Plant Traits in Spring and Winter Canola Genotypes Under Salinity
Abstract
1. Introduction
2. Materials and Methods
3. Results
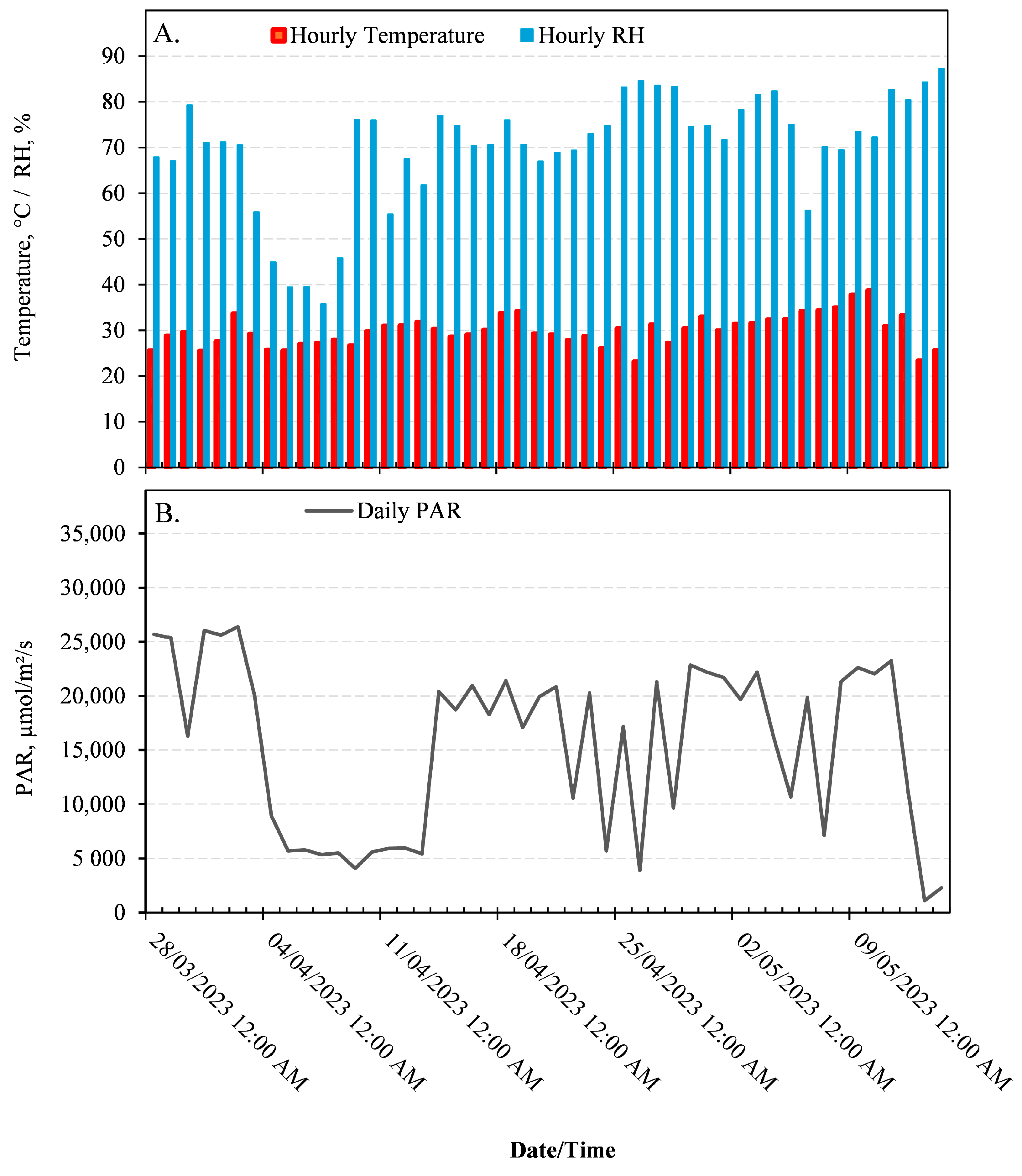
3.1. Contrast Between Canola Types
3.2. Salinity Impacts and Biometrics Relationships
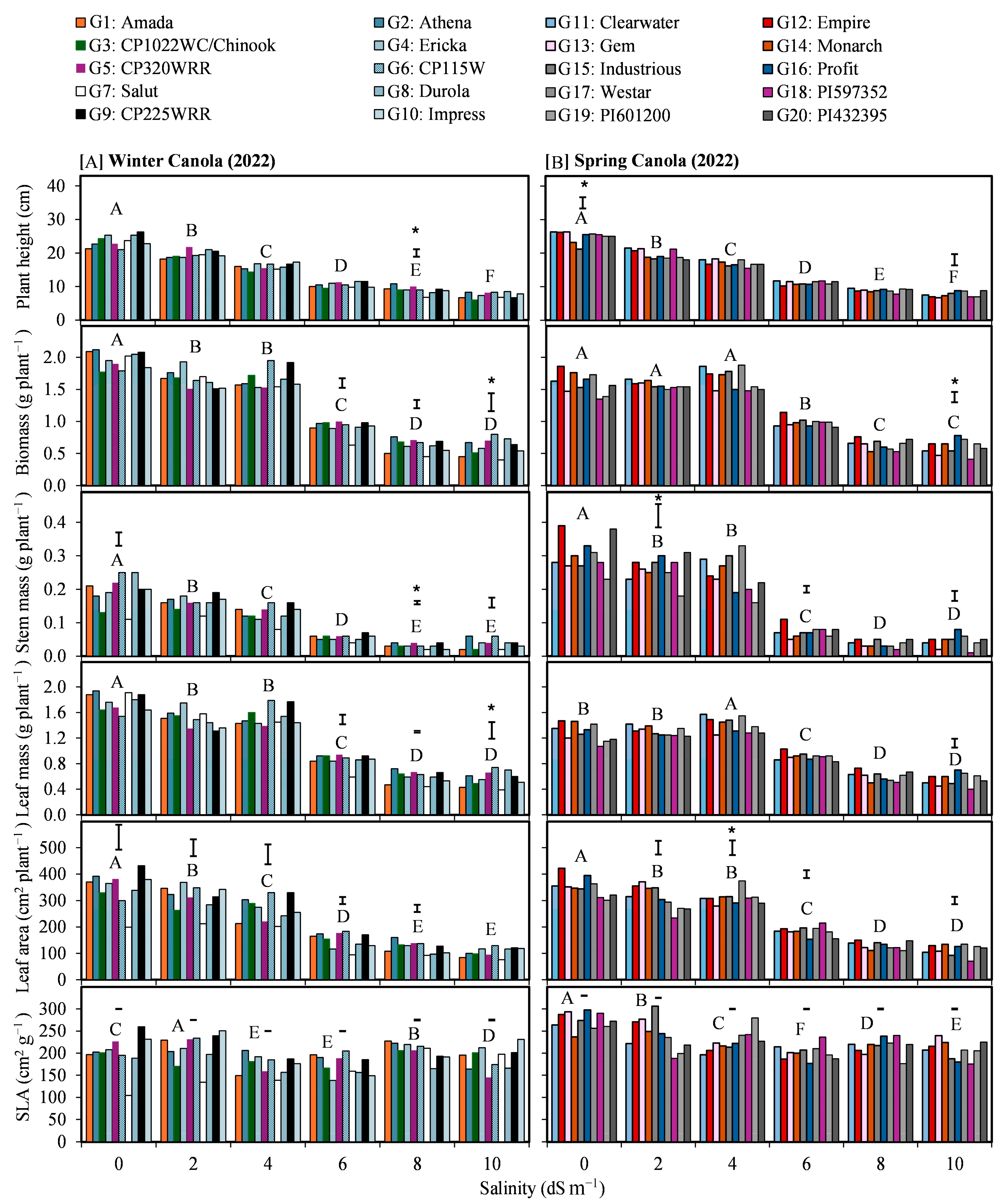
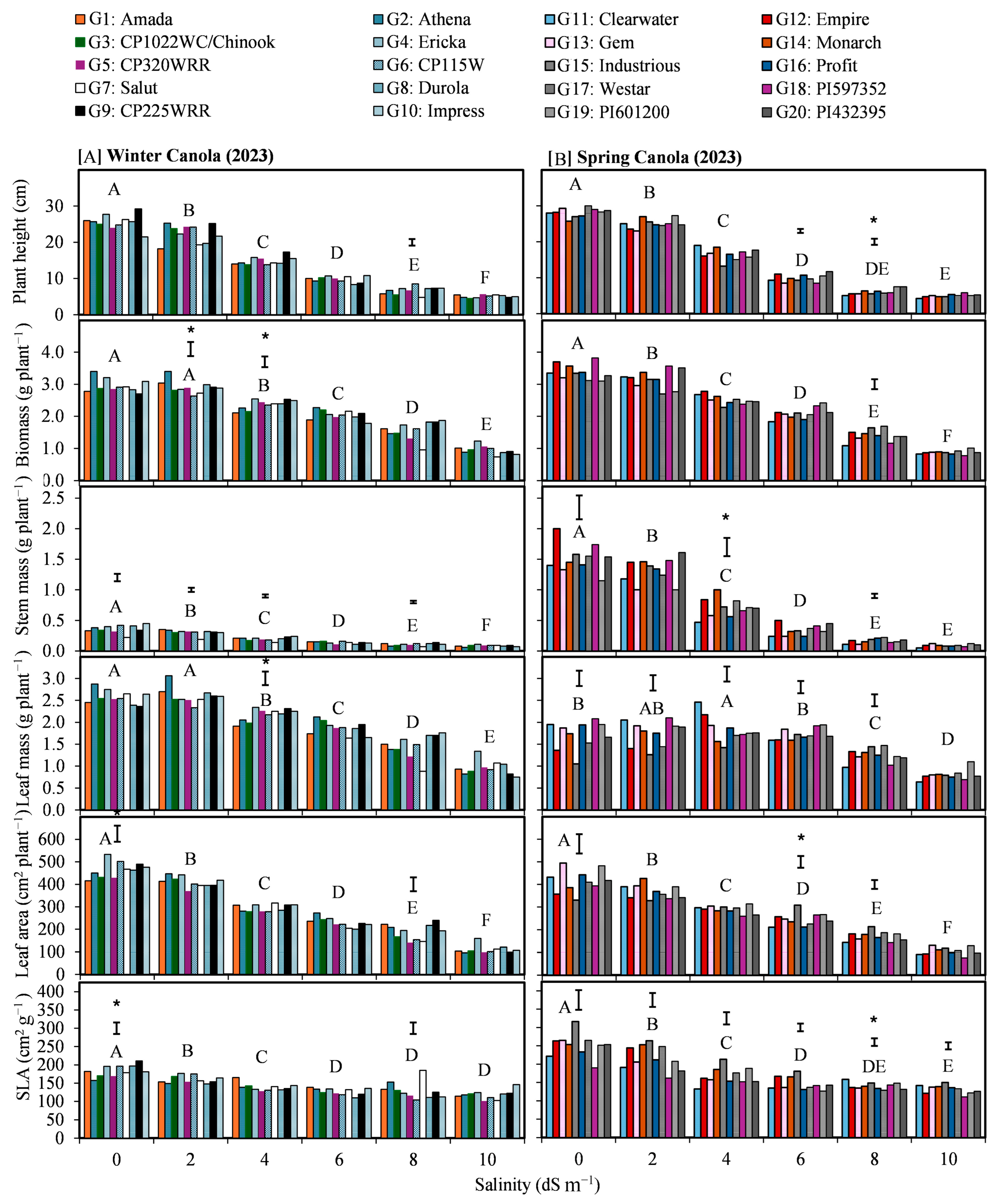
3.3. Genotypic Variations Under Salinity
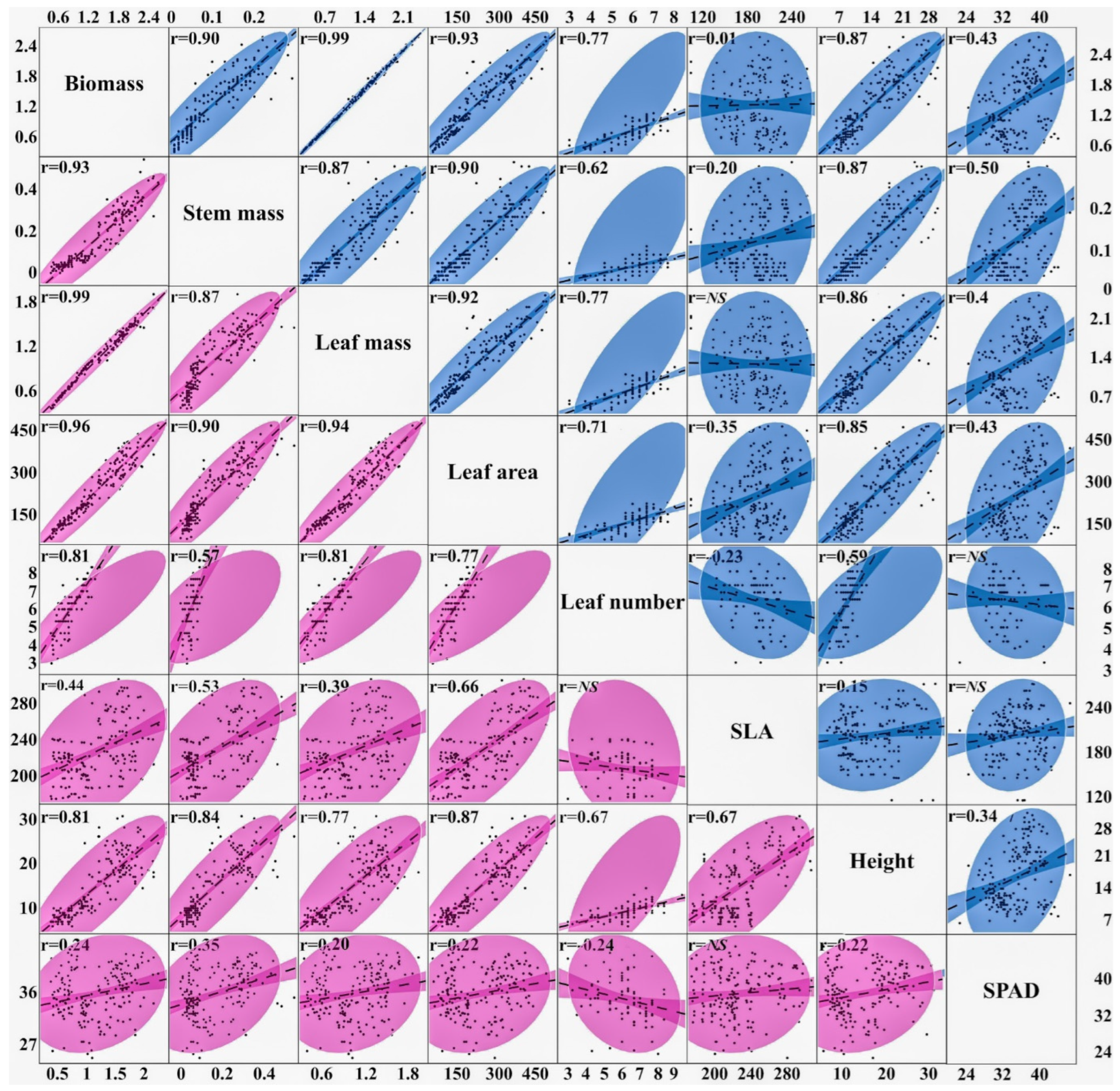
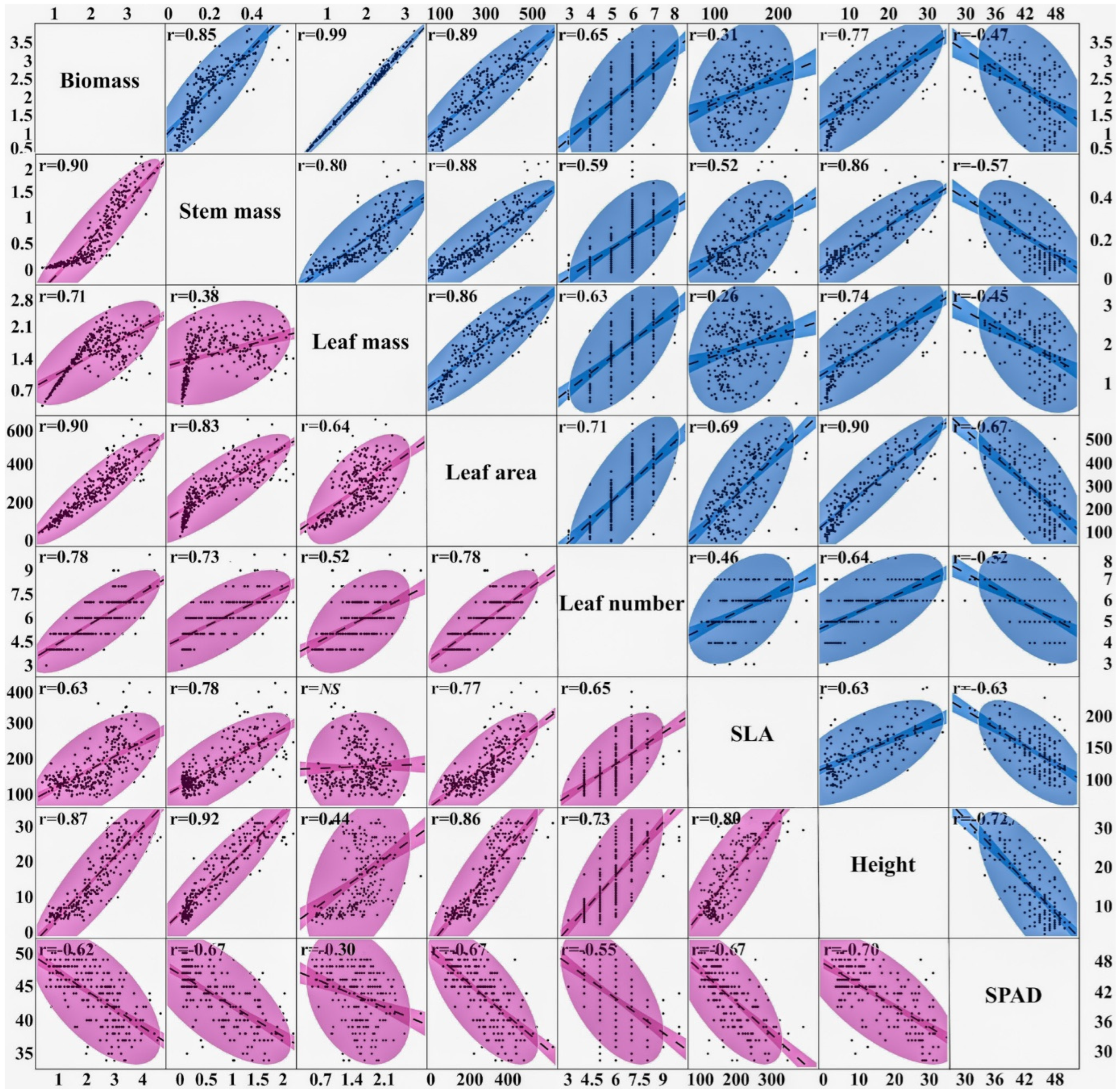
3.4. Correlations and Heritability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Control treatment |
| DAT | Days after transplanting |
| EC | Electrical conductivity |
| LSD | Least Significant Difference |
| SLA | Specific leaf area |
| ST | Salinity treatment |
References
- Cox, C.; Jin, L.; Ganjegunte, G.; Borrok, D.; Lougheed, V.; Ma, L. Soil quality changes due to flood irrigation in agricultural fields along the Rio Grande in western Texas. Appl. Geochem. 2018, 90, 87–100. [Google Scholar] [CrossRef]
- Flynn, R.; Ulery, A. An Introduction to Soil Salinity and Sodium Issues in New Mexico; Circular 656; New Mexico State University: Las Cruces, NM, USA, 2011; pp. 1–12. [Google Scholar]
- Sabagh, A.E.; Hossain, A.; Barutçular, C.; Islam, M.S.; Ratnasekera, D.; Kumar, N.; Meena, R.S.; Gharib, H.S.; Saneoka, H.; Silva, J.A.T.D. Drought and salinity stress management for higher and sustainable canola (Brassica napus L.) production: A critical review. Aust. J. Crop Sci. 2019, 13, 88–97. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- FAO. Global Symposium on Salt-Affected Soils: Outcome Document; FAO: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; De Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh water resource, scarcity, water salinity challenges and possible remedies: A review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Mehta, S.; Fryar, A.E.; Brady, R.M.; Morin, R.H. Modeling regional salinization of the Ogallala aquifer, Southern High Plains, TX, USA. J. Hydrol. 2000, 238, 44–64. [Google Scholar] [CrossRef]
- Miyamoto, S. Salinization of Irrigated Urban Soils: A Case Study of El Paso, TX. 2012. Available online: https://oaktrust.library.tamu.edu/server/api/core/bitstreams/bc0c192b-3d26-4853-9dba-40eeba7c5a0e/content (accessed on 28 June 2025).
- Miyamoto, S.; Foster, M.; Trostle, C.; Glenn, E. Salt tolerance of oilseed crops during establishment. J. Arid Land Stud. 2012, 22, 147–151. [Google Scholar]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirb. Agric. 2016, 103, 229–238. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ. Exp. Bot. 2008, 63, 266–273. [Google Scholar] [CrossRef]
- Fehr, W.; Suza, W. Principles of Cultivar Development; Iowa State University Digital Press: Ames, IA, USA, 2025. [Google Scholar] [CrossRef]
- Ashraf, M.; McNeilly, T. Salinity tolerance in Brassica oilseeds. Crit. Rev. Plant Sci. 2004, 23, 157–174. [Google Scholar] [CrossRef]
- Bandehagh, A.; Dehghanian, Z.; Henry, R.; Anwar Hossain, M. Salinity tolerance in canola: Insights from proteomic studies. In Brassica Breeding and Biotechnology; Aminul Islam, A.K.M., Anwar Hossain, M., Mominul Islam, A.K.M., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Iqbal, M.; Athar, H.-U.-R.; Ibrahim, M.; Javed, M.; Zafar, Z.U.; Ashraf, A. Leaf proteome analysis signified that photosynthesis and antioxidants are key indicators of salinity tolerance in canola (Brassica napus L.). Pak. J. Bot. 2019, 51, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ulfat, M.; Athar, H.-R.; Khan, Z.; Kalaji, H.M. RNAseq analysis reveals altered expression of key ion transporters causing differential uptake of selective ions in Canola (Brassica napus L.) grown under NaCl stress. Plants 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.T.; El-Sarkassy, N.M.; Fuller, M.P. Osmoregulators proline and glycine betaine counteract salinity stress in canola. Agron. Sustain. Dev. 2012, 32, 747–754. [Google Scholar] [CrossRef]
- Ali, E.A.; Galal, A.H.; Abou-Elwafa, S.F.; Abd El-Monem, A.M.A.; Saker, D.S.S. Study the response of five canola cultivars to foliar spraying by some antioxidants. J. Plant Prod. 2020, 11, 419–424. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of salt tolerance and interactive impact of Azotobacter chroococcum and/or Alcaligenes faecalis inoculation on Canola (Brassica napus L.) plants grown in saline soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef]
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34. [Google Scholar] [CrossRef]
- Sun, X.; Feng, X.; Li, C.; Zhang, Z.; Wang, L. Study on salt tolerance with YHem1 transgenic canola (Brassica napus). Physiol. Plant. 2015, 154, 223–242. [Google Scholar] [CrossRef]
- Kandil, A.; Sharief, A.; Abido, W.; Ibrahim, M. Response of some canola cultivars (Brassica napus L.) to salinity stress and its effect on germination and seedling properties. J. Crop Sci. 2012, 3, 98–103. [Google Scholar]
- Mousavi, M.; Omidi, H. Seed priming with bio-priming improves stand establishment, seed germination and salinity tolerance in canola cultivar (Hayola 401). Iran. J. Plant Physiol. 2019, 9, 2807–2817. [Google Scholar]
- Ahmadi, S.H.; Ardekani, J.N. The effect of water salinity on growth and physiological stages of eight Canola (Brassica napus) cultivars. Irrig. Sci. 2006, 25, 11–20. [Google Scholar] [CrossRef]
- Anagholi, A.; Tabatabaee, S.A. Salinity tolerance indices of Barley, Cotton, Canola, and Forage sorghum cultivars. Iran. J. Soil Res. 2019, 33, 45–59. [Google Scholar] [CrossRef]
- Jan, S.; Shinwari, Z.; Rabbani, M. Morpho-biochemical evaluation of Brassica rapa sub-species for salt tolerance. Genetika 2016, 48, 323–338. [Google Scholar] [CrossRef]
- Kholghi, M.; Toorchi, M.; Bandeh-Hagh, A.; Shakiba, M.R. An evaluation of canola genotypes under salinity stress at vegetative stage via morphological and physiological traits. Pak. J. Bot. 2018, 50, 447–455. [Google Scholar]
- Shrestha, R.; Xue, Q.; Leiva Soto, A.; Ganjegunte, G.; Palmate, S.S.; Chaganti, V.N.; Kumar, S.; Ulery, A.L.; Flynn, R.P.; Zapata, S. Seedling emergence in winter and spring canola genotypes under salinity stress. Crop Sci. 2025, 65, e70011. [Google Scholar] [CrossRef]
- Bybordi, A. The influence of salt stress on seed germination, growth and yield of canola cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 128–133. [Google Scholar]
- Kamrani, M.H.; Hosseinniya, H.; Chegeni, A.R. Effect of salinity on the growth characteristics of canola (Brassica napus L.). Tech. J. Eng. App. Sci. 2013, 3, 2327–2333. [Google Scholar]
- Steppuhn, H.; Raney, J.P. Emergence, height, and yield of canola and barley grown in saline root zones. Can. J. Plant Sci. 2005, 85, 815–827. [Google Scholar] [CrossRef]
- Grewal, H.S. Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric. Water Manag. 2010, 97, 148–156. [Google Scholar] [CrossRef]
- Gul, H.S.; Ulfat, M.; Zafar, Z.U.; Haider, W.; Ali, Z.; Manzoor, H.; Afzal, S.; Ashraf, M.; Athar, H.-R. Photosynthesis and salt exclusion are key physiological processes contributing to salt tolerance of Canola (Brassica napus L.): Evidence from physiology and transcriptome analysis. Genes 2022, 14, 3. [Google Scholar] [CrossRef]
- Mukhtar, E.; Siddiqi, E.H.; Bhatti, K.; Nawaz, K.; Hussain, K. Gas exchange attributes can be valuable selection criteria for salinity tolerance in canola cultivars (Brassica napus L.). Pak. J. Bot. 2013, 45, 35–40. [Google Scholar]
- Rasheed, R.; Ashraf, M.A.; Parveen, S.; Iqbal, M.; Hussain, I. Effect of salt stress on different growth and biochemical attributes in two canola (Brassica napus L.) cultivars. Commun. Soil Sci. Plant Anal. 2014, 45, 669–679. [Google Scholar] [CrossRef]
- Steppuhn, H.; Volkmar, K.M.; Miller, P.R. Comparing Canola, Field Pea, Dry Bean, and Durum Wheat crops grown in saline media. Crop Sci. 2001, 41, 1827–1833. [Google Scholar] [CrossRef]
- Alsharari, S.F.; Ibrahim, A.A.; Okasha, S.A. Combining ability for yield, oil content, and physio-biochemical characters of canola (Brassica napus L.) under salt stress conditions. SABRAO J. Breed. Genet. 2023, 55, 1003–1024. [Google Scholar] [CrossRef]
- Francois, L.E. Growth, seed yield, and oil content of canola grown under saline conditions. Agron. J. 1994, 86, 233–237. [Google Scholar] [CrossRef]
- Sharifi, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A. Effect of drought and salinity stresses morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2017, 15, 1859–1866. [Google Scholar] [CrossRef]
- Bandehagh, A.; Motienoparvar, P.; Heidari, A. Chlorophyll content and Chlorophyll Fluorescence in canola cultivars in response to salinity. Int. J. Adv. Sci. Eng. Technol. 2015, 5, 90–93. [Google Scholar]
- Huang, J.; Redmann, R.E. Salt tolerance of Hordeum and Brassica species during germination and early seedling growth. Can. J. Plant Sci. 1995, 75, 815–819. [Google Scholar] [CrossRef]
- Rameeh, V. Ions uptake, yield and yield attributes of rapeseed exposed to salinity stress. J. Soil Sci. Plant Nutr. 2012, 12, 851–861. [Google Scholar] [CrossRef]
- Tahmasebpour, B.; Nojadeh, M.S.; Esmaeilpour, M. Salt stress tolerance of spring canola (Brassica napus L.) cultivars. Int. J. Plant Biol. Res. 2018, 6, 1098. [Google Scholar]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, H.; Naeini, M. Effects of salinity stress on the morphology and yield of two cultivars of canola (Brassica napus L.). J. Agron. 2007, 6, 409–414. [Google Scholar] [CrossRef]

| ID. | Genotype | Type | Source/Origin |
|---|---|---|---|
| G1 | Amanda | Winter | University of Idaho, Moscow, ID, USA |
| G2 | Athena | Winter | University of Idaho, USA |
| G3 | CP1022WC/Chinook | Winter | University of Idaho, USA |
| G4 | Ericka—PI 597353 | Winter | University of Idaho, USA |
| G5 | CP320WRR | Winter | CROPLAN-WinField United, Arden Hills, MN, USA |
| G6 | CP115W | Winter | CROPLAN-WinField United, Minnesota, USA |
| G7 | Salut | Winter | Sweden |
| G8 | Durola | Winter | University of Idaho, USA |
| G9 | CP225WRR | Winter | CROPLAN-WinField United, Minnesota, USA |
| G10 | Impress | Winter | University of Idaho, USA |
| G11 | Clearwater | Spring | University of Idaho, USA |
| G12 | Empire | Spring | University of Idaho, USA |
| G13 | Gem | Spring | University of Idaho, USA |
| G14 | Monarch | Spring | University of Idaho, USA |
| G15 | Industrious | Spring | University of Idaho, USA |
| G16 | Profit | Spring | Canada |
| G17 | Westar—PI 649150 | Spring | Canada |
| G18 | Sunrise—PI597352 | Spring | University of Idaho, USA |
| G19 | Global—PI601200 | Spring | Sweden |
| G20 | PI432395 | Spring | Bangladesh |
| Target Salinity Level (EC, dS m−1) | 2022 | 2023 | ||
|---|---|---|---|---|
| NaCl g/L H2O | Measured EC (dS m−1) | NaCl g/L H2O | Measured EC (dS m−1) | |
| 0 (Control) | 0.00 (tap H2O) | 0.72 | 0.00 (tap H2O) | 0.66 |
| 2 | 0.65 | 2.27 | 0.78 | 1.89 |
| 4 | 1.65 | 3.82 | 1.95 | 3.94 |
| 6 | 2.65 | 6.03 | 3.12 | 5.78 |
| 8 | 3.65 | 7.71 | 4.29 | 7.99 |
| 10 | 4.65 | 9.69 | 5.46 | 10.3 |
| Source | Plant Height | Biomass | Stem Mass | Leaf Mass | Leaf Area | Leaves Number | SLA | SPAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | p-Value | df | p-Value | df | p-Value | df | p-Value | df | p-Value | df | p-Value | df | p-Value | df | p-Value | |
| 2022 | ||||||||||||||||
| Winter type | ||||||||||||||||
| Replication (R) | 2 | 0.9668 | 2 | 0.5962 | 2 | 0.1769 | 2 | 0.6917 | 2 | 0.8856 | 2 | 0.0077 | 2 | 0.788 | 2 | 0.246 |
| Salinity (S) | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 2 | 0.0001 | 5 | <0.0001 | 5 | 0.0061 |
| R × S | 10 | <0.0001 | 10 | <0.0001 | 10 | <0.0001 | 10 | <0.0001 | 10 | <0.0001 | 4 | 0.9643 | 10 | 0.9056 | 10 | <0.0001 |
| Genotype (G) | 9 | 0.0525 | 9 | 0.0901 | 9 | <0.0001 | 9 | 0.2082 | 9 | <0.0001 | 9 | <0.0001 | 9 | <0.0001 | 9 | 0.0001 |
| G × S | 45 | 0.408 | 45 | 0.4371 | 45 | 0.0183 | 45 | 0.2178 | 45 | 0.0004 | 18 | 0.6157 | 45 | <0.0001 | 45 | 0.658 |
| Spring type | ||||||||||||||||
| R | 2 | 0.8801 | 2 | 0.2237 | 2 | 0.3927 | 2 | 0.1917 | 2 | 0.174 | 2 | 0.4812 | 2 | 0.058 | 2 | 0.091 |
| S | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 2 | 0.0017 | 5 | <0.0001 | 5 | 0.011 |
| R × S | 10 | <0.0001 | 10 | 0.0002 | 10 | <0.0001 | 10 | 0.0036 | 10 | 0.0441 | 4 | 0.3167 | 10 | 0.7505 | 10 | 0.0003 |
| G | 9 | 0.0364 | 9 | 0.0061 | 9 | 0.011 | 9 | 0.0014 | 9 | 0.0007 | 9 | 0.0128 | 9 | <0.0001 | 9 | 0.003 |
| G × S | 45 | 0.2915 | 45 | 0.6273 | 45 | 0.3828 | 45 | 0.4916 | 45 | 0.0018 | 18 | 0.2845 | 45 | <0.0001 | 45 | 0.391 |
| 2023 | ||||||||||||||||
| Winter type | ||||||||||||||||
| R | 2 | 0.0161 | 3 | 0.0021 | 3 | 0.014 | 3 | 0.0067 | 3 | 0.2913 | 2 | 0.3303 | 3 | 0.0002 | 2 | 0.013 |
| S | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 |
| R × S | 10 | 0.0139 | 15 | 0.0453 | 15 | 0.1158 | 15 | 0.0026 | 15 | 0.0009 | 10 | 0.2437 | 15 | 0.0012 | 10 | 0.010 |
| G | 9 | 0.3208 | 9 | 0.2087 | 9 | 0.0002 | 9 | 0.2254 | 9 | 0.0009 | 9 | <0.0001 | 9 | 0.1255 | 9 | 0.533 |
| G × S | 45 | 0.5697 | 45 | 0.2665 | 45 | 0.0061 | 45 | 0.1611 | 45 | 0.1246 | 45 | 0.0292 | 45 | 0.0033 | 45 | 0.293 |
| Spring type | ||||||||||||||||
| R | 2 | 0.1698 | 3 | 0.0028 | 3 | 0.0248 | 3 | 0.0038 | 3 | 0.0238 | 2 | 0.1751 | 3 | 0.1718 | 2 | 0.598 |
| S | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 | 5 | <0.0001 |
| R × S | 10 | 0.7119 | 15 | 0.0149 | 15 | 0.2511 | 15 | 0.0043 | 15 | 0.4567 | 10 | 0.6299 | 15 | 0.0007 | 10 | 0.893 |
| G | 9 | 0.7692 | 9 | 0.2784 | 9 | <0.0001 | 9 | <0.0001 | 9 | 0.0005 | 9 | 0.0025 | 9 | <0.0001 | 9 | 0.176 |
| G × S | 45 | 0.9704 | 45 | 0.0354 | 45 | 0.0071 | 45 | <0.0001 | 45 | 0.0003 | 45 | 0.5583 | 45 | <0.0001 | 45 | 0.862 |
| Parameter | Canola Type | 2022 | 2023 | Genotypic Heritability (H2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (CT) | Salinity (ST) | ST Impact (% CT) | CT vs. ST (p-Value) | CT | ST | ST Impact (% CT) | CT vs. ST | |||
| (p-Value) | ||||||||||
| Biomass (g plant−1) | Winter (W) | 1.96 a | 1.10 a | −43.9 | <0.0001 | 2.96 b | 2.00 b | −32.4 | <0.0001 | 0.43 |
| Spring (S) | 1.59 b | 1.09 a | −31.4 | <0.0001 | 3.33 a | 2.09 a | −37.2 | <0.0001 | 0.38 | |
| Stem mass (g plant−1) | W | 0.19 b | 0.08 b | −57.9 | <0.0001 | 0.36 b | 0.17 b | −52.8 | <0.0001 | 0.12 |
| S | 0.30 a | 0.13 a | −56.7 | <0.0001 | 1.48 a | 0.52 a | −64.9 | <0.0001 | 0.28 | |
| Leaf mass (g plant−1) | W | 1.77 a | 1.02 a | −42.4 | <0.0001 | 2.57 a | 1.83 a | −28.8 | <0.0001 | 0.45 |
| S | 1.29 b | 0.96 b | −25.6 | <0.0001 | 1.62 b | 1.51 b | −6.8 | 0.0003 | 0.39 | |
| Leaf area (cm2 plant−1) | W | 345.89 a | 192.31 b | −44.4 | <0.0001 | 466.07 a | 247.52 a | −46.9 | <0.0001 | 0.41 |
| S | 351.13 a | 208.10 a | −40.7 | <0.0001 | 420.16 b | 237.36 b | −43.5 | <0.0001 | 0.38 | |
| Leaves number (plant−1) | W | - | 5.93 a | - | - | 6.57 b | 5.42 a | −17.5 | <0.0001 | 0.46 |
| S | - | 6.13 a | - | - | 7.05 a | 5.54 a | −21.4 | <0.0001 | 0.45 | |
| Height (cm) | W | 23.57 b | 12.55 a * | −46.8 | <0.0001 | 25.58 b | 11.81 b | −53.8 | <0.0001 | 0.44 |
| S | 24.98 a | 12.85 a * | −48.6 | <0.0001 | 27.95 a | 12.69 a | −54.6 | <0.0001 | 0.45 | |
| SPAD-Chlorophyll | W | 37.78 a | 34.16 b | −9.6 | <0.0001 | 38.13 a | 44.87 a | 17.7 | <0.0001 | 0.54 |
| S | 37.97 a | 35.35 a | −6.9 | <0.0001 | 38.64 a | 44.52 a | 15.2 | <0.0001 | 0.44 | |
| SLA (cm2 g−1) | W | 199.33 b | 190.04 b | −4.7 | <0.0001 | 184.35 b | 134.43 b | −27.1 | <0.0001 | 0.67 |
| S | 273.40 a | 217.42 a | −20.5 | <0.0001 | 269.37 a | 158.49 a | −41.2 | <0.0001 | 0.29 | |
| ID | Plant Height | Biomass | Stem Mass | Leaf Mass | Leaf Area | Specific Leaf Area | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | a | b | c | R2 | a | b | R2 | a | b | c | R2 | a | b | R2 | a | b | R2 | |
| Winter type | ||||||||||||||||||||
| G1 | 21.1 | −1.5 | 0.97 | 2.1 | −0.21 | 0.003 | 0.93 | 0.2 | 0.0 | 0.96 | 1.9 | −0.18 | 0.002 | 0.93 | 371 | −31.3 | 0.95 | - | - | - |
| G2 | 21.6 | −1.4 | 0.94 | 2.2 | −0.21 | 0.005 | 0.94 | 0.2 | 0.0 | 0.83 | 2.0 | −0.18 | 0.004 | 0.95 | 390 | −29.7 | 0.96 | - | - | - |
| G3 | 22.7 | −1.8 | 0.95 | 1.8 | −0.06 | −0.008 | 0.86 | 0.2 | 0.0 | 0.89 | 1.7 | −0.06 | −0.007 | 0.85 | 331 | −24.0 | 0.90 | - | - | - |
| G4 | 23.6 | −1.8 | 0.95 | 2.1 | −0.18 | 0.002 | 0.88 | 0.2 | 0.0 | 0.89 | 1.9 | −0.15 | 0.001 | 0.88 | 380 | −30.3 | 0.85 | - | - | - |
| G5 | 23.0 | −1.6 | 0.94 | 1.9 | −0.16 | 0.003 | 0.90 | 0.2 | 0.0 | 0.92 | 1.7 | −0.12 | 0.002 | 0.88 | 364 | −28.5 | 0.97 | - | - | - |
| G6 | 21.3 | −1.4 | 0.94 | 1.9 | −0.13 | - | 0.72 | 0.2 | 0.0 | 0.84 | 1.7 | −0.11 | - | 0.68 | 355 | −23.4 | 0.77 | - | - | - |
| G7 | 22.8 | −1.8 | 0.95 | 2.1 | −0.24 | 0.006 | 0.88 | 0.1 | 0.0 | 0.90 | 2.0 | −0.23 | 0.006 | 0.88 | 223 | −15.5 | 0.80 | 106 | 10.3 | 0.89 |
| G8 | 24.1 | −1.8 | 0.94 | 2.1 | −0.21 | 0.006 | 0.83 | 0.2 | 0.0 | 0.88 | 1.8 | −0.16 | 0.004 | 0.79 | 330 | −25.5 | 0.91 | - | - | - |
| G9 | 24.9 | −2.0 | 0.97 | 2.0 | −0.14 | −0.001 | 0.71 | 0.2 | 0.0 | 0.91 | 1.8 | −0.12 | −0.001 | 0.65 | 412 | −32.5 | 0.90 | 242 | −6.1 | 0.54 |
| G10 | 22.4 | −1.6 | 0.93 | 1.9 | −0.13 | −0.001 | 0.86 | 0.2 | 0.0 | 0.93 | 1.7 | −0.11 | −0.002 | 0.85 | 375 | −30.8 | 0.89 | - | - | - |
| Spring type | ||||||||||||||||||||
| G11 | 25.5 | −1.9 | 0.97 | 1.7 | −0.02 | −0.012 | 0.70 | 0.3 | 0.0 | 0.77 | 1.4 | 0.01 | −0.011 | 0.72 | 370 | −27.2 | 0.94 | - | - | - |
| G12 | 24.8 | −2.0 | 0.95 | 1.9 | −0.07 | −0.006 | 0.85 | 0.4 | 0.0 | 0.94 | 1.5 | −0.01 | −0.008 | 0.83 | 416 | −31.4 | 0.97 | 270 | −8.3 | 0.58 |
| G13 | 25.6 | −2.0 | 0.98 | 1.6 | −0.02 | −0.010 | 0.87 | 0.3 | 0.0 | 0.87 | 1.3 | 0.01 | −0.010 | 0.89 | 383 | −29.5 | 0.92 | - | - | - |
| G14 | 22.7 | −1.7 | 0.96 | 1.8 | −0.09 | −0.004 | 0.74 | 0.3 | 0.0 | 0.81 | 1.5 | −0.06 | −0.005 | 0.74 | 375 | −27.2 | 0.87 | - | - | - |
| G15 | 21.0 | −1.4 | 0.96 | 1.6 | 0.02 | −0.014 | 0.74 | 0.3 | 0.0 | 0.75 | 1.3 | 0.04 | −0.013 | 0.78 | 382 | −28.6 | 0.93 | 285 | −10.2 | 0.68 |
| G16 | 23.4 | −1.7 | 0.92 | 1.7 | −0.13 | 0.001 | 0.75 | 0.3 | 0.0 | 0.84 | 1.4 | −0.06 | −0.002 | 0.68 | 376 | −28.5 | 0.91 | 273 | −9.4 | 0.60 |
| G17 | 23.8 | −1.7 | 0.92 | 1.9 | −0.12 | - | 0.73 | 0.3 | 0.0 | 0.73 | 1.5 | −0.09 | - | 0.72 | 378 | −26.3 | 0.77 | 252 | −4.5 | 0.79 |
| G18 | 24.5 | −2.0 | 0.97 | 1.4 | 0.02 | −0.014 | 0.84 | 0.3 | 0.0 | 0.93 | 1.1 | 0.06 | −0.014 | 0.82 | 327 | −23.4 | 0.80 | - | - | - |
| G19 | 23.4 | −1.8 | 0.96 | 1.5 | 0.00 | −0.009 | 0.73 | 0.2 | 0.0 | 0.92 | 1.2 | 0.03 | −0.011 | 0.67 | 323 | −21.2 | 0.79 | - | - | - |
| G20 | 22.9 | −1.6 | 0.92 | 1.6 | −0.06 | −0.005 | 0.85 | 0.4 | 0.0 | 0.92 | 1.2 | 0.00 | −0.008 | 0.81 | 324 | −21.4 | 0.87 | - | - | - |
| ID | Plant Height | Biomass | Stem Mass | Leaf Mass | Leaf Area | Specific Leaf Area | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | a | b | c | R2 | a | b | R2 | a | b | c | R2 | a | b | R2 | a | b | R2 | |
| Winter type | ||||||||||||||||||||
| G1 | 23.5 | −2.05 | 0.94 | 2.9 | −0.11 | −0.008 | 0.86 | 0.3 | −0.03 | 0.91 | 2.6 | −0.08 | −0.008 | 0.86 | 441 | −31.6 | 0.95 | 178 | −6.1 | 0.88 |
| G2 | 26.2 | −2.36 | 0.92 | 3.5 | −0.19 | −0.007 | 0.91 | 0.4 | −0.03 | 0.96 | 3.0 | −0.10 | −0.012 | 0.88 | 471 | −35.6 | 0.94 | - | - | - |
| G3 | 25.3 | −2.30 | 0.94 | 2.9 | −0.08 | −0.011 | 0.93 | 0.3 | −0.03 | 0.91 | 2.6 | −0.04 | −0.013 | 0.93 | 448 | −34.8 | 0.96 | 170 | −5.4 | 0.86 |
| G4 | 26.5 | −2.36 | 0.98 | 3.2 | −0.16 | −0.003 | 1.00 | 0.4 | −0.03 | 0.89 | 2.8 | −0.11 | −0.003 | 0.99 | 505 | −38.2 | 0.95 | 185 | −7.4 | 0.81 |
| G5 | 25.1 | −2.14 | 0.93 | 3.0 | −0.10 | −0.010 | 0.95 | 0.3 | −0.03 | 0.88 | 2.6 | −0.05 | −0.012 | 0.96 | 428 | −34.3 | 0.99 | 165 | −6.6 | 0.95 |
| G6 | 25.0 | −2.14 | 0.91 | 2.9 | −0.08 | −0.010 | 0.99 | 0.4 | −0.03 | 0.90 | 2.5 | −0.03 | −0.012 | 0.99 | 476 | −40.0 | 0.97 | 186 | −9.3 | 0.86 |
| G7 | 24.3 | −2.16 | 0.94 | 2.9 | −0.07 | −0.016 | 0.92 | 0.2 | −0.01 | 0.90 | 2.8 | −0.18 | −0.002 | 0.85 | 462 | −37.6 | 0.98 | - | - | - |
| G8 | 23.8 | −2.08 | 0.94 | 2.9 | −0.03 | −0.017 | 0.92 | 0.4 | −0.03 | 0.90 | 2.5 | 0.01 | −0.015 | 0.90 | 447 | −33.3 | 0.94 | 173 | −7.4 | 0.70 |
| G9 | 28.6 | −2.63 | 0.95 | 2.8 | 0.05 | −0.023 | 0.96 | 0.3 | −0.03 | 0.95 | 2.4 | 0.09 | −0.024 | 0.96 | 472 | −35.9 | 0.95 | 183 | −7.8 | 0.70 |
| G10 | 22.9 | −1.86 | 0.96 | 3.1 | −0.11 | −0.010 | 0.90 | 0.4 | −0.04 | 0.93 | 2.7 | −0.05 | −0.013 | 0.88 | 474 | −37.3 | 0.98 | 171 | −4.8 | 0.59 |
| Spring type | ||||||||||||||||||||
| G11 | 28.6 | −2.70 | 0.95 | 3.5 | −0.22 | −0.007 | 0.95 | 1.3 | −0.15 | 0.89 | 2.0 | 0.13 | −0.028 | 0.80 | 441 | −36.1 | 0.99 | 199 | −7.1 | 0.54 |
| G12 | 27.4 | −2.52 | 0.97 | 3.7 | −0.22 | −0.007 | 1.00 | 1.8 | −0.20 | 0.95 | 1.3 | 0.27 | −0.032 | 0.65 | 384 | −26.2 | 0.95 | 256 | −14.7 | 0.89 |
| G13 | 27.7 | −2.60 | 0.94 | 3.2 | −0.14 | −0.010 | 0.99 | 1.2 | −0.13 | 0.91 | 1.8 | 0.11 | −0.022 | 0.95 | 473 | −36.9 | 0.98 | 235 | −12.5 | 0.78 |
| G14 | 27.9 | −2.51 | 0.92 | 3.7 | −0.24 | −0.004 | 0.98 | 1.6 | −0.16 | 0.91 | 1.7 | 0.05 | −0.013 | 0.92 | 425 | −31.0 | 0.92 | 256 | −13.3 | 0.90 |
| G15 | 26.7 | −2.51 | 0.91 | 3.4 | −0.19 | −0.006 | 0.95 | 1.5 | −0.16 | 0.93 | 1.0 | 0.26 | −0.027 | 0.71 | 366 | −20.0 | 0.79 | 299 | −17.3 | 0.93 |
| G16 | 27.3 | −2.44 | 0.96 | 3.4 | −0.22 | −0.004 | 0.99 | 1.4 | −0.15 | 0.88 | 1.9 | 0.05 | −0.016 | 0.94 | 433 | −34.3 | 0.99 | 220 | −10.6 | 0.79 |
| G17 | 28.3 | −2.67 | 0.94 | 3.1 | −0.10 | −0.011 | 0.98 | 1.5 | −0.15 | 0.96 | 1.4 | 0.16 | −0.021 | 0.72 | 413 | −29.8 | 1.00 | 257 | −15.1 | 0.86 |
| G18 | 28.2 | −2.60 | 0.93 | 3.9 | −0.29 | −0.003 | 0.93 | 1.7 | −0.18 | 0.92 | 2.1 | 0.03 | −0.017 | 0.85 | 401 | −31.0 | 0.95 | 183 | −6.6 | 0.90 |
| G19 | 28.7 | −2.59 | 0.93 | 3.1 | −0.07 | −0.014 | 0.92 | 1.2 | −0.12 | 0.95 | 1.9 | 0.03 | −0.012 | 0.73 | 469 | −34.9 | 0.99 | 238 | −12.7 | 0.87 |
| G20 | 28.4 | −2.50 | 0.98 | 3.5 | −0.16 | −0.011 | 0.92 | 1.6 | −0.17 | 0.89 | 1.7 | 0.11 | −0.021 | 0.97 | 409 | −31.3 | 0.99 | 222 | −11.4 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, R.; Xue, Q.; Soto, A.L.; Ganjegunte, G.; Palmate, S.S.; Chaganti, V.N.; Kumar, S.; Ulery, A.L.; Zapata, S. Plant Traits in Spring and Winter Canola Genotypes Under Salinity. Agronomy 2025, 15, 1657. https://doi.org/10.3390/agronomy15071657
Shrestha R, Xue Q, Soto AL, Ganjegunte G, Palmate SS, Chaganti VN, Kumar S, Ulery AL, Zapata S. Plant Traits in Spring and Winter Canola Genotypes Under Salinity. Agronomy. 2025; 15(7):1657. https://doi.org/10.3390/agronomy15071657
Chicago/Turabian StyleShrestha, Rajan, Qingwu Xue, Andrea Leiva Soto, Girisha Ganjegunte, Santosh Subhash Palmate, Vijayasatya N. Chaganti, Saurav Kumar, April L. Ulery, and Samuel Zapata. 2025. "Plant Traits in Spring and Winter Canola Genotypes Under Salinity" Agronomy 15, no. 7: 1657. https://doi.org/10.3390/agronomy15071657
APA StyleShrestha, R., Xue, Q., Soto, A. L., Ganjegunte, G., Palmate, S. S., Chaganti, V. N., Kumar, S., Ulery, A. L., & Zapata, S. (2025). Plant Traits in Spring and Winter Canola Genotypes Under Salinity. Agronomy, 15(7), 1657. https://doi.org/10.3390/agronomy15071657








