Impact of Basalt Rock Powder on Ryegrass Growth and Nutrition on Sandy and Loamy Acid Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Basalt Dust Origin and Chemical Characteristics Compared with the Soils
2.3. Experimental Design and Monitoring
2.3.1. General Variables Studied
2.3.2. Trial Set-Up, Monitoring and Maintenance of the Crop
2.4. Soil, Basalt and Plant Measurements
2.5. Calculation Formulas
2.5.1. Theoretical Concentrations of Extractable Nutrients Between Soil and Basalt Mixes
2.5.2. Apparent Quantity of Minerals Weathered and Released from the Basalt + Soil Mixes
2.5.3. Nutrient Use Efficiencies
2.5.4. Acid Neutralising Capacity of the Basalt
2.6. Statistical Analysis
3. Results
3.1. Impact of Basalt Dust on Soil Chemical Properties
3.1.1. General Trends in Short-Terms Reactions
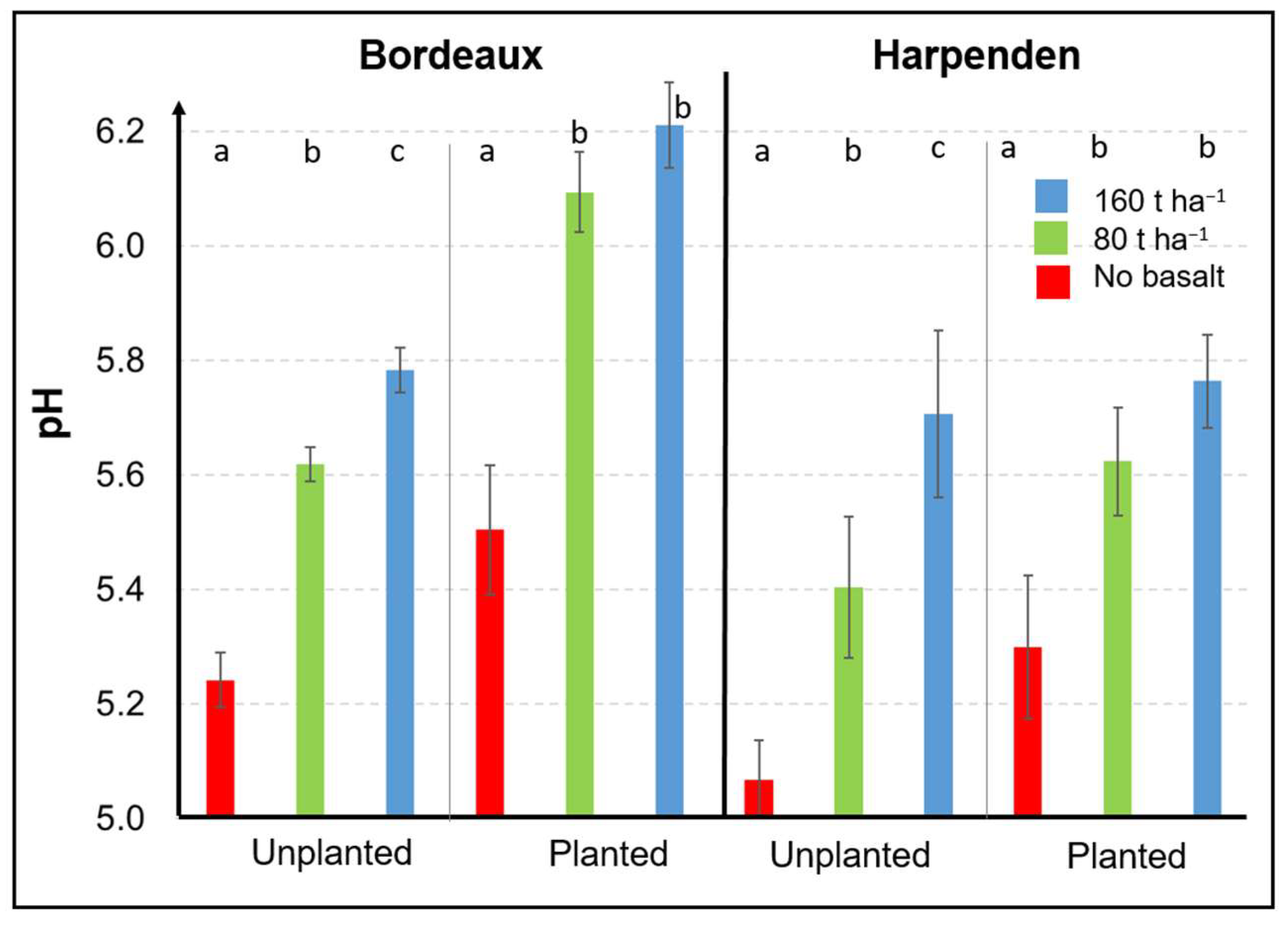
3.1.2. Effects of Basalt on the Soil pH at the End of the Experiment
3.1.3. Effect of Basalt on Extractable Elements at the End of the Experiment
3.2. Impact of Basalt Dust on Plant Response
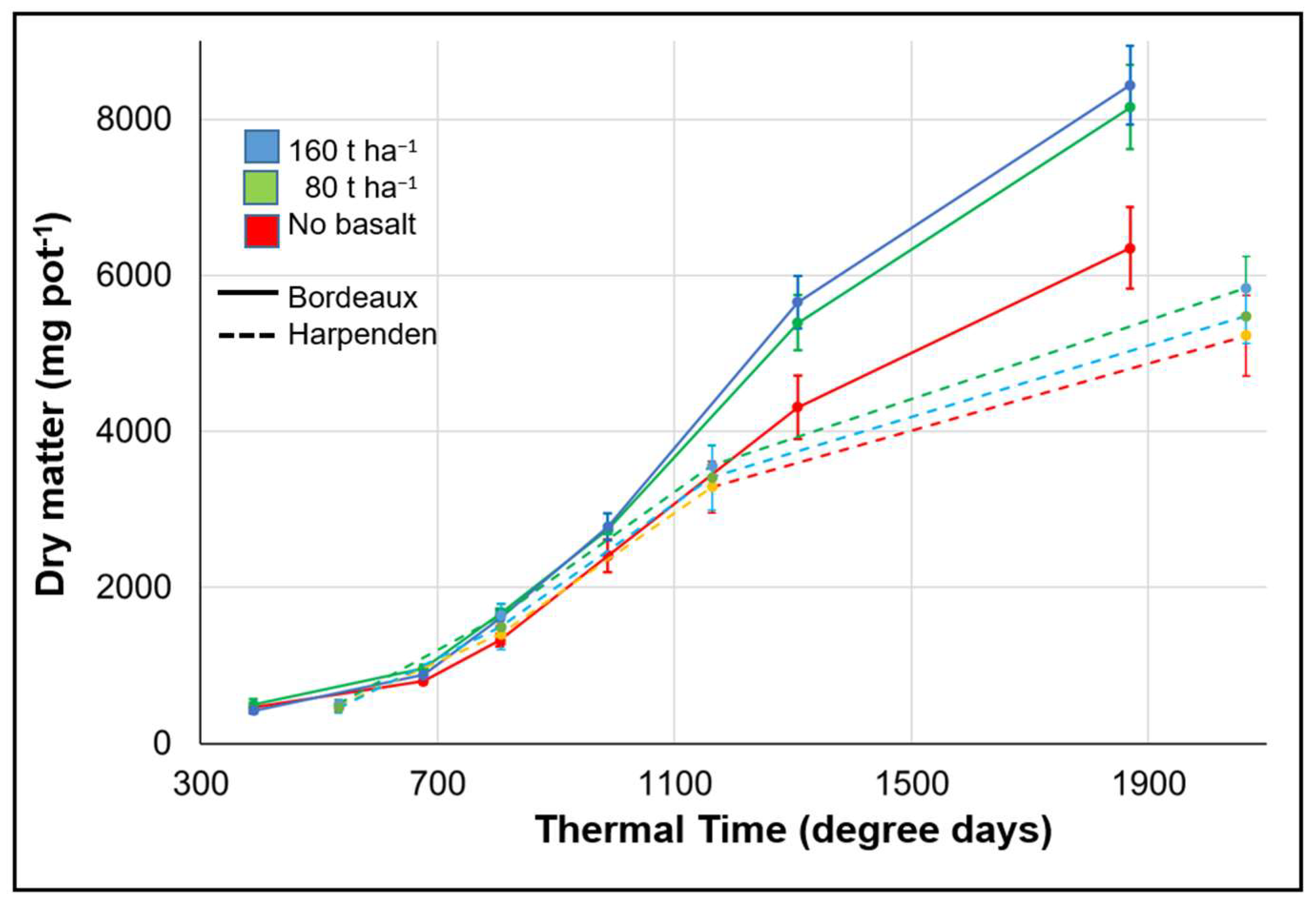
3.2.1. Yield Effect
3.2.2. Concentration and Uptake of Elements in Plants
3.3. Focus on Two Macro- and Two Micronutrients
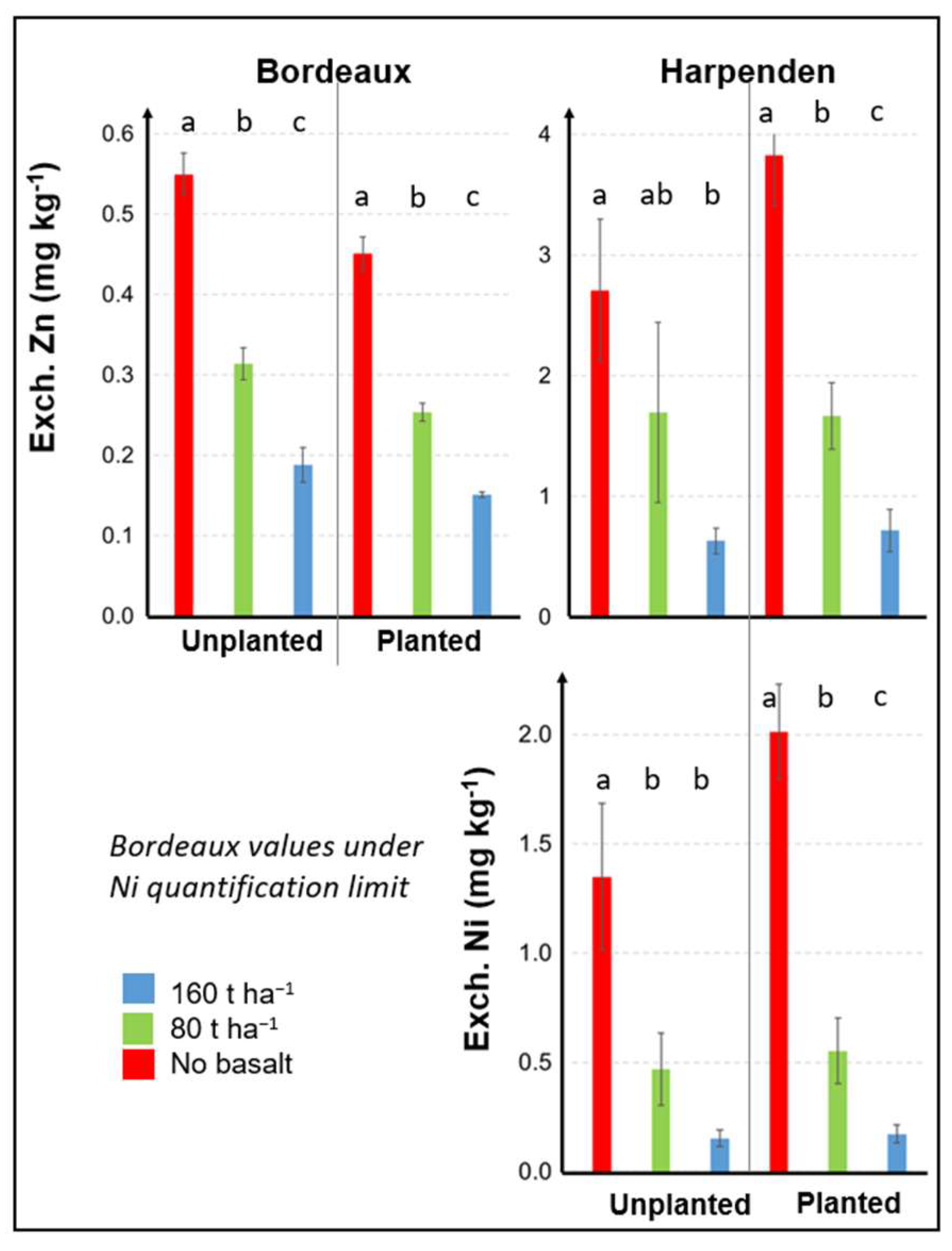
3.3.1. Effects of Basalt on the Soil–Plant P and K Transfer
3.3.2. Effects of Basalt on the Soil–Plant Zn and Ni Transfer
3.4. Overview of Soil–Plant Responses to Basalt Use for the Two Experiments
4. Discussion
4.1. pH Responses in the Short Term, Compared to Conventional Liming Products and Literature on Agricultural ERW
4.2. High K Weathering
4.3. Attenuated Effect on Plant P Nutrition, in Contrast to Literature
4.4. To Which Extent Can a Weathering Result for a Given Element Be Extended to Other Elements, and to Other Soil Contexts?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartmann, J.; West, J.; Renforth, P.; Kölher, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Renforth, P.; Henderson, G. Assessing ocean alkalinity for carbon sequestration. Rev. Geophys. 2017, 55, 636–674. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J.J. Increase in the Export of Alkalinity from North Americas Largest River. Science 2003, 301, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Gastmans, D.; Hutcheon, I.; Menegario, A.; Kiang Chang, H. Geochemical evolution of groundwater in a basaltic aquifer based on chemical and stable isotopic data: Case study from the Northeastern portion of Serra Geral Aquifer, Sao Paulo state (Brazil). J. Hydrol. 2016, 535, 598–611. [Google Scholar] [CrossRef]
- Leonardos, O.H.; Fyfe, W.S.; Kronberg, B.I. The use of ground rocks in laterite systems: An improvement to the use of conventional soluble fertilizers? Chem. Geol. 1987, 60, 361–370. [Google Scholar] [CrossRef]
- Gillman, G.P. The Effect of Crushed Basalt Scoria on the Cation Exchange Properties of a Highly Weathered Soil. Soil Sci. Soc. Am. J. 1980, 44, 465–468. [Google Scholar] [CrossRef]
- Holden, F.J.; Davies, K.; Bird, M.I.; Hume, R.; Green, H.; Beerling, D.J.; Nelson, P.N. In-field carbon dioxide removal via weathering of crushed basalt applied to acidic tropical agricultural soil. Sci. Total Environ. 2024, 955, 176568. [Google Scholar] [CrossRef] [PubMed]
- Harley, A.D.; Gilkes, R.J. Factors influencing the release of plant nutrient elements from silicate rock powders: A geochemical overview. Nutr. Cycl. Agroecosystems 2000, 56, 11–36. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Theodoro, S.H. Enabling food security through use of local rocks and minerals. Extr. Ind. Soc. 2020, 7, 480–487. [Google Scholar] [CrossRef]
- Manning, D.A.C. How will minerals feed the world in 2050? Proc. Geol. Assoc. 2015, 126, 14–17. [Google Scholar] [CrossRef]
- Vandeginste, V.; Lim, C.; Ji, Y. Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method. Minerals 2024, 14, 75. [Google Scholar] [CrossRef]
- Almaraz, M.; Bingham, N.L.; Holzer, I.O.; Geohegan, E.K.; Goertzen, H.; Sohng, J.; Houlton, B.Z. Methods for determining the CO2 removal capacity of enhanced weathering in agronomic settings. Front. Clim. 2022, 4, 970429. [Google Scholar] [CrossRef]
- Fuss, S.; Lamb, W.F.; Callaghan, M.W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; Garcia, W.O.; Hartmann, J.; Khanna, T.; et al. Negative emissions—Part 2: Costs, potentials and side effects. Environ. Res. Lett. 2018, 13, 063002. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- IPCC. IPCC Climate Change 2023: Synthesis Report Contribution of Working Groups, I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Andrews, M.G.; Taylor, L.L. Combating Climate Change Through Enhanced Weathering of Agricultural Soils. Elements 2019, 15, 253–258. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzaz, E.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.J.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Kantzaz, E.; Lomas, M.R.; Taylor, L.L.; Zhang, S.; Kanzaki, Y.; Eufrasio, R.M.; Renforth, P.; Mercure, J.-F.; Politt, H.; et al. Transforming US agriculture for carbon removal with enhanced weathering. Nature 2024, 638, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kantzas, E.P.; Martin, M.V.; Lomas, M.R.; Eufrasio, R.M.; Renforth, P.; Lewis, A.L.; Taylor, L.L.; Mercure, J.-F.; Politt, H.; Vercoulen, P.V.; et al. Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nat. Geosci. 2022, 15, 382–389. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.L.; Kubicki, J.D.; White, A.F. Kinetics of Water-Rock Interaction; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Oelkers, E.H. General kinetic description of multioxide silicate mineral and glass dissolution. Geochim. Cosmochim. Acta 2001, 65, 3703–3719. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralising soils? The agricultural usage of silicate rock powders: A review. Sci. Total. Environ. 2022, 807, 150976. [Google Scholar] [CrossRef] [PubMed]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.E.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef] [PubMed]
- Skov, K.; Wardman, J.; Healey, M.; McBride, A.; Bierowiec, T.; Cooper, J.; Edeh, I.; George, D.; Kelland, M.E.; Mann, J.; et al. Initial agronomic benefits of enhanced weathering using basalt: A study of spring oat in a temperate climate. PLoS ONE 2024, 19, e0295031. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsen, K.C.; Jensen, L.S.; Rosing, M.T.; Dietzen, C. Greenlandic glacial rock flour improves crop yield in organic agricultural production. Nutr. Cycl. Agroecosystems 2023, 126, 51–66. [Google Scholar] [CrossRef]
- WRB IUSS Working Group. World Reference Base for Soil Resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports; FAO: Rome, Italy, 2015; p. 106. [Google Scholar]
- COMIFER. La Fertilisation P-K-Mg: Les Bases du Raisonnement; French Association for Fertilisation Research and Development: Paris, France, 2019; Available online: https://comifer.asso.fr/les-brochures (accessed on 9 July 2025).
- Augusto, L.; Bakker, M.; Morel, C.; Meredieu, C.; Trichet, P.; Badeau, V.; Arrouays, D.; Plassard, C.; Achat, D.; Gallet-Budynec, A.; et al. Is ‘grey literature’ a reliable source of data to characterize soils at the scale of a region? A case study in a maritime pine forest in southwestern France. Eur. J. Soil Sci. 2010, 61, 807–822. [Google Scholar] [CrossRef]
- Agriculture and Horticulture Development Board. RB209 Section 4, Arable Crops in Nutrient Management Guide; Agriculture and Horticulture Development Board: Warwickshire, UK, 2021; p. 52. Available online: https://ahdb.org.uk/knowledge-library/rb209-section-4-arable-crops (accessed on 9 July 2025).
- Mengel, K.; Kirkby, E.A.; Kosearten, H.; Appel, T. Principles of Plant Nutrition; Springer: Dodrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Avery, B.W.; Catt, J.A. The Soil at Rothamsted; Lawes Agricultural Trust: Harpenden, UK, 1995. [Google Scholar] [CrossRef]
- Randal, B.A.O. Dolerite-pegmatites from the Whin Sill near Barrasford, Northumberland. Proc. Yorks. Geol. Soc. 1989, 47, 249–265. [Google Scholar] [CrossRef]
- Wood, C.; Harrison, A.L.; Power, I.M. Impacts of dissolved phosphorus and soil-mineral-fluid interactions on CO2 removal through enhanced weathering of wollastonite in soils. Appl. Geochem. 2023, 148, 105511. [Google Scholar] [CrossRef]
- ISO10390; Soil Quality—Determination of pH. International Organisation for Standardisation: Geneva, Switzerland, 2005.
- McGrath, S.P.; Cunliffe, C.H. A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J. Sci. Food Agric. 1985, 36, 794–798. [Google Scholar] [CrossRef]
- Zeien, H.; Brümmer, G.W. Chemische Extraktionen Zur Bestimmung von chwermetallbindungsformen in Böden. Mitt. Dtsch. Bodenk. Gesell. 1989, 59, 505–510. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction With Sodium Bicarbonate; USDA Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- ISO 23470; Soil Quality—Determination of Effective Cation Exchange Capacity and Exchangeable Cations Using A Hexamminecobalt(III) Chloride Solution. International Organisation for Standardisation: Geneva, Switzerland, 2008.
- Zhao, F.; McGrath, S.P.; Crosland, A.R. Comparison of three wet digestion methods for the determination of plant sulphur by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Commun. Soil Sci. Plant Anal. 1994, 25, 407–418. [Google Scholar] [CrossRef]
- AFNOR. NF 14984: Amendements Minéraux Basiques—Détermination de L’Effet d’un Produit sur le pH d’un Sol—Méthode par Incubation du Sol; Association Française de Normalisation: La Plaine Saint-Denis, France, 2016; Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-en-14984/amendements-mineraux-basiques-determination-de-leffet-dun-produit-sur-le-ph/fa186574/58036 (accessed on 9 July 2025).
- Rémy, J.C.; Marin-Laflèche, A. L’analyse de terre: Réalisation d’un programme automatique. Ann. Agron. 1974, 25, 607–632. [Google Scholar]
- COMIFER. Le Chaulage, Des Bases Pour Le Raisonner; French Association for Fertilisation Research and Development: Paris, France, 2009; Available online: https://comifer.asso.fr/les-brochures (accessed on 9 July 2025).
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localisation of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Meille, L.; Holland, J.E.; McGrath, S.P.; Glendining, M.J.; Thomas, C.L.; Haefele, S.M. The grain mineral composition of barley, oat and wheat on soils with pH and soil phosphorus gradients. Eur. J. Agron. 2021, 126, 126281. [Google Scholar] [CrossRef]
- Reuter, D.; Robinson, J.B. Plant Analysis: An Interpretation Manual; CSIRO Publishing: Canberra, Australia, 1997. [Google Scholar] [CrossRef]
- Richardson, J.B. Basalt Rock Dust Amendment on Soil Health Properties and Inorganic Nutrients—Laboratory and Field Study at Two Organic Farm Soils in New England, USA. Agriculture 2025, 15, 52. [Google Scholar] [CrossRef]
- Verma, T.S.; Minhas, R.S. Zinc and phosphorus interaction in a wheat-maize cropping system. Fertil. Res. 1987, 13, 77–86. [Google Scholar] [CrossRef]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Conceição, L.T.; Silva, G.N.; Silva Holsback, H.M.; de Figueire do Oliveira, C.; Chaves Marcante, N.; de Souza Martins, E.; de Souza Santos, F.L.; Ferreira Santos, E. Potential of basalt dust to improve soil fertility and crop nutrition. J. Agric. Food Res. 2022, 10, 100443. [Google Scholar] [CrossRef]
- Hinsinger, P.; Jaillard, B. Root-induced release of interlayer potassium and vermiculitisation of phlogopite as related to potassium depletion in the rhizosphere of ryegrass. Eur. J. Soil Sci. 1993, 44, 525–553. [Google Scholar] [CrossRef]
- Hervé, A.; Elghali, A.; Ouabid, M.; Raji, O.; Bodinier, J.-L.; El Messbahi, H. Alkali-Hydrothermal Treatment of K-Rich Igneous Rocks for Their Direct Use as Potassic Fertilizers. Minerals 2021, 11, 140. [Google Scholar] [CrossRef]
- Hervé, A.; Khiari, L.; Raji, O.; Elghali, A.; Lajili, A.; Ouabid, M.; Jemo, M.; Bodinier, J.-L. Potassium Fertilizer Value of Raw and Hydrothermally Treated Igneous Rocks. J. Soil Sci. Plant Nutr. 2023, 23, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J. The effects of pH on phosphate uptake from the soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Lewis, A.L.; Sarkar, B.; Wade, P.W.; Kemp, S.J.; Hodson, M.E.; Taylor, L.L.; Yeong, K.L.; Davies, K.; Nelson, P.N.; Bird, M.I.; et al. Effects of mineralogy, chemistry and physical properties of basalts on carbon capture potential and plant-nutrient element release via enhanced weathering. Appl. Geochem. 2021, 132, 105023. [Google Scholar] [CrossRef]

| Units | Soils | Basalt Dust | |||
|---|---|---|---|---|---|
| Bordeaux | Harpenden | ||||
| pH | 5.79 | 5.53 | 8.79 | ||
| CaCO3 | % | 0.001 | 0.01 | 0.16 | |
| Ntot. | % | 0.11 | 0.12 | 0.002 | |
| Corg. | % | 2.18 | 1.37 | 0.19 | |
| eCEC | cmolc kg−1 | 5.7 | 9.2 | 5.9 | |
| Total elements | P | mg kg−1 | 168 | 452 | 952 |
| Ca | 821 | 1388 | 27,021 | ||
| K | 153 | 2036 | 1672 | ||
| Mg | 143 | 1446 | 11,411 | ||
| Na | 54 | 111 | 5070 | ||
| Cu | 2 | 18 | 45 | ||
| Fe | 627 | 22,889 | 47,831 | ||
| Mn | 11 | 1127 | 419 | ||
| Ni | 0.7 | 17 | 26 | ||
| Zn | 6 | 54 | 68 | ||
| Extractable elements | P | mg kg−1 | 31 | 5.5 | 0.7 |
| Ca | 550 | 1064 | 1832 | ||
| K | 52 | 109 | 47 | ||
| Mg | 74 | 49 | 56 | ||
| Na | 8.5 | 7.8 | 49 | ||
| Cu | <DL | <DL | <DL | ||
| Fe | 0.2 | 0.2 | <DL | ||
| Mn | 0.7 | 22.9 | 2.8 | ||
| Ni | (0.01–0.02) | 0.44 | <DL | ||
| Zn | 0.5 | 1.4 | (0.06–0.19) | ||
| Theoretical Initial Mix | Measurement for Initial Mix | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bordeaux | Harpenden | Bordeaux | Harpenden | ||||||
| 80 t ha−1 | 160 t ha−1 | 80 t ha−1 | 160 t ha−1 | 80 t ha−1 | 160 t ha−1 | 80 t ha−1 | 160 t ha−1 | ||
| Extractable elements (mg kg−1) | P | 30.5 | 29.8 | 5.4 | 5.3 | 29.1 | 29.9 | 5.9 | 5.1 |
| Ca | 582 | 614 | 1083 | 1102 | 592 | 625 | 1169 | 1226 | |
| K | 52 | 51 | 107 | 106 | 52 | 51 | 110 | 108 | |
| Mg | 73.2 | 72.8 | 49.6 | 49.7 | 72.4 | 69.3 | 50.8 | 50.4 | |
| Na | 9.5 | 10.5 | 8.8 | 9.8 | 9.6 | 10.2 | 10.1 | 10.9 | |
| Cu | <DL | <DL | <DL | <DL | |||||
| Fe | 0.20 | 0.20 | 0.20 | 0.20 | 0.22 | 0.22 | 0.23 | <DL | |
| Mn | 0.75 | 0.80 | 22.43 | 21.93 | 0.92 | 1.04 | 20.03 | 17.25 | |
| Ni | <DL | <DL | <DL | 0.29 | 0.20 | ||||
| Zn | 0.51 | 0.50 | 1.36 | 1.32 | 0.43 | 0.25 | 0.82 | 0.50 | |
| a | Bordeaux Soil Unplanted | Bordeaux Soil Planted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | Average Final Concentration | p | Average Final Concentration | |||||||
| No Basalt | 80 t ha−1 | 160 t ha−1 | No Basalt | 80 t ha−1 | 160 t ha−1 | |||||
| Major elements | mg kg−1 | P | ** | 35.0 a | 30.0 b | 27.6 b | ** | 27.1 a | 25.5 ab | 22.4 b |
| Ca | *** | 613 a | 699 b | 744 b | *** | 553 a | 656 b | 709 b | ||
| K | NS | 44 | 47 | 41 | *** | 5.85 a | 6.28 a | 8.73 b | ||
| Mg | NS | 75 | 82 | 72 | * | 63.2 a | 69.5 b | 65.5 ab | ||
| Na | NS | 8.5 | 12.4 | 12.2 | * | 2.24 a | 2.24 a | 3.89 b | ||
| Trace elements | mg kg−1 | Cu | NA | <DL | NA | <DL | ||||
| Fe | * | 0.30 a | 0.25 ab | 0.23 b | NS | 0.214 | 0.208 | 0.190 | ||
| Mn | * | 0.92 a | 0.60 b | 0.55 b | *** | 0.651 a | 0.419 b | 0.321 b | ||
| Zn | *** | 0.55 a | 0.31 b | 0.19 c | ** | 0.451 a | 0.254 b | 0.151 c | ||
| Ni | NA | <DL | NA | <DL | ||||||
| b | Harpenden Soil Unplanted | Harpenden Soil Planted | ||||||||
| p | Average final concentration | p | Average final concentration | |||||||
| No basalt | 80 t ha−1 | 160 t ha−1 | No basalt | 80 t ha−1 | 160 t ha−1 | |||||
| Major elements | mg kg−1 | P | NS | 5.34 | 4.84 | 4.93 | NS | 4.51 | 4.40 | 4.48 |
| Ca | *** | 1072 a | 1210 b | 1297 c | *** | 1136 a | 1239 b | 1308 c | ||
| K | ** | 105 a | 112 ab | 117 b | ** | 58.5 a | 62.0 ab | 68.9 b | ||
| Mg | *** | 46 a | 58 b | 61 c | *** | 47.3 a | 56.4 b | 60.1 b | ||
| Na | NS | 28 | 33 | 34 | NS | 41.1 | 45.9 | 46.0 | ||
| Trace elements | mg kg−1 | Cu | NA | <DL | NA | <DL | ||||
| Fe | NA | <DL | NA | <DL | ||||||
| Mn | *** | 26 a | 12 b | 7 b | *** | 30.0 a | 16.2 b | 10.4 c | ||
| Zn | ** | 2.71 a | 1.70 ab | 0.63 b | *** | 3.83 a | 1.67 b | 0.72 c | ||
| Ni | ** | 1.35 a | 0.47 b | 0.15 b | *** | 2.013 a | 0.553 b | 0.173 c | ||
| a | Bordeaux | Harpenden | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | Average Concentration | p | Average Concentration | |||||||
| No Basalt | 80 t ha−1 | 160 t ha−1 | No Basalt | 80 t ha−1 | 160 t ha−1 | |||||
| Major elements | mg kg−1 | Ca | NS | 8900 | 9300 | 8200 | NS | 5700 | 5900 | 6400 |
| K | * | 19,300 a | 20,900 ab | 24,000 b | NS | 35,500 | 36,500 | 36,500 | ||
| Mg | *** | 4600 a | 4000 b | 3700 b | NS | 2000 | 2100 | 2100 | ||
| Na | NS | 3500 | 4200 | 4300 | NS | 400 | 400 | 400 | ||
| P | *** | 5340 a | 4670 b | 4180 b | NS | 1470 | 1480 | 1310 | ||
| Trace elements | mg kg−1 | Cu | ** | 7.86 a | 7.09 b | 7.04 b | NS | 8.54 | 8.29 | 8.48 |
| Fe | ** | 64.0 a | 74.7 b | 81.7 c | NS | 59.3 | 59.6 | 78.1 | ||
| Mn | * | 84.7 a | 69.2 ab | 55.6 b | ** | 177 a | 133 b | 98 b | ||
| Ni | * | 0.78 a | 0.91 ab | 0.95 b | *** | 3.26 a | 2.27 b | 1.89 c | ||
| Zn | NS | 83.2 | 74. | 76.7 | *** | 53.5 a | 50.0 a | 42.6 b | ||
| b | Bordeaux | Harpenden | ||||||||
| p | Average uptake | p | Average uptake | |||||||
| No basalt | 80 t ha−1 | 160 t ha−1 | No basalt | 80 t ha−1 | 160 t ha−1 | |||||
| Major elements | mg | Ca | ** | 57 a | 76 b | 69 b | NS | 30 | 35 | 35 |
| K | *** | 122 a | 169 b | 203 c | NS | 187 | 214 | 200 | ||
| Mg | NS | 30 | 33 | 31 | NS | 11 | 12 | 11 | ||
| Na | * | 22 a | 34 b | 37 b | NS | 2 | 3 | 2 | ||
| P | NS | 34 | 38 | 35 | NS | 7.7 | 87.0 | 7.2 | ||
| Trace elements | mg | Cu | NS | 0.05 | 0.06 | 0.06 | NS | 0.045 | 0.049 | 0.046 |
| Fe | *** | 0.41 a | 0.61 b | 0.69 b | NS | 0.311 | 0.349 | 0.421 | ||
| Mn | * | 0.53 a | 0.56 ab | 0.47 b | * | 0.94 a | 0.78 a | 0.54 b | ||
| Ni | ** | 0.005 a | 0.007 b | 0.008 b | * | 0.017 a | 0.013 ab | 0.01 b | ||
| Zn | ** | 0.52 a | 0.6 b | 0.65 b | * | 0.28 a | 0.29 a | 0.23 b | ||
| Nutrient Use Efficiency (%) | ||||
|---|---|---|---|---|
| Ca | K | Mg | P | |
| Bordeaux 80 t ha−1 | 0.9 | 35.7 | 0.4 | 5.5 |
| Bordeaux 160 t ha−1 | 0.3 | 30.2 | 0.1 | 0.9 |
| Harpenden 80 t ha−1 | 0.3 | 26.4 | 0.2 | 1.7 |
| Harpenden 160 t ha−1 | 0.2 | 6.2 | 0.1 | −0.5 |
| a | Bordeaux | Harpenden | |||||
|---|---|---|---|---|---|---|---|
| No Basalt | 80 t ha−1 | 160 t ha−1 | No Basalt | 80 t ha−1 | 160 t ha−1 | ||
| All values are in mg per pot | |||||||
| Initial state | Exchangeable K in soil mix | 169 | 173 | 174 | 273 | 282 | 283 |
| Exchangeable K in basalt | 0 | 3.8 | 7.5 | 0 | 2.9 | 5.9 | |
| Total K in soil mix | 344 | 437 | 601 | 5096 | 5202 | 5395 | |
| Total K in basalt | 0 | 134 | 268 | 0 | 105 | 209 | |
| Final state | K uptake by plant | 122 | 169 | 203 | 187 | 214 | 200 |
| Exchangeable K in soil mix | 19 | 21 | 30 | 146 | 159 | 181 | |
| Plant uptake + exchangeable in soil | 141 | 190 | 232 | 333 | 373 | 381 | |
| Apparent K released and weathered | 0 | 17 | 58 | 60 | 92 | 98 | |
| (K released + weathered) / (tot K in soil mix) | 0% | 4% | 10% | 1% | 2% | 2% | |
| b | Bordeaux | Harpenden | |||||
| No basalt | 80 t ha−1 | 160 t ha−1 | No basalt | 80 t ha−1 | 160 t ha−1 | ||
| All values are in mg per pot | |||||||
| Initial state | Extractable P in soil mix | 102 | 97 | 102 | 14 | 15 | 13 |
| Extractable P in basalt | 0 | 0.05 | 0.11 | 0 | 0.04 | 0.08 | |
| Total P in soil mix | 547 | 619 | 676 | 1129 | 1241 | 1222 | |
| Total P in basalt | 0 | 76 | 152 | 0 | 60 | 119 | |
| Final state | P uptake by plant | 34 | 38 | 35 | 8 | 9 | 7 |
| Extractable P in soil mix | 88 | 85 | 76 | 11 | 11 | 12 | |
| Plant uptake + Extractable in soil | 122 | 123 | 111 | 19 | 20 | 19 | |
| Apparent P released or weathered | 20 | 26 | 10 | 5 | 5 | 6 | |
| (P released + weathered)/(tot P in soil mix) | 4% | 4% | 1% | 0.5% | 0.4% | 0.5% | |
| P | Ca | K | Mg | Na | Cu | Fe | Mn | Ni | Zn | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | Bdx | Harp | |
| Basalt VS Soil Total elements | >> | > | >> | >> | >> | < | >> | >> | >> | >> | >> | >> | >> | > | >> | < | >> | > | >> | > |
| Basalt VS Soil Extractable elements | << | < | >> | > | ≈ | < | < | ≈ | > | > | NA | NA | << | << | > | << | NA | << | << | << |
| Theoreticalmeasurement VS Initial measurement | ≈ | ≈ | ≈ | > | ≈ | ≈ | ≈ | ≈ | ≈ | ≈ | NA | NA | > | NA | > | < | NA | NA | < | < |
| Final soil concentration Unplanted | ↘ | ≈ | ↗ | ↗ | ≈ | ↗ | ≈ | ↗ | ≈ | ≈ | NA | NA | ↘ | NA | ↘ | ↘ | NA | ↘ | ↘ | ↘ |
| Final soil concentration Planted | ↘ | ≈ | ↗ | ↗ | ↗ | ↗ | ↗ | ↗ | ↗ | ≈ | NA | NA | ≈ | NA | ↘ | ↘ | NA | ↘ | ↘ | ↘ |
| Plant Concentration | ↘ | ≈ | ≈ | ≈ | ↗ | ≈ | ↘ | ≈ | ≈ | ≈ | ↘ | ≈ | ↗ | ≈ | ↘ | ↘ | ↗ | ↘ | ≈ | ↘ |
| Plant uptake | ≈ | ≈ | ↗ | ≈ | ↗ | ≈ | ≈ | ≈ | ↗ | ≈ | ≈ | ≈ | ↗ | ≈ | ↘ | ↘ | ↗ | ↘ | ↗ | ↘ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmalles, C.; Jordan-Meille, L.; Hernandez, J.; Thomas, C.L.; Dunham, S.; Deng, F.; McGrath, S.P.; Haefele, S.M. Impact of Basalt Rock Powder on Ryegrass Growth and Nutrition on Sandy and Loamy Acid Soils. Agronomy 2025, 15, 1791. https://doi.org/10.3390/agronomy15081791
Desmalles C, Jordan-Meille L, Hernandez J, Thomas CL, Dunham S, Deng F, McGrath SP, Haefele SM. Impact of Basalt Rock Powder on Ryegrass Growth and Nutrition on Sandy and Loamy Acid Soils. Agronomy. 2025; 15(8):1791. https://doi.org/10.3390/agronomy15081791
Chicago/Turabian StyleDesmalles, Charles, Lionel Jordan-Meille, Javier Hernandez, Cathy L. Thomas, Sarah Dunham, Feifei Deng, Steve P. McGrath, and Stephan M. Haefele. 2025. "Impact of Basalt Rock Powder on Ryegrass Growth and Nutrition on Sandy and Loamy Acid Soils" Agronomy 15, no. 8: 1791. https://doi.org/10.3390/agronomy15081791
APA StyleDesmalles, C., Jordan-Meille, L., Hernandez, J., Thomas, C. L., Dunham, S., Deng, F., McGrath, S. P., & Haefele, S. M. (2025). Impact of Basalt Rock Powder on Ryegrass Growth and Nutrition on Sandy and Loamy Acid Soils. Agronomy, 15(8), 1791. https://doi.org/10.3390/agronomy15081791







