Abstract
Peach fruit flesh spongy tissue disorder causes dry, porous, and brown areas in the flesh, severely compromising fruit quality and market value. While soil properties and calcium nutrition have been linked to the disorder, the role of rhizosphere microbial communities in disorder resistance remains unclear. This study investigated both the physicochemical properties and the root-associated microbiomes of disordered (CK) and healthy (TT) peach orchards to explore microbial mechanisms underlying disorder suppression. TT soils exhibited higher pH, greater organic matter, increased exchangeable calcium, and more balanced trace elements compared to CK. Microbial analysis revealed significantly higher diversity and enrichment of beneficial taxa in TT associated with plant growth and disorder resistance. Functional gene prediction showed TT was enriched in siderophore production, auxin biosynthesis, phosphate solubilization, and acetoin–butanediol synthesis pathways. Co-occurrence network analysis demonstrated that TT harbored a more complex and cooperative microbial community structure, with 274 nodes and 6013 links. Metagenomic binning recovered high-quality MAGs encoding diverse resistance and growth-promoting traits, emphasizing the ecological roles of Gemmatimonadaceae, Reyranella, Nitrospira, Bacillus megaterium, and Bryobacteraceae. These findings highlight the combined importance of soil chemistry and microbiome structure in disorder suppression and provide a foundation for microbiome-informed soil management to enhance fruit quality and promote sustainable orchard practices.
1. Introduction
Peach (Prunus persica L.) spongy tissue disorder is characterized by the development of dry, porous, brown areas within the fruit flesh, which are difficult to detect from the surface, and it has only been found in southern China [1,2,3]. This disorder is a significant threat to fruit quality, affecting both the texture and nutritional value [2,3]. The disorder is closely linked to soil conditions, tree nutrition, and management practices [3]. Key factors contributing to its onset include calcium deficiency [4], improper water management [5], and poor soil structure [6]. Calcium is essential for maintaining cell wall stability, and its deficiency results in weakened cell walls, leading to the spongy texture [7,8]. Additionally, inadequate or fluctuating water supply during fruit development disrupts cell growth, exacerbating the condition [9,10].
While the relationship between soil properties and peach fruit flesh spongy tissue disorder is well-established, the role of rhizosphere microbial communities in this process remains underexplored [11]. The rhizosphere is a dynamic soil zone surrounding plant roots, harboring diverse microbial communities that play a significant role in nutrient solubilization (e.g., calcium uptake) and directly enhance plant growth [12]. These microorganisms are also critical for growth promotion and pathogen resistance. They alleviate plant stress and suppress harmful pathogens through mechanisms such as siderophore production, phytohormone synthesis, and biofilm formation [13,14,15,16,17]. Despite their importance, the specific characteristics of rhizosphere microbes in disordered (CK) and healthy (TT) soils, as well as their potential for promoting disorder resistance, are poorly understood.
In this study, we aimed to analyze soil physicochemical properties, micronutrient availability, and root-associated microbial community composition and metabolic potential in CK (disordered) and TT (healthy) soils. Using advanced sequencing technologies (e.g., 16S rRNA and metagenomics), we sought to (i) identify soil differential microbes associated with peach fruit flesh spongy tissue disorder, (ii) explore their metabolic potential for disorder suppression and growth promotion, and (iii) develop microbial-based strategies for mitigating the disorder. Our findings are expected to provide valuable insights into the development of microbial agents, soil quality diagnostics, and sustainable management practices to mitigate this emerging threat, ultimately enhancing fruit quality and promoting ecological balance in peach cultivation.
2. Materials and Methods
2.1. Study Site and Sample Collection
This study was conducted in Changsha City, Hunan Province, China. Two peach orchards were selected for comparative analysis: one orchard 80% exhibiting typical symptoms of peach fruit flesh spongy tissue disorder (designated as CK; 28.46° N, 114.02° E) and a nearby orchard with no visible symptoms of the disorder (designated as TT; 28.45° N, 114.02° E). The plants were grown using high-quality horticultural cultivation techniques, standard with disease and pest control. Notably, the TT orchard had not undergone artificial soil improvement or calcium supplementation. The soil samples were collected on 6 September 2024. From each orchard, root samples and bulk soil were collected from three parallel sampling points (n = 5 per group). Bulk soil was sampled at a depth of 0–20 cm within the root zone, and fine roots with adhering rhizosphere soil were carefully collected for further analysis.
2.2. Soil Physicochemical Analysis
Soil samples were air-dried at room temperature (~25 °C) for 5 days, then sieved through a 2 mm mesh to remove stones, large root fragments, and other debris prior to physicochemical analyses. Key indicators measured included soil pH, water-soluble nitrogen (NO3−-N and NH4+-N), available phosphorus (Olsen-P), available potassium (extracted with NH4OAc), available sulfur (extracted with Ca(H2PO4)2), and organic matter (via potassium dichromate oxidation) [18,19,20,21]. Exchangeable elements such as calcium, magnesium, iron, boron, manganese, copper, zinc, and molybdenum were determined using atomic absorption spectroscopy following extraction with 1 M ammonium acetate (pH 7.0) [22].
2.3. Separation of Root-Associated Microbes
Bulk soil was defined as loosely adhering soil removed by vigorous shaking. Rhizoplane microbial fractions were obtained by scraping root-adhering soil with sterile scalpel blades. The remaining roots were surface sterilized using 75% ethanol, followed by five rinses with sterile water, then mechanically ground under aseptic conditions to isolate endophytic microbes. All isolation steps were performed on ice to minimize microbial degradation and were adapted from previously described established protocols [23].
2.4. 16S rRNA Gene Amplicon Sequencing and Bioinformatic Analysis
Genomic DNA from bulk soil, rhizoplane, and endosphere samples was extracted using the DNeasy PowerSoil Kit (Qiagen, Dresden, Germany) according to the manufacturer’s instructions. The V3–V4 hypervariable region of the 16S rRNA gene was amplified using primers 338F/806R for bulk soil and rhizoplane samples, while the V5–V7 region was amplified using primers 799F/1193R for endosphere samples to minimize chloroplast contamination [23,24]. Sequencing was performed on the Illumina MiSeq PE250 platform (Personal Biotechnology, Shanghai, China) [25]. Raw reads were processed using QIIME2 (QIIME2 2021.2), including adapter trimming, quality filtering, merging, and chimera removal. Denoising and ASV (amplicon sequence variant) inference were conducted via the DADA2 pipeline [26]. Taxonomic classification was assigned using the SILVA 138 reference database. Downstream microbial diversity and composition analyses were conducted using MicrobiomeAnalyst (MicrobiomeAnalyst 2.0) [27]. Microbial functional profiles were predicted using PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2) using the representative ASV sequences [28]. Predicted gene family abundances (KEGG Orthologs) were generated based on ancestral-state reconstruction using hidden-state prediction and subsequently categorized into functional pathways. The output was normalized and filtered to retain functions with peach growth. Differences in functional profiles between groups were assessed using non-parametric statistical tests (e.g., Wilcoxon rank-sum test), and significantly enriched functions (p < 0.05) were visualized across compartments.
2.5. Shotgun Metagenomic Sequencing and Metagenomic Binning
Shotgun metagenomic sequencing of bulk soil, rhizoplane, and endosphere samples was conducted using the Illumina NovaSeq PE150 platform (Personal Biotechnology, Shanghai, China). After quality control, high-quality reads were de novo assembled using MEGAHIT (MEGAHIT v1.0, k-mer = 21–121, step size = 10). Metagenome-assembled genomes (MAGs) were generated using the MetaWRAP pipeline with integrated binning tools MetaBAT2, MaxBin2, and CONCOCT, followed by bin_refinement [29]. MAG quality was assessed using CheckM, retaining bins with completeness > 50% and contamination < 10%. Taxonomic annotation was performed using the Genome Taxonomy Database Toolkit (GTDB-Tk), while open reading frames (ORFs) were predicted using Prokka (Prokka v1.12) [30]. Functional gene annotation was carried out via KofamKOALA with default parameters based on the KEGG database [31].
2.6. Data Visualization
All data visualizations were generated using the ggplot2 package in R (R v4.5). All raw amplicon and metagenomic sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA1254973. Microbial taxa serving as potential biomarkers were identified using Linear Discriminant Analysis Effect Size (LEfSe) on the Galaxy web platform (http://huttenhower.sph.harvard.edu/galaxy/) (MicrobiomeAnalyst 2.0), which combines non-parametric Kruskal–Wallis and pairwise Wilcoxon tests with linear discriminant analysis (LDA). Taxa with LDA scores > 2.0 and p < 0.05 were considered significant indicators for either disordered or healthy samples.
3. Results
3.1. Physicochemical Differences in Soils with and Without Fruit Flesh Spongy Tissue Disorder of Peach
This study examined the physico-chemical properties of two distinct soil types—CK (disordered soil) and TT (disorder-free soil)—and identified significant differences in key physico-chemical indicators (Figure 1). Compared to CK soil, TT soil showed notable advantages in pH, exchangeable calcium, organic matter content, and trace element balance, providing a more favorable environment for peach growth, preventing fruit flesh spongy tissue disorder, and enhancing fruit quality. Conversely, the low pH, calcium deficiency, and reduced organic matter content in CK soil likely contribute to the poor quality of peaches and the development of disorder. Specifically, CK soil has a pH of 4.61, indicating high acidity, while TT soil has a more neutral pH of 5.96 (Figure 1a). TT soil contains 7.31 cmol/kg of exchangeable calcium, significantly higher than CK soil’s 1.76 cmol/kg (Figure 1f). Organic matter content in TT soil (34.40 g/kg) is also higher than in CK soil (22.45 g/kg) (Figure 1e). In terms of trace elements, CK soil contains much higher levels of effective iron (169.00 mg/kg) than TT soil (64.82 mg/kg), potentially restricting the uptake of other micronutrients such as zinc and boron, thereby diminishing the fruit’s resistance and quality (Figure 1h). Additionally, the available potassium content in CK soil (188.50 mg/kg) exceeds that of TT soil (136.50 mg/kg) (Figure 1d). While potassium is necessary for plant growth, excessive potassium may interfere with calcium absorption, further decreasing calcium accumulation in fruit and negatively impacting its quality.
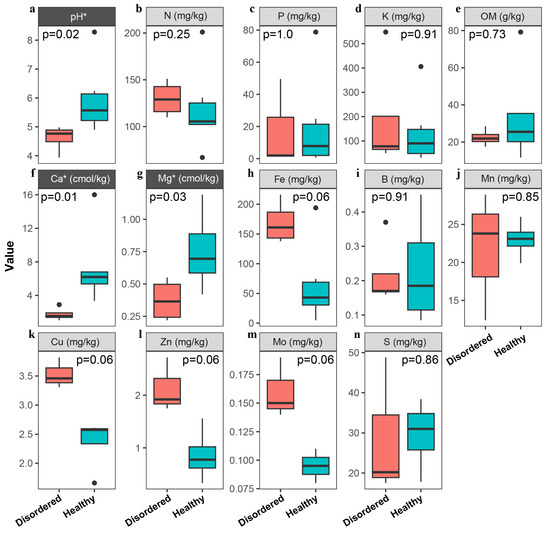
Figure 1.
Comparison of soil physicochemical properties (a–n) between disordered (CK) and healthy (TT) peach orchard soils. Parameters showing statistically significant differences (*, p < 0.05) are highlighted with dark gray shading. (a) Soil pH; (b) water-soluble nitrogen; (c) available phosphorus; (d) available potassium; (e) organic matter; (f) calcium; (g) magnesium; (h) iron; (i) boron; (j) manganese; (k) copper; (l) zinc; (m) molybdenum; (n) available sulfur. Black dots represent outliers in the box plots.
3.2. Diversity and Composition of Root-Associated Microbial Communities
On average, each sample yielded 133,129 high-quality reads, resulting in a total of 16,408 ASVs across all samples. Taxonomic annotation of these ASVs covered a broad spectrum: 28 phyla, 114 classes, 58 orders, 227 families, 365 genera, and 219 species. At the genus level, the proportion of unassigned sequences varied across compartments: 19–30% in the endosphere, 63–70% in the rhizosphere, and 66–72% in bulk soil. Microbial diversity and composition were analyzed using 16S rRNA gene amplicon sequencing to examine bacteria from different root regions (bulk soil, rhizosphere, and endosphere) in CK and TT orchards. The results revealed significant differences in microbial α-diversity between TT and CK soils. TT orchards exhibited lower microbial diversity in both rhizosphere and bulk soil compared to CK soils (Figure 2). In contrast, the endosphere bacteria diversity in TT orchards (4.5) was significantly higher than that in CK soils (3.8).
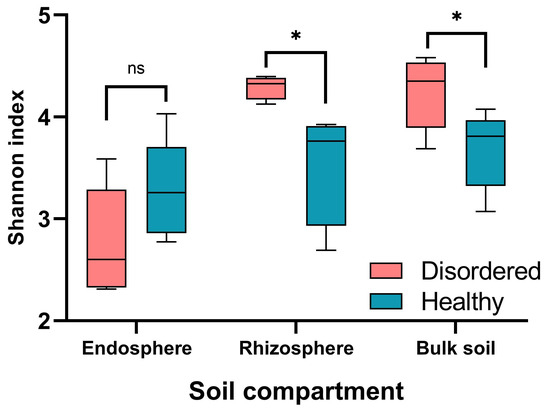
Figure 2.
Comparison of α-diversity (Shannon index) across three root-associated compartments (endosphere, rhizosphere, and bulk soil) between disordered (CK) and healthy (TT) peach orchard soils. Statistically significant differences (*, p < 0.05) are indicated based on pairwise comparisons. “ns” indicates the none-significant differences (p > 0.05).
Using Bray–Curtis distance-based β-diversity analysis (Figure 3), we found significant differences in microbial community composition between TT and CK orchards across all habitats (bulk, rhizosphere, and endosphere) (PERMANOVA test, p < 0.05). These findings highlight that the physicochemical properties of the soils strongly influence the composition of rhizosphere microbial communities. For example, TT soil’s higher exchangeable calcium, richer organic matter, and more suitable pH likely promote the colonization of specific microbial communities. In contrast, CK soil’s higher acidity and elevated effective iron content may support more tolerant but functionally limited microbial communities. These communities may exhibit more functional specificity and form closer symbiotic relationships with plants, potentially influencing nutrient absorption efficiency and improving fruit quality.
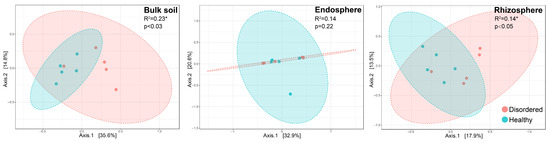
Figure 3.
Comparison of β-diversity (Bray–Curtis distance) across three root-associated compartments (endosphere, rhizosphere, and bulk soil) between disorderd (CK) and healthy (TT) peach orchard soils. Differences were assessed using PERMANOVA test. (* indicates a significant difference).
Taxonomy annotation using the SILVA database further revealed significant differences in microbial composition between TT and CK orchards (Figure 4). In bulk soil, Acidibacter and Acidothermus were slightly more abundant in TT soil (1.6% vs. 1.4%, 1.8% vs. 1.1%), and Bryobacter was also slightly higher (1.8% vs. 1.4%). Similarly, Candidatus_Solibacter showed a significantly higher relative abundance in TT soil (4.6% vs. 3.8%). In contrast, Roseiflexus had a significantly higher relative abundance in CK soil (3.2% vs. 0.2%), and Nitrospira was more abundant in CK soil (1.7% vs. 1.4%).
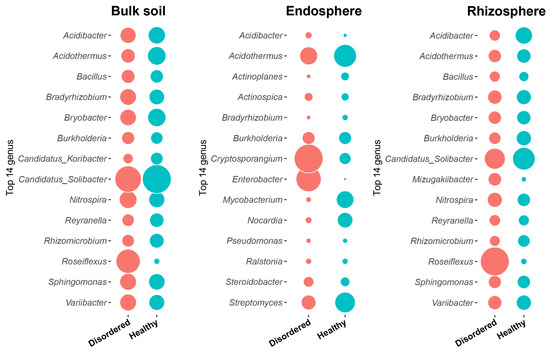
Figure 4.
Microbial relative abundance at the genus level across three root-associated compartments (bulk soil, endosphere, rhizosphere) between disordered (CK) and healthy (TT) peach orchard soils. The top 14 most abundant genera are displayed. Bubble sizes are scaled proportionally to their relative abundance values.
In the endosphere, Acidothermus was significantly more abundant in TT orchard (14.9% vs. 9.1%), and the relative abundance of Mycobacterium also significantly increased in TT orchard (8.7% vs. 0.7%). Additionally, Nocardia and Streptomyces had higher relative abundances in TT orchard (6.7% vs. 1.1%, 12.0% vs. 6.1%). Conversely, Cryptosporangium and Enterobacter were significantly enriched in CK soil (24.2% vs. 3.8%, 18.4% vs. 0.1%), and Acidibacter was slightly more abundant in CK soil (1.2% vs. 0.3%). Furthermore, TT orchard had a higher proportion of unclassified microbial taxa (30.4% vs. 19.5%), indicating the presence of potentially unknown microbial populations with uncharacterized functions.
In the rhizosphere, Acidibacter exhibited significantly higher relative abundance in TT orchard (2.1% vs. 0.9%), while Burkholderia and Candidatus_Solibacter were also more abundant in TT orchard (1.6% vs. 1.1%, 3.8% vs. 3.2%). In contrast, Roseiflexus had a significantly higher relative abundance in CK soil (6.2% vs. 0.2%), and Mizugakiibacter was more abundant in CK soil (1.3% vs. 0.2%). Some low-abundance genera, such as Rhizomicrobium and Variibacter, were more abundant in TT orchard, suggesting their potential role in maintaining the microecological balance in the rhizosphere.
3.3. Identification of Core Differential Bacteria in Rhizosphere
The distinct physicochemical properties of TT and CK soils have shaped their microbial community structures, which may be a core factor influencing differences in peach quality. To further investigate the microbial differences, LEfSe analysis (LDA score > 2) was performed, comparing microbial community compositions in the endosphere, rhizosphere, and bulk soil between CK and TT orchards (Figure 5). This analysis revealed significant differential microbiota that reflects soil microecological variations and potentially uncovers mechanisms linked to peach fruit flesh spongy tissue disorder, providing critical insights for soil health diagnostics and improvement.
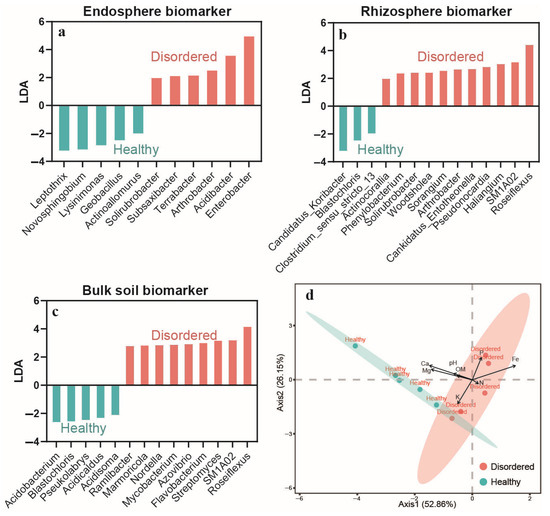
Figure 5.
Biomarker taxa (a–c) identified across root-associated compartments (endosphere, rhizoplane, and bulk soil) using LEfSe analysis (LDA score > 2). Differential taxa are indicative of either disordered (CK) or healthy (TT) peach orchard soils. (d) Canonical correspondence analysis (CCA) was conducted to examine the relationship between compartment-specific biomarkers and environmental variables under two soil health conditions.
LEfSe identified a range of disease-resistant and growth-promoting microbes enriched in TT orchard. These microbes may help suppress the occurrence of fruit flesh spongy tissue disorder by regulating nutrient cycling, enhancing plant resistance, and optimizing the rhizosphere microecological balance. For example, biomarkers in the endosphere, such as Actinoallomurus, Geobacillus, Novosphingobium, and Leptothrix, may be involved in secondary metabolite production, organic matter decomposition, and heavy metal stabilization (Figure 5a). In the rhizosphere, Candidatus_Koribacter and Blastochloris are closely linked to soil carbon cycling, nitrogen metabolism, and photosynthesis (Figure 5b). Biomarkers in bulk soil, such as Acidobacterium and Acidisoma, likely play roles in acid adaptation and nutrient breakdown (Figure 5c). These microbial communities contribute to a stable and healthy microbiome in TT orchard, promoting peach tree growth and fruit quality.
Conversely, LEfSe identified microbial communities enriched in CK soil, which may be associated with fruit flesh spongy tissue disorder. These communities could exacerbate disease through pathogenic substance secretion or occupation of ecological niches. Endosphere biomarkers such as Enterobacter, Acidibacter, and Terrabacter may facilitate pathogen colonization, compromising plant health (Figure 5a). In the rhizosphere, Roseiflexus, Microlunatus, and Phenylobacterium exhibit higher disease potential and better adaptation to acidic conditions (Figure 5b). Bulk soil biomarkers like Rhizobium and Lysobacter are known for their role in plant disease induction (Figure 5c). The enrichment of these microbial communities in CK soil reflects its poor physicochemical properties (e.g., high acidity, low calcium), which may further enhance disease occurrence and spread.
The results showed that these disease-resistant microbes in TT orchard, such as Candidatus_Koribacter, Blastochloris, and Actinoallomurus, may serve as positive indicators of soil health, supporting the development and application of disease-resistant microbes. In contrast, disease-associated microbes in CK soil, such as Enterobacter, Roseiflexus, and Phenylobacterium, can act as negative indicators of disease risk, aiding early diagnostics and soil improvement. Future research should experimentally validate the disease-resistant and growth-promoting functions of these biomarkers (e.g., through secretion analysis and metabolic predictions). Introducing disease-resistant microbial communities can improve the rhizosphere environment and reduce disease incidence, while these microbial groups can serve as indicators to monitor soil microbial dynamics and optimize the growth environment for peach trees.
To explore whether the identified biomarkers from three soil compartments (endosphere, rhizosphere, and bulk soil) are influenced by soil physicochemical properties, canonical correspondence analysis (CCA) was conducted to examine the relationship between compartment-specific biomarkers and environmental variables under two soil health conditions (Figure 5d). The results showed that the first and second canonical axes explained 52.86% and 26.15% of the total variation in community structure, respectively. Biomarkers from the healthy and disordered groups formed two distinct clusters, indicating a clear separation between the two soil conditions. Notably, biomarkers from the healthy group were strongly associated with higher levels of Ca, Mg, pH, OM, and K, suggesting that these environmental factors play a dominant role in shaping microbial community composition in healthy soils. This pattern is consistent with previous findings, further supporting the notion that improved soil physicochemical conditions selectively enrich beneficial microbial taxa.
3.4. Co-Occurrence Networks of Rhizosphere Microbes
To investigate the influence of CK and TT soils on rhizosphere microbial interactions, co-occurrence networks (|R| > 0.85, p < 0.05) were constructed, revealing notable differences in their structural and functional dynamics (Figure 6). The TT network exhibited greater complexity, with 274 nodes and 6013 links compared to 190 nodes and 4251 links in CK, reflecting a richer and more interconnected microbial community. Although the average degree was slightly higher in CK (44.747) than in TT (43.891), the broader distribution of connections in TT suggests more diverse interactions. CK displayed a higher clustering coefficient (0.75) and network density (0.237) than TT (0.678 and 0.161, respectively), indicating tighter clustering of interactions. However, TT had lower modularity (0.328 vs. 0.384 in CK), reflecting a more distributed network structure that facilitates broader microbial cooperation. Despite its larger size, the TT network maintained efficient connectivity with an average path length of 2.365, similar to CK’s 2.343. Additionally, the statistical inference value was markedly higher in TT (13,107.281) than in CK (8489.479), highlighting the enhanced structural complexity and functional potential of the TT microbial community. These findings suggest that the TT soil supports a more diverse and cooperative microbial network, enhancing resilience and ecological stability, whereas the CK soil, with its tightly clustered but less diverse community, may be less adaptive to environmental changes. This highlights the critical role of microbial diversity and cooperation in maintaining soil health and mitigating peach fruit flesh spongy tissue disorder.
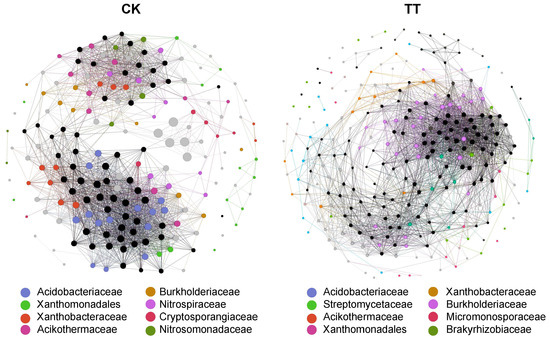
Figure 6.
Co-occurrence network of root-associated microbial communities in disordered (CK) and healthy (TT) peach orchard soils. Colored nodes represent the top eight most highly connected taxa (evaluated at the family level), while gray nodes indicate other taxa with lower connectivity, and black nodes represent unassigned taxa.
3.5. Metabolic Potential of Rhizosphere Microbes
To further explore their metabolic capabilities, PICRUSt2 was used to predict functional gene compositions (Figure 7). The functional analysis of the rhizosphere microbial community suggested that TT soil harbors microbes with strong disease resistance and growth-promoting potential. For the significant enrichment of genes associated with plant disease suppression in the TT group. These included pchB (isochorismate pyruvate lyase), gluA (glutamate transport system ATP-binding protein), hcnABC (hydrogen cyanide synthase), and licABC (surfactin/lichenysin synthetase), which play key roles in pathogen inhibition and plant defense [32,33,34,35]. Additionally, genes involved in plant growth promotion were more abundant in TT soil, particularly those related to acetoin and butanediol synthesis (poxB, alsD, budA, alsS, budC), which enhance stress tolerance and support plant development [36,37,38,39,40], and glycine–betaine production (opuD, opuC, opuBD, opuA), which helps maintain cellular osmotic balance under environmental stress [41,42]. The increased presence of phosphate solubilization genes (pstB, pstC, pstA) suggested improved nutrient acquisition, while the enrichment of ipdC, involved in auxin biosynthesis, indicated a potential role in stimulating root growth [43,44,45,46]. Siderophore production genes (acrA, mbtH, pvd, fhuF, fpvA, fhuE, acrB), essential for iron acquisition, were also more prevalent, reflecting enhanced microbial interactions that support plant health [47,48,49,50,51,52]. Moreover, the TT group exhibited higher expression of heat shock (dnaJ, dnaK, groE) [53,54,55] and cold shock (cspE, cspD, cspC, cspA) [56,57,58] proteins, suggesting an increased ability to adapt to temperature fluctuations, along with elevated levels of superoxide dismutase genes (sodB, sodC) [59,60], which play a crucial role in oxidative stress mitigation. These findings highlight the metabolic potential of TT rhizosphere microbes in enhancing plant resilience, improving nutrient uptake, and maintaining soil health, offering valuable insights for developing sustainable strategies to mitigate peach fruit flesh spongy tissue disorder.
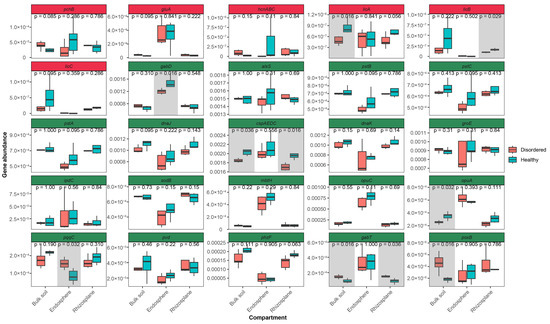
Figure 7.
Comparison of microbial functional gene abundance across three root-associated compartments (bulk soil, endosphere, and rhizoplane) between disordered (CK) and healthy (TT) peach orchard soils. Genes with statistically significant differences (p < 0.05) are highlighted with gray shading. Selected genes are associated with plant-disordered module (indicated in red panel) and plant growth promotion module (indicated in green panel).
3.6. Metabolic Mechanisms of Key Taxa Revealed by Metagenome Binning
To better link key taxa with their metabolic pathways, a metagenomic binning approach was employed to assemble 76 metagenome-assembled genomes (MAGs) from rhizosphere metagenomic data, representing a diverse range of rhizosphere microorganisms spanning 13 phyla and 29 orders (Table S1). Taxonomy annotation using the GTDB database identified several key microorganisms linked to fruit flesh spongy tissue disorder, as well as disease-resistant and growth-promoting microorganisms.
Among the identified MAGs, six microorganisms were associated with fruit flesh spongy tissue disorder, including bin.79 (SCGC-AG-212-J23), bin.64 (Bryobacteraceae), bin.24 (Pantoea dispersa), bin.20 (SLND01), bin.18 (Kosakonia cowanii), and bin.15 (Agrobacterium tumefaciens) (Figure 8). Analysis of their gene content revealed distinct pathogenic mechanisms, such as toxin secretion, cell wall degradation, and environmental disruption. Notably, bin.79 and bin.15 exhibited a higher number of pathogenic genes, particularly related to toxin secretion and cell wall degradation. Both bin.79 and bin.15 contained genes associated with toxin secretion systems (e.g., hop and hrp) that utilize T3SS to transfer toxins into plant cells, altering gene expression and suppressing immune responses, which induce disease. Additionally, bin.15 showed a prominent presence of toxin-antitoxin systems (e.g., RelE), suggesting that these microorganisms may promote plant decline by interfering with immune responses and disrupting plant metabolism. Bin.24 and bin.18 were also rich in genes for cell wall-degrading enzymes (e.g., bglA and pelA), which degrade plant cell wall components like β-glucose and pectin, leading to the breakdown of plant cell structure and disease onset. Moreover, bin.24 and bin.18 demonstrated higher gene content in acidification and environmental disruption, particularly the ureC gene, which acidifies the soil and decreases calcium availability, thereby disrupting the absorption of essential minerals by plants. These pathogenic microorganisms exert their effects on peach health through multiple mechanisms, including toxin secretion, cell wall degradation, acidification, and ROS production, all of which compromise plant cell structure, metabolic processes, and immune defenses, ultimately leading to plant decline and disease. Particularly, the pathogenicity of bin.79 and bin.15 is likely a major contributing factor to the occurrence of fruit flesh spongy tissue disorder.
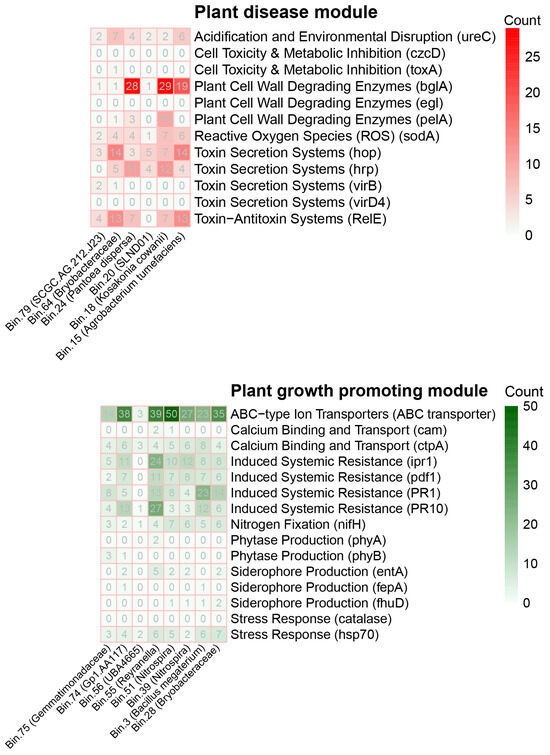
Figure 8.
Metabolic potential of rhizosphere-associated plant pathogens (red module) and plant growth-promoting bacteria (green module) based on metagenome-assembled genomes (MAGs).
On the other hand, eight MAGs associated with peach rhizosphere disease-resistant and growth-promoting microorganisms were also retrieved, including bin.75 (Gemmatimonadaceae), bin.74 (Gp1-AA117), bin.56 (UBA4665), bin.55 (Reyranella), bin.51 (Nitrospira), bin.39 (Nitrospira), bin.3 (Bacillus megaterium), and bin.28 (Bryobacteraceae). These microorganisms exhibited high gene content, particularly related to siderophore production, disease resistance, stress response, and nitrogen fixation, all contributing to improved plant growth and enhanced disease resistance. Specifically, bin.75 and bin.55 contained genes such as fepA and entA, which play crucial roles in enhancing iron absorption and boosting plant immune responses [61,62]. Additionally, bin.75 contained the hsp70 gene, which supports stress resistance and adaptation to adverse conditions [63]. Bin.74 and bin.28 exhibited significant involvement in induced systemic resistance (ISR), containing multiple genes like PR1, PR10, and ipr1, which strengthen plant immunity and help resist pathogen attacks [64,65,66]. All MAGs showed high levels of nifH genes, which enable nitrogen fixation, contributing to plant nutrition and soil quality [67]. Furthermore, bin.39 and bin.28 contained genes related to calcium binding and transport (e.g., cam and ctpA), indicating their potential to enhance calcium absorption and improve root health [68,69]. These rhizosphere microorganisms help plants grow and enhance disease resistance through various mechanisms such as siderophore production, stress response, nitrogen fixation, and calcium transport. By improving nutrient absorption and regulating immune responses, they enable plants to better withstand pathogen invasion, making these microorganisms promising candidates for use as biofertilizers and biocontrol agents to improve the disease resistance and growth performance of peaches and other crops.
4. Discussion
Peach fruit flesh spongy tissue disorder significantly impacts fruit quality, causing a soft, spongy texture that reduces its marketability and nutritional value [1]. Our study indicates that CK soil’s physicochemical properties contribute to an environment conducive to disease development. High acidity, low calcium, and insufficient organic matter in CK soil hinder calcium absorption by peach trees, destabilizing the cell wall structure and potentially leading to the spongy texture observed in the fruit. Pathogenic microbial communities in CK soil further exacerbate the disease by secreting toxins and degrading plant cell walls [70,71].
This study suggests that the physicochemical properties of two distinct soil types are likely key factors influencing variations in peach quality and the occurrence of physiological disorders (Figure 1). For instance, acidic soils can reduce the availability of essential cations, such as calcium, thereby limiting plant uptake and indirectly contributing to quality issues, including the development of spongy tissue disorder in peach flesh [2]. Calcium is essential for maintaining the structural integrity of fruit cell walls, and lower calcium levels in CK soil may weaken cell wall stability, increasing susceptibility to spongy tissue formation [3]. Inadequate calcium levels may also negatively affect fruit sweetness and overall quality [72]. Furthermore, organic matter plays a critical role in soil fertility by supporting microbial activity and facilitating nutrient cycling [73]. Its deficiency in CK soil likely exacerbates calcium uptake limitations, further contributing to poor fruit development.
Evidence from microbial community composition and diversity suggests that rhizosphere microbes also play a critical role in plant health by influencing nutrient uptake, pathogen resistance, and overall growth [74,75]. In our study, the microbial communities in CK soil promote disease by supporting pathogenic microbes, while those in TT soil enhance plant health by improving nutrient absorption and boosting disease resistance.
Microbial diversity patterns further revealed that the endosphere bacteria diversity in TT orchard was higher than that in CK soil. This suggests that TT orchard conditions are more favorable for the colonization of beneficial microbes that symbiotically associate with peach trees. The higher exchangeable calcium, neutral pH, and abundant organic matter in TT soil likely provide an optimal environment for these endophytic microbes. These microbes may support nutrient and calcium uptake, thereby improving fruit quality.
Additionally, LEfSe analysis identified key microbial taxa associated with disease resistance in TT soil and disease promotion in CK soil. Beneficial microbes such as Candidatus_Koribacter, Blastochloris, and Actinoallomurus in TT soil enhance nutrient cycling, nitrogen metabolism, and rhizo-sphere balance, contributing to disease resistance [76]. In contrast, pathogenic microbes like Enterobacter, Roseiflexus, and Phenylobacterium in CK soil promote disease through toxin secretion and cell wall degradation, weakening the plant’s immune system [71].
Importantly, these microbial biomarkers are linked to disease risk in CK soil (Enterobacter, Roseiflexus, Phenylobacterium) and disease resistance in TT soil (Candidatus_Koribacter, Blastochloris, Actinoallomurus) [75]. Therefore, these biomarkers provide valuable tools for early disease detection and guiding soil management practices aimed at improving peach quality.
Metagenome binning allowed us to link key microbial species to their respective metabolic pathways, identifying pathogenic and growth-promoting pathways [77]. Pathogenic species like Agrobacterium tumefaciens and Pantoea dispersa carry genes for toxin secretion and cell wall degradation, directly contributing to disease onset [78]. Conversely, beneficial microbes like Bacillus megaterium and Reyranella in TT soil contain genes for nitrogen fixation, siderophore production, and stress response, promoting nutrient availability and supporting healthy plant growth [79]. Microbial communities in TT soil suppress fruit flesh spongy tissue disorder through various mechanisms, including enhancing nutrient absorption, regulating soil pH, and producing antimicrobial compounds [80]. Microbes like Reyranella and Bacillus megaterium improve calcium uptake, while Candidatus_Koribacter and Blastochloris optimize nitrogen metabolism and carbon cycling [81]. These beneficial microbial activities create a rhizosphere environment less favorable to disease-causing pathogens. The functional potential of TT soil’s microbial communities is closely linked to improved soil health.
Our study is one of the first papers correlating the microbial community characterization to fruit flesh spongy tissue disorder of peach, highlighting the need for targeted soil management strategies that introduce beneficial microbes to enhance soil health. Future research should focus on experimentally validating the disorder suppression functions of identified biomarkers, conducting field trials to apply these findings, and assessing the long-term effects of soil amendments on peach health and sustainable orchard management.
5. Conclusions
This study demonstrates the critical role of soil physicochemical conditions and rhizosphere microbial communities in suppressing spongy tissue disorder in peach fruit. Healthy orchard soils provided more favorable rhizosphere conditions—higher pH, richer organic matter, and greater exchangeable calcium—that supported beneficial root-microbial assembly.
More importantly, we revealed that healthy soils harbor a functionally enriched and ecologically stable microbiome. These microbial communities were characterized by enhanced plant-beneficial traits, including siderophore production, auxin biosynthesis, phosphate solubilization, and acetoin–butanediol pathways, all of which contribute to improved nutrient uptake and stress tolerance.
Beyond identifying potential microbial biomarkers and functional genes linked to disorder suppression, our findings offer practical implications by providing a scientific basis for developing microbiome-based diagnostic tools and biocontrol strategies. This research advances the understanding of microbe-mediated plant health and points toward microbiome-informed soil management as a sustainable solution to relieve physiological disorders in high-value fruit crops, which benefits both agricultural productivity and food quality in the face of increasing environmental stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071697/s1, Table S1: Summary for recovered bins.
Author Contributions
Conceptualization, W.C. and L.Z.; Validation, Y.Y.; Formal analysis, W.C., D.T., J.H. and Y.Y.; Investigation, W.C., D.T., J.H. and Y.Y.; Resources, W.C. and L.Z.; Data curation, W.C., D.T., J.H. and Y.Y.; Writing-original draft, W.C.; Writing-review and editing, L.Z.; Visualization, W.C., D.T., J.H. and Y.Y.; Supervision, L.Z.; Funding acquisition, W.C., D.T. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hunan Provincial Science and Technology Innovation Fund (2024CX95, 2024CX28 and 2024CX27) and the Natural Science Foundation of Changsha (kq2502279).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liao, Y.; Wang, L.; Zhang, Y.; Huang, S.; Li, C.; Liu, W. Investigation on Disease Condition of Spongy Tissue in Yingzui Peach. J. Anhui Agri. Sci. 2016, 44, 123–124. (In Chinese) [Google Scholar] [CrossRef]
- Lu, J.; Lin, X.; Liao, Y. Transcriptome sequencing analysis of differentially-expressed genes involved in the spongy tissue of Olecranon peach (Prunus persica L.). J. Fruit Sci. 2023, 40, 2524–2535. (In Chinese) [Google Scholar] [CrossRef]
- Ma, X.W.; Liu, B.; Zhang, Y.H.; Su, M.Q.; Zheng, B.; Wang, S.B.; Wu, H.X. Unraveling correlations between calcium deficiency and spongy tissue in mango fruit flesh. Sci. Hortic. 2023, 309, 111694. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Alcobendas, R.; Alarcón, J.J.; Valsesia, P.; Génard, M.; Nicolás, E. Assessment of the water stress effects on peach fruit quality and size using a fruit tree model, QualiTree (vol 128C, pg 1, 2013). Agric. Water Manag. 2013, 130, 178. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, S.; Eyni-Nargeseh, H.; Ahmadi, S.A.K.; Azizi, K. Towards withholding irrigation regimes and drought-resistant genotypes as strategies to increase canola production in drought-prone environments: A modeling approach. Agric. Water Manag. 2021, 243, 106487. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Oak, P.; Jha, V.; Deshpande, A.; Tanpure, R.; Dawkar, V.; Mundhe, S.; Ghuge, S.; Prabhudesai, S.; Krishanpal, A.; Jere, A.; et al. Transcriptional and translational perturbation in abiotic stress induced physiological activities and metabolic pathway networks in spongy tissue disorder of mango fruit. Postharvest Biol. Technol. 2022, 188, 111880. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H.; Labavitch, J.M. Cell wall modifications in chilling-injured plum fruit (Prunus salicina). Postharvest Biol. Technol. 2008, 48, 77–83. [Google Scholar] [CrossRef]
- Campi, P.; Gaeta, L.; Mastrorilli, M.; Losciale, P. Innovative Soil Management and Micro-Climate Modulation for Saving Water in Peach Orchards. Front. Plant Sci. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Shi, Y.N.; Li, B.J.; Grierson, D.; Chen, K.S. Insights into cell wall changes during fruit softening from transgenic and naturally occurring mutants. Plant Physiol. 2023, 192, 1671–1683. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, Z.; Wang, Y.; Wang, J.; Zhai, R.; Lin-Wang, K.; Espley, R.; Ma, F.; Li, P. Competition between anthocyanin and kaempferol glycosides biosynthesis affects pollen tube growth and seed set of Malus. Hortic. Res. 2021, 8, 173. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Albizua, A.; Williams, A.; Hedlund, K.; Pascual, U. Crop rotations including ley and manure can promote ecosystem services in conventional farming systems. Appl. Soil Ecol. 2015, 95, 54–61. [Google Scholar] [CrossRef]
- Kong, Q.S.; Gao, L.Y.; Cao, L.; Liu, Y.; Saba, H.; Huang, Y.; Bie, Z.L. Assessment of Suitable Reference Genes for Quantitative Gene Expression Studies in Melon Fruits. Front. Plant Sci. 2016, 7, 1178. [Google Scholar] [CrossRef]
- Long, Q.X.; Yan, R.; Hu, J.L.; Cai, D.W.; Mitra, B.; Kim, E.S.; Marchetti, A.; Zhang, H.; Wang, S.J.; Liu, Y.J.; et al. The role of host DNA ligases in hepadnavirus covalently closed circular DNA formation. PLoS Pathog. 2017, 13, e1006784. [Google Scholar] [CrossRef]
- Raval, S.S.; Mahatma, M.K.; Chakraborty, K.; Bishi, S.K.; Singh, A.L.; Rathod, K.J.; Jadav, J.K.; Sanghani, J.M.; Mandavia, M.K.; Gajera, H.P.; et al. Metabolomics of groundnut (Arachis hypogaea L.) genotypes under varying temperature regimes. Plant Growth Regul. 2018, 84, 493–505. [Google Scholar] [CrossRef]
- Wu, W.K.; Panyod, S.; Liu, P.Y.; Chen, C.C.; Kao, H.L.; Chuang, H.L.; Chen, Y.H.; Zou, H.B.; Kuo, H.C.; Kuo, C.H.; et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome 2020, 8, 162. [Google Scholar] [CrossRef]
- Shamrikova, E.V.; Kondratenok, B.M.; Tumanova, E.A.; Vanchikova, E.V.; Lapteva, E.M.; Zonova, T.V.; Lu-Lyan-Min, E.I.; Davydova, A.P.; Libohova, Z.; Suvannang, N. Transferability between soil organic matter measurement methods for database harmonization. Geoderma 2022, 412, 115547. [Google Scholar] [CrossRef]
- Elsgaard, L. Dynamics of mineral nitrogen, water-soluble carbon and potential nitrification in band-steamed arable soil. Biol. Fertil. Soils 2010, 46, 883–889. [Google Scholar] [CrossRef]
- Milham, P.J.; Jill, K.C.; and Holford, P. Selective Measurement of Phosphate in 0.5 M Sodium Bicarbonate Soil Extracts. Commun. Soil Sci. Plan 2024, 55, 529–535. [Google Scholar] [CrossRef]
- Ullah, R.; Abbas, Z.; Bilal, M.; Habib, F.; Iqbal, J.; Bashir, F.; Noor, S.; Qazi, M.A.; Niaz, A.; Baig, K.S.; et al. Method development and validation for the determination of potassium (K2O) in fertilizer samples by flame photometry technique. J. King Saud Univ. Sci. 2022, 34, 102070. [Google Scholar] [CrossRef]
- Caravajal, G.S.; Mahan, K.I.; Goforth, D.; Leyden, D.E. Evaluation of methods based on acid extraction and atomic absorption spectrometry for multi-element determinations in rivers sidements. Anal. Chim. Acta 1983, 147, 133–150. [Google Scholar] [CrossRef]
- Gao, H.B.; Guo, Z.H.; Xu, R.; He, X.; Fernio, J.U.; Li, S.K.; Liu, X.C.; Liu, H.X.; Xue, W.J. Chemolithoautotrophic Antimonite Oxidation Coupled Nitrogen Fixation in the Rhizosphere of Local Plant in Antimony Tailing Area. Environ. Sci. Technol. 2025, 59, 12703–12716. [Google Scholar] [CrossRef]
- Xu, Y.M.; Zhang, X.Y.; Yang, H.; Lu, D.L. Effects of Exogenous Brassinolide Application at the Silking Stage on Nutrient Accumulation, Translocation and Remobilization of Waxy Maize Under Post-Silking Heat Stress. Agriculture 2022, 12, 572. [Google Scholar] [CrossRef]
- Guo, Z.H.; Cao, J.; Xu, R.; Zhang, H.L.; He, L.L.; Gao, H.B.; Zhu, L.N.; Jia, M.Y.; Yang, Z.H.; Xiong, W.P. Novel Photoelectron-Assisted Microbial Reduction of Arsenate Driven by Photosensitive Dissolved Organic Matter in Mine Stream Sediments. Environ. Sci. Technol. 2024, 58, 22170–22182. [Google Scholar] [CrossRef]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. In Microbiome Analysis: Methods and Protocols; Beiko, R.G., Hsiao, W., Parkinson, J., Eds.; Springer: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, G.Y.; Xia, J.G. Using Microbiome Analyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Uritskiy, G.V.; DiRuggiero, J.; Taylor, J. MetaWRAP—A flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 2018, 6, 158. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Serino, L.; Reimmann, C.; Visca, P.; Beyeler, M.; Chiesa, V.D.; Haas, D. Biosynthesis of pyochelin and dihydroaeruginoic acid requires the iron-regulated pchDCBA operon in Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 248–257. [Google Scholar] [CrossRef]
- Davidson, A.L.; Chen, J. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 2004, 73, 241–268. [Google Scholar] [CrossRef]
- Laville, J.; Blumer, C.; Von Schroetter, C.; Gaia, V.; Défago, G.; Keel, C.; Haas, D. Characterization of the hcnABC Gene Cluster Encoding Hydrogen Cyanide Synthase and Anaerobic Regulation by ANR in the Strictly Aerobic Biocontrol Agent Pseudomonas fluorescens CHA0. J. Bacteriol. 1998, 180, 3187–3196. [Google Scholar] [CrossRef]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biot. 1999, 51, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, S.; Shimizu, K. Effect of poxB gene knockout on metabolism in Escherichia coli based on growth characteristics and enzyme activities. World J. Microbiol. Biotechnol. 2007, 23, 573–580. [Google Scholar] [CrossRef]
- Wang, M.; Fu, J.; Zhang, X.; Chen, T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnol. Lett. 2012, 34, 1877–1885. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.; Yang, J.; Jeong, D.; Shin, S.H.; Kook, J.H.; Yang, K.S.; Lee, J. The influence of budA deletion on glucose metabolism related in 2,3-butanediol production by Klebsiella pneumoniae. Enzym. Microb. Technol. 2015, 73–74, 1–8. [Google Scholar] [CrossRef]
- Kanekura, K.; Hashimoto, Y.; Niikura, T.; Aiso, S.; Matsuoka, M.; Nishimoto, I. Alsin, the Product of ALS2 Gene, Suppresses SOD1 Mutant Neurotoxicity Through RhoGEF Domain by Interacting with SOD1 Mutants. J. Biol. Chem. 2004, 279, 19247–19256. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.; Reder, A.; Hoffmann, T.; Hammer, E.; Seubert, A.; Bremer, E.; Völker, U.; Mäder, U. Management of Osmoprotectant Uptake Hierarchy in Bacillus subtilis via a SigB-Dependent Antisense RNA. Front. Microbiol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef]
- Cox, G.B.; Webb, D.; Rosenberg, H. Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J. Bacteriol. 1989, 171, 1531–1534. [Google Scholar] [CrossRef]
- Braibant, M.; De Wit, L.; Peirs, P.; Kalai, M.; Ooms, J.; Drowart, A.; Huygen, K.; Content, J. Structure of the Mycobacterium tuberculosis antigen 88, a protein related to the Escherichia coli PstA periplasmic phosphate permease subunit. Infect. Immun. 1994, 62, 849–854. [Google Scholar] [CrossRef]
- Malhotra, M.; Srivastava, S. An ipdC gene knock-out of Azospirillum brasilense strain SM and its implications on indole-3-acetic acid biosynthesis and plant growth promotion. Antonie Van Leeuwenhoek 2008, 93, 425–433. [Google Scholar] [CrossRef]
- Tikhonova, E.B.; Zgurskaya, H.I. AcrA, AcrB, and TolC of Escherichia coli Form a Stable Intermembrane Multidrug Efflux Complex. J. Biol. Chem. 2004, 279, 32116–32124. [Google Scholar] [CrossRef]
- Krithika, R.; Marathe, U.; Saxena, P.; Ansari, M.Z.; Mohanty, D.; Gokhale, R.S. A genetic locus required for iron acquisition in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2006, 103, 2069–2074. [Google Scholar] [CrossRef]
- Lamont, I.L.; Beare, P.A.; Ochsner, U.; Vasil, A.I.; Vasil, M.L. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2002, 99, 7072–7077. [Google Scholar] [CrossRef]
- Müller, K.; Matzanke, B.F.; Schünemann, V.; Trautwein, A.X.; Hantke, K. FhuF, an iron-regulated protein of Escherichia coli with a new type of [2Fe-2S] center. Eur. J. Biochem. 1998, 258, 1001–1008. [Google Scholar] [CrossRef]
- Shen, J.; Meldrum, A.; Poole, K. FpvA Receptor Involvement in Pyoverdine Biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 2002, 184, 3268–3275. [Google Scholar] [CrossRef]
- Bitter, W.; van Leeuwen, I.S.; de Boer, J.; Zomer, H.W.M.; Koster, M.C.; Weisbeek, P.J.; Tommassen, J. Localization of functional domains in the Escherichia coli coprogen receptor FhuE and the Pseudomonas putida ferric-pseudobactin 358 receptor PupA. Mol. Gen. Genet. MGG 1994, 245, 694–703. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef]
- Braig, K.; Otwinowski, Z.; Hegde, R.; Boisvert, D.C.; Joachimiak, A.; Horwich, A.L.; Sigler, P.B. The crystal structure of the bacterial chaperonln GroEL at 2.8 Å. Nature 1994, 371, 578–586. [Google Scholar] [CrossRef]
- Michaux, C.; Holmqvist, E.; Vasicek, E.; Sharan, M.; Barquist, L.; Westermann, A.J.; Gunn, J.S.; Vogel, J. RNA target profiles direct the discovery of virulence functions for the cold-shock proteins CspC and CspE. Proc. Natl. Acad. Sci. USA 2017, 114, 6824–6829. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Zheng, W.; Crooke, E.; Wang, Y.-H.; Inouye, M. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 2001, 39, 1572–1584. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, Y.; Inouye, M. CspA, the Major Cold-shock Protein of Escherichia coli, Is an RNA Chaperone. J. Biol. Chem. 1997, 272, 196–202. [Google Scholar] [CrossRef]
- Keyer, K.; Imlay, J.A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 1996, 93, 13635–13640. [Google Scholar] [CrossRef]
- Imlay, K.R.; Imlay, J.A. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J. Bacteriol. 1996, 178, 2564–2571. [Google Scholar] [CrossRef]
- Newton, S.M.C.; Igo, J.D.; Scott, D.C.; Klebba, P.E. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 1999, 32, 1153–1165. [Google Scholar] [CrossRef]
- Crosa Jorge, H.; Walsh Christopher, T. Genetics and Assembly Line Enzymology of Siderophore Biosynthesis in Bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Yap, W.H.; Li, X.; Soong, T.W.; Davies, J.E. Genetic diversity of soil microorganisms assessed by analysis of hsp70 (dnaK) sequences. J. Ind. Microbiol. 1996, 17, 179–184. [Google Scholar] [CrossRef]
- Beatrice, C.; Linthorst, J.M.H.; Cinzia, F.; Luca, R. Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae. Eur. J. Plant Pathol. 2017, 148, 163–179. [Google Scholar] [CrossRef]
- Sikorski, M.M.; Biesiadka, J.; Kasperska, A.E.; Kopcińska, J.; Łotocka, B.; Golinowski, W.; Legocki, A.B. Expression of genes encoding PR10 class pathogenesis-related proteins is inhibited in yellow lupine root nodules. Plant Sci. 1999, 149, 125–137. [Google Scholar] [CrossRef]
- He, X.N.; Su, F.; Lou, Z.Z.; Jia, W.Z.; Song, Y.L.; Chang, H.Y.; Wu, Y.H.; Lan, J.; He, X.Y.; Zhang, Y. Ipr1 Gene Mediates RAW 264.7 Macrophage Cell Line Resistance to Mycobacterium bovis. Scand. J. Immunol. 2011, 74, 438–444. [Google Scholar] [CrossRef]
- Kizilova, A.K.; Titova, L.V.; Kravchenko, I.K.; Iutinskaya, G.A. Evaluation of the diversity of nitrogen-fixing bacteria in soybean rhizosphere by nifH gene analysis. Microbiology 2012, 81, 621–629. [Google Scholar] [CrossRef]
- Wärngård, L.; Fransson, R.; Drakenberg, T.B.; Flodström, S.; Ahlborg, U.G. Calmodulin involvement in TPA and DDT induced inhibition of intercellular communication. Chem. Biol. Interact. 1988, 65, 41–49. [Google Scholar] [CrossRef]
- Dong, J.; Signo, K.S.L.; Vanderlinde, E.M.; Yost, C.K.; Dahms, T.E.S. Atomic force microscopy of a ctpA mutant in Rhizobium leguminosarum reveals surface defects linking CtpA function to biofilm formation. Microbiology 2011, 157, 3049–3058. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmolle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.M.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.F.; Chen, Y.; Wang, R.; Guo, S.L. Thirty-year dryland crop rotation improves soil multifunctionality and shifts soil fungal community. Plant Soil 2025, 507, 11–24. [Google Scholar] [CrossRef]
- Levy, A.; Gonzalez, I.S.; Mittelviefhaus, M.; Clingenpeel, S.; Paredes, S.H.; Miao, J.M.; Wang, K.R.; Devescovi, G.; Stillman, K.; Monteiro, F.; et al. Genomic features of bacterial adaptation to plants. Nat. Genet. 2018, 50, 138. [Google Scholar] [CrossRef]
- Fu, X.P.; Liu, S.; Ru, J.R.; Tang, B.Y.; Zhai, Y.J.; Wang, Z.G.; Wang, L.C. Biological control of potato late blight by sp. FXP04 and potential role of secondary metabolites. Biol. Control 2022, 169, 104891. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.; Rodríguez-Vázquez, R.; Duarte, B.; Caviedes, M.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Caçador, M.I.; Rodríguez-Llorente, I.D.; Pajuelo, E. Investigating the mechanisms underlying phytoprotection by plant growth-promoting rhizobacteria in under metal stress. Plant Biol. 2018, 20, 497–506. [Google Scholar] [CrossRef]
- Cao, Y.F.; Shen, Z.Z.; Zhang, N.; Deng, X.H.; Thomashow, L.S.; Lidbury, I.; Liu, H.J.; Li, R.; Shen, Q.R.; Kowalchuk, G.A. Phosphorus availability influences disease-suppressive soil microbiome through plant-microbe interactions. Microbiome 2024, 12, 185. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple Replant Disease: Role of Microbial Ecology in Cause and Control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).