Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum lycopersicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setting
2.2. Chemical Synthesis and Characterization of ZnO NPs
2.3. Estimation of Enzyme Activities
2.3.1. Extraction of the Enzymes
2.3.2. Estimation of Peroxidase Activity
2.3.3. Estimation of Glutathione Reductase’s Activity
2.3.4. Estimation of Glutathione-S-Transferase’s Activity
2.3.5. Estimation of Superoxide Dismutase’s Activity
2.3.6. Estimation of Catalase’s Activity
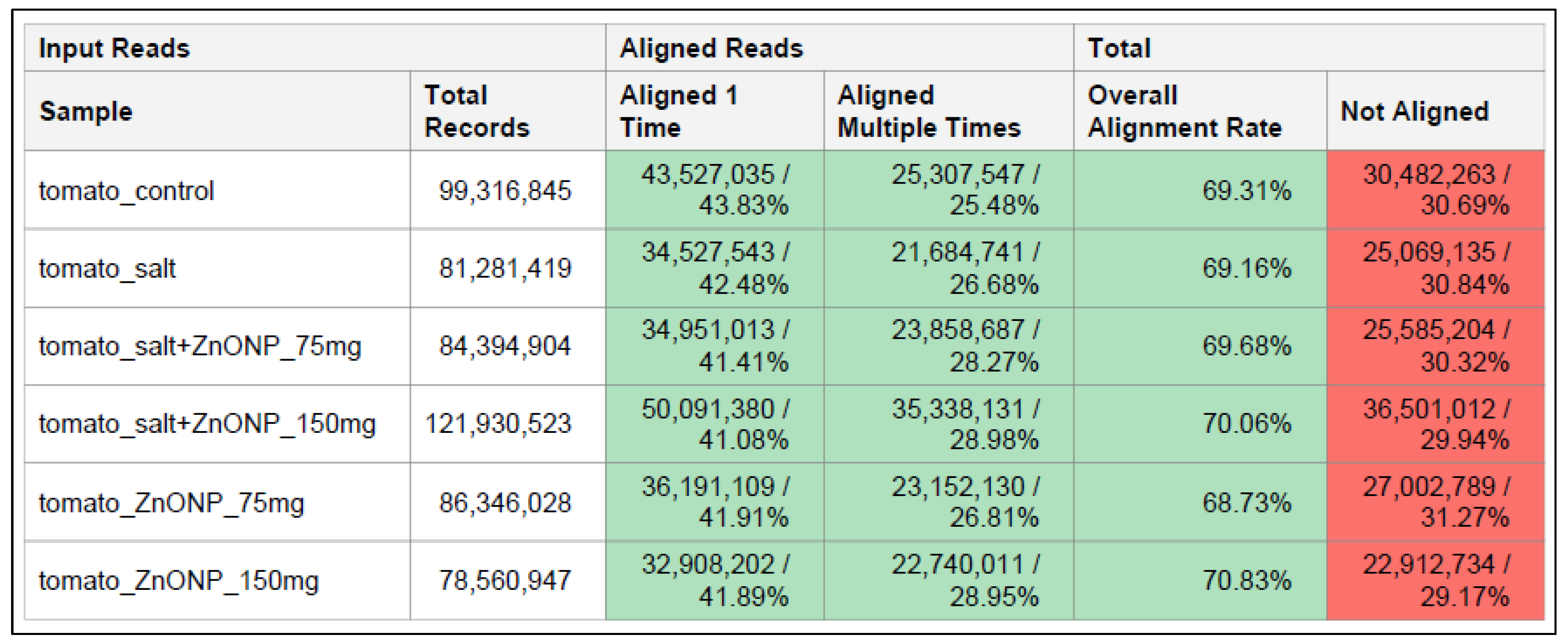
2.4. Transcriptomic Analysis
2.5. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of ZnO NPs
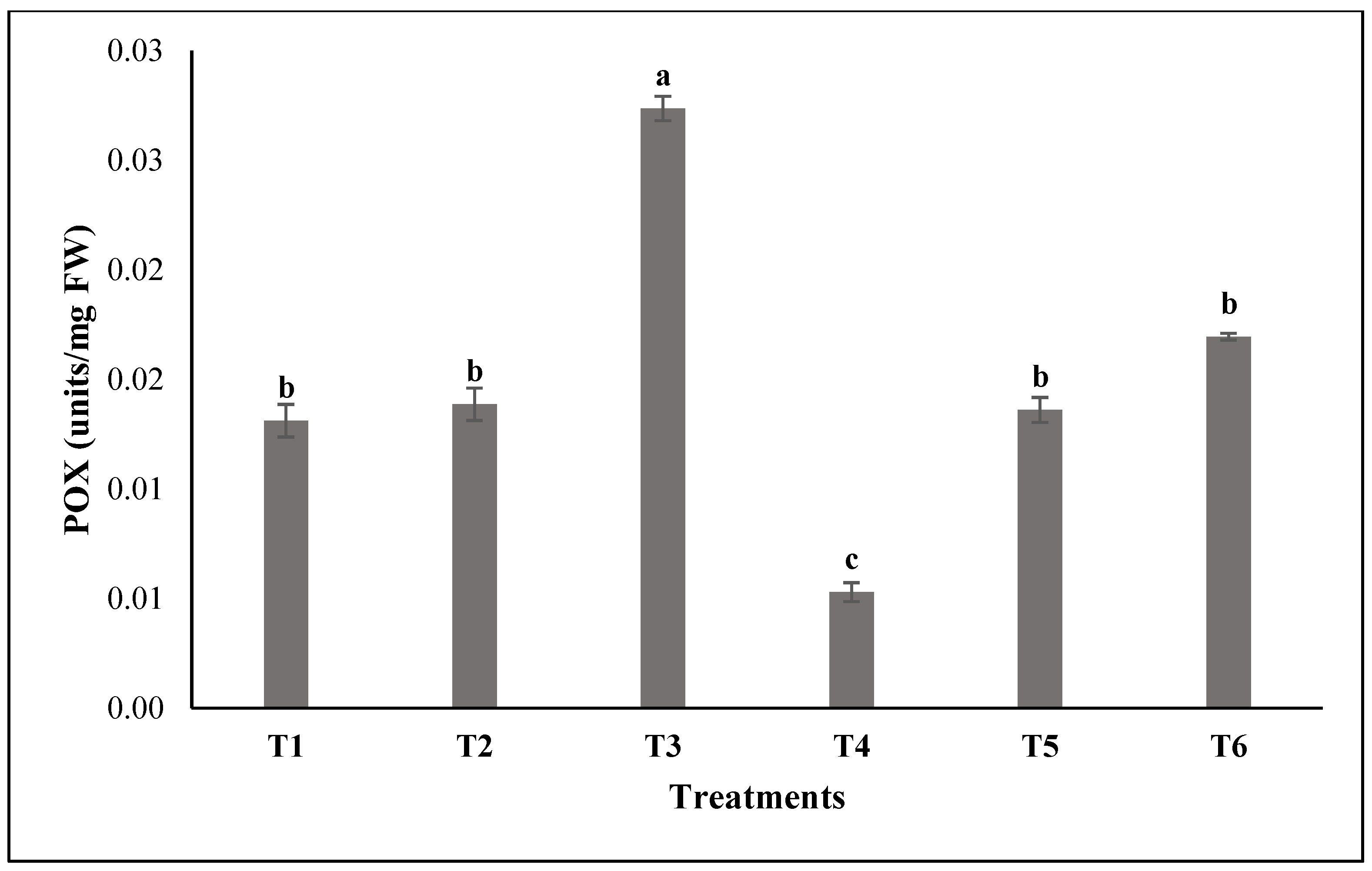
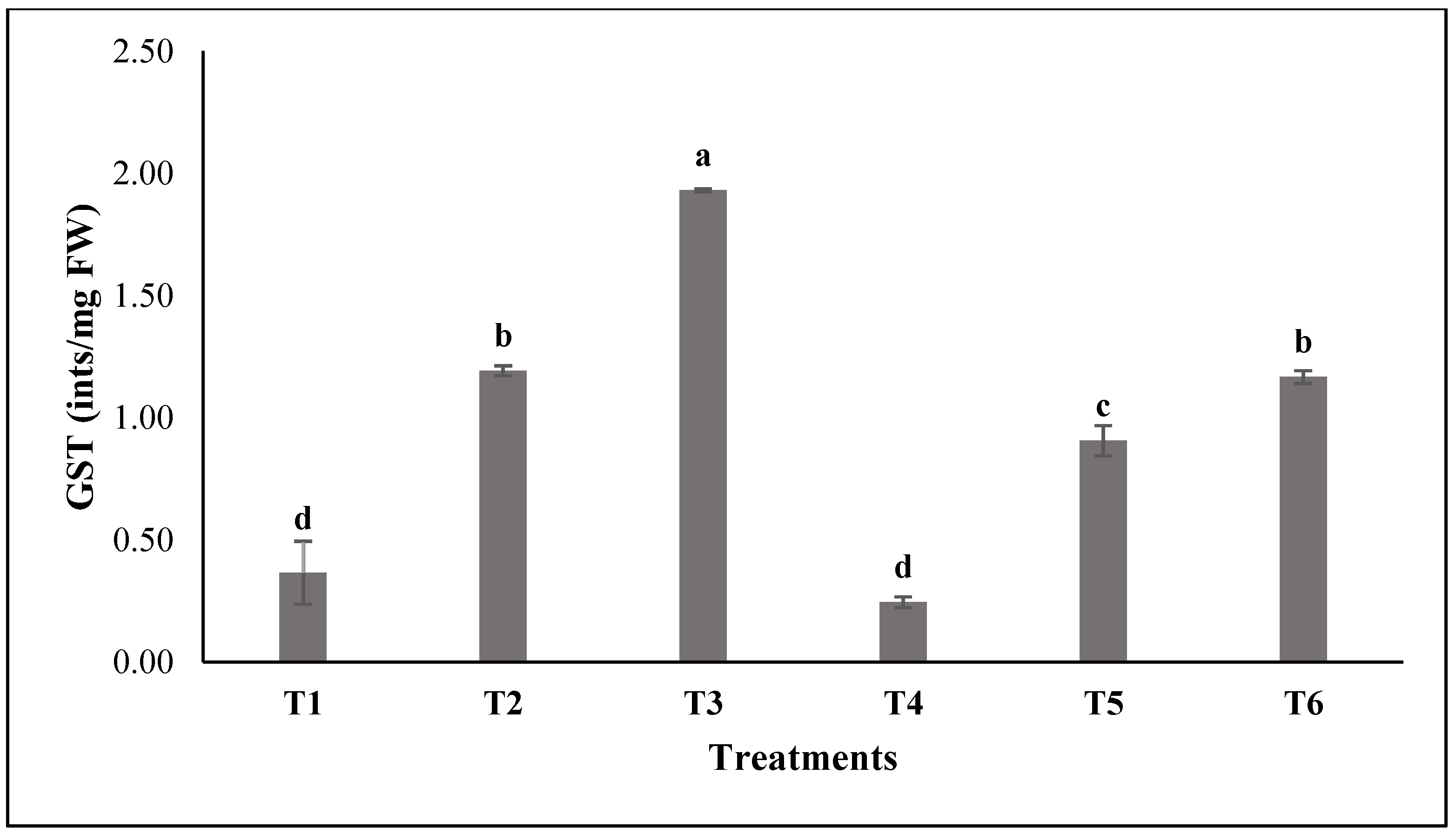
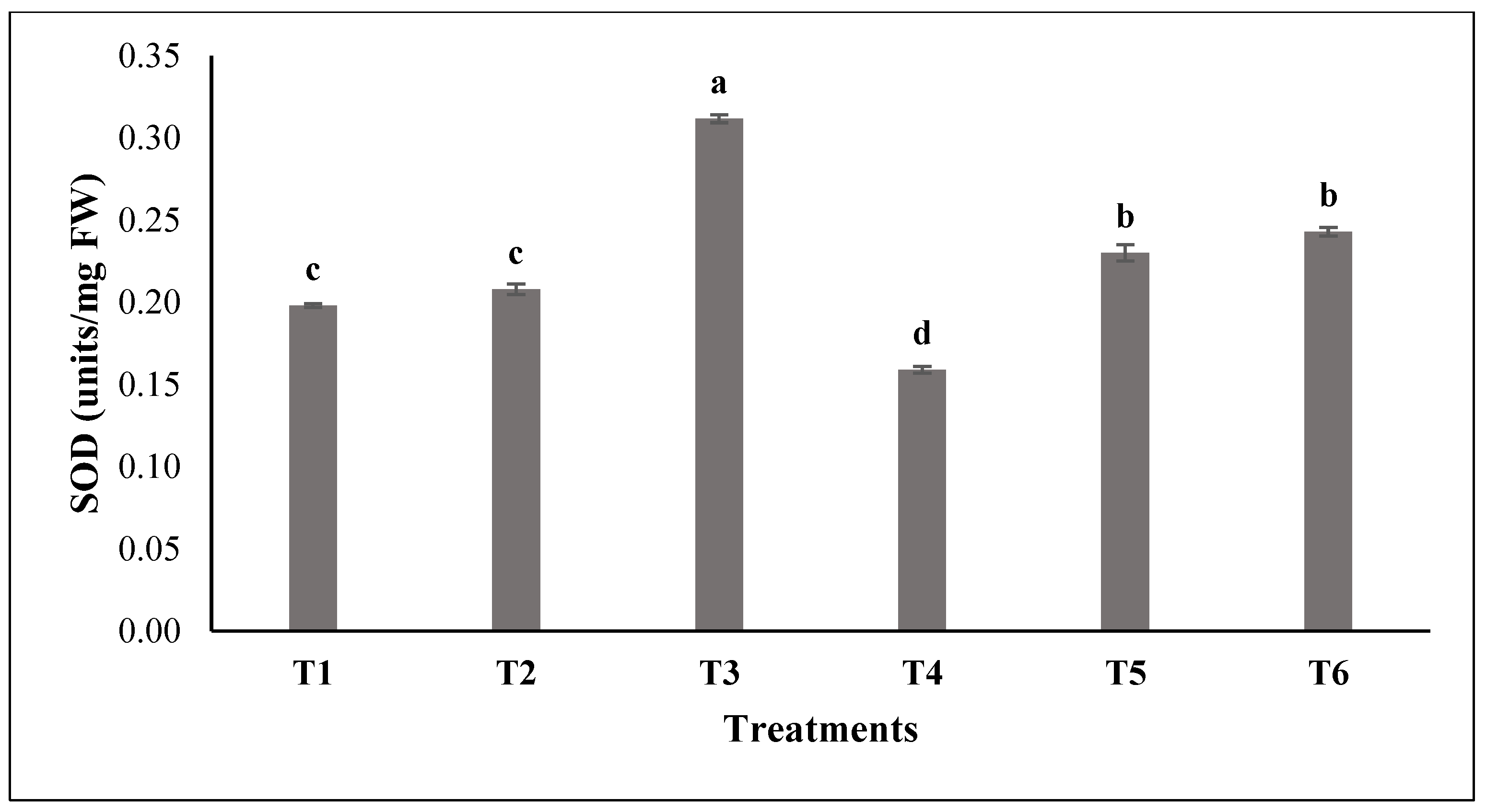
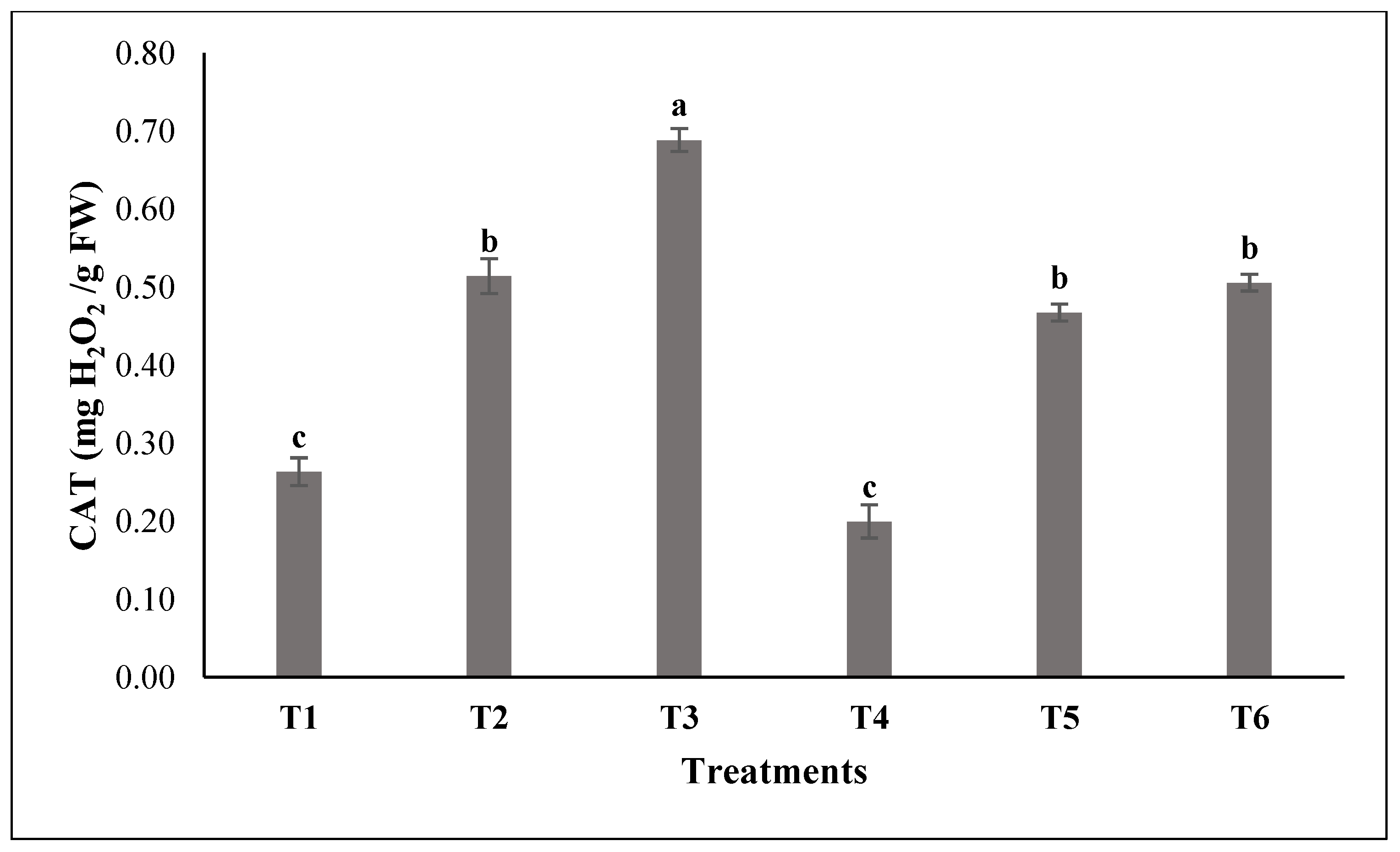
3.2. Assessment of the Enzymatic Activities
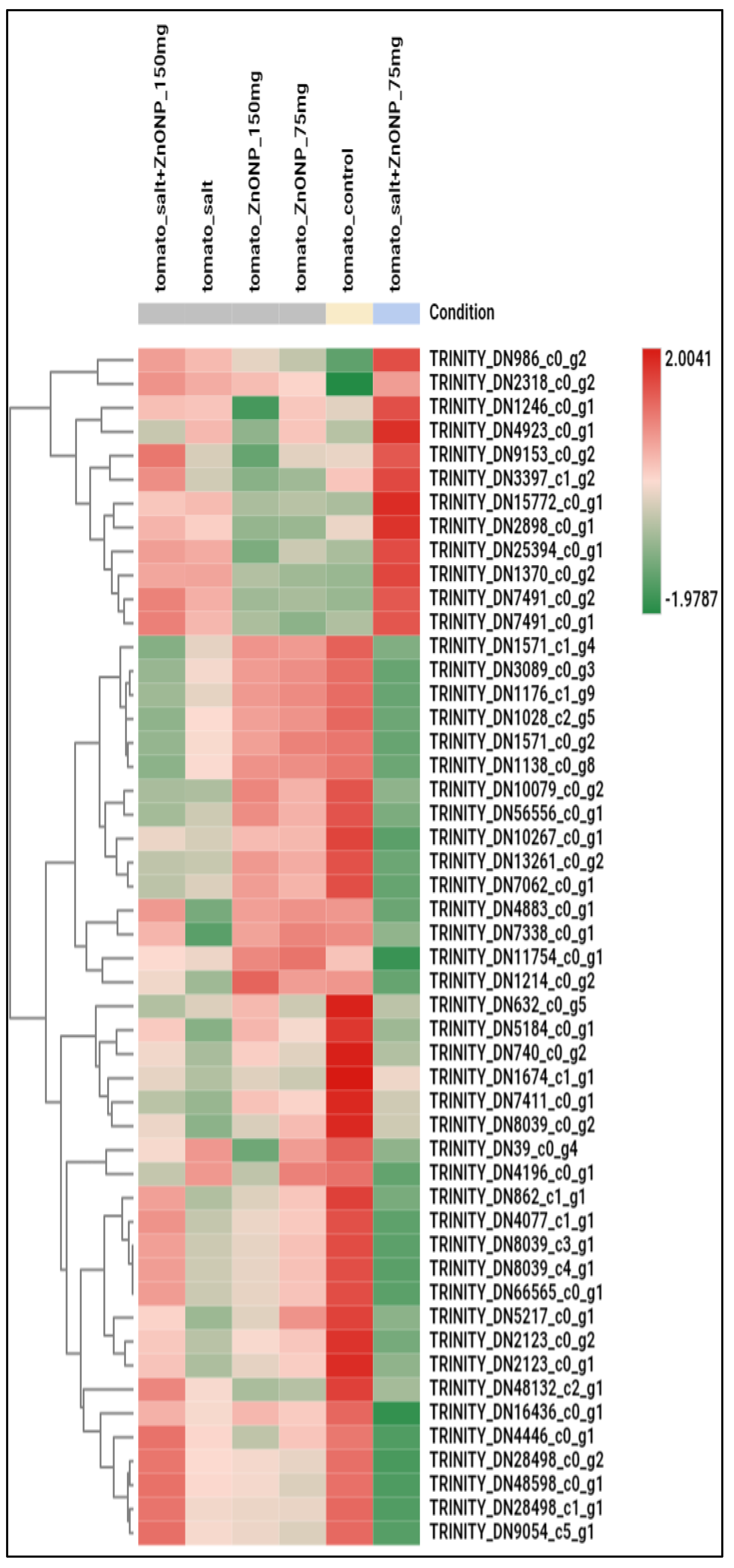
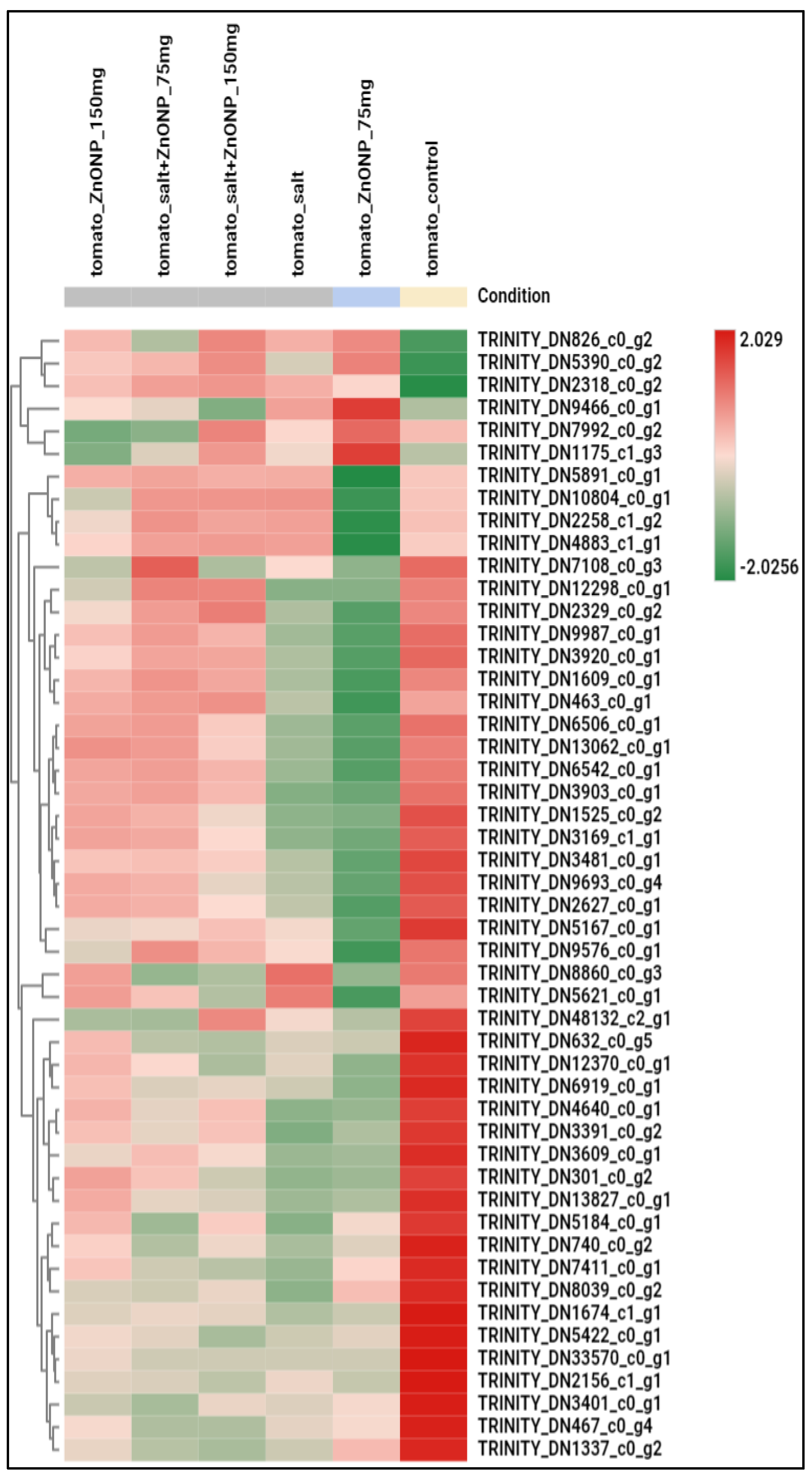
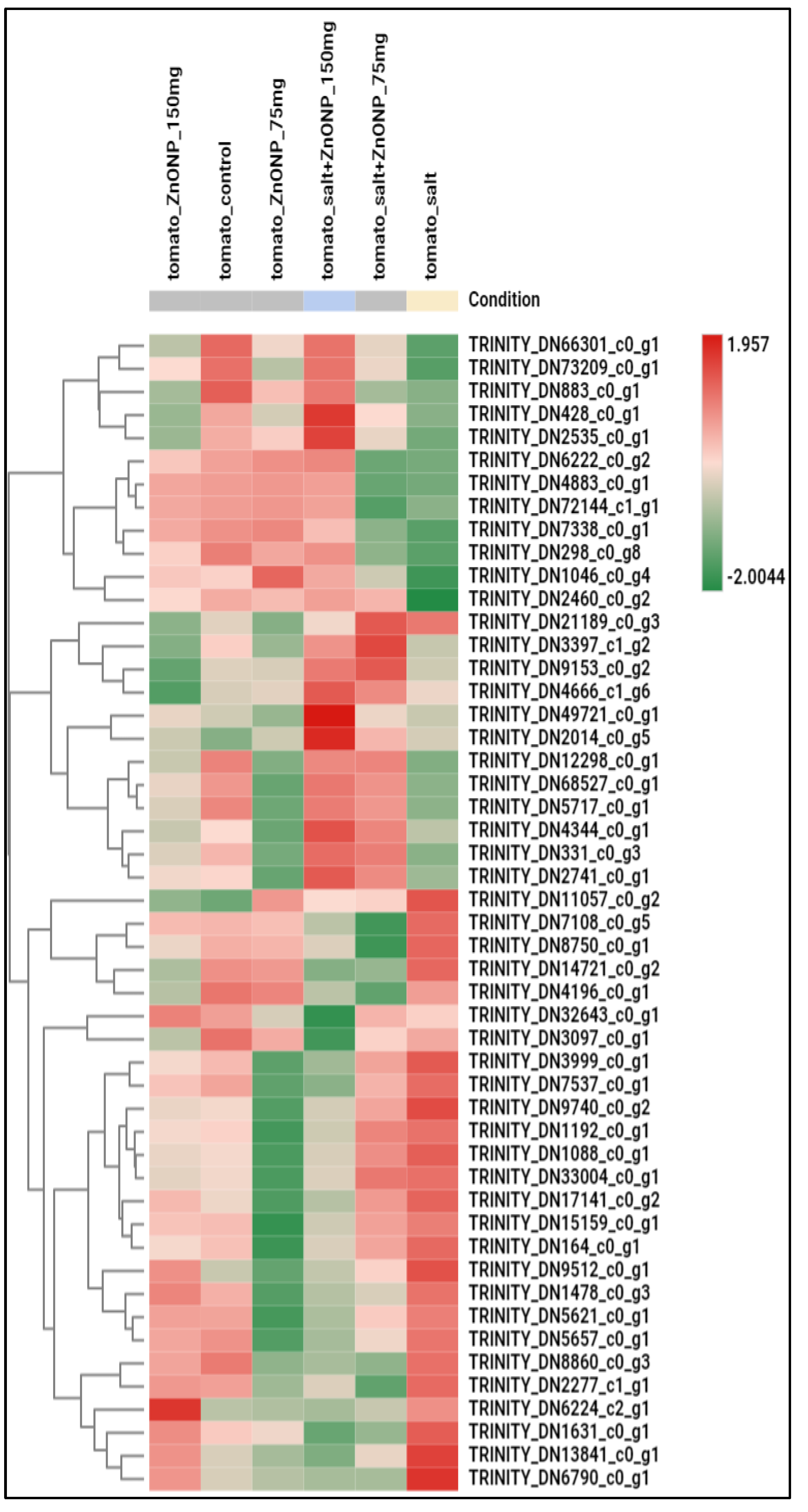
3.3. Genome-Wide Transcriptomic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Remzi Becer, C. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Mazzei, R.; Papaioannou, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection. Membranes 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in Plant Immunity and Plant Protection: Roles and Potential Application as Foliar Sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Freitag, S.; Bryan, G.J.; Stewart, D.; Taylor, M.A. Environmental and Genetic Factors Associated with Solanesol Accumulation in Potato Leaves. Front. Plant Sci. 2016, 7, 1263. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Fraser, P.D. Solanesol: Added Value from Solanaceous Waste. Phytochemistry 2011, 72, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Herth, S. Plant Nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Zedan, M.; Abdul-Hamid, D.; Decsi, K.; Kovács, G.P.; Tóth, Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum Lycopersicum L. Plants. Plants 2024, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Abdul-Hamid, D.; Tóth, Z.; Decsi, K. Interventional Effect of Zinc Oxide Nanoparticles with Zea mays L. Plants When Compensating Irrigation Using Saline Water. Nanomaterials 2024, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Tóth, Z.; Decsi, K. The Impact of Salinity on Crop Yields and the Confrontational Behavior of Transcriptional Regulators, Nanoparticles, and Antioxidant Defensive Mechanisms under Stressful Conditions: A Review. Int. J. Mol. Sci. 2024, 25, 2654. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.C.; Sahi, S.V. Zinc Oxide Nanoparticles (ZnONPs) Alleviate Heavy Metal-Induced Toxicity in Leucaena leucocephala Seedlings: A Physiochemical Analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Decsi, K.; Tóth, Z. Different Tactics of Synthesized Zinc Oxide Nanoparticles, Homeostasis Ions, and Phytohormones as Regulators and Adaptatively Parameters to Alleviate the Adverse Effects of Salinity Stress on Plants. Life 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, R.; Diab, R.; Halawa, M.; Elshanshory, A.; El-Shaer, A.; Hamouda, M. Role of Zinc Oxide Nanoparticles in Ameliorating Salt Tolerance in Soybean. Egypt. J. Bot. 2020, 60, 733–747. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Jaszczyszyn, Y.; Thermes, C. Library Preparation Methods for Next-Generation Sequencing: Tone down the Bias. Exp. Cell Res. 2014, 322, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome Analysis by Strand-Specific Sequencing of Complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic, and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Ahmed, M.; Tóth, Z.; Marrez, D.A.; Rizk, R.; Abdul-Hamid, D.; Decsi, K. Transcriptome Datasets of Salt-Stressed Tomato Plants Treated with Zinc Oxide Nanoparticles. Data Brief 2025, 58, 111282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bharati, R.; Kubes, J.; Popelkova, D.; Praus, L.; Yang, X.; Severova, L.; Skalicky, M.; Brestic, M. Zinc Oxide Nanoparticles Application Alleviates Salinity Stress by Modulating Plant Growth, Biochemical Attributes and Nutrient Homeostasis in Phaseolus vulgaris L. Front. Plant Sci. 2024, 15, 1432258. [Google Scholar] [CrossRef] [PubMed]
- Venisse, J.-S.; Gullner, G.; Brisset, M.-N. Evidence for the Involvement of an Oxidative Stress in the Initiation of Infection of Pear by Erwinia Amylovora. Plant Physiol. 2001, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1955; Volume 2, pp. 764–775. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bonnichsen, R.K.; Chance, B.; Theorell, H. Catalase Activity. Acta Chem. Scand. 1947, 1, 685–709. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Wiley: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar]
- Wingett, S.W.; Andrews, S. FastQ Screen: A Tool for Multi-Genome Mapping and Quality Control. F1000Res 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- OmicsBox. Bioinformatics Made Easy; BioBam Bioinformatics: Valencia, Spain, 2019. [Google Scholar]
- Berkhout, S.W.; Haaf, J.M.; Gronau, Q.F.; Heck, D.W.; Wagenmakers, E.-J. A Tutorial on Bayesian Model-Averaged Meta-Analysis in JASP. Behav. Res. 2024, 56, 1260–1282. [Google Scholar] [CrossRef]
- Hoffmann, T.; Hofman, A.; Wagenmakers, E.-J. Bayesian Tests of Two Proportions: A Tutorial With R and JASP. Methodology 2022, 18, 239–277. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving Abiotic Stress Tolerance in Plants through Antioxidative Defense Mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Shaheen, M.R.; Ihsan, M.Z.; Masood, S.; Zubair, M.; Shehzad, F.; Khalid, A.-U.-H. Growth, Photosynthesis and Antioxidant Enzyme Modulations in Broccoli (Brassica oleracea L. Var. Italica) under Salinity Stress. South Afr. J. Bot. 2022, 148, 104–111. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar Sprayed Green Zinc Oxide Nanoparticles Mitigate Drought-Induced Oxidative Stress in Tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Plant Resistance to Environmental Stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Beers-Mulroy, B.; Tidmarsh, G.F. Therapeutic Opportunities for Hepcidin in Acute Care Medicine. Crit. Care Clin. 2019, 35, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Vokkaliga, T.H.; Wu, T.-M.; Hong, C.-Y. Glutathione Reductase and Abiotic Stress Tolerance in Plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2017; pp. 265–286. ISBN 978-3-319-66681-5. [Google Scholar]
- Csiszár, J.; Horváth, E.; Váry, Z.; Gallé, Á.; Bela, K.; Brunner, S.; Tari, I. Glutathione Transferase Supergene Family in Tomato: Salt Stress-Regulated Expression of Representative Genes from Distinct GST Classes in Plants Primed with Salicylic Acid. Plant Physiol. Biochem. 2014, 78, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Edwards, R. Glutathione Transferases. Arab. Book Am. Soc. Plant Biol. 2010, 8, e0131. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity Up-regulates the Antioxidative System in Root Mitochondria and Peroxisomes of the Wild Salt-tolerant Tomato Species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Shalata, A.; Neumann, P.M. Exogenous Ascorbic Acid (Vitamin C) Increases Resistance to Salt Stress and Reduces Lipid Peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, S.; Navari-Izzo, F.; Izzo, R. Antioxidative Responses of Shoots and Roots of Wheat to Increasing NaCl Concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- Decsi, K.; Ahmed, M.; Abdul-Hamid, D.; Rizk, R.; Tóth, Z. Verification of Seed-Priming-Induced Stress Memory by Genome-Wide Transcriptomic Analysis in Wheat (Triticum aestivum L.). Agronomy 2025, 15, 1365. [Google Scholar] [CrossRef]
- Li, W.; Mi, X.; Jin, X.; Zhang, D.; Zhu, G.; Shang, X.; Zhang, D.; Guo, W. Thiamine Functions as a Key Activator for Modulating Plant Health and Broad-Spectrum Tolerance in Cotton. Plant J. 2022, 111, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Z.N.B. Thiamine and Its Role in Protection Against Stress in Plants (Enhancement in Thiamine Content for Nutritional Quality Improvement). In Nutritional Quality Improvement in Plants; Jaiwal, P.K., Chhillar, A.K., Chaudhary, D., Jaiwal, R., Eds.; Springer International Publishing: Cham, Germany, 2019; pp. 177–186. ISBN 978-3-319-95354-0. [Google Scholar]
- Goyer, A. Thiamine in Plants: Aspects of Its Metabolism and Functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozdemir, M.; Miller, G.; Song, L.; Kim, J.; Sodek, A.; Koussevitzky, S.; Misra, A.N.; Mittler, R.; Shintani, D. Thiamin Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis. Plant Physiol. 2009, 151, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Z.; Xiang, J.; Li, S.; Zheng, Z.; Li, Y.; Fang, Y.; Zhang, X.; Chen, X.; Xue, D. Purine Nucleotide Metabolism Response to Drought Stress in Rice. Plant Growth Regul. 2025, 105, 821–832. [Google Scholar] [CrossRef]
- Sun, L.R.; Zhao, Z.J.; Hao, F.S. NADPH Oxidases, Essential Players of Hormone Signalings in Plant Development and Response to Stresses. Plant Signal. Behav. 2019, 14, 1657343. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and Purine Biosynthesis and Degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Matsumoto, M.; Hakomori, Y.; Takagi, H.; Shimada, H.; Sakamoto, A. The Purine Metabolite Allantoin Enhances Abiotic Stress Tolerance through Synergistic Activation of Abscisic Acid Metabolism. Plant Cell Environ. 2014, 37, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.K.; Witte, C.-P. The Biochemistry of Nitrogen Mobilization: Purine Ring Catabolism. Trends Plant Sci. 2011, 16, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]






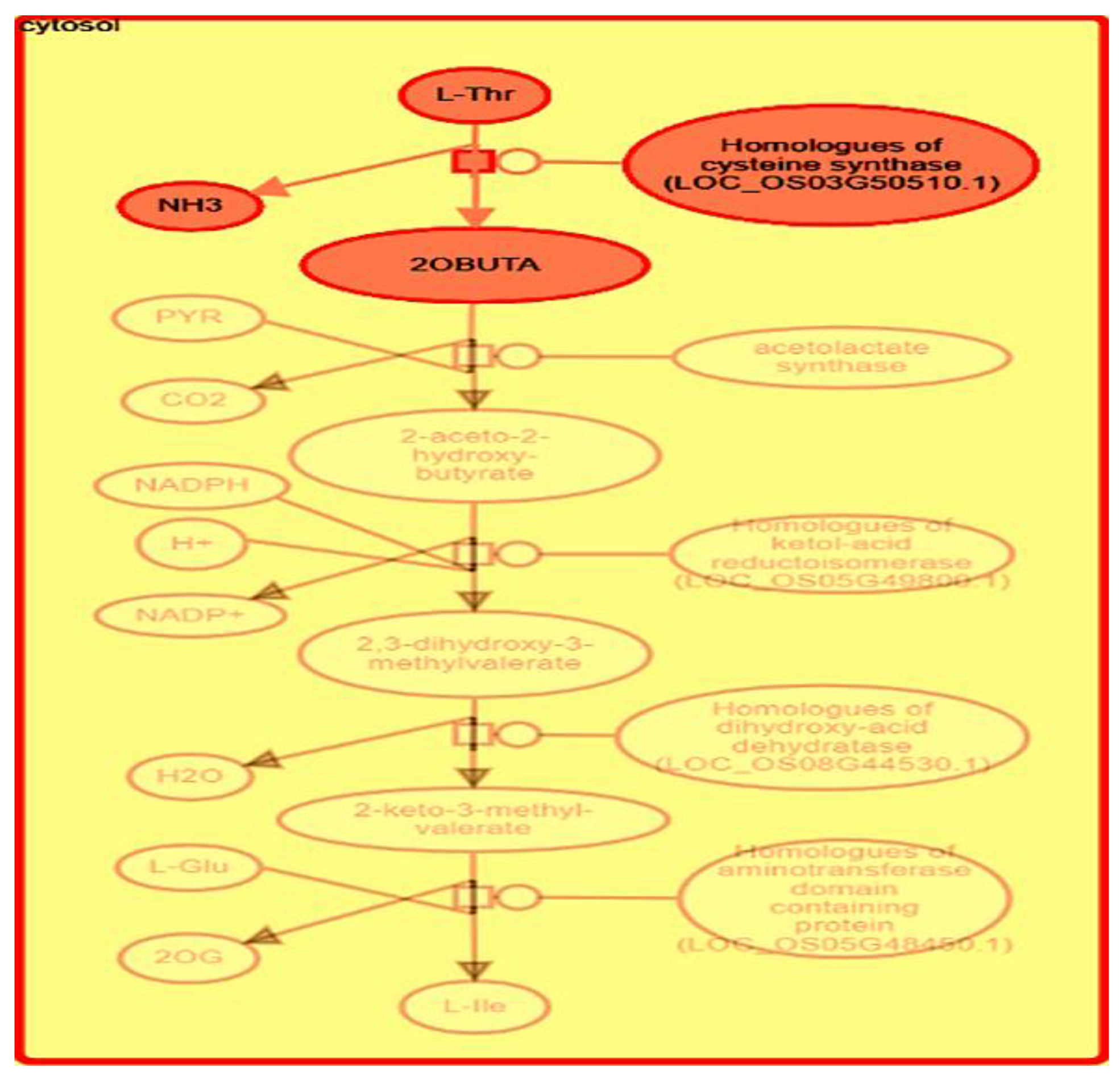


| Comparison | Pathways Linked to KEGG | No. of Sequences Linked to KEGG Database |
|---|---|---|
| T1 vs. T4 | Fatty acid elongation, Biosynthesis of unsaturated fatty acids, Brassinosteroid and Phenylpropanoid biosynthesis, Steroids and steroid hormone biosynthesis, Metabolism of Phenylalanine, Ascorbate and aldarate, Purine, Glycerolipid, Inositol phosphate, Riboflavin, and Thiamine. | 19 |
| T1 vs. T2 | Metabolism of Purine, Glycerolipid, Riboflavin, and Thiamine. Steroid degradation, Terpenoid backbone biosynthesis, Steroid hormone biosynthesis, and Brassinosteroid biosynthesis. | 10 |
| T1 vs. T3 | Metabolism of Nicotinate and nicotinamide, Porphyrin, Glyoxylate and dicarboxylate, Phenylalanine, Ascorbate and aldarate, Purine and pyrimidine, Glycerolipid, Inositol phosphate, Riboflavin, and Thiamine. Pantothenate and CoA biosynthesis. | 20 |
| T1 vs. T5 | Metabolism of Purine and Pyrimidine, Ascorbate and aldarate, Methane, Nicotinate and nicotinamide, Inositol phosphate, Riboflavin, Drugs/other enzymes, Thiamine, Pyruvate, Starch and sucrose, Oxidative phosphorylation, Citrate cycle, Glycolysis, Pantothenate and CoA biosynthesis, Carbon fixation pathways in prokaryotes, Gluconeogenesis, and Carbon fixation in photosynthetic organisms. | 26 |
| T1 vs. T6 | Metabolism of Thiamine, Starch and sucrose, Galactose, Glycerolipid, and Riboflavin. Phenylpropanoid biosynthesis, Oxidative phosphorylation, and Photosynthesis, | 10 |
| T4 vs. T5 | Metabolism of xenobiotics by cytochrome P450, Thiamine, Drugs/other enzymes, Glutathione, Purine, and Amino sugar and nucleotide sugar. Tight junction, Longevity-regulating pathway—worm, MAPK signaling pathway—yeast, Endocytosis, and Ubiquitin-mediated proteolysis. | 11 |
| T4 vs. T6 | Cysteine and methionine metabolism, Arachidonic acid, Linoleic acid, Tyrosine, Purine, Sulfur metabolism, and Thiamine. Synthesis and secretion of cortisol, Prolactin signaling pathway, Steroid hormone biosynthesis, Ovarian steroidogenesis, Serotonergic synapse, Isoquinoline alkaloid biosynthesis, Inflammatory mediator regulation of TRP channels, Tight junction, Biosynthesis of various antibiotics, MAPK signaling pathway—yeast, Endocytosis, Monoterpenoid biosynthesis, Ubiquitin-mediated proteolysis, and Diterpenoid biosynthesis. | 24 |
| Comparison | Pathways Linked to the KEGG Database and Their Responsible Sequences | |
|---|---|---|
| Increased Sequences | Reduced Sequences | |
| T1 vs. T4 (Table S7-1) |
|
|
| T1 vs. T2 (Table S7-2) |
|
|
| T1 vs. T3 (Table S7-3) |
|
|
| T1 vs. T5 (Table S7-4) |
|
|
| T1 vs. T6 (Table S7-5) |
|
|
| T4 vs. T5 (Table S7-6) |
|
|
| T4 vs. T6 (Table S7-7) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Tóth, Z.; Rizk, R.; Abdul-Hamid, D.; Decsi, K. Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum lycopersicum. Agronomy 2025, 15, 1715. https://doi.org/10.3390/agronomy15071715
Ahmed M, Tóth Z, Rizk R, Abdul-Hamid D, Decsi K. Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum lycopersicum. Agronomy. 2025; 15(7):1715. https://doi.org/10.3390/agronomy15071715
Chicago/Turabian StyleAhmed, Mostafa, Zoltán Tóth, Roquia Rizk, Donia Abdul-Hamid, and Kincső Decsi. 2025. "Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum lycopersicum" Agronomy 15, no. 7: 1715. https://doi.org/10.3390/agronomy15071715
APA StyleAhmed, M., Tóth, Z., Rizk, R., Abdul-Hamid, D., & Decsi, K. (2025). Investigation of Antioxidative Enzymes and Transcriptomic Analysis in Response to Foliar Application of Zinc Oxide Nanoparticles and Salinity Stress in Solanum lycopersicum. Agronomy, 15(7), 1715. https://doi.org/10.3390/agronomy15071715







