Seasonal Changes in the Soil Microbiome on Chernozem Soil in Response to Tillage, Fertilization, and Cropping System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Processing
2.3. Measurement of Soil Physico-Chemical Parameters
2.4. Measurement of Soil Microbial Biomass and Composition
2.5. Statistical Analysis
3. Results
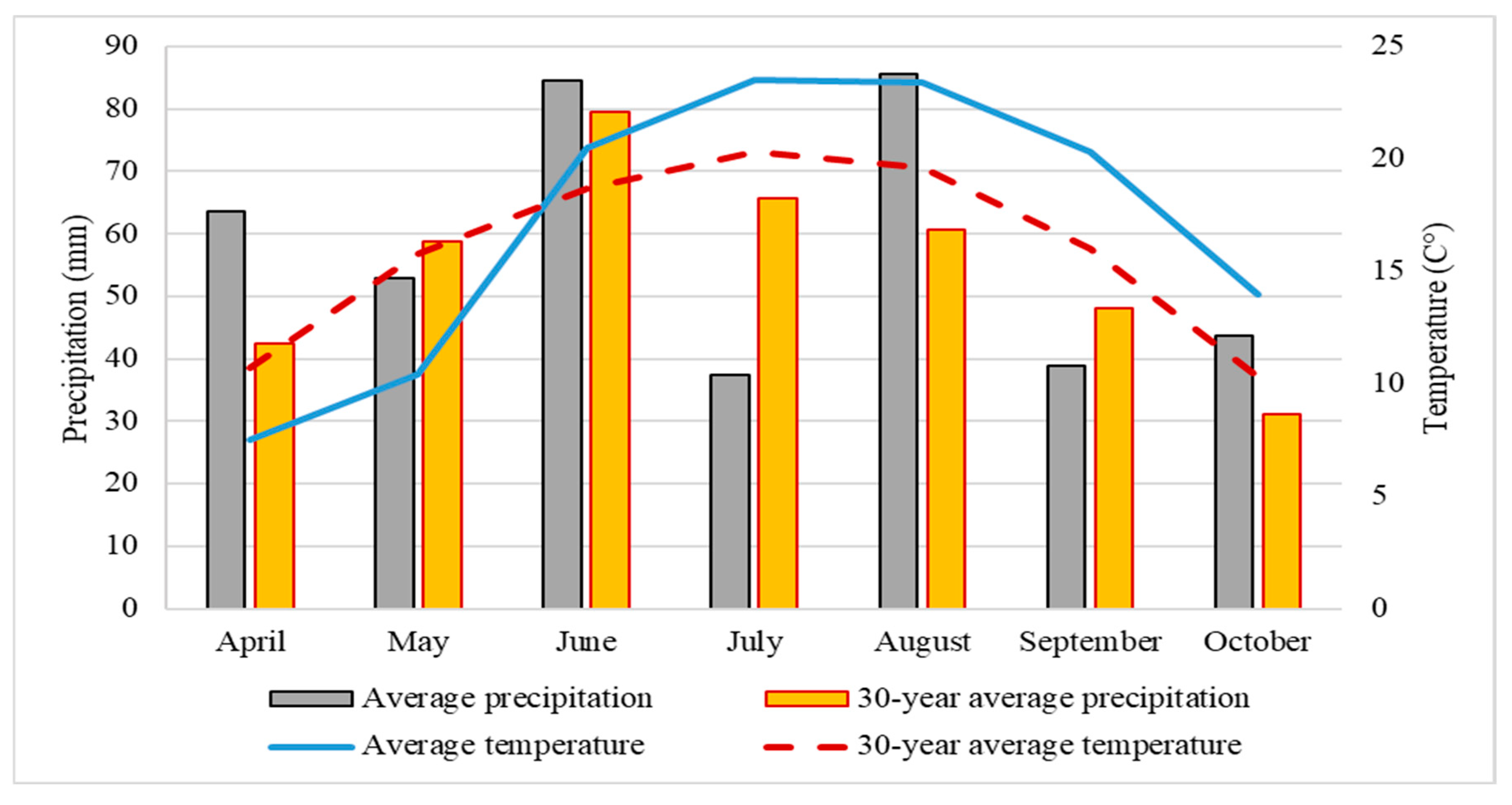
3.1. The Physico-Chemical Parameters of Soil
3.2. Soil Microbial Biomass and Community Composition
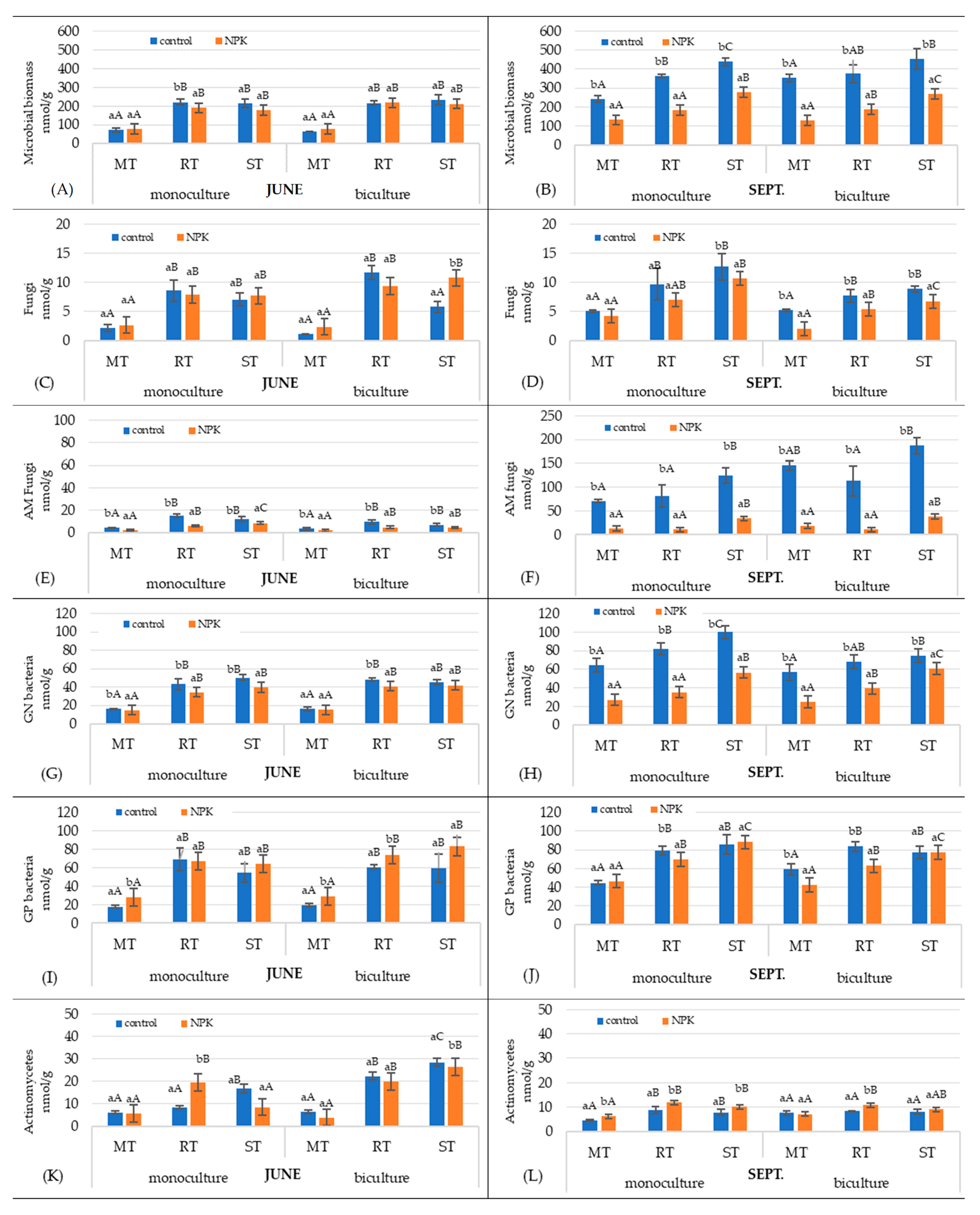
3.2.1. Microbial Biomass
3.2.2. Microbial Community Composition
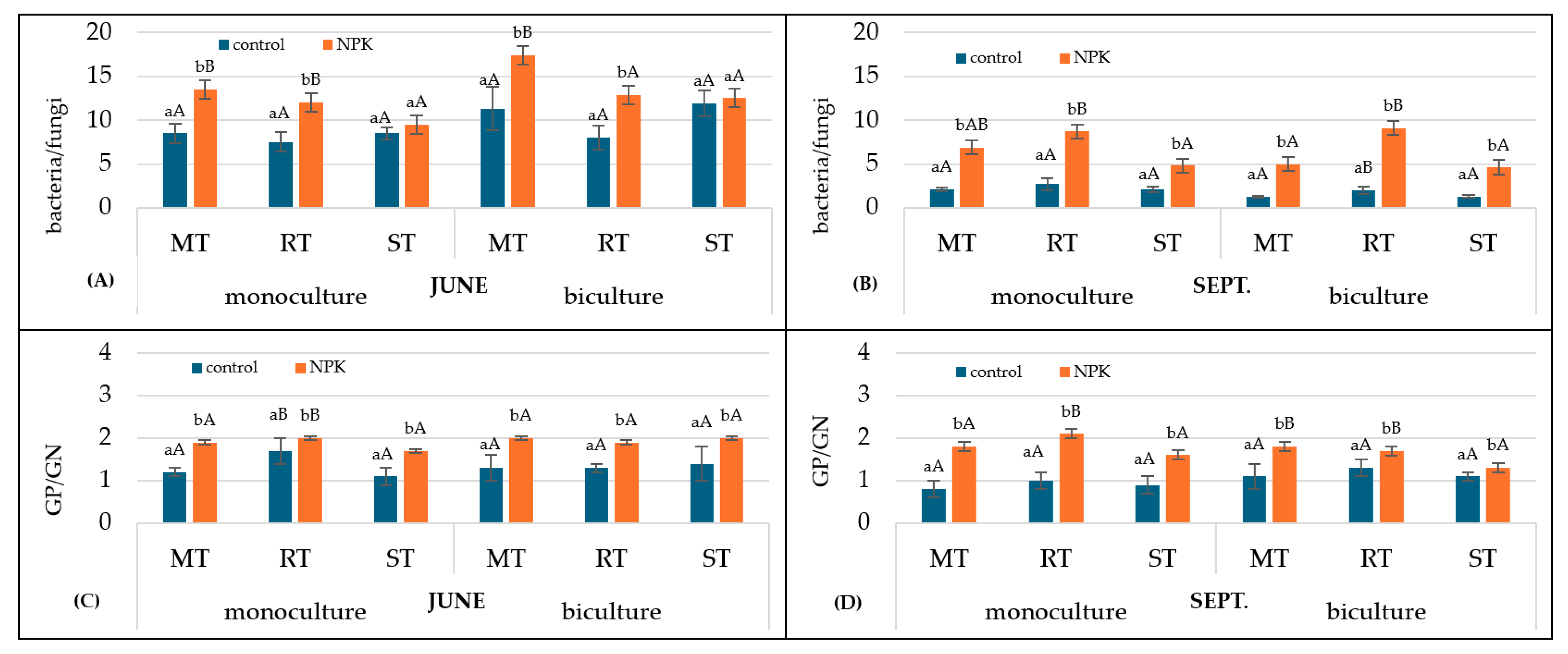
3.2.3. Ratios of Microbial Groups
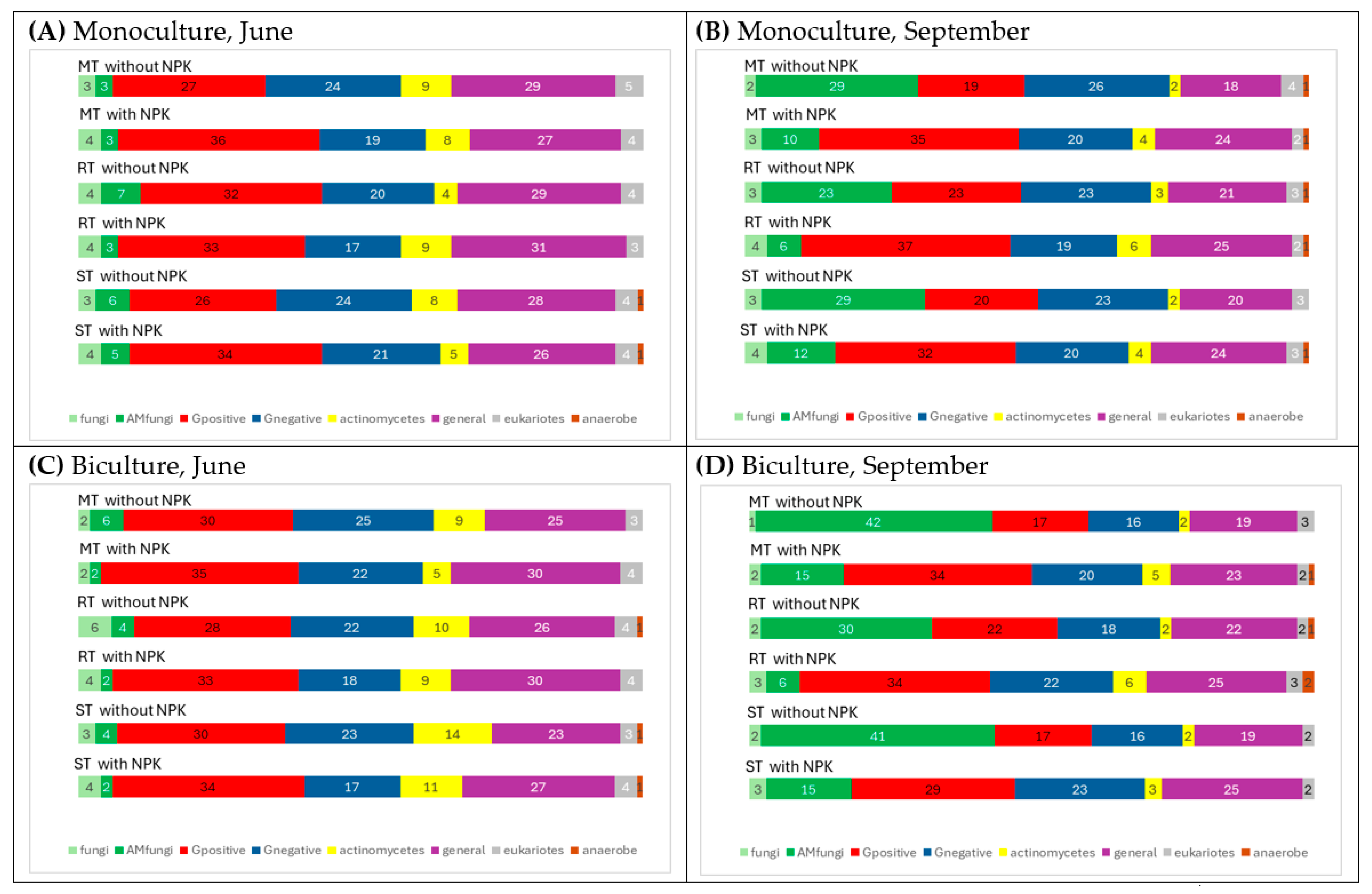
3.2.4. Relative Abundance of Microbial Groups
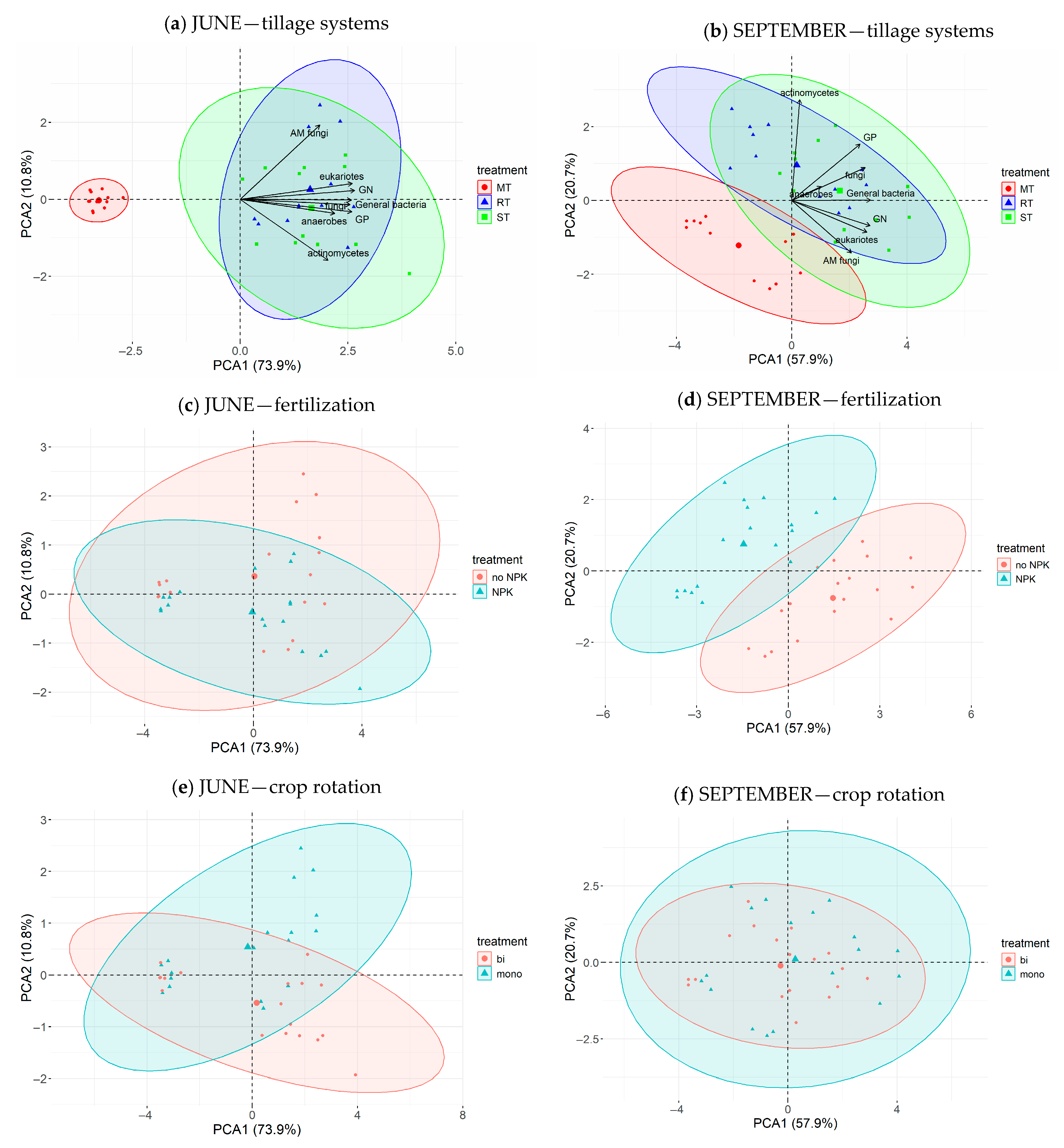
3.2.5. The Results of PCA and RDA
4. Discussion
4.1. Changes in the Physico-Chemical Parameters of Soil
4.2. Changes in Soil Microbial Biomass and Community Composition
5. Conclusions
- Reduced tillage systems, particularly ST, should be adopted to enhance microbial stability and promote beneficial fungal groups such as arbuscular mycorrhizal fungi, thereby providing effective soil health conservation.
- The long-term use of NPK (N: 160; P: 26; K: 74 kg/ha) fertilizers should be carefully managed due to their acidifying effect and negative impact on microbial biomass.
- Crop diversification is advisable to improve microbial balance and soil nutrient dynamics.
- Seasonal variability must be taken into account when assessing soil health, as microbial biomass and community structure showed pronounced seasonal variation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MT | moldboard tillage |
| RT | ripped tillage |
| ST | strip tillage |
| SOC | soil organic carbon |
| TN | total nitrogen |
| PLFA | phospholipid fatty acid |
| AM | arbuscular mycorrhizal |
| NPK | nitrogen, phorphorus, potassium |
| DNA | deoxyribonucleic acid |
| NT | no-till |
| IUSS WG WRB | International Union of Soil Sciences, Working Group, World Reference Base for Soil Resources |
| CAN | calcium–ammonium–nitrate |
| MAP | monoammonium–phosphate |
| KCl | potassium chloride |
| UAN | urea–ammonium–nitrate |
| FID | flame ionization detector |
| K2HPO4 | dipotassium phosphate |
| GP | Gram-positive bacteria |
| GN | Gram-negative bacteria |
| F | fungi |
| B | total bacteria |
| B/F | the ratio of total bacteria to total fungi |
| GP/GN | the ratio of Gram-positive to Gram-negative bacteria |
| SEM | standard error of the mean |
| Till. | tillage |
| Crop r. | crop rotation |
| n.s. | non-significant |
| PCA | principal component analysis |
| RDA | redundancy analysis |
| C/N | ratio of carbon to nitrogen |
References
- Karlen, D.L.; Veum, K.S.; Sudduth, K.A.; Obrycki, J.F.; Nunes, M.R. Soil health assessment: Past accomplishments, current activities, and future opportunities. Soil Tillage Res. 2019, 195, 104365. [Google Scholar] [CrossRef]
- Almási, C.; Orosz, V.; Tóth, T.; Mansour, M.M.; Demeter, I.; Henzsel, I.; Bogdányi, Z.; Szegi, T.A.; Makádi, M. Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment. Agronomy 2025, 15, 143. [Google Scholar] [CrossRef]
- Abdu, A.; Laekemariam, F.; Gidago, G.; Getaneh, L. Explaining the soil quality using different assessment techniques. Appl. Environ. Soil Sci. 2023, 2023, 6699154. [Google Scholar] [CrossRef]
- Şeker, C.; Özaytekin, H.H.; Negiş, H.; Gümüş, İ.; Dedeoğlu, M.; Atmaca, E.; Karaca, Ü. Identification of regional soil quality factors and indicators: A case study on an alluvial plain (central Turkey). Solid. Earth 2017, 8, 583–595. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.d.; Alves, P.R.L.; Paula, A.M.d.; Nakatani, A.S.; Pereira, J.d.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Csontos, P.; Mucsi, M.; Ragályi, P.; Tamás, J.; Kalapos, T.; Pápay, G.; Mjazovszky, Á.; Penksza, K.; Szili-Kovács, T. Standing vegetation exceeds soil microbial communities in soil type indication: A procrustes test of four salt-affected pastures. Agronomy 2021, 11, 1652. [Google Scholar] [CrossRef]
- Allen, D.E.; Singh, B.P.; Dalal, R.C. Soil health indicators under climate change: A review of current knowledge. In Soil Health and Climate Change. Soil Biology; Singh, B., Cowie, A., Chan, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 29, pp. 25–45. [Google Scholar] [CrossRef]
- Willers, C.; Jansen van Rensburg, P.J.; Claassens, S. Phospholipid fatty acid profiling of microbial communities–a review of interpretations and recent applications. J. Appl. Microbiol. 2015, 119, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, X.; Lin, Q.; Li, G.; Kong, W. Using a combination of PLFA and DNA-based sequencing analyses to detect shifts in the soil microbial community composition after a simulated spring precipitation in a semi-arid grassland in China. Sci. Total Environ. 2019, 657, 1237–1245. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Mizik, T.; Rádai, Z. The Significance of the Hungarian Maize Production in Relation to the Common Agricultural Policy. Rev. Agric. Rural Dev. 2021, 10, 44–51. [Google Scholar] [CrossRef]
- Ibáñez, J.; Pérez-Gómez, R.; Martínez, F.S.J. The spatial distribution of soils across Europe: A fractal approach. Ecol. Complex. 2009, 6, 294–301. [Google Scholar] [CrossRef]
- Domnariu, H.; Trippe, K.M.; Botez, F.; Partal, E.; Postolache, C. Long-term impact of tillage on microbial communities of an Eastern European Chernozem. Sci. Rep. 2025, 15, 642. [Google Scholar] [CrossRef]
- Fageria, N. The Role of Soil Organic Matter in Maintaining Sustainability of Cropping Systems. Commun. Soil Sci. Plant Anal. 2012, 43, 2063–2113. [Google Scholar] [CrossRef]
- Jakab, G.; Madarász, B.; Masoudi, M.; Karlik, M.; Király, C.; Zacháry, D.; Filep, T.; Dekemati, I.; Centeri, C.; Al-Graiti, T.; et al. Soil organic matter gain by reduced tillage intensity: Storage, pools, and chemical composition. Soil Tillage Res. 2023, 226, 105584. [Google Scholar] [CrossRef]
- Kurganova, I.N.; de Gerenyu, V.O.L.; Smolentseva, E.N.; Semenova, M.P.; Lichko, V.I.; Smolentsev, B.A. Influence of Land Use on the Physical Properties of Chernozems in the Forest-Steppe Zone of Western Siberia. Eurasian Soil Sci. 2021, 54, 1337–1349. [Google Scholar] [CrossRef]
- Labaz, B.; Kowalska, J.B.; Kabala, C.; Kobierski, M.; Waroszewski, J.; Dudek, M.; Szopka, K.; Gruszka, D. Distribution and Pools of Soil Organic Carbon in Chernozemic Soils Impacted by Intensive Farming and Erosion in the Loess Plateau in South-East Poland. Agronomy 2024, 14, 2544. [Google Scholar] [CrossRef]
- Dupraz, P.; Guyomard, H. Environment and Climate in the Common Agricultural Policy. EuroChoices 2019, 18, 18–25. [Google Scholar] [CrossRef]
- Priori, S.; Zanini, M.; Falcioni, V.; Casa, R. Topsoil vertical gradient in different tillage systems: An analytical review. Soil Tillage Res. 2024, 236, 105947. [Google Scholar] [CrossRef]
- Galka, B.; Kabala, C.; Karczewska, A.; Sowinski, J.; Jakubiec, J. Variability of soil properties in an intensively cultivated experimental field. Soil Sci. Annu. 2016, 67, 10. [Google Scholar] [CrossRef]
- Breza-Boruta, B.; Kotwica, K.; Bauza-Kaszewska, J. Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation. Energies 2021, 14, 7451. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative Agriculture—A Literature Review on the Practices and Mechanisms Used to Improve Soil Health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Rhodes, C.J. The Imperative for Regenerative Agriculture. Sci. Prog. 2017, 100, 80–129. [Google Scholar] [CrossRef]
- Jat, M.L.; Dagar, J.C.; Sapkota, T.B.; Govaerts, B.; Ridaura, S.; Saharawat, Y.S.; Sharma, R.K.; Tetarwal, J.; Jat, R.K.; Hobbs, H. Climate change and agriculture: Adaptation strategies and mitigation opportunities for food security in South Asia and Latin America. Adv. Agron. 2016, 137, 127–235. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Jørgensen, H.B.; Isberg, P.-E. How does tillage intensity affect soil organic carbon? A systematic review. Environ. Evid. 2017, 6, 30. [Google Scholar] [CrossRef]
- Govednik, A.; Potočnik, Ž.; Eler, K.; Suhadolc, M. Combined effects of long-term tillage and fertilisation regimes on soil organic carbon, microbial biomass, and abundance of the total microbial communities and N-functional guilds. Appl. Soil Ecol. 2023, 188, 104876. [Google Scholar] [CrossRef]
- Srour, A.Y.; Ammar, H.A.; Subedi, A.; Pimentel, M.; Cook, R.L.; Bond, J.; Fakhoury, A.M. Microbial communities associated with long-term tillage and fertility treatments in a corn-soybean cropping system. Front. Microbiol. 2020, 11, 1363. [Google Scholar] [CrossRef]
- Lampurlanés, J.; Cantero-Martínez, C. Soil Bulk Density and Penetration Resistance under Different Tillage and Crop Management Systems and Their Relationship with Barley Root Growth. Agron. J. 2003, 95, 526–536. [Google Scholar] [CrossRef]
- Romaneckas, K.; Kimbirauskienė, R.; Sinkevičienė, A. Impact of Tillage Intensity on Planosol Bulk Density, Pore Size Distribution, and Water Capacity in Faba Bean Cultivation. Agronomy 2022, 12, 2311. [Google Scholar] [CrossRef]
- Tamm, K.; Nugis, E.; Edesi, L.; Lauringson, E.; Talgre, L.; Viil, P.; Plakk, T.; Võsa, T.; Vettik, R.; Penu, P. Impact of cultivation method on the soil properties in cereal production. Agron. Res. 2016, 14, 280–289. [Google Scholar]
- Dadalto, J.P.; Fernandes, H.C.; Teixeira, M.M.; Cecon, P.R.; Matos, A.T.d. Tilage influence on soil microbial activity. Eng. Agrícola 2015, 35, 506–513. [Google Scholar] [CrossRef]
- Vilkiene, M.; Mockeviciene, I.; Karcauskiene, D.; Suproniene, S.; Doyeni, M.O.; Ambrazaitiene, D. Biological indicators of soil quality under different tillage systems in retisol. Sustainability 2021, 13, 9624. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Yang, C.H. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 2004, 261, 199–208. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Jiao, Y.; Yuan, L. Positive effects of increasing crop diversity in land use on soil microbial biomass, enzyme activity and bacterial community composition. Soil Res. 2019, 57, 779–787. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Li, T.; Chen, L.; Chen, Y.; Sui, P. Changes in soil microbial biomass, diversity, and activity with crop rotation in cropping systems: A global synthesis. Appl. Soil Ecol. 2023, 186, 104815. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Grządziel, J.; Księżak, J. Effect of different agricultural management practices on soil biological parameters including glomalin fraction. Plant Soil Environ. 2017, 63, 300–306. [Google Scholar] [CrossRef]
- Dóka Fülöp, P.P. Role of watersupply in monoculture maize (Zea mays L.) production. Cereal Res. Commun. 2007, 35, 353–356. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, S.; Hua, K.; Yin, Y.; Chu, H.; Wang, D.; Guo, X. Fertilization regime has a greater effect on soil microbial community structure than crop rotation and growth stage in an agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Zhao, C.; Hu, X.; Yin, C. Nitrogen Fertilization Increases Soil Microbial Biomass and Alters Microbial Composition Especially Under Low Soil Water Availability. Microb. Ecol. 2023, 86, 536–548. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Esperschütz, J.; Gattinger, A.; Mäder, P.; Schloter, M.; Fließbach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Wu, M.; Jiang, C.; Chen, X.; Cai, Z.; Wang, B.; Zhang, J.; Zhang, T.; Li, Z. Soil pH rather than nutrients drive changes in microbial community following long-term fertilization in acidic Ultisols of southern China. J. Soils Sediments 2018, 18, 1853–1864. [Google Scholar] [CrossRef]
- Balla Kovács, A.; Juhász, E.K.; Béni, Á.; Kincses, I.; Tállai, M.; Sándor, Z.; Kátai, J.; Rátonyi, T.; Kremper, R. Changes in Microbial Community and Activity of Chernozem Soil under Different Management Systems in a Long-Term Field Experiment in Hungary. Agronomy 2024, 14, 745. [Google Scholar] [CrossRef]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World reference base for soil resources. World Soil Resour. Rep. 2006, 103, 1–128. [Google Scholar]
- Ellis, S.; Ritz, K. A modified high-throughput analysis of PLFAs in soil. MethodsX 2018, 5, 1491–1497. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Kujur, M.; Patel, A.K. PLFA Profiling of soil microbial community structure and diversity in different dry tropical ecosystems of Jharkhand. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 556–575. [Google Scholar]
- Gude, A.; Kandeler, E.; Gleixner, G. Input related microbial carbon dynamic of soil organic matter in particle size fractions. Soil Biol. Biochem. 2012, 47, 209–219. [Google Scholar] [CrossRef]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Kádár, I.; Ragályi, P.; Murányi, A.; Radimszky, L.; Gajdó, A. Effect of Gérce alginit on the fertility of an acid sandy soil. Agrokémia és Talajt. Agrokem. 2015, 64, 437–452. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Pearson Education India: Noida, India, 2016. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.W.K.; Zhang, Y.; Han, Y.; Rong, J.; Cai, X.; Chen, L.; Shi, C.; Zheng, Y. Patterns of soil microorganisms and enzymatic activities of various forest types in coastal sandy land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]
- Santangeli, M.; Steininger-Mairinger, T.; Vetterlein, D.; Hann, S.; Oburger, E. Maize (Zea mays L.) root exudation profiles change in quality and quantity during plant development—A field study. Plant Sci. 2024, 338, 111896. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Wakelin, S.A.; Liang, Y.; Chu, G. Soil microbial activity and community structure as affected by exposure to chloride and chloride-sulfate salts. J. Arid. Land. 2018, 10, 737–749. [Google Scholar] [CrossRef]
- Toledo, S.; Gargaglione, V.; Peri, P.L. Mineral fertilization impacts microbial activity and endophytic fungi but not microbial biomass in semiarid grasslands. Pedobiologia 2024, 102, 150929. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Li, J.; Jia, L.; Struik, P.C.; An, Z.; Wang, Z.; Xu, Z.; Ji, L.; Yao, Y.; Lv, J.; Zhou, T. Plant and soil responses to tillage practices change arbuscular mycorrhizal fungi populations during crop growth. Front. Microbiol. 2024, 15, 1394104. [Google Scholar] [CrossRef]
- Gu, S.; Wu, S.; Guan, Y.; Zhai, C.; Zhang, Z.; Bello, A.; Guo, X.; Yang, W. Arbuscular mycorrhizal fungal community was affected by tillage practices rather than residue management in black soil of northeast China. Soil Tillage Res. 2020, 198, 104552. [Google Scholar] [CrossRef]
- de la Cruz-Ortiz, Á.V.; Álvarez-Lopeztello, J.; Robles, C.; Hernández-Cuevas, L.V. Tillage intensity reduces the arbuscular mycorrhizal fungi attributes associated with Solanum lycopersicum, in the Tehuantepec Isthmus (Oaxaca), Mexico. Appl. Soil Ecol. 2020, 149, 103519. [Google Scholar] [CrossRef]
- Sándor, Z.; Tállai, M.; Kincses, S.; László, Z.; Kátai, J.; Vágó, I. Effect of various soil cultivation methods on some microbial soil properties. DRC Sustain. Future J. Environ. Agric. Energy 2020, 1, 14–20. [Google Scholar] [CrossRef]
- Malik, A.A.; Chowdhury, S.; Schlager, V.; Oliver, A.; Puissant, J.; Vazquez, P.G.; Jehmlich, N.; Von Bergen, M.; Griffiths, R.I.; Gleixner, G. Soil fungal: Bacterial ratios are linked to altered carbon cycling. Front. Microbiol. 2016, 7, 1247. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Li, X.; Yang, S.; Rengel, Z. Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecific interactions on nutrients. Field Crops Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]


| Treatment | Soil Physico-Chemical Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| June | September | |||||||
| Moisture Content | pHKCl | SOC | TN | Moisture Content | pHKCl | SOC | TN | |
| Tillage | 0.396 n.s | 0.001 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| NPK | 0.151 n.s | 0.000 *** | 0.255 n.s | 0.827 n.s | 0.007 * | 0.000 *** | 0.000 *** | 0.294 n.s |
| Crop rotation | 0.304 n.s | 0.07 n.s | 0.000 *** | 0.000 *** | 0.032 * | 0.028 * | 0.000 *** | 0.000 *** |
| NPK × till. | 0.744 n.s | 0.539 n.s | 0.001 *** | 0.007 * | 0.000 *** | 0.433 n.s | 0.000 *** | 0.302 n.s |
| Crop r.× till. | 0.160 n.s | 0.607 n.s | 0.080 n.s | 0.07 n.s | 0.113 n.s | 0.572 n.s | 0.037 n.s | 0.017 n.s |
| Crop r. × NPK | 0.192 n.s | 0.368 n.s | 0.216 n.s | 0.007 * | 0.014 * | 0.447 n.s | 0.061 n.s | 0.524 n.s |
| Crop r.× NPK × Till | 0.777 n.s | 0.684 n.s | 0.093 n.s | 0.084 n.s | 0.592 n.s | 0.488 n.s | 0.138 n.s | 0.126 n.s |
| Monoculture | Biculture | |||
|---|---|---|---|---|
| Control | NPK | Control | NPK | |
| Moisture content % (June) | ||||
| MT | 12.3 ± 0.8 aA | 11.4 ± 1.4 aA | 11.7 ± 0.9 aA | 10.3 ± 2.4 aA |
| RT | 9.6 ± 0.9 aA | 10.6 ± 3.3 aA | 11.7 ± 0.9 aA | 10.1 ± 3.3 aA |
| ST | 10.6 ± 3.1 aA | 10.1 ± 2.3 aA | 13.4 ± 0.4 aB | 11.3 ± 2.2 aA |
| Moisture content % (September) | ||||
| MT | 15.5 ± 0.4 aA | 16.5 ± 0.5 aA | 16.2 ± 1.9 aAB | 16.3 ± 0.5 aA |
| RT | 18.5 ± 1.8 aB | 18.3 ± 0.7 aAB | 18.0 ± 0.6 aB | 16.6 ± 0.5 aA |
| ST | 15.1 ± 0.8 aA | 18.9 ± 1.2 aB | 15.2 ± 1.1 aA | 16.8 ± 0.4 aA |
| pHKCl (June) | ||||
| MT | 5.4 ± 0.4 bA | 4.6 ± 0.1 aA | 5.3 ± 0.5 bA | 4.6 ± 0.2 aB |
| RT | 4.9 ± 0.1 bA | 4.4 ± 0.2 aA | 4.9 ± 0.5 bA | 4.1 ± 0.2 aA |
| ST | 5.4 ± 0.4 bA | 4.6 ± 0.2 aA | 5.2 ± 0.4 bA | 4.3 ± 0.1 aB |
| pHKCl (September) | ||||
| MT | 5.5 ± 0.2 bB | 4.8 ± 0.1 aB | 5.2 ± 0.4 bA | 4.5 ± 0.2 aA |
| RT | 4.9 ± 0.3 bA | 4.4 ± 0.3 aA | 4.7 ± 0.1 bA | 4.2 ± 0.4 aA |
| ST | 5.4 ± 0.3 bB | 4.6 ± 0.2 aAB | 5.1 ± 0.5 bA | 4.7 ± 0.2 aA |
| SOC % (June) | ||||
| MT | 1.8 ± 0.2 aA | 1.7 ± 0.1 aA | 2.0 ± 0.2 aA | 1.8 ± 0.1 aA |
| RT | 1.8 ± 0.1 aA | 2.0 ± 0.1 aA | 2.2 ± 0.2 aA | 2.1 ± 0.2 aB |
| ST | 2.0 ± 0.2 aA | 2.1 ± 0.2 aB | 2.0 ± 0.1 aA | 2.2 ± 0.2 aB |
| SOC % (September) | ||||
| MT | 1.7 ± 0.1 aA | 1.7 ± 0.1 aA | 1.7 ± 0.1 aA | 1.8 ± 0.1 aA |
| RT | 2.0 ± 0.1 aB | 2.0 ± 0.1 aB | 2.3 ± 0.1 aC | 2.2 ± 0.1 aB |
| ST | 1.8 ± 0.2 aAB | 2.2 ± 0.2 bB | 2.0 ± 0.1 aB | 2.3 ± 0.1 bC |
| TN % (June) | ||||
| MT | 0.21 ± 0.02 aA | 0.19 ± 0.01 aA | 0.21 ± 0.01 aA | 0.20 ± 0.01 aA |
| RT | 0.20 ± 0.01 aA | 0.21 ± 0.01 aB | 0.23 ± 0.02 aA | 0.22 ± 0.01 aB |
| ST | 0.21 ± 0.01 aA | 0.22 ± 0.01 aB | 0.22 ± 0.01 aA | 0.24 ± 0.02 aB |
| TN% (September) | ||||
| MT | 0.19 ± 0.01 aA | 0.19 ± 0.01 aA | 0.20 ± 0.01 aA | 0.20 ± 0.01 aA |
| RT | 0.21 ± 0.01 aB | 0.22 ± 0.01 aB | 0.24 ± 0.01 aB | 0.23 ± 0.01 aB |
| ST | 0.20 ± 0.02 aAB | 0.20 ± 0.01 aAB | 0.23 ± 0.01 aB | 0.23 ± 0.02 aAB |
| Independent Variables | Microbial Biomass | Fungi | Am Fungi | GN Bacteria | GP Bacteria | Actinomycetes |
|---|---|---|---|---|---|---|
| June | ||||||
| Tillage | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| NPK fertilization | 0.042 * | 0.131 n.s | 0.000 *** | 0.000 *** | 0.001 *** | 0.506 n.s |
| Crop rotation | 0.038 * | 0.085 n.s | 0.000 *** | 0.145 n.s | 0.129 n.s | 0.000 *** |
| NPK × Tillage | 0.028 * | 0.004 ** | 0.000 *** | 0.021 n.s | 0.274 n.s | 0.008 * |
| Crop rotation × NPK | 0.09 n.s | 0.051 n.s | 0.000 *** | 0.017 * | 0.161 n.s | 0.000 *** |
| Crop rotation × Tillage | 0.106 n.s | 0.227 n.s | 0.01 * | 0.149 n.s | 0.089 n.s | 0.164 n.s |
| Crop rot. × NPK × Tillage | 0.663 n.s | 0.053 n.s | 0.066 n.s | 0.311 n.s | 0.469 n.s | 0.006 * |
| September | ||||||
| Tillage | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| NPK fertilization | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** | 0.000 *** |
| Crop rotation | 0.011 * | 0.000 *** | 0.000 *** | 0.003 ** | 0.238 n.s | 0.359 n.s |
| NPK × Tillage | 0.718 n.s | 0.872 n.s | 0.007 * | 0.191 n.s | 0.003 * | 0.016 * |
| Crop rotation × NPK | 0.027 * | 0.030 * | 0.058 n.s | 0.415 n.s | 0.004 * | 0.001 * |
| Crop rotation × Tillage | 0.105 n.s | 0.452 n.s | 0.000 *** | 0.000 *** | 0.005 * | 0.024 * |
| Crop rot. × NPK × Tillage | 0.022 * | 0.383 n.s | 0.190 n.s | 0.063 n.s | 0.161 n.s | 0.547 n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.B.; Juhász, E.K.; Béni, Á.; Gumisiriya, C.; Tállai, M.; Szabó, A.; Kincses, I.; Novák, T.; Tamás, A.; Kremper, R. Seasonal Changes in the Soil Microbiome on Chernozem Soil in Response to Tillage, Fertilization, and Cropping System. Agronomy 2025, 15, 1887. https://doi.org/10.3390/agronomy15081887
Kovács AB, Juhász EK, Béni Á, Gumisiriya C, Tállai M, Szabó A, Kincses I, Novák T, Tamás A, Kremper R. Seasonal Changes in the Soil Microbiome on Chernozem Soil in Response to Tillage, Fertilization, and Cropping System. Agronomy. 2025; 15(8):1887. https://doi.org/10.3390/agronomy15081887
Chicago/Turabian StyleKovács, Andrea Balla, Evelin Kármen Juhász, Áron Béni, Costa Gumisiriya, Magdolna Tállai, Anita Szabó, Ida Kincses, Tibor Novák, András Tamás, and Rita Kremper. 2025. "Seasonal Changes in the Soil Microbiome on Chernozem Soil in Response to Tillage, Fertilization, and Cropping System" Agronomy 15, no. 8: 1887. https://doi.org/10.3390/agronomy15081887
APA StyleKovács, A. B., Juhász, E. K., Béni, Á., Gumisiriya, C., Tállai, M., Szabó, A., Kincses, I., Novák, T., Tamás, A., & Kremper, R. (2025). Seasonal Changes in the Soil Microbiome on Chernozem Soil in Response to Tillage, Fertilization, and Cropping System. Agronomy, 15(8), 1887. https://doi.org/10.3390/agronomy15081887







