Abstract
Sugarcane (Saccharum spp.) is crucial for sweetener production but is highly susceptible to diseases such as orange rust, caused by Puccinia kuehnii. In the northwestern Colombian Amazon, sugarcane is increasingly cultivated, mainly for panela production, a traditional sweetener. However, the introduction of sugarcane has occurred without systematic planning, resulting in limited knowledge about cultivars’ characteristics and disease susceptibility. This study aimed to characterize sugarcane cultivars in the region and assess the occurrence and field-level impact of orange rust, while also confirming the identity of the pathogen using molecular and morphological analysis. We identified five sugarcane cultivars, with only CP 57-603 having an official designation, while the others were known by local names: Regional Without Fuzz, Hairy Purple, and two unnamed genotypes (Cultivar-1 and -2). CP 57-603 and Regional Without Fuzz were the most commonly cultivated (by 49.2% and 74.5% of farms, respectively), while Cultivar-1 (11.8%), Cultivar-2 (7.8%), and Hairy Purple (1.96%) were less frequent. Orange rust was detected in 72% of farms with CP 57-603, 66% with Regional Without Fuzz, and 50% with Cultivar-1, but was absent in farms growing Cultivar-2 and Hairy Purple. Molecular analysis of the ITS1/2 region revealed a single haplotype within the P. kuehnii population, indicating low diversity at this locus in the region. Phylogenetic analysis grouped our ITS1/2 haplotype within a clade alongside isolates from the Americas. Morphological characterization of the pathogen showed no significant trait variation among samples. These findings confirm the presence of P. kuehnii in the region and highlight the urgent need for cultivar diversification and improved disease management to safeguard sugarcane production.
1. Introduction
Sugarcane (Saccharum spp.) is important for producing sweeteners for both domestic and industrial use. Over the decades, breeding programs across different countries have developed cultivars aimed at improving yield, stress tolerance, and disease resistance [1]. Some of the most widely recognized cultivars were developed by pioneering sugarcane breeding programs, including Proefstation Java (POJ), Puerto Rico (PR), Cuba (My), the Dominican Republic (RD), Coimbatore, India (Co), and Canal point in Florida, USA (CP) [2,3,4]. Among these, CP 57-603, developed in 1957 by the Canal Point sugarcane breeding program in Florida, USA, stands out for its agronomic performance and adaptability, leading to its widespread adoption across Latin America [5,6,7].
In remote regions, far from major sugarcane production centers, cultivars are often introduced informally and without systematic planning. As a result, there is limited information regarding the identity, genetic background, and disease susceptibility of the varieties that are currently in use. One of the key challenges in sugarcane production is the progressive breakdown of varietal resistance, which often results in significant yield losses and increased vulnerability to disease [8]. For example, in Colombia, the severity of brown rust in CP 57-603 increased from 1.5% to approximately 30% between 1981 and 1984 [9]. Similarly, RD 75-11, a variety developed in the Dominican Republic from a cross between CB 38-22 and CP 57-603, was adopted in some Latin American countries, including Colombia, particularly by smallholders, but its cultivation has declined due to its susceptibility to rust diseases [5,6,7,10].
Among sugarcane diseases, orange rust, caused by the fungus Puccinia kuehnii (W. Krüger) E.J. Butler, is particularly impactful [11,12,13,14,15,16]. This disease is characterized by orange powdery pustules on the abaxial part of the leaf, ranging from 2 to 10 mm in length and 1 to 3 mm in width, which helps to differentiate it from other fungal diseases [17,18]. The presence of orange rust has been reported to reduce photosynthetic efficiency and overall yield, with reported losses ranging from 10% to 40% in Florida, USA; up to 50% in Australia; around 25% in La Réunion, France; and above 40% in Brazil [16,18,19,20,21]. Orange rust has a wide geographical distribution, being reported in various regions of Asia, Africa, Oceania, and the Americas [22]. In 2007, P. kuehnii was confirmed for the first time in Florida (United States) [20]. Since then, it has spread to other countries in Central America, the Caribbean, and South America [23,24,25,26,27]. In Colombia, it was first detected in 2010 in the southwest region, where the main sugarcane production is concentrated [28].
In addition to industrial use, sugarcane in Colombia is widely used to produce a traditional sweetener known as panela, also referred to as non-centrifugal sugarcane, especially in less-developed regions such as the northwestern Colombian Amazon, where it is produced using artisanal methods [29]. This region has approximately 10,101.5 ha planted with sugarcane, representing 4.6% of the national area [30]. In the department of Caquetá, the largest producer in this region with 5090 ha and an annual production of 285,949 tons, nearly 2992 smallholders benefit from sugarcane cultivation [31,32].
However, sugarcane cultivars in this region have been introduced and distributed informally by farmers, without systematic planning. There are no official records specifying the cultivars that are grown, which are often generically referred to as sugarcane. As a result, there is limited information on planting materials and their susceptibility to disease. These issues are further exacerbated by the region’s agroclimatic conditions, characterized by alternating rainfall cycles, temperature fluctuations, and high relative humidity [33], which favor the development of rust pathogens [34,35,36,37]. Prior to this study, the presence of orange rust in the region had been suspected based on visual symptoms but had not been formally confirmed. Characterizing the cultivars that are present is essential for maintaining their identity, preventing unnecessary individualization, and improving traceability for production and phytosanitary purposes [38]. Thus, identifying and characterizing the sugarcane cultivars in this region is necessary for promoting sustainable production and implementing effective disease management strategies.
In this context, the present study aimed to (i) identify and characterize the sugarcane cultivars that are currently grown by farmers in the northwestern Colombian Amazon and (ii) assess the occurrence and severity of orange rust infection caused by P. kuehnii across these cultivars under field conditions. To achieve this, we combined field-level observations with morphological and molecular analyses to confirm the presence of P. kuehnii. The molecular identification was based on the ITS1/2 region of ribosomal DNA, a widely accepted marker for species-level identification within the Puccinia genus [39,40,41,42,43]. We hypothesized that the predominant sugarcane cultivars in the northwestern Colombian Amazon would exhibit varying levels of susceptibility to orange rust and that the absence of systematic cultivar selection has contributed to the spread of highly susceptible varieties, potentially facilitating disease dissemination.
2. Materials and Methods
2.1. Study Area
This study was carried out in the Caquetá department, located in the northwestern Colombian Amazon, involving 51 sugarcane-producing farmers (supplemental Table S1) across four municipalities, El Paujil, El Doncello, Puerto Rico, and San Vicente del Caguán (Figure 1), during the years 2021 to 2023. These municipalities are responsible for approximately 25% of the sugarcane production in the region [29]. In this region, sugarcane producers typically plant their crops between March and April, leveraging the increased rainfall during this period to benefit plant growth. Additionally, farmers implement the “entresaque” system, a cultural practice where mature stalks are harvested while leaving immature ones in the field. This practice results in a continuous presence of young plants aged 2–6 months, which are more susceptible to rust compared to mature plants [37].
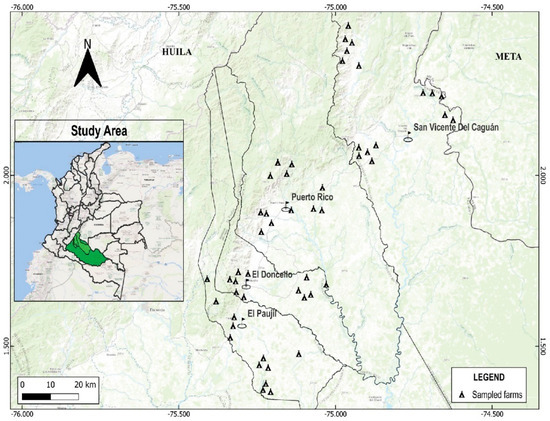
Figure 1.
Geographic distribution of sugarcane-producing farms included in the study, located in the northwestern Colombian Amazon, Caquetá department. The inset map shows the location of the study region within Colombia; the Caquetá department is highlighted in green.
The study area lies in the transition zone between the foothills of the eastern Andes Mountain and the Amazon Plain, with altitudes ranging from 235 to 1414 m above sea level (m.a.s.l.). Based on climatic parameters, the area is classified as a tropical rainforest zone type Af according to the Koppen classification, with an average temperature of 26 °C, annual precipitation of 2809 mm, and relative humidity of 90.5% (Agrometeorological Station WS-ZL6B, 2022).
2.2. Morphological Characterization of the Sugarcane Cultivars
To better understand the sugarcane cultivars that are grown in the region, we initially conducted a morphological characterization of the material that is present in the sugarcane-producing farmers’ plantations, following the methodology described by Victoria et al. [44] (Supplemental Figure S1). Five plants with millable stems were randomly selected from each plantation for characterization. The following variables were considered: stem growth habit, stem shape, internode shape, internode wax content, presence of bud canal, apical bud shape, leaf position, fluff content in the leaf sheath, type of defoliation, internode color, leaf sheath color, stem length (cm), internode diameter (mm), internode length (cm), and leaf length and width (cm). The percentage of farms cultivating each sugarcane cultivar was calculated by dividing the number of farms where a given cultivar was identified by the total number of farms surveyed (n = 51) and multiplying by 100.
2.3. Assessment of Orange Rust Incidence and Severity in the Sugarcane Cultivars
In each farm where the cultivars were present, disease evaluation was conducted on 20 randomly selected plants per cultivar. The third fully expanded leaf from the top with a visible collar (leaf + 3) was selected from each plant. When rust symptoms were observed, the leaf was classified as symptomatic. The disease incidence was then calculated as the proportion of symptomatic plants out of the total number of plants evaluated for each cultivar across all surveyed farms, expressed as a percentage. Subsequently, two parameters were recorded exclusively on symptomatic leaves, following the method proposed by Purdy and Dean (1981) [45] and adopted by the Colombian Sugarcane Research Center—Cenicaña [46,47]. (i) Disease severity (S) was estimated as the percentage of the total leaf area that was affected by lesions (ranging from 0 to 100%); and (ii) the type of pustule (TP), originally termed “TP” by Purdy and Dean [45] and referred to as the reaction grade by Cenicaña [47], was assessed using a visual scale from 0 to 9, which describes the stage of pustule development and sporulation as follows: 0 = no visible symptoms; 1 = small chlorotic stripes; 2 = necrotic stripes; 3 = small to large irregular reddish or brown spots with no pustules; 4 = individual chlorotic or reddish lesions with unopened pustules; 5 = individual lesions with open pustules producing spores; 6 = large reddish or necrotic lesions with spore-producing pustules; 7 = coalescing reddish or brown lesions with sporulating pustules crossing the leaf blade; 8 = actively sporulating pustules on chlorotic tissue; and 9 = actively sporulating pustules on green tissue. Based on these values, a damage index (DI) was calculated using the formula DI = (TP × 100) + S, where TP is the pustule type score and S is the severity percentage. According to Victoria et al. [37], cultivars with a DI greater than 512 were classified as susceptible to orange rust.
2.4. Morphological Characterization of Orange Rust
For the morphological characterization of the orange rust, samples were collected from leaves with S greater than 50%, presenting open pustules and germinating uredospores, which allowed for the differentiation from other fungal diseases of sugarcane. The samples were transported to the facilities of the Laboratorio de Agrobiotencología at Centro de Investigaciones Amazónicas CIMAZ Macagual of the Universidad de la Amazonia for further processing.
Subsequently, samples were dried at 45 °C for 36 h prior to morphological characterization. Biometric traits of pustules and uredospores were assessed using light microscopy at 10×, 40× and 60× magnification. For pustules, quantitative parameters included the lesion length (mm), while qualitative traits included the lesion shape and position on the leaf. For uredospores, quantitative measurements included the apical wall thickness (µm) and diameter (µm), and qualitative features included the spore shape, apical wall morphology, and color [17,48]. Additionally, the presence of paraphyses, as well as teliospores, was recorded.
2.5. Molecular Identification and Phylogenetic Analysis of Orange Rust
A total of 14 samples were collected by scrapings leaves showing symptoms of orange rust with visible uredospores. These samples were obtained from the identified sugarcane cultivars in which the presence of the pathogen was detected and used for DNA extraction. Genomic DNA was extracted using the CTAB (cetyl trimethyl ammonium bromide) method. A ribosomal DNA (rDNA) locus (527 bp) that included the Internal Transcribed Spacer (ITS) 1 and ITS2 (ITS1/2) regions was targeted for DNA amplifications using the specific primers Pk1F (5′ AAGAGAGTGCACTTAATTGTGGCTC 3′) and Pk1R (5′ CAGGTAACACACCTTCCTTGATGTG 3′), as previously described by Glynn et al. [41]. Amplicons were purified and sequenced in the forward direction using an ABI Prism 3730 automated DNA sequencer (PE Applied Biosystems, Foster city, CA, USA), following standard protocols.
The chromatograms from each amplicon were examined utilizing FinchTV 1.4.0 (https://digitalworldbiology.com/FinchTV, accessed on 23 July 2024). Low-quality bases at the 5′ and 3′ ends (Quality score ≤ Q25) were trimmed from individual sequences. The resulting curated sequences were aligned to detect variations and generate a consensus sequence. The consensus sequence was subsequently compared against the GenBank database using BLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 29 May 2023) to confirm its identity.
The phylogenetic relationships of the ITS1/2 haplotype found was constructed using P. kuehnii (W. Krüger) E.J. Butler sequences from different geographical areas, sourced from GenBank and the Barcode of Life Data System (BOLD Systems—https://www.boldsystems.org/, accessed on 18 May 2023) [49]. Sequences were aligned with the MAFFT version 7.526 tool [50], using the L-INS-i method, and refined with trimAl v.1.2 using automated cut-off parameters (-automated1) [51]. The evolutionary model for the nucleotide alignment matrix was calculated using MEGA v 11 [52], based on the Akaike Information Criterion (AIC). Phylogenetic analyses were conducted using the maximum likelihood (ML) method with IQ-TREE [39], estimating 1000 bootstrap replicates using the HKY+F model. The consensus tree topology was visualized in FigTree v 1.4.4 and edited with Adobe Illustrator v 2.8.0.27. Puccinia melanocephala (GenBank accession: FJ009328), the causal agent of brown rust in sugarcane, was included as the outgroup to root the tree, following its use in previous phylogenetic studies of sugarcane rust pathogens [39,43].
2.6. Statistical Analysis
Compliance with the assumptions of normality and homoscedasticity was verified for the quantitative variables related to the morphological characterization of the cultivars, including the stem length (cm), stem diameter (cm), internode length (mm), and leaf length (cm) and width (cm). These variables were analyzed using analysis of variance (ANOVA), followed by the Duncan mean comparison test (p ˂ 0.05), using the statistical packages “stats” and “agricolae” (https://CRAN.R-project.org/package=agricolae, accessed on 22 October 2023).
In contrast, for the disease-related variables, including the disease severity (S, %), type of pustule (TP), and damage index (DI), and for the morphological traits of the causal agent (lesion size (mm), length (μm), thickness of the apical wall (μm), and diameter of the uredospore (μm)), the data did not meet the parametric assumptions. Therefore, these were analyzed using the Kruskal–Wallis test, followed by Dunn’s multiple comparisons test with Bonferroni correction (p ˂ 0.05), using the “stats” package R Core Team.
All analyses were performed using R statistical software version 4.0.4 and RStudio version 1.3.1.
3. Results
3.1. Identification and Characterization of Sugarcane Cultivars Grown in the Region
We identified five sugarcane cultivars that were grown by farmers in the region, based on the morphological characterization of the plant material that we found in the 51 assessed fields. These included the well-known cultivar CP 57-603; two cultivars traditionally known in the region as Regional Without Fuzz (Tradicional Sin Pelusa in Spanish) and Hairy Purple (Morada Peluzosa in Spanish); and two genotypes that we refer to as Cultivar-1 and Cultivar-2, which are used by farmers but known by different local names, and whose origin remains unidentified. Regional Without Fuzz and CP 57-603 were the most predominant cultivars, found on 74.5% (38 of 51) and 49.2% (25 of 51) of the farms, respectively. Cultivar-1 (11.8%; 6 of 51 farms), Cultivar-2 (7.8%; 4 of 51 farms), and Hairy Purple (1.96%; 1 of 51 farms) were less commonly grown by farmers.
The morphological characterization of the genotypes revealed distinct features that allowed for their distinction (Table 1 and Figure 2). Regarding qualitative characteristics, all cultivars shared an erect stem growth habit and a cylindrical internode shape. However, CP 57-603 and Cultivar-1 exhibited a smooth zig-zag stem shape, superficial bud channel, and erect leaf positions. In contrast, Regional Without Fuzz, Hairy Purple, and Cultivar-2 presented an erect stem shape, absent bud channel, and arched leaf positions. The fluff contents in the leaf sheath also varied, ranging from little or absent in CP 57-603 to abundant in Hairy Purple and Cultivar-2, and absent in R. Without Fuzz. The defoliation pattern was generally easy across cultivars, except for CP 57-603, which exhibited regular defoliation. The internode colors differed across cultivars, with CP 57-603 presenting a yellow-purple coloration, while Regional Without Fuzz and Cultivar-2 displayed a greenish-yellow. Hairy Purple was characterized by a yellowish-purple internode color, and Cultivar-1 exhibited green-yellow internodes. The leaf sheath color was predominantly green-yellow, except for Hairy Purple and Cultivar-1, which displayed green with purple veins.

Table 1.
Qualitative morphological characteristics of sugarcane cultivars present in the northwestern Colombian Amazon.
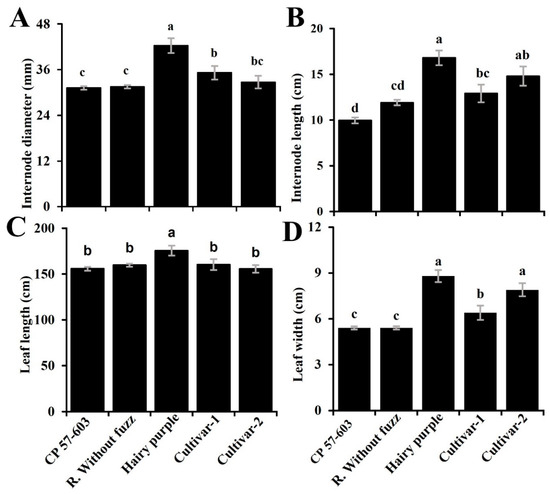
Figure 2.
Quantitative morphological characteristics of sugarcane cultivars present in the northwestern Colombian Amazon. (A) Internode diameter (mm), (B) internode length (cm), (C) leaf length (cm), and (D) leaf width (cm). Means were compared using Duncan’s test, and values with a common letter are not significantly different (p > 0.05).
The quantitative morphological characteristics also varied significantly among cultivars (Figure 2). Hairy Purple consistently exhibited the largest morphological traits. CP 57-603 and Regional Without Fuzz showed similar morphological patterns across multiple characteristics, indicating potential commonalities in their growth habits. The largest internode diameter and length were observed in Hairy Purple, which differed notably from CP 57-603 and Regional Without Fuzz. In terms of leaf size, Hairy Purple also exhibited the longest leaves (175.6 ± 11.9 cm), while the other four cultivars presented similar shorter leaf lengths (157.9 ± 3.4 cm). The leaf width also varied, with Hairy Purple having the widest leaves (8.8 ± 0.9 cm), while CP 57-603 and Regional Without Fuzz had the narrowest leaves (5.4 ± 0.9 cm and 5.4 ± 1.0 cm, respectively). The stem length presented similar characteristics among varieties, with an average value of 187.3 ± 13.9 cm.
Interestingly, Regional Without Fuzz displayed several morphological traits that resemble those reported for RD 75-11, a cultivar that was previously introduced in Colombia for smallholder panela production and whose cultivation has progressively declined due to increasing susceptibility to rust diseases [5,53,54,55]. However, due to the absence of molecular data and the high degree of morphological similarity among RD cultivars, we were unable to confirm its identity with certainty. Therefore, we opted to refer to it by its locally recognized name.
3.2. Incidence of Orange Rust in Predominant Sugarcane Cultivars
Orange rust symptoms were observed in the most widely grown cultivars (CP 57-603, Regional Without Fuzz, and Cultivar-1), particularly on the abaxial side of the leaf blade, where small orange pustules were visible (Figure 3A,B). Specifically, orange rust symptoms were recorded in 18 out of 25 farms growing CP 57-603, in 25 out of 38 farms growing Regional Without Fuzz, and in 3 out of 6 farms growing Cultivar-1 (Figure 4A). When analyzing the disease incidence, we found that CP 57-603 and Regional Without Fuzz exhibited infection levels exceeding 70% of the analyzed plants, while Cultivar-1 showed a lower disease incidence of less than 25% (Figure 4B).
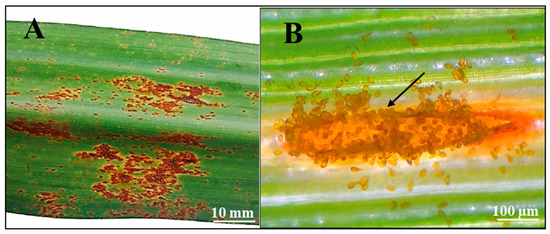
Figure 3.
Representative images of the symptoms of P. kuehnii (A) on the abaxial part of the leaf blade and (B) open pustule releasing spores (arrow). The images were taken from infected leaves of the Regional Without Fuzz cultivar.
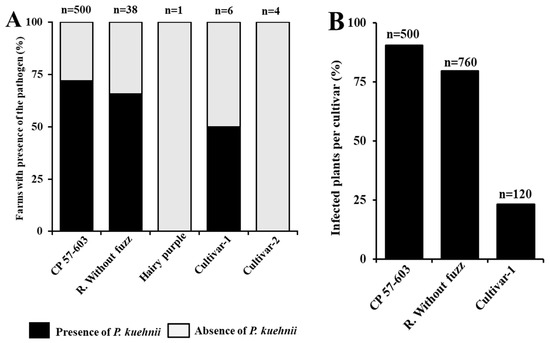
Figure 4.
(A) Percentages of farms where P. kuehnii was detected, grouped by cultivar. (B) Percentage of infected plants within each cultivar.
In terms of disease severity (S), we observed significantly higher values in Regional Without Fuzz and CP 57-603 than in Cultivar-1. However, no significant differences were detected regarding the type of pustule (TP) among the cultivars (Table 2). Regarding the damage index (DI), all three infected cultivars showed values exceeding 512, indicating that all cultivars are susceptible according to the scale proposed by Victoria et al. [37]. However, it is worth noting that the DI varied widely across cultivars, with Cultivar-1 presenting the lowest maximum values.

Table 2.
Mean values of severity (%), type of pustule, and damage index of the cultivars.
3.3. Results of the Morphological Characterization of Orange Rust
Since orange rust was present in most of the cultivars grown in the region (CP 57-603, Regional Without Fuzz, and Cultivar-1), we investigated its morphological features. Despite some variability in lesion size (1.0 to 8.9 mm) and pustule length (10 to 25 µm), there were no significant differences in the mean values across cultivars (Table 3). Similarly, the uredospore wall thickness and diameter ranged from 0.5 to 2.5 µm and 7 to 15 µm, respectively, but these differences were not statistically significant.

Table 3.
Quantitative morphological variables of the pustules and uredispores of P. kuehnii with respect to the host cultivar.
In terms of qualitative features, we observed variation in pustule shapes among the cultivars (Figure 5). The shape of the uredospores was entirely obovoid in R. Without Fuzz and Cultivar-1 (Figure 6C), while in CP 57-603, 89% were obovoid and 11% were obpyriform (Figure 6C and 6B, respectively). This morphological variation in uredospores could be attributed to differences in the developmental stages of the spores, as they can vary in shape and size depending on their maturity [48]. In all cases, the apical wall shape of the uredospores was coarse (Figure 6D (arrow)). The presence of paraphyses and teliospores was not observed.
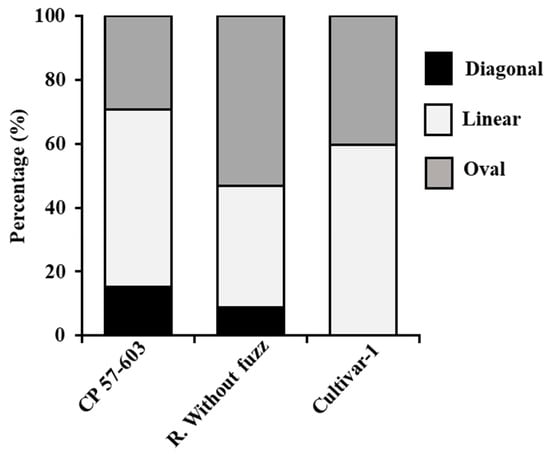
Figure 5.
Shapes of pustules of P. kuehnii by cultivar.
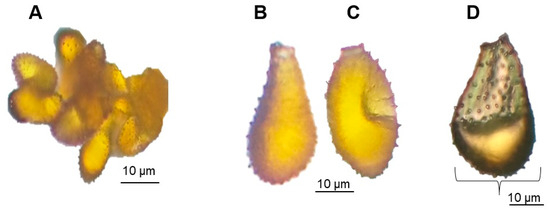
Figure 6.
(A) Uredospore population, (B) obpyriform uredospore form, (C) obovoid uredospore form, and (D) morphology of the apical wall of the thick uredospore (curly bracket).
3.4. Molecular Identification of Orange Rust
To confirm the identify of P. kuehnii uredospore, molecular analyses were conducted using DNA sequences of the rDNA locus ITS1/2. A total of 14 samples from the three cultivars (CP 57-603, Regional Without Fuzz, and Cultivar-1) where P. kuehnii was identified were used for amplification and sequencing analysis. The targeted locus covered a 474 pb region. The sequencing results revealed that all samples shared the same haplotype, with no sequence variation observed the among the analyzed isolates, indicating 100% identity. This haplotype has been uploaded to GenBank with the access code PQ452303.
To explore the genetic similarity between the identified P. kuehnii haplotype and others reported globally, we conducted a phylogenetic analysis based on ITS1/2 sequences retrieved from GenBank and BOLD Systems. The resulting phylogenetic tree topology revealed five main clades, with the P. kuehnii from Caquetá, Colombia, clustering within clade 2 (Figure 7), alongside sequences from Brazil, the United Sates, and Cuba. Clade 2 also includes isolates from other regions such as Central America, Asia, Oceania, and Africa.
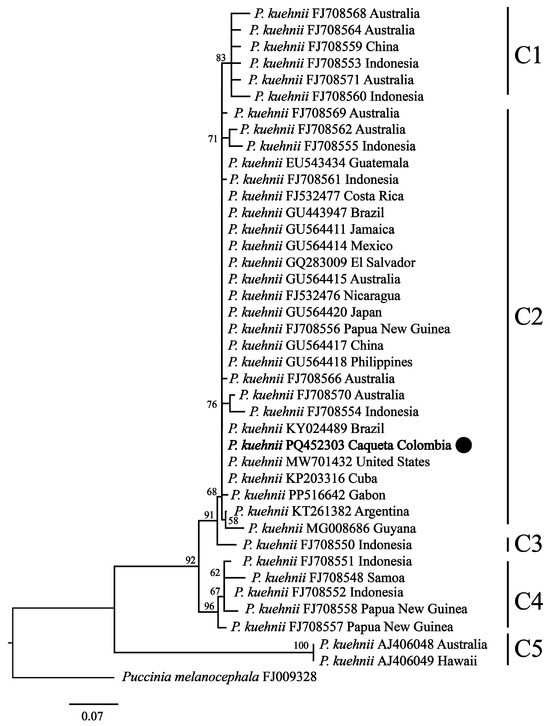
Figure 7.
Maximum likelihood phylogenetic tree based on partial sequences of the ITS1/2 region of Puccinia kuehnii (orange rust). Bootstrap support values are indicated at each node (bootstrap < 50% are not shown). The black circle represents the sequence from this study. The tree was rooted using Puccinia melanocephala as the outgroup. Sequences are labeled by species names, followed by the GenBank accession number and the country of origin. The scale bar represents 0.07 substitutions per site.
4. Discussion
Our study focused on the identification and characterization of sugarcane cultivars grown in the northwestern Colombian Amazon and their susceptibility to orange rust caused by P. kuehnii. We observed a significant presence of orange rust in the most widely grown cultivars (CP 57-603, Regional Without Fuzz, and Cultivar-1) in the region.
This research provides valuable insights into sugarcane cultivation in the northwestern Colombian Amazon, a region where sugarcane is traditionally grown and is gaining popularity among farmers, particularly for panela production. However, the introduction of sugarcane into this region has largely occurred without formal planning or systemic evaluation [29]. In the study area, we found a predominance of the cultivar CP 57-603 and the traditionally known Regional Without Fuzz, which displays certain morphological traits that are reminiscent of RD 75-11, although we could not confirm its identity. Additionally, we identified three other genotypes that could not be matched to any well-known genotypes described by breeding programs. Notably, two of these genotypes are known by different names among local farmers, suggesting that their introduction may have occurred informally.
We hypothesize that the cultivars that are currently grown in the region were likely introduced from neighboring departments such as Huila and Tolima, which have a stronger tradition of sugarcane cultivation. Farmers in the northwestern Amazon may have initially acquired these cultivars from sellers in those regions, facilitating their spread. As a colonizer crop, sugarcane may also have been brought into the region by migrating farmers who were familiar with these cultivars from their areas of origin. The lack of systematic planning in sugarcane cultivation has likely contributed to the confusion between genotypes and a loss of identity, exacerbated by the use of regionalisms or nicknames.
While these genotypes have become widespread in the region, probably due to shared morphological traits, historical use, and suitability for local environmental conditions, the reliance on a few predominant cultivars presents challenges, particularly in terms of disease susceptibility. Indeed, our study confirms a high prevalence of orange rust in the region, especially affecting CP 57-603 and Regional Without Fuzz. The environmental conditions in the northwestern Colombian Amazon, such as high humidity, significant rainfall, and drastic temperature fluctuations, create an ideal environment for Puccinia kuehnii to thrive. This is consistent with findings from other tropical regions, where similar environmental conditions have been associated with increased rust severity [35,56].
The molecular analysis of the ITS1/2 region revealed a single haplotype among the analyzed P. kuehnii isolates, aligning with previous studies reporting low genetic variability at this locus across geographically populations [39,43,48,57]. This lack of variation may reflect the highly conserved nature of the ITS region, which represents only a small portion of the genome and may not be sufficiently resolutive to resolve intraspecific genetic differences. Additionally, the low genetic recombination observed in the pathogen could contribute to this homogeneity [43,48].
Our phylogenetic analysis, based on ITS1/2 sequences, grouped isolates into five main clades. The haplotype identified in this study clustered within clade 2 (C2), alongside ones isolated from the Americas, Asia, Oceania, and Africa. While the presence of isolates from diverse regions within a single clade may suggest long-distance dispersal patterns, such inferences should be made with caution given the limited resolution of single-locus analyses. The ITS-based phylogeny serves here primarily as a comparative framework for situating the Colombian haplotype within the global diversity reported for P. kuehnii, rather than as a basis for inferring evolutionary relationships or pathogen migration routes. To better understand the genetic structure and evolutionary history of P. kuehnii, future studies should incorporate multiple molecular markers or whole-genome sequencing approaches. Such strategies would offer improved resolution and enable the detection of fine-scale genetic differentiation. Furthermore, integrating high-resolution environmental data could enhance our understanding of the ecological and epidemiological drivers influencing the pathogen distribution and disease severity within the northwestern Colombian Amazon.
Interestingly, Hairy Purple and Cultivar-2 exhibited no infection, and Cultivar-1 showed a lower disease incidence, which may suggest some degree of resistance. However, caution is required when interpreting these results, as these cultivars were the least commonly grown in the region, leading to smaller sample sizes. The limited number of plants that were assessed could have influenced the observed disease incidence, and it is possible that the absence or lower incidence of infection may result from factors unrelated to inherent resistance, such as better management practices or simply the absence of orange rust in the specific crops sampled. Field characterization alone cannot confirm resistance, and controlled studies with larger and more evenly distributed sample sizes are necessary to gain clearer insights into the potential resistance of these cultivars.
The significant susceptibility of CP 57-603 and Regional Without Fuzz to orange rust underscores the need for greater cultivar diversification in the region. Relying on only a few predominant cultivars increases the vulnerability of sugarcane production to disease outbreaks. An integrated disease management approach, including crop rotation, early planting dates, weed removal within the crop, seed treatment and balanced nutrition with potassium contents, and the introduction of more resistant cultivars, could help mitigate the impact of rust on the crop [35,58,59]. Adjusting planting schedules might also influence disease dynamics, contributing to more effective management strategies. Currently, the Universidad de la Amazonia in Colombia is developing a program to evaluate new sugarcane cultivars produced by Cenicaña-Colombia [60]. These cultivars are being assessed both agronomically and phytosanitarily for their potential use in the northwestern Amazon region. This initiative aims to address the current issues of disease susceptibility and reduce the reliance on local cultivars, offering farmers more resilient and sustainable options for sugarcane production.
5. Conclusions
Our study highlights the high susceptibility of the predominant sugarcane cultivars, CP 57-603 and Regional Without Fuzz, to orange rust in the northwestern Colombian Amazon. This heightened susceptibility increases the region’s vulnerability to disease outbreaks and emphasizes the need for greater cultivar diversification and improved disease management strategies. The molecular analysis of the ITS1/2 region revealed the presence of a single haplotype across samples, suggesting low genetic diversity at this locus within the orange rust population in the region. However, future research should employ more sensitive techniques to better assess the orange rust’s genetic diversity.
To mitigate orange rust’s impact, we recommend a systematic approach to introducing and evaluating new sugarcane cultivars. Comprehensive agronomic and phytosanitary assessments are crucial to ensure that the selected cultivars are resilient to local conditions and diseases. Furthermore, similar studies should be conducted in other regions within the region, as local environmental conditions and disease dynamics may differ. Expanding this research will provide a broader understanding of cultivar performance and pathogen diversity, contributing to a sustainable sugarcane production system across the region.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15071695/s1: Table S1. Information on sugarcane-producing plantations; Figure S1. Morphological characteristics of sugarcane: (A) stem growth habits; (B) stem shape; (C) leaf position; (D) internode color; (E) leaf sheath color. Source: Adapted from Victoria et al. 2013 [44] for this research.
Author Contributions
Conceptualization, S.R.-V., D.A.J.-C., and F.A.O.-M.; Methodology, S.R.-V., D.A.J.-C., and F.A.O.-M.; Software, S.R.-V., D.A.T.-A., D.A.J.-C., and F.A.O.-M.; Validation, S.R.-V., D.A.T.-A., D.A.J.-C., and F.A.O.-M.; Formal analysis, S.R.-V., J.C.Á.-S., and F.A.O.-M.; Investigation, S.R.-V., D.A.T.-A., G.R.-V., and D.A.J.-C.; Data curation, S.R.-V., D.A.T.-A., G.R.-V., D.A.J.-C., and F.A.O.-M.; Writing—original draft, S.R.-V., and F.A.O.-M.; Writing—review and editing, S.R.-V., J.C.Á.-S., D.A.T.-A., D.A.J.-C., and F.A.O.-M.; Visualization, J.C.Á.-S., and D.A.T.-A.; Supervision, J.C.Á.-S., D.A.J.-C., and F.A.O.-M.; Project administration, S.R.-V., and D.A.J.-C.; Funding acquisition, S.R.-V., D.A.J.-C., and F.A.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was developed with resources from the Research Project “Optimization of the production system in the sugarcane sector in the department of Caquetá”/BPIN 2020000100034, executed by the Universidad de la Amazonia (Colombia) with resources from the Science, Technology and Innovation Fund of the Sistema General de Regalias—SGR. D.A.J.-C. also acknowledges the scholarship support provided by the Universidad de la Amazonia.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Wu, Q.; Li, A.; Zhao, P.; Xia, H.; Zhang, Y.; Que, Y. Theory to Practice: A Success in Breeding Sugarcane Variety YZ08–1609 Known as the King of Sugar. Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R. Resources Management for Sustainable Sugarcane Production. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 647–693. ISBN 978-981-15-6953-1. [Google Scholar]
- Cursi, D.; Castillo, R.; Tarumoto, Y.; Umeda, M.; Tippayawat, A.; Ponragdee, W.; Racedo, J.; Perera, M.; Hoffmann, H.; Carneiro, M. Origin, Genetic Diversity, Conservation, and Traditional and Molecular Breeding Approaches in Sugarcane. In Cash Crops: Genetic Diversity, Erosion, Conservation and Utilization; Springer: Cham, Switzerland, 2022; pp. 83–116. ISBN 978-3-030-74925-5. [Google Scholar]
- Sandu, H.; Gilbert, R.; Shine, J.; Rice, R.; Odero, D.C. Maturity Curves and Harvest Schedule Recommendations for CP Sugarcane Varieties. Ask IFAS-Powered EDIS 2004. [Google Scholar] [CrossRef]
- Deantonio-Florido, L.; Ramírez Durán, J.; López González, X.; Lesmes Suárez, J.C. Capítulo 1. Avances Conceptuales Sobre Semilla de Caña de Azúcar Para Panela. In Avances de Investigación Para la Agroindustria Panelera; Agrosavia: Mosquera, Colombia, 2024; pp. 57–85. ISBN 978-958-740-750-1. [Google Scholar]
- Insuasty Burbano, O.; Manrique, R.; Palacio, O. Catálogo de Variedades de Caña Para La Producción de Panela En La Hoya Del Río Suárez; Corpoica: Bogotá, Colombia, 2003. [Google Scholar]
- Murcia-Pardo, M. Manejo Agronómico de La Caña de Azúcar Para Panela En Énfasis En Fertilización; Federación Nacional de Productores de Panela: Bogotá, Colombia, 2022; ISBN 978-958-99218-8-3. [Google Scholar]
- Viswanathan, R. Degeneration in Sugarcane Varieties: Does the Sugar Industry Realize It? Sugar Tech. 2024, 26, 1501–1504. [Google Scholar] [CrossRef]
- Rincón, E.; Saavedra-Díaz, C.; Ángel, J.; Garcés, F.; Riascos, J.; Aguilar, F. Finding Novel Sources of Resistance to Orange Rust and Brown Rust in the Colombian Sugarcane Breeding Program. Proc. Int. Soc. Sugar Cane Technol. 2023, 31, 907–916. [Google Scholar]
- Ramírez Durán, J.; Insuasty Burbano, O.; Viveros Valens, C.A. Comportamiento agroindustrial de diez variedades de caña de azúcar para producción de panela en Santander, Colombia. Corpoica Cienc. Tecnol. Agropecu. 2014, 15, 183–195. [Google Scholar] [CrossRef]
- Correr, F.H.; Hosaka, G.K.; Gómez, S.G.P.; Cia, M.C.; Vitorello, C.B.M.; Camargo, L.E.A.; Massola, N.S.; Carneiro, M.S.; Margarido, G.R.A. Time-Series Expression Profiling of Sugarcane Leaves Infected with Puccinia Kuehnii Reveals an Ineffective Defense System Leading to Susceptibility. Plant Cell Rep. 2020, 39, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, Z.; Xu, F.; Pan, Y.B.; Grisham, M.P.; Xu, L. Sugarcane Mosaic Disease: Characteristics, Identification and Control. Microorganisms 2021, 9, 1984. [Google Scholar] [CrossRef]
- Maurya, S.K.; Vandana, P.; Kumar, S.; Singh, V.; Kumar, S.; Singh, D. Screening of Varieties Resistant to Sugarcane Smut Disease Caused by Sporosorium scitamineum under Sub-Tropical India. J. Exp. Agric. Int. 2024, 46, 131–139. [Google Scholar] [CrossRef]
- Thilagavathi, K.; Kavitha, K.; Praba, R.D.; Arina, S.V.; Sahana, R.C. Detection of Diseases in Sugarcane Using Image Processing Techniques Classification. Biosci. Biotechnol. Res. Commun. 2020, 13, 109–115. [Google Scholar] [CrossRef]
- Viswanathan, R. Fusarium Diseases Affecting Sugarcane Production in India. Indian Phytopathol. 2020, 73, 415–424. [Google Scholar] [CrossRef]
- Dijoux, J.; Dumont, T.; Paysan, M.; Legrand, C.; Hervouet, C.; Barau, L.; Rott, P.; Hoarau, J.-Y. Effects of Orange Rust on Sugarcane Yield Traits in a Multi-Environment Breeding Program. Euphytica 2023, 219, 49. [Google Scholar] [CrossRef]
- Martin, L.A.; Lloyd Evans, D.; Castlebury, L.A.; Sifundza, J.T.; Comstock, J.C.; Rutherford, R.S.; McFarlane, S.A. Macruropyxis fulva Sp. Nov., a New Rust (Pucciniales) Infecting Sugarcane in Southern Africa. Australas. Plant Pathol. 2017, 46, 63–74. [Google Scholar] [CrossRef]
- Rott, P.; Sood, S.; Comstock, J.C.; Raid, R.N.; Glynn, N.C.; Gilbert, R.A.; Sandhu, H.S. Sugarcane Orange Rust: SS-AGR-378/SC099, 6/2014. UF/IFAS Ext. Univ. Fla. 2014. [Google Scholar] [CrossRef]
- Araújo, K.L.; Canteri, M.G.; Gilio, T.A.S.; Neubauer, R.A.; Sanches, P.B.; Sumida, C.H.; Giglioti, É.A. Resistência genotípica e monitoramento da favorabilidade para ocorrência da ferrugem alaranjada da cana-de-açúcar. Summa Phytopathol. 2013, 39, 271–274. [Google Scholar] [CrossRef][Green Version]
- Comstock, J.C.; Glynn, N.C.; Davidson, R.W. Sugarcane Rusts in Florida. Proc. Int. Soc. Sugar Cane Technol. 2010, 27, 1–9. [Google Scholar][Green Version]
- Magarey, R.C. Orange Rust Disease of Sugracane. In Proceedings of the International Society of Sugar Cane Technologists: Proceedings of the XXVIIth Congress, Veracruz, Mexico, 7–11 March 2010; Volume 27, p. 9. [Google Scholar]
- CABI Puccinia kuehnii (Orange Rust). Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.45818 (accessed on 19 March 2025).
- Aday Díaz, O.d.l.C.; Pérez Vicente, L.; Oloriz Ortega, M.I.; Rodríguez Lema, E.L.; Montalván Delgado, J.; Puchades Izaguirre, Y.; Delgado Padrón, J.; Martínez de la Parte, E. Vigilancia Fitosanitaria y Caracterización de La Enfermedad Roya Naranja de La Caña de Azúcar En Cuba. An. Acad. Cienc. Cuba 2022, 12, 2304–2308. Available online: http://ref.scielo.org/ppmmrb (accessed on 17 March 2025).[Green Version]
- Barbasso, D.; Jordão, H.; Maccheroni, W.; Boldini, J.; Bressiani, J.; Sanguino, A. First Report of Puccinia kuehnii, Causal Agent of Orange Rust of Sugarcane, in Brazil. Plant Dis. 2010, 94, 1170. [Google Scholar] [CrossRef]
- Chavarría, E.; Subirós, F.; Vega, J.; Ralda, G.; Glynn, N.C.; Comstock, J.C.; Castlebury, L.A. First Report of Orange Rust of Sugarcane Caused by Puccinia kuehnii in Costa Rica and Nicaragua. Plant Dis. 2009, 93, 425. [Google Scholar] [CrossRef]
- Flores, R.C.; Loyo, J.R.; Ojeda, R.A.; Rangel, O.C.A.; Cerón, F.A.; Márquez, W.; Guerra-Moreno, A.S.; Hernandez-Ibarra, H.M.; González, R.E.; Castlebury, L.A.; et al. First Report of Orange Rust of Sugarcane Caused by Puccinia Kuehnii in Mexico, El Salvador, and Panama. Plant Dis. 2009, 93, 1347. [Google Scholar] [CrossRef]
- Ovalle, W.; Comstock, J.C.; Glynn, N.C.; Castlebury, L.A. First Report of Puccinia kuehnii, Causal Agent of Orange Rust of Sugarcane, in Guatemala. Plant Dis. 2008, 92, 973. [Google Scholar] [CrossRef]
- Cadavid, M.; Ángel, J.C.; Victoria, J.I. First Report of Orange Rust of Sugarcane Caused by Puccinia kuehnii in Colombia. Plant Dis. 2012, 96, 143. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Carvajal, D.A.; Sánchez-Avilés, A.M.; Hernández-Núñez, H.E.; Gutiérrez-García, G.A.; Rojas-Vargas, S.; Hembuz-Falla, G.D.; Salamanca-Falla, C.H.; Ortiz-Morea, F.A. Role of Sugarcane Cultivation for Panela Production in the Livelihood Strategies of Peasant Families in the Colombian Amazon. Hum. Ecol. 2024, 52, 409–423. [Google Scholar] [CrossRef]
- Agronet Área, Producción y Rendimiento Nacional Por Cultivo. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=1 (accessed on 17 March 2025).
- Agronet Participación Departamental En La Producción y En El Área Cosechada. Available online: https://www.agronet.gov.co/estadistica/Paginas/home.aspx?cod=2 (accessed on 27 March 2025).
- Rodriguez Borray, G.A.; Polo Murcia, S.M.; Riveros, M.Á.; Buitrago Ardila, A.M. La Agroindustria Panelera Impulsando El Desarrollo Rural En Colombia: Un Diagnóstico de La Cadena Productiva; Roffapint Editores S.A.S: Bogotá, Colombia, 2019; ISBN 978-958-99218-7-6. [Google Scholar]
- Zapata, C.A.; Morea, E.G.O.; Mora-Motta, D.A.; Ojeda, D.M.M.; Quiceno-Mayo, E.J.; Toro, D.A.; Ortiz-Morea, F.A. Characterization and Seasonal Dynamics of Tick Populations in Dairy Cattle Production Systems of Northwestern Colombian Amazon. Vet. Sci. 2024, 11, 244. [Google Scholar] [CrossRef]
- Barrera, W.; Hoy, J.; Li, B. Effects of Temperature and Moisture Variables on Brown Rust Epidemics in Sugarcane. J. Phytopathol. 2013, 161, 98–106. [Google Scholar] [CrossRef]
- Infante, D.; Martínez, B.; González, E.; González, N. Puccinia kuehnii (KRÜGER) BUTLER Y Puccinia Melanocephala H. SYDOW Y P. SYDOW. En El Cultivo de La Caña de Azúcar. Rev. Prot. Veg. 2009, 24, 22–28. Available online: http://ref.scielo.org/57dqtv (accessed on 19 March 2025).
- Sumida, C.H.; Fantin, L.H.; Gonçalves, R.M.; Canteri, M.G.; Araújo, K.L.; Giglioti, É.A. A System to Map the Risk of Infection by Puccinia kuehnii in Brazil. Acta Sci. Agron. 2018, 41, e39905. [Google Scholar] [CrossRef]
- Victoria, J.; Moreno, C.; Casslett, C. Genotype Environment Interaction and Its Effect on Sugarcane Rust Incidence. Sugar Cane 1990, 4, 13–17. [Google Scholar]
- Shrestha, A.; Thapa, B. Characterization and Evaluation of Sugarcane Genotypes. J. Genet. Genom. Plant Breed. 2021, 5, 53–62. [Google Scholar]
- Braithwaite, K.S.; Croft, B.J.; Magarey, R.C.; Scharaschkin, T. Phylogenetic Placement of the Sugarcane Orange Rust Pathogen Puccinia kuehnii in a Historical and Regional Context. Australas. Plant Pathol. 2009, 38, 380–388. [Google Scholar] [CrossRef]
- Dijoux, J.; Blondin, L.; Minko, H.A.S.; Raïvire, E.; Daugrois, J.H.; Girard, J.-C.; Hoarau, J.-Y.; Rott, P. Identification of Puccinia Kuehnii, the Causal Agent of Orange Rust of Sugarcane, in Gabon. Sugar Tech. 2024, 26, 1823–1826. [Google Scholar] [CrossRef]
- Glynn, N.C.; Dixon, L.J.; Castlebury, L.A.; Szabo, L.J.; Comstock, J.C. PCR Assays for the Sugarcane Rust Pathogens Puccinia kuehnii and P. melanocephala and Detection of a SNP Associated with Geographical Distribution in P. kuehnii. Plant Pathol. 2010, 59, 703–711. [Google Scholar] [CrossRef]
- McFarlane, S.A.; Kistan, C.J.; Naude, A.; Koch, A.C.; Rutherford, R.S. First Report of Orange Rust Caused by Puccinia kuehnii on Sugarcane in South Africa. Plant Dis. 2022, 107, 953. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Nogueira Junior, A.F.; Gonçalves, C.R.N.B.; Souza, N.A.; Bergamin Filho, A. Pathogenic and Molecular Comparison of Puccinia kuehnii Isolates and Reactions of Sugarcane Varieties to Orange Rust. Plant Pathol. 2018, 67, 1687–1696. [Google Scholar] [CrossRef]
- Victoria, J.I.; Salazar, F.; López, J.; Bustillo, A.E. Catálogo de Variedades de Caña de Azúcar. Catálogo de Variedades de Caña de Azúcar, 3rd ed.; Cenicaña: Cali, Colombia, 2013. [Google Scholar]
- Purdy, L.H.; Dean, J.L. A System for Recording Data about the Sugarcane Rust/Host Interactions. Sugarcane Pathol. Newsl. 1981, 27, 35–40. [Google Scholar]
- Ángel, J.C.; Cadavid, M.; Victoria, J.I. Presencia de La Roya Naranja (Puccinia kuehnii) En El Valle Del Río Cauca, Colombia. Sanid. Veg. 2010, 32, 24–29. [Google Scholar]
- Cenicaña Guía Visual Para La Evaluación Del Grado de Reacción. Available online: https://www.cenicana.org/wp-content/uploads/2024/02/Guia-Visual-para-la-evalucacion-V-25-01-2024.pdf (accessed on 5 June 2025).
- Perera, M.F.; Bertani, R.P.; Arias, M.E.; Hechavarria, M.d.l.L.L.; Zardon Navarro, M.d.l.A.; Debes, M.A.; Luque, A.C.; Cuenya, M.I.; Rojas, R.A.; Castagnaro, A.P. Morphological and Molecular Characterization of Puccinia kuehnii, the Causal Agent of Sugarcane Orange Rust, in Cub. Sci. Agric. 2019, 77, e20180038. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Cenicaña Paneleros Le Apuestan a Mejorar Su Productividad Con Variedades CC. Available online: https://www.cenicana.org/paneleros-le-apuestan-a-mejorar-su-productividad-con-variedades-cc/ (accessed on 6 June 2025).
- Rojas, J. La Técnología Del Cultivo de La Caña Panelera; Corpoica: Florencia, Colombia, 1999. [Google Scholar]
- Vargas Berdugo, A.M.; Deantonio-Florido, L.Y.; Jiménez Vargas, J.; del Carmen Barreto-Triana, N. Capítulo 4 Enfermedades Prevalentes En Variedades de Caña de Azúcar Para Panela y Su Relación Con Factores Abióticos En Boyacá y Santander. In Avances de Investigación Para la Agroindustria Panelera; Agrosavia: Mosquera, Colombia, 2024; pp. 137–167. ISBN 978-958-740-750-1. [Google Scholar]
- Delgado, J.M.; Terry, I.A.; Lema, E.R.; Sierra, I.P.; Galves, Y.F.; Cristina, I.; Varela, T. Evaluation of New Sugarcane Cultivars against Brown Rust, with the Use of Qualitative and Quantitative Scales. Cent. Agríc. 2020, 47, 13–21. [Google Scholar]
- Virtudazo, E.V.; Nakamura, H.; Kakishima, M. Phylogenetic Analysis of Sugarcane Rusts Based on Sequences of ITS, 5.8 S rDNA and D1/D2 Regions of LSU rDNA. J. Gen. Plant Pathol. 2001, 67, 28–36. [Google Scholar] [CrossRef]
- Díaz, O.A.; Mujica, F.D.; Tríada, E.L.M.; Vicente, L.P.; Terry, I.A.; Milián, J.P.; Melillo, J.B. Presencia de la roya naranja Puccinia kuehnii (krüger) Butler en áreas experimentales de Caña de Azúcar (Saccharum Spp. Híbrido) de la Región Central de Cuba. Fitosanidad 2010, 14, 83–89. Available online: http://ref.scielo.org/zktm6r (accessed on 19 March 2025).
- Morales, C.; Hamada, E.; Madariaga, H.L.; Rago, A.M. Impacto Del Cambio Climático Sobre La Enfermedad Roya Marrón de La Caña de Azúcar de Argentina. J. Hyperspectral Remote Sens. 2021, 12, 28–37. [Google Scholar] [CrossRef]
- Jimenez Carvajal, D.; Rojas Vargas, S.; Alexánder, J.; Arturo, C.; Valens, V.; Arbey, C.; Ortega, M. Evaluación Agroindustrial de Variedades de Caña de Azúcar (Saccharum Spp. L.) Para La Producción de Panela En Florencia, Caquetá; Cenicaña: Cali, Colombia, 2019; ISBN 978-958-99218-6-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).