Y. Catalytic Efficiency of Soil Enzymes Explains Temperature Sensitivity: Insights from Physiological Theory. Sci. Total Environ. 2022, 822, 153365. [Google Scholar] [CrossRef]
- Woo, D.K.; Seo, Y. Effects of Elevated Temperature and Abnormal Precipitation on Soil Carbon and Nitrogen Dynamics in a Pinus Densiflora Forest. Front. For. Glob. Change 2022, 5, 1051210. [Google Scholar] [CrossRef]
- Adekanmbi, A.A.; Dale, L.; Shaw, L.; Sizmur, T. Differential Temperature Sensitivity of Intracellular Metabolic Processes and Extracellular Soil Enzyme Activities. Biogeosciences 2023, 20, 2207–2219. [Google Scholar] [CrossRef]
- Min, K.; Buckeridge, K.; Ziegler, S.E.; Edwards, K.A.; Bagchi, S.; Billings, S.A. Temperature Sensitivity of Biomass-Specific Microbial Exo-Enzyme Activities and CO2 Efflux Is Resistant to Change across Short- and Long-Term Timescales. Glob. Change Biol. 2019, 25, 1793–1807. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Heinzle, J.; Salas, E.; Kwatcho-Kengdo, S.; Borken, W.; Schindlbacher, A.; Wanek, W. Long-Term Soil Warming Decreases Soil Microbial Necromass Carbon by Adversely Affecting Its Production and Decomposition. Glob. Change Biol. 2024, 30, e17379. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, V.; Sharma, A.D.; Srinivasan, K. Evaluation of Seed Quality of Barley Varieties through Controlled Deterioration Test. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3123–3131. [Google Scholar] [CrossRef][Green Version]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S.; et al. Soil Enzymes in Response to Climate Warming: Mechanisms and Feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Mayes, M.A.; Allison, S.D.; Frey, S.D.; Shi, Z.; Hu, X.-M.; Luo, Y.; Melillo, J.M. Reduced Carbon Use Efficiency and Increased Microbial Turnover with Soil Warming. Glob. Change Biol. 2019, 25, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial Temperature Sensitivity and Biomass Change Explain Soil Carbon Loss with Warming. Nat. Clim. Change. 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, B.; Liang, M.; Wu, Y.; Li, Y.; Zhao, Z.; Liu, G.; Xue, S. Temperature Sensitivity Response of Soil Enzyme Activity to Simulated Climate Change at Growth Stages of Winter Wheat. Agronomy 2025, 15, 106. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Yang, C.; Li, H.; Wu, J. Effects of Water Stress on Nutrients and Enzyme Activity in Rhizosphere Soils of Greenhouse Grape. Front. Microbiol. 2024, 15, 1376849. [Google Scholar] [CrossRef]
- Kumari, J.A.; Rao, P.C.; Padmaja, G.; Madhavi, M. Effect of Temperature on Soil Enzyme Acid Phosphatase. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2830–2845. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Wang, H.; Gao, D.; Hu, G.; Xu, W.; Zhuge, Y.; Bai, E. Drying–Rewetting Events Enhance the Priming Effect on Soil Organic Matter Mineralization by Maize Straw Addition. Catena 2024, 238, 107872. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A.; Niedźwiecki, J. Changes in Soil Enzymatic Activity Causedby Hydric Stress. Pol. J. Environ. Stud. 2020, 29, 2653–2660. [Google Scholar] [CrossRef]
- Dowdeswell-Downey, E.; Grabowski, R.C.; Rickson, R.J. Do Temperature and Moisture Conditions Impact Soil Microbiology and Aggregate Stability? J. Soils Sediments 2023, 23, 3706–3719. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; Van Nostrand, J.D.; Wu, L.; et al. Warming Enhances Old Organic Carbon Decomposition through Altering Functional Microbial Communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.M.; Hayer, M.; Koch, B.J.; Mau, R.L.; Blazewicz, S.J.; Dijkstra, P.; Mack, M.C.; Marks, J.C.; Morrissey, E.M.; Pett-Ridge, J.; et al. Decreased Growth of Wild Soil Microbes after 15 Years of Transplant-Induced Warming in a Montane Meadow. Glob. Change Biol. 2022, 28, 128–139. [Google Scholar] [CrossRef]
- Ruan, Y.; Kuzyakov, Y.; Liu, X.; Zhang, X.; Xu, Q.; Guo, J.; Guo, S.; Shen, Q.; Yang, Y.; Ling, N. Elevated Temperature and CO2 Strongly Affect the Growth Strategies of Soil Bacteria. Nat. Commun. 2023, 14, 391. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.; Sun, W.; Zhao, Q.; Liang, M.; Chen, W.; Guo, Z.; Jiang, Y.; Jiang, Y.; Liu, G.; et al. Temperature Sensitivity of Soil Enzyme Kinetics under N and P Fertilization in an Alpine Grassland, China. Sci. Total Environ. 2022, 838, 156042. [Google Scholar] [CrossRef]
- Gałęzewski, L.; Jaskulska, I.; Kotwica, K.; Lewandowski, Ł. The Dynamics of Soil Moisture and Temperature—Strip-Till vs. Plowing—A Case Study. Agronomy 2023, 13, 83. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Chen, J.; Castellano, M.J.; Ye, C.; Zhang, N.; Miao, Y.; Zheng, H.; Li, J.; Ding, W. Oxygen Availability Regulates the Quality of Soil Dissolved Organic Matter by Mediating Microbial Metabolism and Iron Oxidation. Glob. Change Biol. 2022, 28, 7410–7427. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial Carbon Use Efficiency Promotes Global Soil Carbon Storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.E.; Breecker, D.O.; Blackwood, C.B.; Gallagher, T.M. A New Approach to Continuous Monitoring of Carbon Use Efficiency and Biosynthesis in Soil Microbes from Measurement of CO2 and O2. Biogeosciences 2025, 22, 87–101. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture Is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Warren, C.R.; Swarbrick, B.; Minasny, B.; McBratney, A.B.; Young, I.M. Microbial Decomposition of Organic Matter and Wetting–Drying Promotes Aggregation in Artificial Soil but Porosity Increases Only in Wet-Dry Condition. Geoderma 2024, 447, 116924. [Google Scholar] [CrossRef]
- Murthy, R.K.; Raut, M. Impact of Climate Change on Soil Properties and Functions. Mysore J. Agric. Sci. 2024, 59, 34–49. [Google Scholar]
- Horodecki, P.; Jagodziński, A.M. Site Type Effect on Litter Decomposition Rates: A Three-Year Comparison of Decomposition Process between Spoil Heap and Forest Sites. Forests 2019, 10, 353. [Google Scholar] [CrossRef]
- Piaszczyk, W.; Lasota, J.; Błońska, E.; Foremnik, K. How Habitat Moisture Condition Affects the Decomposition of Fine Woody Debris from Different Species. Catena 2022, 208, 105765. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Zheng, L.; Yu, Y.; Mushinski, R.M.; Zhou, Y.; Xiao, C. Greater Soil Water and Nitrogen Availability Increase C:N Ratios of Root Exudates in a Temperate Steppe. Soil Biol. Biochem. 2021, 161, 108384. [Google Scholar] [CrossRef]
- Bian, H.; Li, C.; Zhu, J.; Xu, L.; Li, M.; Zheng, S.; He, N. Soil Moisture Affects the Rapid Response of Microbes to Labile Organic C Addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Runkov, R.A.; Ilyasov, D.V. Spatial Variability of Methane Emissions from Soils of Wet Forests: A Brief Review. Environ. Dyn. Glob. Clim. Change 2023, 14, 167–180. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, I. Sustainable Development Goal 13: Recent Progress and Challenges to Climate Action. Environ. Sustain. 2023, 6, 297–301. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, R.; Li, T.; Zhou, R.; Hou, Z.; Zhang, X. Interactions among Microorganisms Functionally Active for Electron Transfer and Pollutant Degradation in Natural Environments. Eco-Environ. Health 2023, 2, 3–15. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, S.; Niu, B.; Pei, Y.; Song, S.; Lei, T.; Yun, H. Soil Texture Influences Soil Bacterial Biomass in the Permafrost-Affected Alpine Desert of the Tibetan Plateau. Front. Microbiol. 2022, 13, 1007194. [Google Scholar] [CrossRef]
- Naylor, D.; Sadler, N.; Bhattacharjee, A.; Graham, E.B.; Anderton, C.R.; McClure, R.; Lipton, M.; Hofmockel, K.S.; Jansson, J.K. Soil Microbiomes Under Climate Change and Implications for Carbon Cycling. Annu. Rev. Environ. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Salinas, J.; Guardia, G.; Monistrol, A.; Iglesias-Díez, S.M.; García-Gutiérrez, S.; Jiménez-Horcajada, R.; Espín, G.; Vallejo, A. Can Soluble Organic Fertilizers and/or Manure Reach the Agronomic and Environmental Performance of Synthetic Fertilizers in Drip-Fertigated Crops? Soil Use Manag. 2024, 40, e13136. [Google Scholar] [CrossRef]
- Rashid, M.A.; Bastami, M.S.; Bakar, N.A.A.; Jumat, F.; Suptian, M.F.M.; Rahman, M.H.A.; Azmin, A.A.; Talib, S.A.A. Development of National Emission Factor for Rice Production System in Malaysia: A Step Toward Achieving Sustainable Development Goals. J. Lifestyle SDGs Rev. 2025, 5, e02774. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Mapfumo, P.; Kirchmann, H. Decomposition of Organic Matter in Soil as Influenced by Texture and Pore Size Distribution. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Academy Science Publishers: Karen, Nairobi, Kenya, 2004; Volume 261. [Google Scholar]
- Wahdan, S.F.M.; Ji, L.; Schädler, M.; Wu, Y.-T.; Sansupa, C.; Tanunchai, B.; Buscot, F.; Purahong, W. Future Climate Conditions Accelerate Wheat Straw Decomposition alongside Altered Microbial Community Composition, Assembly Patterns, and Interaction Networks. ISME J. 2023, 17, 238–251. [Google Scholar] [CrossRef]
- Lu, D.; Mao, Z.; Tang, Y.; Feng, B.; Xu, L. Driving Factors Influencing Soil Microbial Community Succession of Coal Mining Subsidence Areas during Natural Recovery in Inner Mongolia Grasslands. Microorganisms 2023, 12, 87. [Google Scholar] [CrossRef]
- Xie, S.; Tran, H.-T.; Pu, M.; Zhang, T. Transformation Characteristics of Organic Matter and Phosphorus in Composting Processes of Agricultural Organic Waste: Research Trends. Mater. Sci. Energy Technol. 2023, 6, 331–342. [Google Scholar] [CrossRef]
- Chirol, C.; Séré, G.; Redon, P.-O.; Chenu, C.; Derrien, D. Depth Dependence of Soil Organic Carbon Additional Storage Capacity in Different Soil Types by the 2050 Target for Carbon Neutrality. SOIL 2025, 11, 149–174. [Google Scholar] [CrossRef]
- Miguel, M.A.; Kim, S.-H.; Lee, S.-S.; Cho, Y.-I. Impact of Soil Microbes and Oxygen Availability on Bacterial Community Structure of Decomposing Poultry Carcasses. Animals 2021, 11, 2937. [Google Scholar] [CrossRef]
- Leul, Y.; Assen, M.; Damene, S.; Legass, A. Effects of Land-Use Dynamics on Soil Organic Carbon and Total Nitrogen Stock, Western Ethiopia. Appl. Environ. Soil Sci. 2023, 2023, 5080313. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil Carbon Debt of 12,000 Years of Human Land Use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Welty, A.; Blazewicz, S.J.; Bru, D.; Rouard, N.; Breuil, M.-C.; Gessler, A.; Galiano, L.; Miranda, J.C.; Spor, A.; et al. Depth Matters: Effects of Precipitation Regime on Soil Microbial Activity upon Rewetting of a Plant-Soil System. ISME J. 2018, 12, 1061–1071. [Google Scholar] [CrossRef]
- Merloti, L.F.; Mendes, L.W.; Pedrinho, A.; de Souza, L.F.; Ferrari, B.M.; Tsai, S.M. Forest-to-Agriculture Conversion in Amazon Drives Soil Microbial Communities and N-Cycle. Soil Biol. Biochem. 2019, 137, 107567. [Google Scholar] [CrossRef]
- Mandal, D.; Chandrakala, M.; Alam, N.M.; Roy, T.; Mandal, U. Assessment of Soil Quality and Productivity in Different Phases of Soil Erosion with the Focus on Land Degradation Neutrality in Tropical Humid Region of India. Catena 2021, 204, 105440. [Google Scholar] [CrossRef]
- Gao, T.; Tian, H.; Wang, Z.; Shi, J.; Yang, R.; Wang, F.; Xiang, L.; Dai, Y.; Megharaj, M.; He, W. Effects of Atrazine on Microbial Metabolic Limitations in Black Soils: Evidence from Enzyme Stoichiometry. Chemosphere 2023, 334, 139045. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Li, F.; Sun, M.; Yang, X.; Wang, G.; Ma, J.; Sun, W. Exchangeable Ca2+ Content and Soil Aggregate Stability Control the Soil Organic Carbon Content in Degraded Horqin Grassland. Ecol. Indic. 2022, 134, 108507. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Li, X.; Su, D.; He, J. Biotic Interactions Contribute More than Environmental Factors and Geographic Distance to Biogeographic Patterns of Soil Prokaryotic and Fungal Communities. Front. Microbiol. 2023, 14, 1134440. [Google Scholar] [CrossRef]
- Doetterl, S.; Berhe, A.A.; Heckman, K.; Lawrence, C.; Schnecker, J.; Vargas, R.; Vogel, C.; Wagai, R. A Landscape-Scale View of Soil Organic Matter Dynamics. Nat. Rev. Earth Environ. 2025, 6, 67–81. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, P.; Zheng, J.; Xue, L.; Zhu, Q.; Cai, X.; Cheng, S.; Zhang, Z.; Kong, F.; Zhang, J. Effects of Soil Texture and Nitrogen Fertilisation on Soil Bacterial Community Structure and Nitrogen Uptake in Flue-Cured Tobacco. Sci. Rep. 2021, 11, 22643. [Google Scholar] [CrossRef]
- Turner, B.L. Variation in pH Optima of Hydrolytic Enzyme Activities in Tropical Rain Forest Soils. Appl. Environ. Microbiol. 2010, 76, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, N.; Heitman, J.L.; Shi, W. Soil Pore Size Distribution Shaped Not Only Compositions but Also Networks of the Soil Microbial Community. Appl. Soil Ecol. 2022, 170, 104273. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil Microbial Diversity and Composition: Links to Soil Texture and Associated Properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Dowd, S.E.; Bell, C.W.; Lascano, R.; Booker, J.D.; Zobeck, T.M.; Upchurch, D.R. Microbial Community Composition as Affected by Dryland Cropping Systems and Tillage in a Semiarid Sandy Soil. Diversity 2010, 2, 910–931. [Google Scholar] [CrossRef]
- Chiba, A.; Uchida, Y.; Kublik, S.; Vestergaard, G.; Buegger, F.; Schloter, M.; Schulz, S. Soil Bacterial Diversity Is Positively Correlated with Decomposition Rates during Early Phases of Maize Litter Decomposition. Microorganisms 2021, 9, 357. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.-C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the Dynamics of Soil Aggregation as Affected by Arbuscular Mycorrhizal Fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium Promotes Persistent Soil Organic Matter by Altering Microbial Transformation of Plant Litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N Ratio Determines Spatial Variations in Soil Microbial Communities and Enzymatic Activities of the Agricultural Ecosystems in Northeast China: Jilin Province Case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Ullah, S.; Raza, M.M.; Abbas, T.; Guan, X.; Zhou, W.; He, P. Responses of Soil Microbial Communities and Enzyme Activities under Nitrogen Addition in Fluvo-Aquic and Black Soil of North China. Front. Microbiol. 2023, 14, 1249471. [Google Scholar] [CrossRef]
- Masson, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef] [PubMed]
- Carrino-Kyker, S.R.; Coyle, K.P.; Kluber, L.A.; Burke, D.J. Fungal and Bacterial Communities Exhibit Consistent Responses to Reversal of Soil Acidification and Phosphorus Limitation over Time. Microorganisms 2020, 8, 1. [Google Scholar] [CrossRef]
- Bai, T.; He, S.; Li, Y.; Deng, J.; Sang, F.; Zhao, Y.; Wang, H.; He, T.; Zhang, K.; Qiu, Y.; et al. Unexpected Suppressive Fungal Diversity and Stimulative Soil Carbon Loss under Soil Acidification in an Alkaline Grassland. Funct. Ecol. 2025, 39, 114–127. [Google Scholar] [CrossRef]
- Breugem, A.; Kros, H.; de Vries, W. Impacts of pH on Mechanisms and Rates of Carbon and Nitrogen Mineralisation: A Review; Wageningen Environmental Research: Wageningen, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, H.; Liu, B.; Zhang, M.; Zhang, C.; Chen, P.; Yang, B. Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil. Microorganisms 2022, 10, 977. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Wu, J.; Ma, D.; Zhang, C.; Zhang, B.; Liu, Y.; Zhang, Z.; Tian, F.; Zhao, H.; et al. Soil Fungal Community Is More Sensitive than Bacterial Community to Modified Materials Application in Saline–Alkali Land of Hetao Plain. Front. Microbiol. 2024, 15, 1255536. [Google Scholar] [CrossRef]
- Tang, S.; Pan, W.; Zhou, J.; Ma, Q.; Yang, X.; Wanek, W.; Marsden, K.A.; Kuzyakov, Y.; Chadwick, D.R.; Wu, L.; et al. Soil Nitrogen and Phosphorus Regulate Decomposition of Organic Nitrogen Compounds in the Rothamsted Experiment. Soil Biol. Biochem. 2024, 196, 109502. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, J.; Hao, J.; Li, C.; Quan, W. Decomposition Characteristics of Lignocellulosic Biomass in Subtropical Rhododendron Litters under Artificial Regulation. Metabolites 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Lankiewicz, T.S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington, S.P.; Leggieri, P.A.; Brown, J.L.; Swift, C.L.; Lipzen, A.; Na, H.; et al. Lignin Deconstruction by Anaerobic Fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef]
- Subedi, P.; Jokela, E.J.; Vogel, J.G.; Bracho, R.; Inglett, K.S. The Effects of Nutrient Limitations on Microbial Respiration and Organic Matter Decomposition in a Florida Spodosol as Influenced by Historical Forest Management Practices. For. Ecol. Manag. 2021, 479, 118592. [Google Scholar] [CrossRef]
- Pan, Y.; Fang, F.; Tang, H. Patterns and Internal Stability of Carbon, Nitrogen, and Phosphorus in Soils and Soil Microbial Biomass in Terrestrial Ecosystems in China: A Data Synthesis. Forests 2021, 12, 1544. [Google Scholar] [CrossRef]
- Soler-Bistué, A.; Couso, L.L.; Sánchez, I.E. The Evolving Copiotrophic/Oligotrophic Dichotomy: From Winogradsky to Physiology and Genomics. Environ. Microbiol. 2023, 25, 1232–1237. [Google Scholar] [CrossRef]
- Stone, B.W.G.; Dijkstra, P.; Finley, B.K.; Fitzpatrick, R.; Foley, M.M.; Hayer, M.; Hofmockel, K.S.; Koch, B.J.; Li, J.; Liu, X.J.A.; et al. Life History Strategies among Soil Bacteria—Dichotomy for Few, Continuum for Many. ISME J. 2023, 17, 611–619. [Google Scholar] [CrossRef]
- Lladó, S.; Baldrian, P. Community-Level Physiological Profiling Analyses Show Potential to Identify the Copiotrophic Bacteria Present in Soil Environments. PLoS ONE 2017, 12, e0171638. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.-B.; Zhang, X.-Y.; Dang, Y.-R.; Sun, L.-L.; Zhang, W.-P.; Fu, H.-H.; Yang, G.-P.; Wang, M.; McMinn, A.; et al. Experimental Evidence for Long-Term Coexistence of Copiotrophic and Oligotrophic Bacteria in Pelagic Surface Seawater. Environ. Microbiol. 2021, 23, 1162–1173. [Google Scholar] [CrossRef]
- Ntonta, S.; Zengeni, R.; Muchaonyerwa, P.; Chaplot, V. Variability in Decomposition Rate of Sorghum Cultivar Residues Linked to Lignin Content. Rhizosphere 2024, 29, 100850. [Google Scholar] [CrossRef]
- Wang, S.; Uhlgren, O.; Salonen, A.-R.; Heinonsalo, J. Soil Contents and Stoichiometry of Carbon, Nitrogen, and Phosphorus in Finnish Farmland and Feedbacks on Management Patterns. In Proceedings of the Copernicus Meetings, Online, 19–30 April 2021. [Google Scholar]
- Cheng, W.; Zhang, S.; Wang, Y.; Hong, L.; Qiu, M.; Wang, Y.; Luo, Y.; Zhang, Q.; Wang, T.; Jia, X.; et al. Dahongpao Mother Tree Affects Soil Microbial Community and Nutrient Cycling by Increasing Rhizosphere Soil Characteristic Metabolite Content. Front. Plant Sci. 2025, 16, 1508622. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B. Effects of Different Vegetation on Soil Microbial Biomass Carbon and Nitrogen in Newly Cultivated Land. Bangladesh J. Bot. 2021, 50, 879–886. [Google Scholar] [CrossRef]
- Manral, V.; Bargali, K.; Bargali, S.S.; Karki, H.; Chaturvedi, R.K. Seasonal Dynamics of Soil Microbial Biomass C, N and P along an Altitudinal Gradient in Central Himalaya, India. Sustainability 2023, 15, 1651. [Google Scholar] [CrossRef]
- Chakrawal, A.; Calabrese, S.; Herrmann, A.M.; Manzoni, S. Interacting Bioenergetic and Stoichiometric Controls on Microbial Growth. Front. Microbiol. 2022, 13, 859063. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Weintraub, M.N.; Moorhead, D. Estimating Microbial Carbon Use Efficiency in Soil: Isotope-Based and Enzyme-Based Methods Measure Fundamentally Different Aspects of Microbial Resource Use. Soil Biol. Biochem. 2022, 169, 108677. [Google Scholar] [CrossRef]
- Angst, G.; Angst, Š.; Frouz, J.; Jabinski, S.; Jílková, V.; Kukla, J.; Li, M.; Meador, T.B.; Angel, R. Stabilized Microbial Necromass in Soil Is More Strongly Coupled with Microbial Diversity than the Bioavailability of Plant Inputs. Soil Biol. Biochem. 2024, 190, 109323. [Google Scholar] [CrossRef]
- Wu, S.; Konhauser, K.O.; Chen, B.; Huang, L. “Reactive Mineral Sink” Drives Soil Organic Matter Dynamics and Stabilization. npj Mater. Sustain. 2023, 1, 3. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Yan, J.; Ma, Y.; Zhou, X.; Yu, W.; Kan, H.; Meng, Q.; Xie, R.; Dong, P. A Review on Adsorption Characteristics and Influencing Mechanism of Heavy Metals in Farmland Soil. RSC Adv. 2023, 13, 3505–3519. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qu, C.; Huang, Q.; Cai, P. Microbial Extracellular Polymeric Substances (EPS) in Soil: From Interfacial Behaviour to Ecological Multifunctionality. Geo-Bio Interfaces 2024, 1, e4. [Google Scholar] [CrossRef]
- Wang, X.-B.; Azarbad, H.; Leclerc, L.; Dozois, J.; Mukula, E.; Yergeau, É. A Drying-Rewetting Cycle Imposes More Important Shifts on Soil Microbial Communities than Does Reduced Precipitation. mSystems 2022, 7, e0024722. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y.; et al. Formation of Soil Organic Carbon Pool Is Regulated by the Structure of Dissolved Organic Matter and Microbial Carbon Pump Efficacy: A Decadal Study Comparing Different Carbon Management Strategies. Glob. Change Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, K.; Yang, Y.; Gao, B.; Zheng, H. Effects of Biochar on the Accumulation of Necromass-Derived Carbon, the Physical Protection and Microbial Mineralization of Soil Organic Carbon. Crit. Rev. Environ. Sci. Technol. 2024, 54, 39–67. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, Y.; Morrissey, E.; Liu, Y.; Sun, L.; Qu, L.; Sang, C.; Zhang, H.; Li, G.; et al. Integrating Microbial Community Properties, Biomass and Necromass to Predict Cropland Soil Organic Carbon. ISME Commun. 2023, 3, 86. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, G.; Zhao, B.; Cong, N.; Zheng, Z.; Zhu, J.; Duan, X.; Zhang, Y. Climate Warming Alters the Relative Importance of Plant Root and Microbial Community in Regulating the Accumulation of Soil Microbial Necromass Carbon in a Tibetan Alpine Meadow. Glob. Change Biol. 2023, 29, 3193–3204. [Google Scholar] [CrossRef]
- Campeau, A.B.; Lafleur, P.M.; Humphreys, E.R. Landscape-Scale Variability in Soil Organic Carbon Storage in the Central Canadian Arctic. Can. J. Soil. Sci. 2014, 94, 477–488. [Google Scholar] [CrossRef]
- Qu, R.; Liu, G.; Yue, M.; Wang, G.; Peng, C.; Wang, K.; Gao, X. Soil Temperature, Microbial Biomass and Enzyme Activity Are the Critical Factors Affecting Soil Respiration in Different Soil Layers in Ziwuling Mountains, China. Front. Microbiol. 2023, 14, 1105723. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bhattacharyya, R.; Jiménez-Ballesta, R.; Dey, A.; Saha, N.D.; Kumar, S.; Nath, C.P.; Prakash, V.; Jatav, S.S.; Patra, A. Conventional and Zero Tillage with Residue Management in Rice–Wheat System in the Indo-Gangetic Plains: Impact on Thermal Sensitivity of Soil Organic Carbon Respiration and Enzyme Activity. Int. J. Environ. Res. Public Heal. 2023, 20, 810. [Google Scholar] [CrossRef]
- Valentine, K.; Herbert, E.R.; Walters, D.C.; Chen, Y.; Smith, A.J.; Kirwan, M.L. Climate-driven tradeoffs between landscape connectivity and the maintenance of the coastal carbon sink. Nat. Commun. 2023, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.C.; Ryu, D.; Sheridan, G.J.; Lane, P.N.J. Modeling Vegetation Water Stress over the Forest from Space: Temperature Vegetation Water Stress Index (TVWSI). Remote Sens. 2021, 13, 4635. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, L.; Bao, Y.; Zhang, J.; Yao, F.; Bai, L.; Zheng, P. Prediction of Soil Organic Carbon Content in Complex Vegetation Areas Based on CNN-LSTM Model. Land 2024, 13, 915. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chen, X. Land Use Transitions and the Associated Impacts on Carbon Storage in the Poyang Lake Basin, China. Remote Sens. 2023, 15, 2703. [Google Scholar] [CrossRef]
- Jiang, Q.; Jia, L.; Chen, Y.; Yang, Z.; Chen, S.; Xiong, D.; Liu, X.; Wang, X.; Yao, X.; Chen, T.; et al. Substrate and Adenylate Limit Subtropical Tree Fine-Root Respiration under Soil Warming. Plant Cell Environ. 2023, 46, 2827–2840. [Google Scholar] [CrossRef]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz-Horta, L.A.; Yang, X.; Du, H.; Wang, K.; Li, D. Tree Species Diversity Increases Soil Microbial Carbon Use Efficiency in a Subtropical Forest. Glob. Change Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Feng, J.; Li, X.; Zhang, Z.; He, J.-S.; Schimel, J.P.; Zhu, B. Whole-Soil-Profile Warming Does Not Change Microbial Carbon Use Efficiency in Surface and Deep Soils. Proc. Natl. Acad. Sci. USA 2023, 120, e2302190120. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Shen, Q.; Ling, N.; Nan, Z. Microbial Carbon Use Efficiency in Different Ecosystems: A Meta-Analysis Based on a Biogeochemical Equilibrium Model. Glob. Change Biol. 2023, 29, 4758–4774. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, G.; Zhang, T.; Fan, X.; Liu, Z.; Bai, E. Erosion Effects on Soil Microbial Carbon Use Efficiency in the Mollisol Cropland in Northeast China. Soil Ecol. Lett. 2023, 5, 230176. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, W.; Wang, W.; Li, X.; Sheng, Z. Microbial Assemblies with Distinct Trophic Strategies Drive Changes in Soil Microbial Carbon Use Efficiency along Vegetation Primary Succession in a Glacier Retreat Area of the Southeastern Tibetan Plateau. Sci. Total Environ. 2023, 867, 161587. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Su, Y.; Wang, J.; Lin, S.; Huang, Z.; Huang, G. Elevational Patterns of Microbial Carbon Use Efficiency in a Subtropical Mountain Forest. Biol. Fertil. Soils 2024, 60, 5–15. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; McNamara, N.P.; Ostle, N.; Puissant, J.; Goodall, T.; Griffiths, R.I.; Stott, A.W.; Whitaker, J. Environmental and Microbial Controls on Microbial Necromass Recycling, an Important Precursor for Soil Carbon Stabilization. Commun. Earth Environ. 2020, 1, 36. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.-J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial Diversity Drives Carbon Use Efficiency in a Model Soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining Trait-Based Microbial Strategies with Consequences for Soil Carbon Cycling under Climate Change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Ding, J.; Li, C.-Y.; Yan, Z.-Z.; He, J.-Z.; Hu, H.-W. Microbial Functional Attributes, Rather than Taxonomic Attributes, Drive Top Soil Respiration, Nitrification and Denitrification Processes. Sci. Total Environ. 2020, 734, 139479. [Google Scholar] [CrossRef] [PubMed]
- Raczka, N.C.; Piñeiro, J.; Tfaily, M.M.; Chu, R.K.; Lipton, M.S.; Pasa-Tolic, L.; Morrissey, E.; Brzostek, E. Interactions between Microbial Diversity and Substrate Chemistry Determine the Fate of Carbon in Soil. Sci. Rep. 2021, 11, 19320. [Google Scholar] [CrossRef]
- Qi, Q.; Haowei, Y.; Zhang, Z.; Van Nostrand, J.D.; Wu, L.; Guo, X.; Feng, J.; Wang, M.; Yang, S.; Zhao, J.; et al. Microbial Functional Responses Explain Alpine Soil Carbon Fluxes under Future Climate Scenarios. mBio 2021, 12, e00761-20. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Tarkka, M.; Knief, C.; Koller, R.; Peth, S.; Schmidt, V.; Spielvogel, S.; Uteau, D.; Weber, M.; Razavi, B.S. Bridging Microbial Functional Traits With Localized Process Rates at Soil Interfaces. Front. Microbiol. 2021, 12, 625697. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Shinfuku, M.; Junier, P.; Poirier, S.; Verrecchia, E.; Sebag, D.; DeAngelis, K.M. Direct Evidence for the Role of Microbial Community Composition in the Formation of Soil Organic Matter Composition and Persistence. ISME Commun. 2021, 1, 64. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wang, Y.; Chen, X.; Tan, H.; Jin, X.; Lu, Q.; He, S.; Long, M.-X. Cover Cropping Increases Soil Fungal-Bacterial Community Diversity and Network Complexity in Apple Orchards on the Loess Plateau, China. Front. Environ. Sci. 2022, 10, 916288. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, J.; Du, T.; Ju, X.; Gan, Y.; Li, S.; Xia, L.; Shen, Y.; Pacenka, S.; Steenhuis, T.S.; et al. Diversifying Crop Rotation Increases Food Production, Reduces Net Greenhouse Gas Emissions and Improves Soil Health. Nat. Commun. 2024, 15, 198. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Qiu, G.; Yu, H.; Liu, F.; Wang, G.; Duan, Y. Conservation Tillage Facilitates the Accumulation of Soil Organic Carbon Fractions by Affecting the Microbial Community in an Eolian Sandy Soil. Front. Microbiol. 2024, 15, 1394179. [Google Scholar] [CrossRef]
- Joshi, D.R.; Clay, D.E.; Alverson, R.; Clay, S.A.; Westhoff, S.; Johnson, J.M.F.; Wang, T.; Sieverding, H. Tillage Intensity Reductions When Combined with Yield Increases May Slow Soil Carbon Saturation in the Central United States. Sci. Rep. 2025, 15, 10697. [Google Scholar] [CrossRef]
- López i Losada, R.; Hedlund, K.; Haddaway, N.R.; Sahlin, U.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Jørgensen, H.B.; Isberg, P.-E. Synergistic Effects of Multiple “Good Agricultural Practices” for Promoting Organic Carbon in Soils: A Systematic Review of Long-Term Experiments. Ambio 2025. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Li, Y.; Jiao, Y.; Fan, L.; Jiang, Y.; Qu, C.; Filimonenko, E.; Jiang, Y.; Tian, X.; et al. Nitrogen Fertilizer Builds Soil Organic Carbon under Straw Return Mainly via Microbial Necromass Formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanski, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and A Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, C.; Zhang, R.; Jiao, N.; Tian, J.; Lambers, H.; Liang, C.; Cong, W.-F.; Zhang, F. Intercropping Increases Soil Macroaggregate Carbon through Root Traits Induced Microbial Necromass Accumulation. Soil Biol. Biochem. 2023, 185, 109146. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of Rhizobium-Soybean Symbiosis and Nitrogen Fixation under Drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef]
- Yang, X.; Hu, H.-W.; Yang, G.-W.; Cui, Z.-L.; Chen, Y.-L. Crop rotational diversity enhances soil microbiome network complexity and multifunctionality. Geoderma 2023, 436, 116562. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil Organic Carbon Sequestration Rates in Vineyard Agroecosystems under Different Soil Management Practices: A Meta-Analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-Analysis of the Impacts of Global Change Factors on Soil Microbial Diversity and Functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, W.; Zhang, Y.; Liu, X.; Zhang, Y.; Zheng, X.; Luo, J.; Zou, J. Effects of No-till on Upland Crop Yield and Soil Organic Carbon: A Global Meta-Analysis. Plant Soil 2024, 499, 363–377. [Google Scholar] [CrossRef]
- Prairie, A.M.; King, A.E.; Cotrufo, M.F. Restoring Particulate and Mineral-Associated Organic Carbon through Regenerative Agriculture. Proc. Natl. Acad. Sci. USA 2023, 120, e2217481120. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Gao, H.; Jia, A.; Gao, Q. Long-Term Conservation Tillage Increases Soil Organic Carbon Stability by Modulating Microbial Nutrient Limitations and Aggregate Protection. Agronomy 2025, 15, 1571. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-Term Use of Cover Crops and No-till Shift Soil Microbial Community Life Strategies in Agricultural Soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef] [PubMed]
- Seabloom, E.W.; Borer, E.T.; Hobbie, S.E.; MacDougall, A.S. Soil Nutrients Increase Long-Term Soil Carbon Gains Threefold on Retired Farmland. Glob. Change Biol. 2021, 27, 4909–4920. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, M.; Wang, S.; Lu, X. Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield. Agronomy 2024, 14, 1348. [Google Scholar] [CrossRef]
- Nghia, N.K.; Robatjazi, J.; Vy, V.D.T.; Tecimen, H.B.; Lasar, H.G.W.; Lesueur, D.; Bai, S.H.; Tran, H.-T.; Thien, N.H.; Luan, D.T. Effect of Organic Farming Practices on Soil Health Improvement of Coconut Farms. Environ. Technol. Innov. 2025, 38, 104067. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Chapter 2—Influence of Synthetic Fertilizers and Pesticides on Soil Health and Soil Microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. ISBN 978-0-08-103017-2. [Google Scholar]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The Effect of Application of Organic Manures and Mineral Fertilizers on the State of Soil Organic Matter and Nutrients in the Long-Term Field Experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Mcinga, S.; Muzangwa, L.; Janhi, K.; Mnkeni, P.N.S. Conservation Agriculture Practices Can Improve Earthworm Species Richness and Abundance in the Semi-Arid Climate of Eastern Cape, South Africa. Agriculture 2020, 10, 576. [Google Scholar] [CrossRef]
- Sazykina, M.; Minkina, T.; Konstantinova, E.; Khmelevtsova, L.; Azhogina, T.; Antonenko, E.; Karchava, S.; Klimova, M.; Sushkova, S.; Polienko, E.; et al. Pollution Impact on Microbial Communities Composition in Natural and Anthropogenically Modified Soils of Southern Russia. Microbiol. Res. 2022, 254, 126913. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Geng, S.; Cao, W.; Zuo, R.; Teng, Y.; Ding, A.; Fan, F.; Dou, J. Vertical Distribution Characteristics and Interactions of Polycyclic Aromatic Compounds and Bacterial Communities in Contaminated Soil in Oil Storage Tank Areas. Chemosphere 2022, 301, 134695. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Russo, F.; Ceci, A.; Pinzari, F.; Siciliano, A.; Guida, M.; Malusà, E.; Tartanus, M.; Miszczak, A.; Maggi, O.; Persiani, A.M. Bioremediation of Dichlorodiphenyltrichloroethane (DDT)-Contaminated Agricultural Soils: Potential of Two Autochthonous Saprotrophic Fungal Strains. Appl. Environ. Microbiol. 2019, 85, e01720-19. [Google Scholar] [CrossRef]
- Ebsa, G.; Gizaw, B.; Admassie, M.; Degu, T.; Alemu, T. The Role and Mechanisms of Microbes in Dichlorodiphenyltrichloroethane (DDT) and Its Residues Bioremediation. Biotechnol. Rep. 2024, 42, e00835. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Liu, P.; Wen, S.; Zhu, S.; Hu, X.; Wang, Y. Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes 2025, 13, 916. [Google Scholar] [CrossRef]
- Sekiya, N.; Mae, A.; Murai, A.; Peter, M.A.; Goto, M.; Kato, H.; Ichikawa, S.; Watanabe, K. Soil Microbes and Organic Fertilizer Efficiency Are Associated with Rice Field Topography. Sci. Rep. 2025, 15, 24939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).