Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil
Abstract
1. Introduction
2. Material and Methods
2.1. Biological Material
2.2. Inoculation and Experimental Setup in Calcareous Soil
- Plants grown in previously sterilized calcareous soil.
- Plants grown in non-sterilized calcareous soil.
- Plants inoculated by root immersion and grown in sterilized calcareous soil.
- Plants inoculated by root immersion and grown in non-sterilized calcareous soil.
- Plants inoculated by surface irrigation and grown in sterilized calcareous soil.
- Plants inoculated by surface irrigation and grown in non-sterilized calcareous soil.
2.2.1. Root Inoculation
2.2.2. Irrigation Inoculation
2.2.3. Chlorophyll Content (SPAD)
2.2.4. Growth Promotion and Yield Production
2.2.5. Elemental Analysis of Leaves
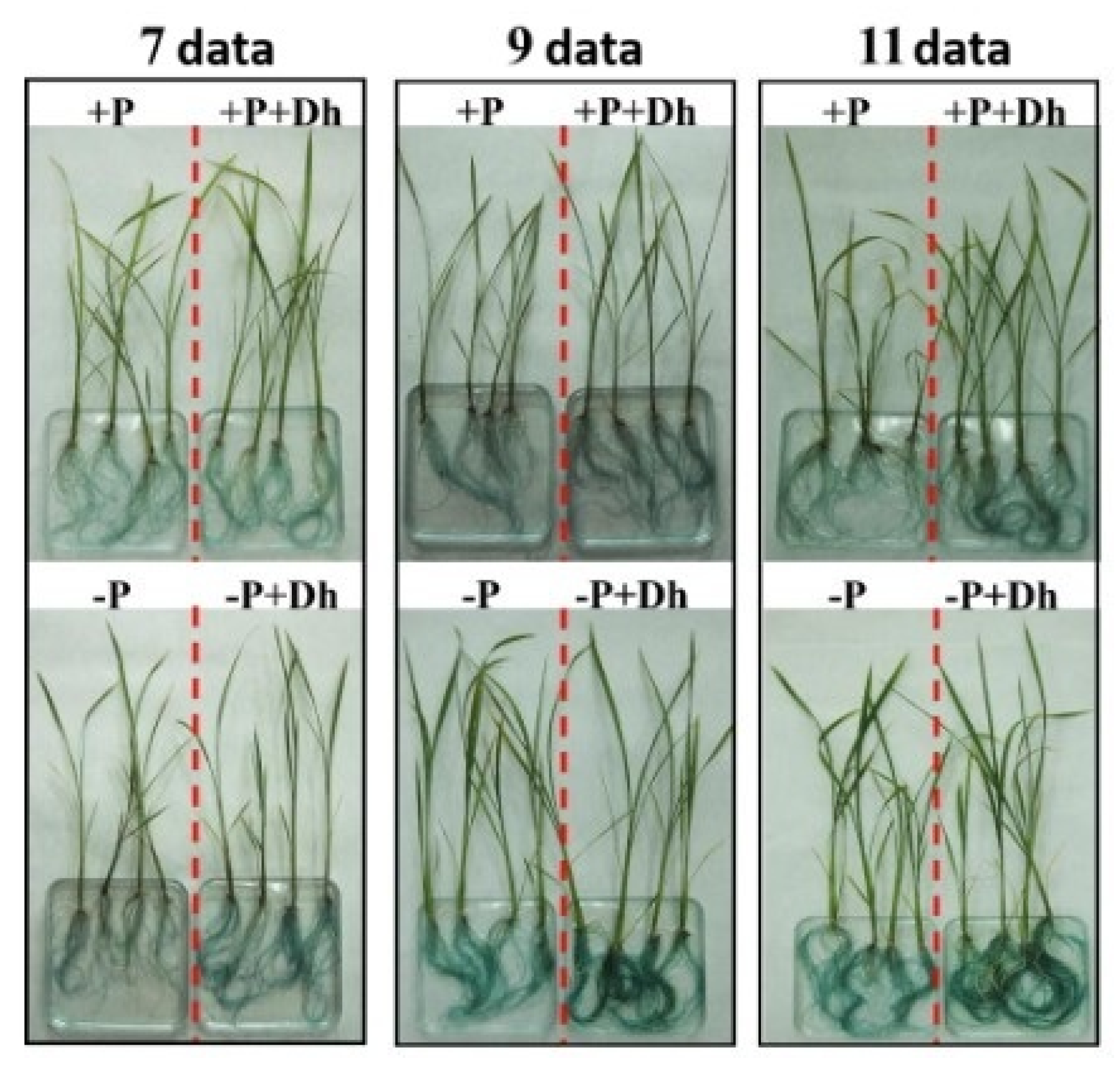
2.3. Inoculation in Hydroponic System
- Plants grown in a complete nutrient solution.
- Plants grown in a complete nutrient solution plus inoculum.
- Plants grown in a P-deficient nutrient solution.
- Plants grown in a P-deficient nutrient solution plus inoculum.
2.3.1. Acid Phosphatase Determination
2.3.2. Gene Expression Analysis by qRT-PCR
2.4. Statistical Analysis
3. Results
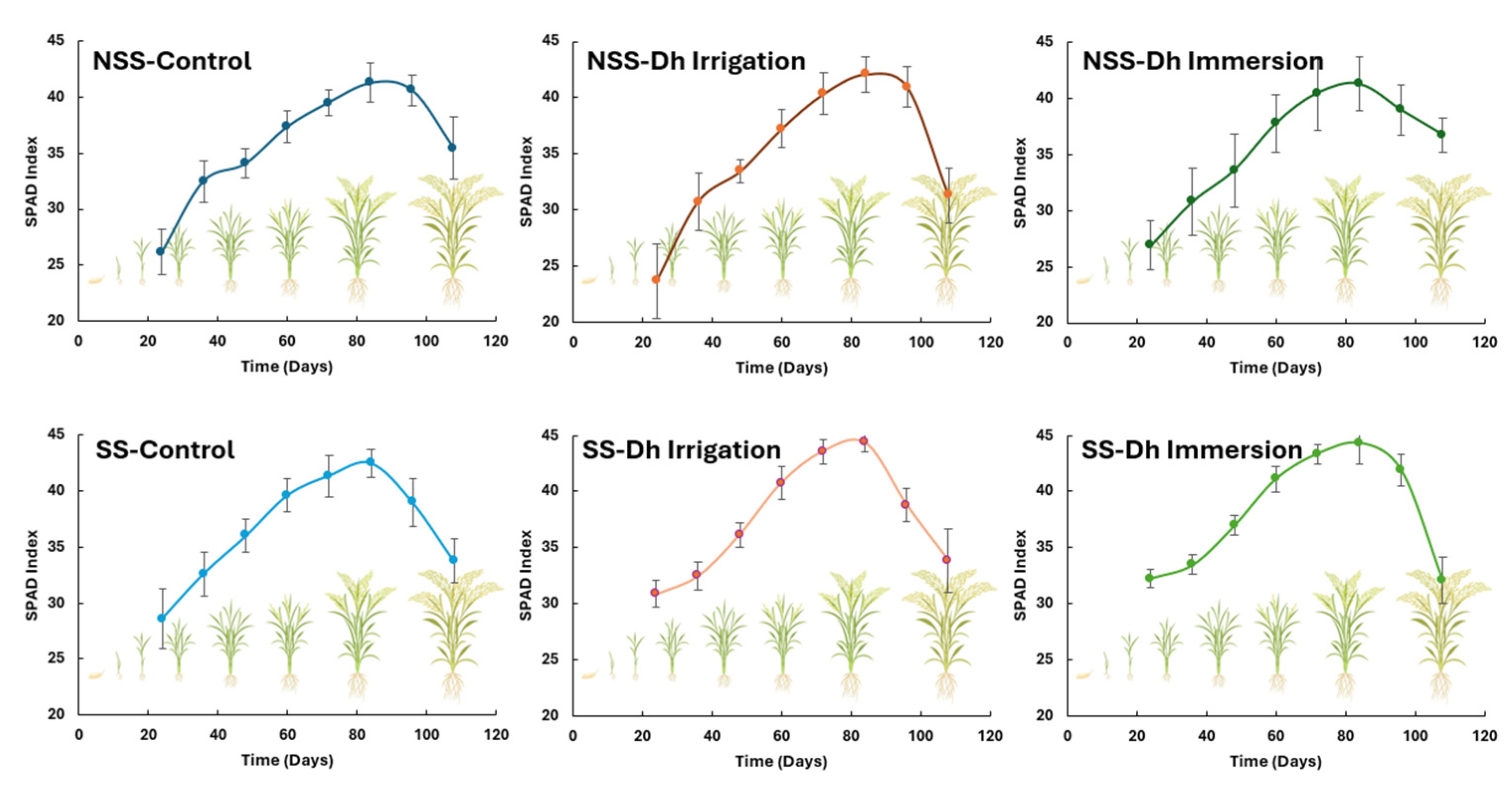
3.1. Effect of Debaryomyces hansenii Inoculation on SPAD Index in Calcareous Soil
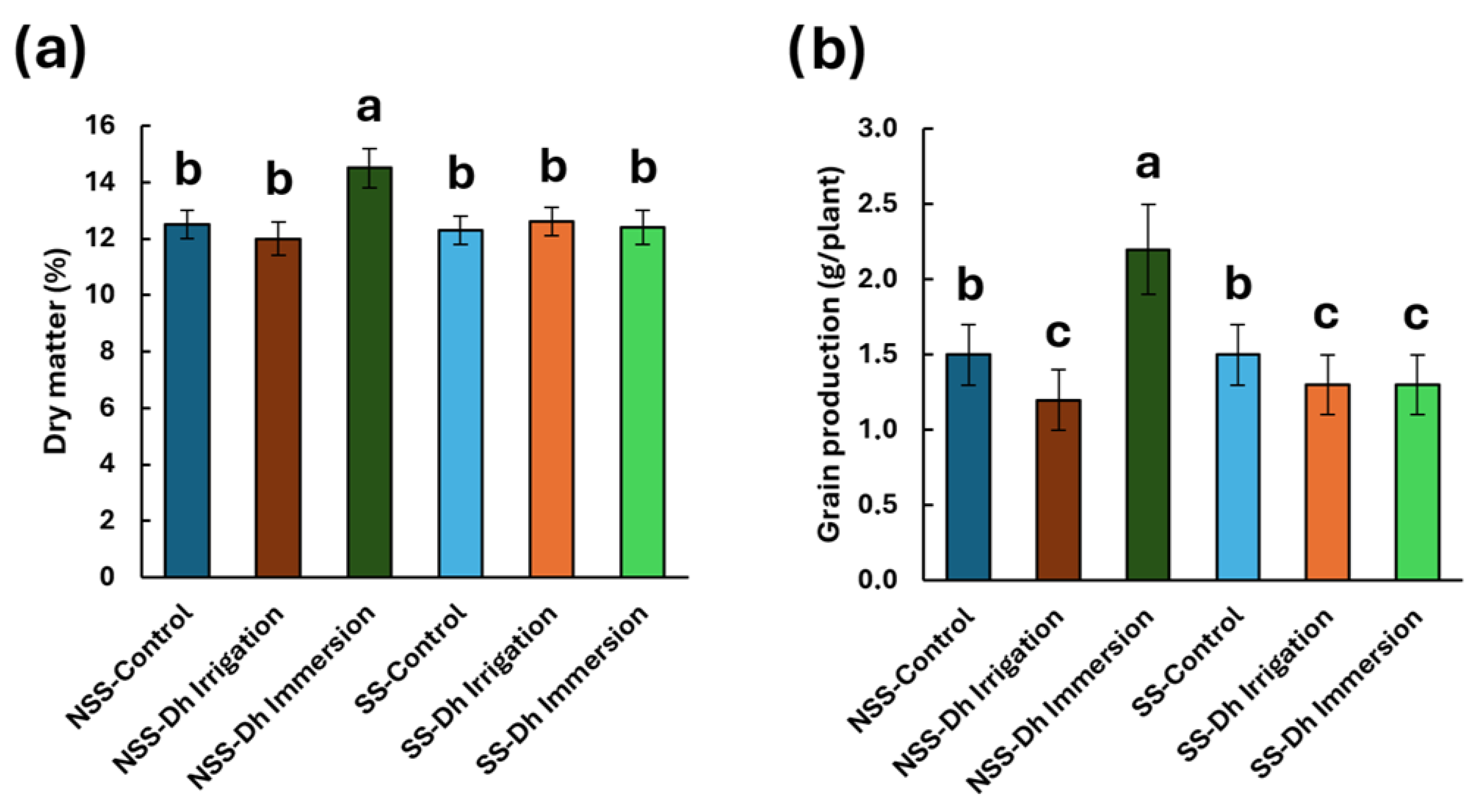
3.2. Effect of Debaryomyces hansenii on Dry Matter Content, Plant Height, and Grain Yield in Calcareous Soil
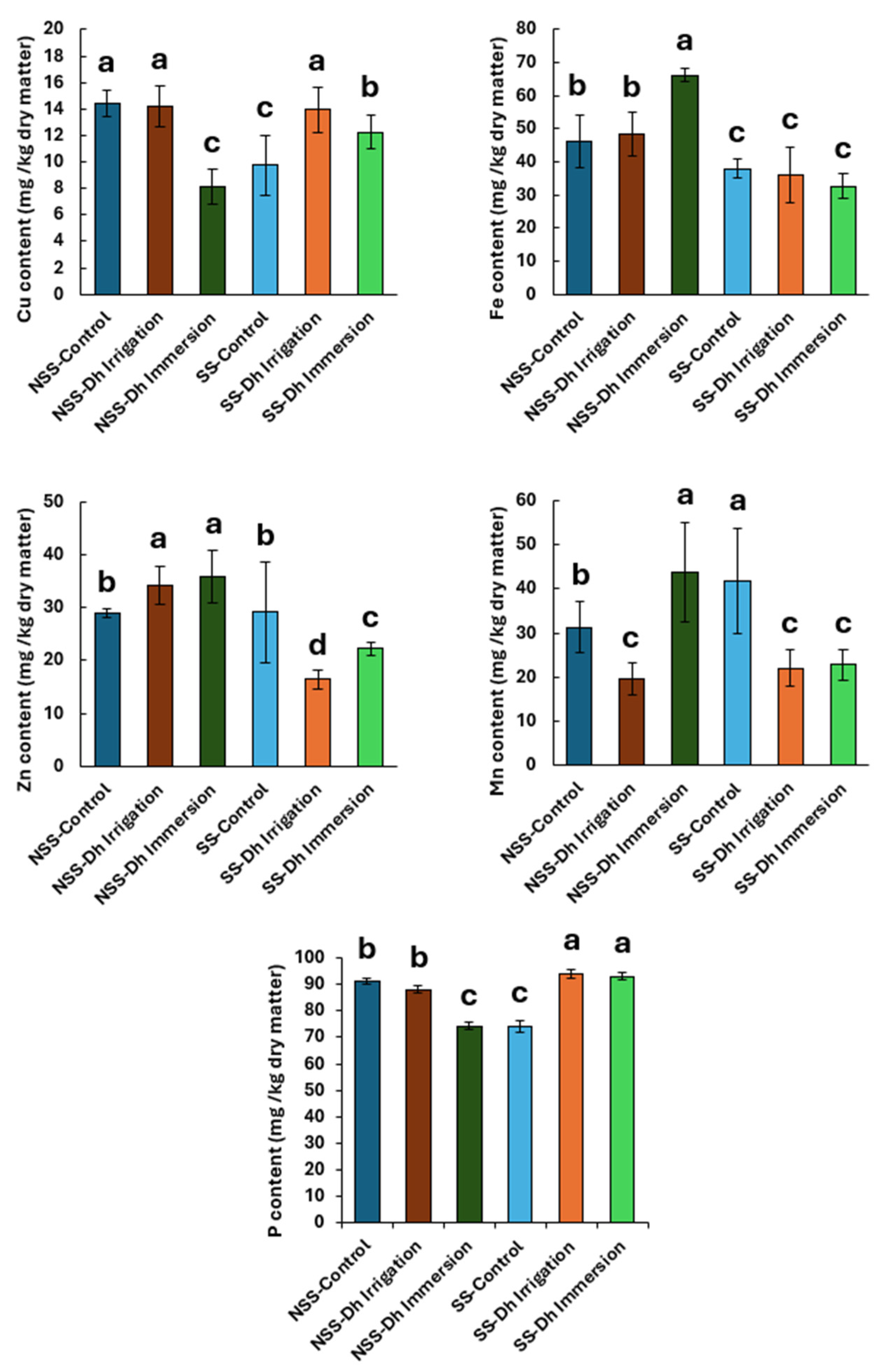
3.3. Effect of Debaryomyces hansenii on Leaf Content of Cu, Fe, Zn, Mn, and P in Rice Plants in Calcareous Soil
3.4. Effect of Debaryomyces hansenii on the Physiological Mechanism of Acid Phosphatase Activity in Rice Plants in a Hydroponic System
3.5. Effect of Debaryomyces hansenii on the Expression of Genes Related to Acid Phosphatase Activity and Phosphorus Transport in Hydroponic System
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and Reduced Soil Micronutrient Availability in Global Drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef]
- Abel, S.; Ticconi, C.A.; Delatorre, C.A. Phosphate Sensing in Higher Plants. Physiol. Plant. 2002, 115, 1–8. [Google Scholar] [CrossRef]
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals-role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Srivastava, P.; Rao, C.S.; Satyanaraya, P.V.; Anderson, G.C.; Bolan, S.; Nortjé, G.P.; Kronenberg, R.; Bardhan, S.; Abbott, L.K.; et al. Distribution, characteristics and management of calcareous soils. Adv. Agron. 2023, 81–130. [Google Scholar] [CrossRef]
- Lucena, C.; Porras, R.; Romera, F.J.; Alcántara, E.; García, M.J.; Pérez-Vicente, R. Similarities and Differences in the Acquisition of Fe and P by Dicot Plants. Agronomy 2018, 8, 148. [Google Scholar] [CrossRef]
- Lucena, C.; Alcalá-Jiménez, M.T.; Romera, F.J.; Ramos, J. Several Yeast Species Induce Iron Deficiency Responses in Cucumber Plants (Cucumis Sativus L.). Microorganisms 2021, 9, 2603. [Google Scholar] [CrossRef]
- Sevillano-Cano, J.; García, M.J.; Córdoba-Galván, C.; Luque-Cruz, C.; Agustí-Brisach, C.; Lucena, C.; Ramos, J.; Pérez-Vicente, R.; Romera, F.J. Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. Int. J. Mol. Sci. 2024, 25, 5729. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and Fertilizer Phosphorus and Crop Responses in the Dryland Mediterranean Zone. In Soil Restoration; Lal, R., Stewart, B.A., Eds.; Advances in Soil Science; Springer: New York, NY, USA, 1992; Volume 17, pp. 81–146. ISBN 978-1-4612-7684-5. [Google Scholar]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M.C. Phosphate Deficiency Promotes Modification of Iron Distribution in Arabidopsis Plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef]
- Mori, S. Iron Acquisition by Plants. Curr. Opin. Plant Biol. 1999, 2, 250–253. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; ISBN 978-0-12-473542-2. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-32714-1. [Google Scholar]
- Mori, S.; Nishizawa, N.; Hayashi, H.; Chino, M.; Yoshimura, E.; Ishihara, J. Why Are Young Rice Plants Highly Susceptible to Iron Deficiency? In Iron Nutrition and Interactions in Plants; Chen, Y., Hadar, Y., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 175–188. ISBN 978-94-010-5455-3. [Google Scholar]
- Takagi, S. Naturally Occurring Iron-Chelating Compounds in Oat- and Rice-Root Washings: I. Activity Measurement and Preliminary Characterization. Soil Sci. Plant Nutr. 1976, 22, 423–433. [Google Scholar] [CrossRef]
- Marschner, H.; Romheld, V.; Kissel, M. Different Strategies in Higher Plants in Mobilization and Uptake of Iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Nimsi, K.A.; Manjusha, K.; Kathiresan, K.; Arya, H. Plant Growth-Promoting Yeasts (PGPY), the Latest Entrant for Use in Sustainable Agriculture: A Review. J. Appl. Microbiol. 2023, 134, lxac088. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sen, S.K. Exploration of Novel Rhizospheric Yeast Isolate as Fertilizing Soil Inoculant for Improvement of Maize Cultivation. J. Sci. Food Agric. 2015, 95, 1491–1499. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, V.; Srivastava, S.; Bist, V.; Tripathi, P.; Naseem, M.; Nand, S.; Anshu; Khare, P.; Srivastava, P.K.; et al. Yeast Strain Debaryomyces Hansenii for Amelioration of Arsenic Stress in Rice. Ecotoxicol. Environ. Saf. 2020, 195, 110480. [Google Scholar] [CrossRef]
- Mundra, S.; Arora, R.; Stobdan, T. Solubilization of Insoluble Inorganic Phosphates by a Novel Temperature-, pH-, and Salt-Tolerant Yeast, Rhodotorula Sp. PS4, Isolated from Seabuckthorn Rhizosphere, Growing in Cold Desert of Ladakh, India. World J. Microbiol. Biotechnol. 2011, 27, 2387–2396. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Mohamed, H.M. Molecular Genetic Identification of Yeast Strains Isolated from Egyptian Soils for Solubilization of Inorganic Phosphates and Growth Promotion of Corn Plants. J. Microbiol. Biotechnol. 2011, 21, 55–61. [Google Scholar] [CrossRef]
- Fu, S.-F.; Sun, P.-F.; Lu, H.-Y.; Wei, J.-Y.; Xiao, H.-S.; Fang, W.-T.; Cheng, B.-Y.; Chou, J.-Y. Plant Growth-Promoting Traits of Yeasts Isolated from the Phyllosphere and Rhizosphere of Drosera Spatulata Lab. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Magri, M.M.; Avansini, S.H.; Lopes-Assad, M.L.; Tauk-Tornisielo, S.M.; Ceccato-Antonini, S.R. Release of Potassium from Rock Powder by the Yeast Torulaspora globosa. Braz. Arch. Biol. Technol. 2012, 55, 577–582. [Google Scholar] [CrossRef]
- Mohamed, H.M.; El-Homosy, R.F.; Abd-Ellatef, A.-E.H.; Salh, F.M.; Hussein, M.Y. Identification of Yeast Strains Isolated from Agricultural Soils for Releasing Potassium-Bearing Minerals. Geomicrobiol. J. 2017, 34, 261–266. [Google Scholar] [CrossRef]
- Nutaratat, P.; Amsri, W.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Indole-3-Acetic Acid Production by Newly Isolated Red Yeast Rhodosporidium paludigenum. J. Gen. Appl. Microbiol. 2015, 61, 1–9. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Kildegaard, K.R.; Cernuda Pastor, M.; Jayachandran, S.; Kristensen, M.; Borodina, I. Yarrowia Lipolytica Strains Engineered for the Production of Terpenoids. Front. Bioeng. Biotechnol. 2020, 8, 945. [Google Scholar] [CrossRef]
- Aparicio, M.A.; Lucena, C.; García, M.J.; Ruiz-Castilla, F.J.; Jiménez-Adrián, P.; López-Berges, M.S.; Prieto, P.; Alcántara, E.; Pérez-Vicente, R.; Ramos, J.; et al. The Nonpathogenic Strain of Fusarium Oxysporum FO12 Induces Fe Deficiency Responses in Cucumber (Cucumis Sativus L.) Plants. Planta 2023, 257, 50. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V.; Marschner, H. Iron Deficiency Stress Induced Morphological and Physiological Changes in Root Tips of Sunflower. Physiol. Plant. 1981, 53, 354–360. [Google Scholar] [CrossRef]
- Sherman, F. Getting Started with Yeast. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 194, pp. 3–21. ISBN 978-0-12-182095-4. [Google Scholar]
- Navarro-Velasco, G.Y.; Prados-Rosales, R.C.; Ortíz-Urquiza, A.; Quesada-Moraga, E.; Di Pietro, A. Galleria Mellonella as Model Host for the Trans-Kingdom Pathogen Fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Zakhleniuk, O.V.; Raines, C.A.; Lloyd, J.C. Pho3: A Phosphorus-Deficient Mutant of Arabidopsis Thaliana (L.) Heynh. Planta 2001, 212, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association (IZA): Durham, NC, USA, 2008. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Bergen, NJ, USA, 2008. [Google Scholar]
- Yoshida, S. Fundamentals of Rice Crop Science; International Rice Research Institute: Los Baños, Philippines, 1981. [Google Scholar]
- Juliano, B.O. Criteria and Tests for Rice Grain Qualities. In Rice Chemistry and Technology, 2nd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1985; pp. 443–524. [Google Scholar]
- Savary, S. Direct and Indirect Effects of Nitrogen Supply and Disease Source Structure on Rice Sheath Blight Spread. Phytopathology 1995, 85, 959. [Google Scholar] [CrossRef]
- Pingali, P.L. From Subsistence to Commercial Production Systems: The Transformation of Asian Agriculture. Am. J. Agric. Econ. 1997, 79, 628–634. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Sharma, A.; Johri, B.N.; Sharma, A.K.; Glick, B.R. Plant Growth-Promoting Bacterium Pseudomonas Sp. Strain GRP3 Influences Iron Acquisition in Mung Bean (Vigna Radiata L. Wilzeck). Soil Biol. Bioch. 2003, 35, 887–894. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Zamioudis, C.; Korteland, J.; Van Pelt, J.A.; Van Hamersveld, M.; Dombrowski, N.; Bai, Y.; Hanson, J.; Van Verk, M.C.; Ling, H.; Schulze-Lefert, P.; et al. Rhizobacterial Volatiles and Photosynthesis-related Signals Coordinate MYB 72 Expression in Arabidopsis Roots during Onset of Induced Systemic Resistance and Iron-deficiency Responses. Plant J. 2015, 84, 309–322. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Streletskii, R.A.; Kachalkin, A.V.; Glushakova, A.M.; Yurkov, A.M.; Demin, V.V. Yeasts Producing Zeatin. PeerJ 2019, 7, e6474. [Google Scholar] [CrossRef]
- Kushner, D. Microbial Life in Extreme Environments; Academic Press: London, UK, 1978; ISBN 978-0-12-430250-1. [Google Scholar]
- Prista, C.; Michán, C.; Miranda, I.M.; Ramos, J. The Halotolerant Debaryomyces Hansenii, the Cinderella of Non-conventional Yeasts. Yeast 2016, 33, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, L.; Gao, H.; Zhang, M.; Guo, C. Enhancement of Alfalfa Yield and Quality by Plant Growth-promoting Rhizobacteria under Saline-alkali Conditions. J. Sci. Food Agric. 2019, 99, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant Growth-Promoting Rhizobacteria (Pgpr) Increase Yield, Growth and Nutrition of Strawberry Under High-Calcareous Soil Conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Marschner, H. Phosphatase Activity in the Rhizosphere and Hyphosphere of VA Mycorrhizal Wheat Supplied with Inorganic and Organic Phosphorus. Soil Biol. Bioch. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.-M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.-Y.; et al. Bacterial Endophyte Sphingomonas Sp. LK11 Produces Gibberellins and IAA and Promotes Tomato Plant Growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]
- Qetrani, S.; Bouray, M.; Oukarroum, A. Phosphorus mobilization and acquisition in the alkaline-calcareous rhizosphere: A synthesis. Rhizosphere 2024, 30, 100907. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Tian, J.; Li, K.; Shou, H. Identification of Rice Purple Acid Phosphatases Related to Posphate Starvation Signalling. Plant Biol. 2011, 13, 7–15. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant Regulation of Rice Arbuscular Mycorrhizal Symbiosis by Two Members of the PHOSPHATE TRANSPORTER1 Gene Family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]


| Clay g kg−1 | Organic Carbon g kg−1 | CaCO3 g kg−1 | pH1:2.5 | EC1:5 dS m−1 | CEC cmol kg−1 | POlsen mg kg−1 | FeDTPA mg kg−1 |
|---|---|---|---|---|---|---|---|
| 370 | 9.3 | 338 | 7.9 | 1.5 | 31.3 | 13.4 | 4.3 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| OsPHT1;6 | CCGCCGCCTCACAAACTGTA | GAACTGGGCGGTTTTCCTGA |
| OsPAP9 | ACCTACGTAGAGACAACATCAGGC | CATATACGTGTTGCCGGTAGTGA |
| OsPAP3 | TCATACCATGAGGAGTGAGTGATG | GTCTTCGTTTTGTGAAAATGGC |
| OsACTIN | TGCATGTAGTACAGTGC CATCCAG | AATGAGTAACCACGCTCCGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Cano, J.; Ruiz-Castilla, F.J.; Ramos, J.; Romera, F.J.; Lucena, C. Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil. Agronomy 2025, 15, 1696. https://doi.org/10.3390/agronomy15071696
Núñez-Cano J, Ruiz-Castilla FJ, Ramos J, Romera FJ, Lucena C. Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil. Agronomy. 2025; 15(7):1696. https://doi.org/10.3390/agronomy15071696
Chicago/Turabian StyleNúñez-Cano, Jorge, Francisco J. Ruiz-Castilla, José Ramos, Francisco J. Romera, and Carlos Lucena. 2025. "Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil" Agronomy 15, no. 7: 1696. https://doi.org/10.3390/agronomy15071696
APA StyleNúñez-Cano, J., Ruiz-Castilla, F. J., Ramos, J., Romera, F. J., & Lucena, C. (2025). Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil. Agronomy, 15(7), 1696. https://doi.org/10.3390/agronomy15071696








