Silver Nanoparticles Embedded in Sodium Alginate: Antibacterial Efficacy and Effects on Red Cabbage Seedling Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents Used for the Synthesis of Polymer Composites with Silver Nanoparticles
2.2. Synthesis of Polymer Gels with Silver Nanoparticles
2.3. Physicochemical Analysis of Alginate Gels with Silver Nanoparticles
2.4. Experimental Treatments
- C–control–sterile water (experiment with bacteria) or deionized water (experiment with plants)
- Alg(20)–sodium alginate solution without silver nanoparticles diluted 7.5-fold
- Alg(60)–sodium alginate solution without silver nanoparticles diluted 2.5-fold
- 20 mg/L AgNPs–solution with silver nanoparticles at a concentration of 20 mg/L
- 60 mg/L AgNPs–solution with silver nanoparticles at a concentration of 60 mg/L.
2.5. Antibacterial ActivityAssay of Silver Nanoparticles
2.6. Growth of Red Cabbage Seedlings in the Presence of Silver Nanoparticles
2.7. Antioxidant Properties Assessment
2.8. Statistical Analysis
3. Results
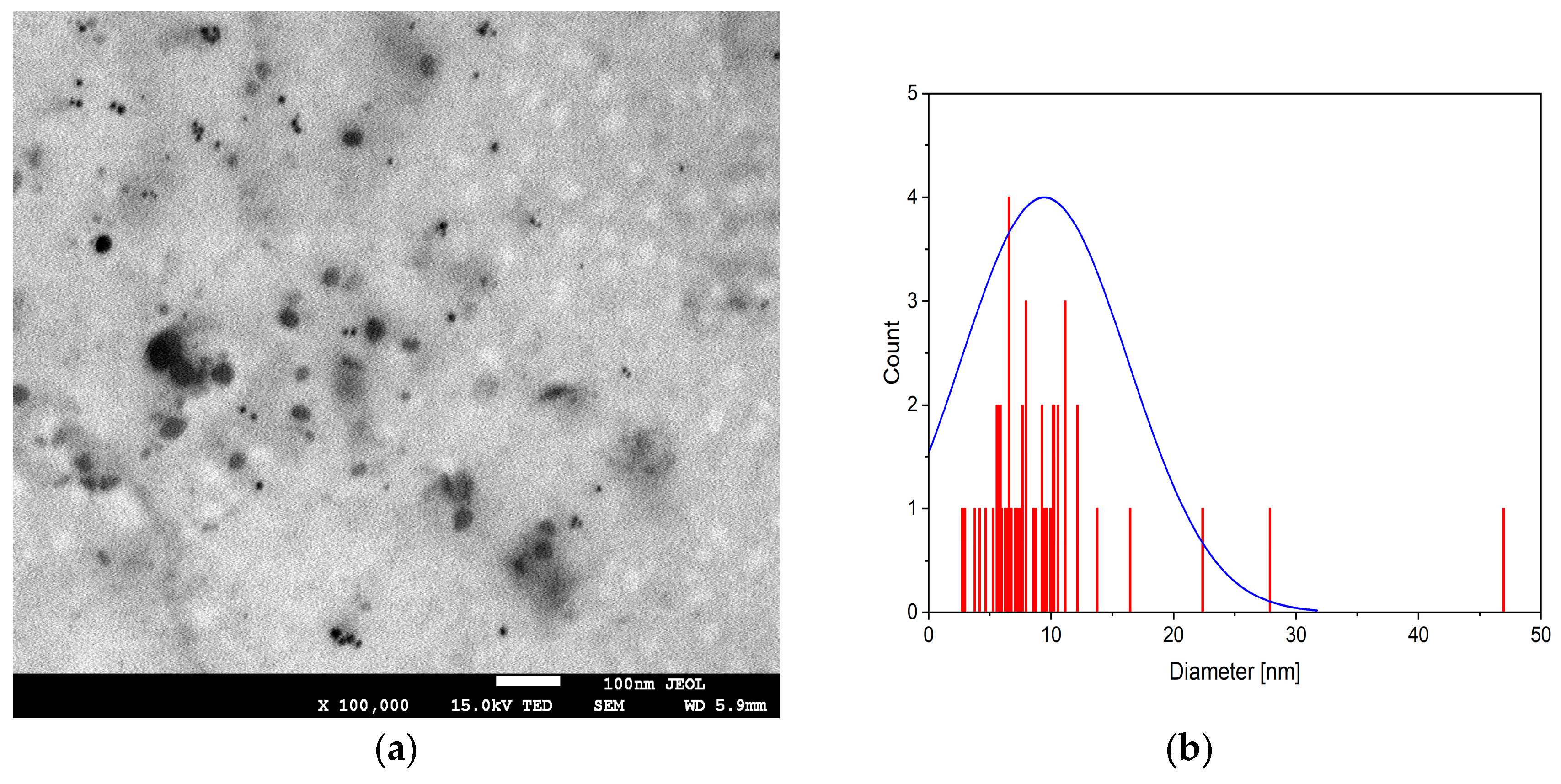
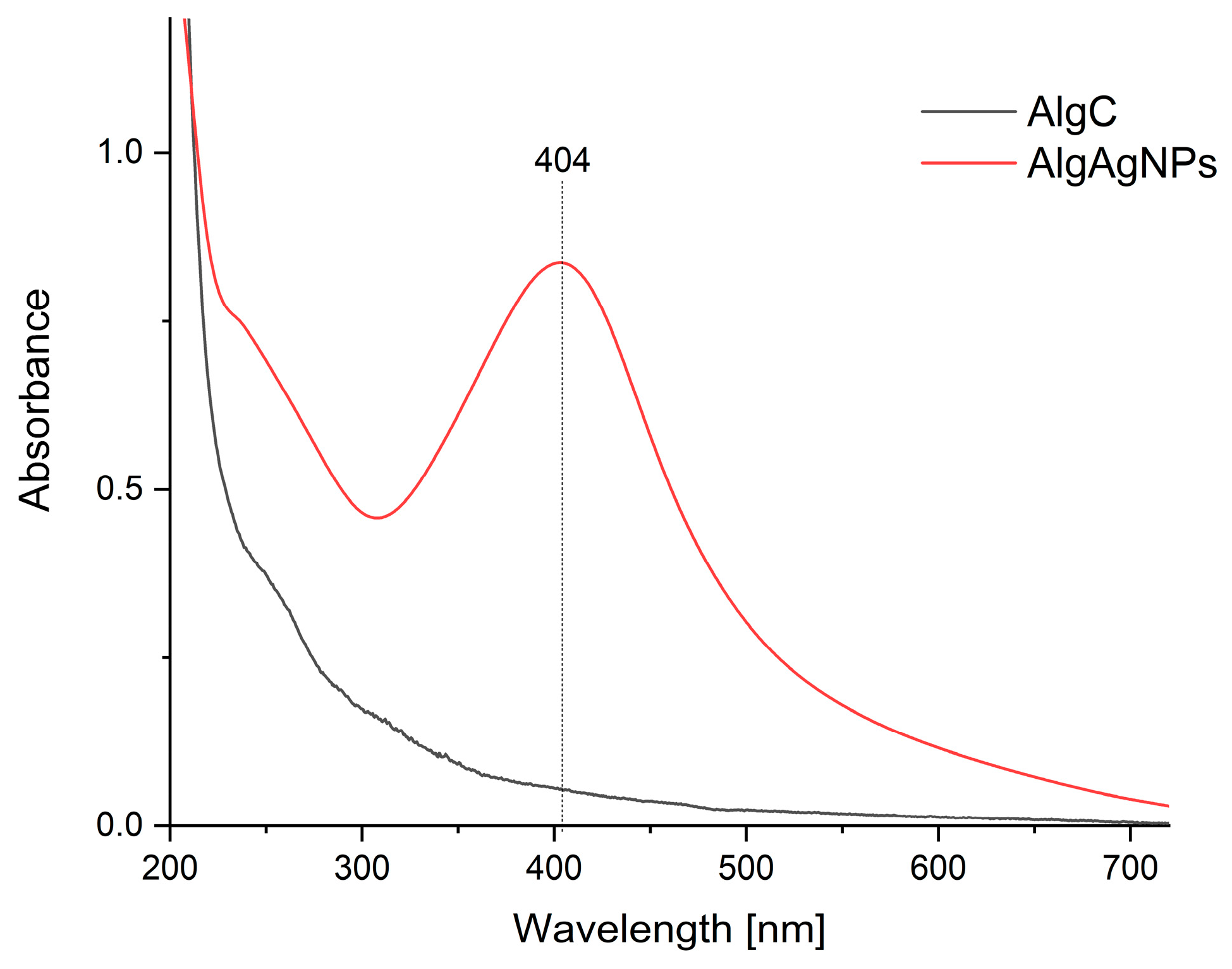
3.1. Physicochemical Characteristics of Alginate Gels with Silver Nanoparticles
3.2. Antibacterial Activity of Silver Nanoparticles
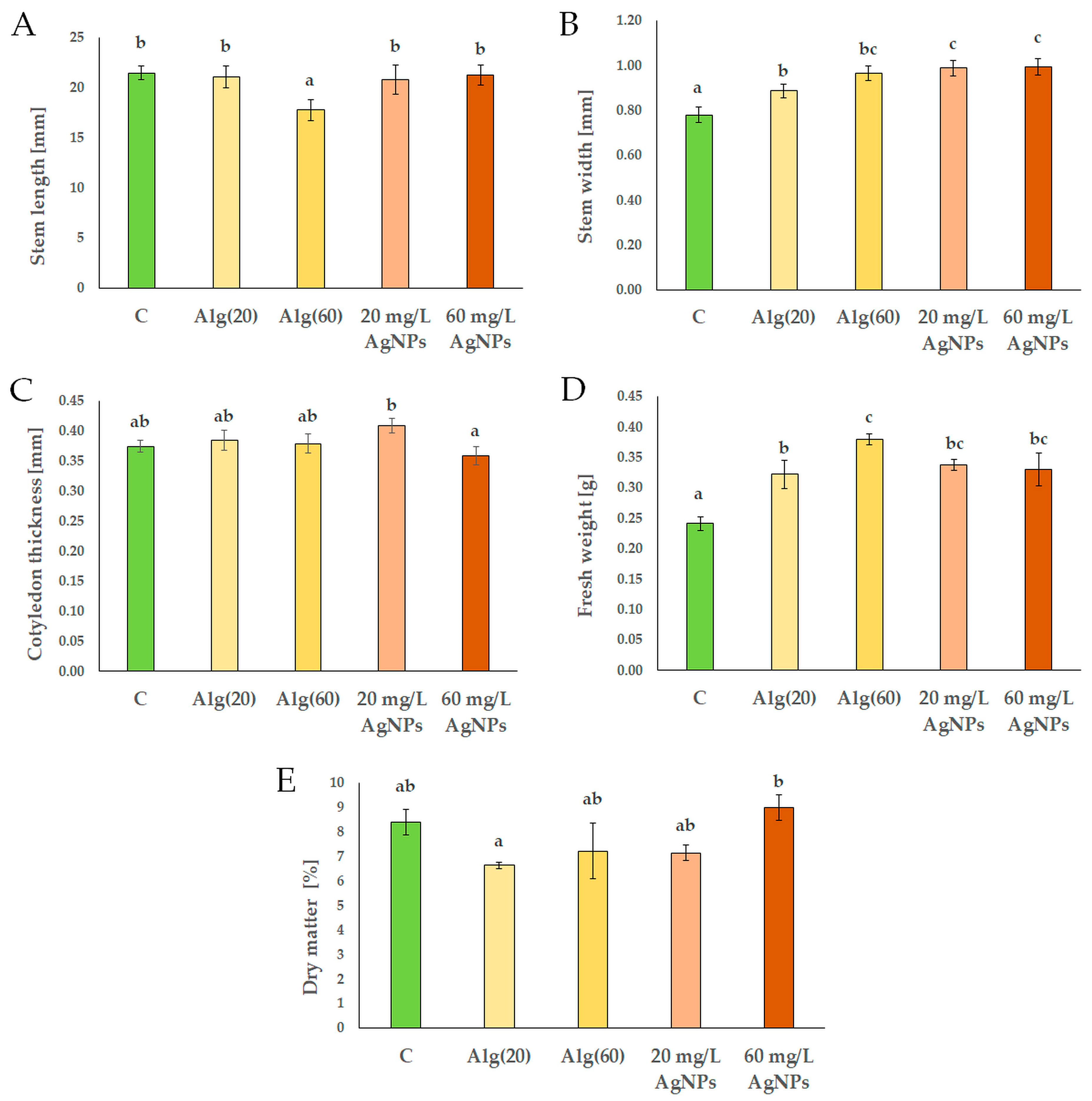
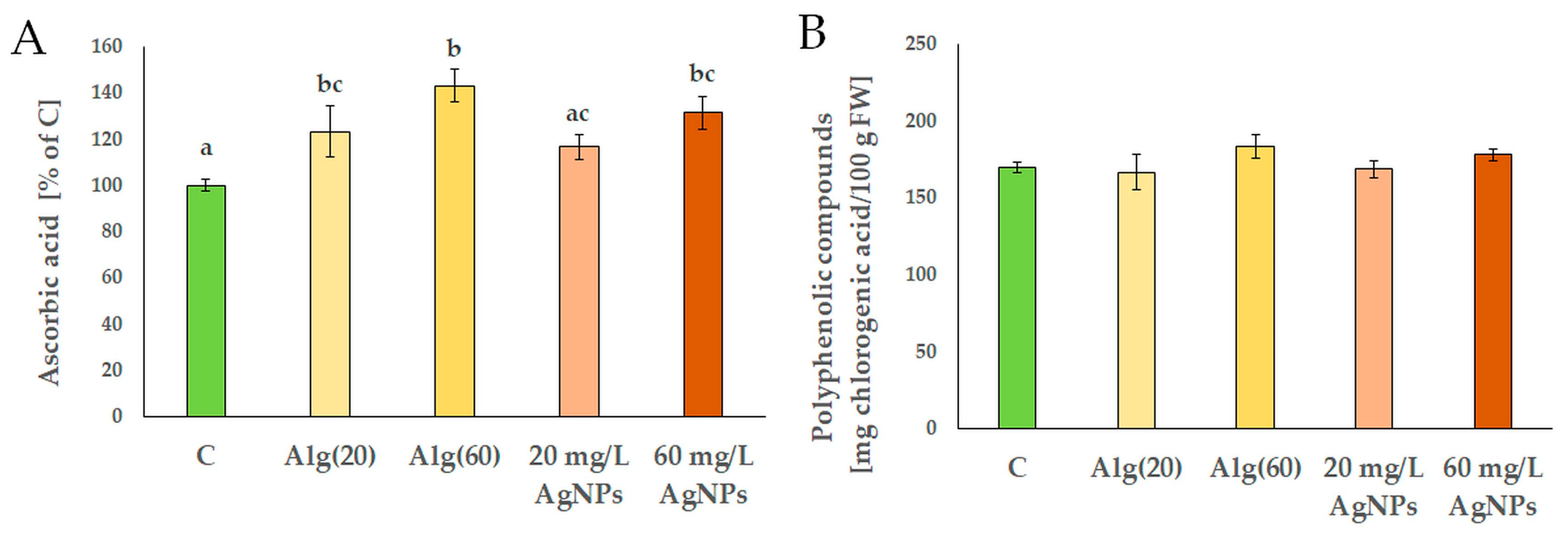
3.3. Features of Red Cabbage Seedlings in the Presence of Silver Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alfei, S.; Zuccari, G. Last Fifteen Years of Nanotechnology Application with Our Contribute. Nanomaterials 2025, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, U.T.; Velidandi, A. An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability 2025, 17, 1250. [Google Scholar] [CrossRef]
- Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Černe, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. [Google Scholar] [CrossRef]
- Ragasová, L.; Peňázová, E.; Gazdík, F.; Pečenka, J.; Čechová, J.; Pokluda, R.; Baránek, M.; Grzebelus, D.; Eichmeier, A. The Change of Bacterial Spectrum after Storage of X. campestris pv. campestris Inoculated Cabbage Heads (Brassica oleracea var. capitata L.). Agronomy 2020, 10, 443. [Google Scholar] [CrossRef]
- Gai, Y.; Wang, H. Plant Disease: A Growing Threat to Global Food Security. Agronomy 2024, 14, 1615. [Google Scholar] [CrossRef]
- Esmael, A.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Filimban, A.A.R.; Alseghayer, M.S.; Almaneea, A.M.; Alhadlaq, M.A.; Ayubu, J.; Teklemariam, A.D. Fresh Produce as a Potential Vector and Reservoir for Human Bacterial Pathogens: Revealing the Ambiguity of Interaction and Transmission. Microorganisms 2023, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Wrońska, N.; Płaczkowska, S.; Niedziałkowska, K.; Lisowska, K. The Synergistic Effect of Biosynthesized Silver Nanoparticles and Phytocompound as a Novel Approach to the Elimination of Pathogens. Molecules 2023, 28, 7921. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kabanov, D.; Kepinska, M.; Narayanan, V.H.B.; Parikesit, A.A.; Fernandez, C.; Bjørklund, G.; Nguyen, H.V.; Farid, A.; Sochor, J.; et al. Effect of Biosynthesized Silver Nanoparticles on Bacterial Biofilm Changes in S. aureus and E. coli. Nanomaterials 2022, 12, 2183. [Google Scholar] [CrossRef]
- Alfeqy, M.M.; El-Hawary, S.S.; El-Halawany, A.M.; Rabeh, M.A.; Alshehri, S.A.; Abdelmohsen, U.R.; Safwat, N.A.; Serry, A.M.; Fahmy, H.A.; Ezzat, M.I. Biosynthesis and Characterization of Aeonium arboreum-Derived Silver Nanoparticles: Antimicrobial Activity, Biofilm Inhibition, Antihemolytic Activity, and In Silico Studies. Int. J. Mol. Sci. 2024, 25, 8039. [Google Scholar] [CrossRef]
- Rutkowski, M.; Krzemińska-Fiedorowicz, L.; Khachatryan, G.; Bulski, K.; Kołton, A.; Khachatryan, K. Biodegradable Silver Nanoparticles Gel and Its Impact on Tomato Seed Germination Rate in In Vitro Cultures. Appl. Sci. 2022, 12, 2722. [Google Scholar] [CrossRef]
- Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers. Molecules 2024, 29, 1937. [Google Scholar] [CrossRef]
- Nam, H.-B.; Lee, K.H.; Yoo, H.Y.; Park, C.; Lim, J.-M.; Lee, J.H. Rapid and High-Yield Recovery of Sodium Alginate from Undaria pinnatifida via Microwave-Assisted Extraction. Processes 2024, 12, 208. [Google Scholar] [CrossRef]
- Sharma, R.; Malviya, R.; Singh, S.; Prajapati, B. A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels 2023, 9, 430. [Google Scholar] [CrossRef]
- Rutkowski, M.; Krzemińska-Fiedorowicz, L.; Khachatryan, K.; Khachatryan, G.; Kalisz, A.; Sękara, A. Impact of silver nanoparticles in alginate gels on seed germination, growth and stress biochemical parameters of cucumber seedlings. Plant Stress 2024, 12, 100491. [Google Scholar] [CrossRef]
- Stoica, R.; Ganciarov, M.; Constantinescu-Aruxandei, D.; Capră, L.; Șuică-Bunghez, I.-R.; Senin, R.-M.; Pricope, G.D.; Ivan, G.-R.; Călin, C.; Oancea, F. Sustainable Recovery of Anthocyanins and Other Polyphenols from Red Cabbage Byproducts. Foods 2023, 12, 4157. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Gomez-Perez, L.S.; Zepeda, F.; Vidal, R.L.; Grunenwald, F.; Mejías, N.; Pasten, A.; Araya, M.; Ah-Hen, K.S. Assessment of Bio-Compounds Content, Antioxidant Activity, and Neuroprotective Effect of Red Cabbage (Brassica oleracea var. capitata rubra) Processed by Convective Drying at Different Temperatures. Antioxidants 2023, 12, 1789. [Google Scholar] [CrossRef]
- Mejías, N.; Vega-Galvez, A.; Gomez-Perez, L.S.; Pasten, A.; Uribe, E.; Cortés, A.; Valenzuela-Barra, G.; Camus, J.; Delporte, C.; Bernal, G. Health-Promoting Properties of Processed Red Cabbage (Brassica oleracea var. capitata f. rubra): Effects of Drying Methods on Bio-Compound Retention. Foods 2024, 13, 830. [Google Scholar] [CrossRef]
- Youssef, E.A.; Abdelbaset, M.M.; Dewedar, O.M.; Molina-Martínez, J.M.; El-Shafie, A.F. Crop Coefficient Estimation and Effect of Abscisic Acid on Red Cabbage Plants (Brassica oleracea var. capitata) under Water-Stress Conditions. Agriculture 2023, 13, 610. [Google Scholar] [CrossRef]
- Aloo, S.-O.; Ofosu, F.-K.; Daliri, E.-B.-M.; Oh, D.-H. UHPLC-ESI-QTOF-MS/MS Metabolite Profiling of the Antioxidant and Antidiabetic Activities of Red Cabbage and Broccoli Seeds and Sprouts. Antioxidants 2021, 10, 852. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.V.; George, N.; Walsh, L.J. An in vitro study of the antimicrobial activity of some endodontic medicaments and their bases using an agar well diffusion assay. Aust. Dent. J. 2009, 54, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lee, J.; Durst, W.R.; Wrolstad, E.R. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 5. [Google Scholar] [CrossRef]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, T.M.; Paraggio, M.; Viggiano, M.; Lattanzi, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Songca, S.P.; Jayachandran, V.; Rouxel, D.; Joubert, O.; Kalarikkal, N.; Thomas, S. Synthesis, antibacterial, cytotoxicity and sensing properties of starch-capped silver nanoparticles. J. Mol. Liq. 2016, 213, 75–81. [Google Scholar] [CrossRef]
- Hovhannisyan, A.; Janik, M.; Woszczak, L.; Khachatryan, G.; Krystyjan, M.; Lenart-Boroń, A.; Stankiewicz, K.; Czernecka, N.; Duraczyńska, D.; Oszczęda, Z.; et al. The Preparation of Silver and Gold Nanoparticles in Hyaluronic Acid and the Influence of Low-Pressure Plasma Treatment on Their Physicochemical and Microbiological Properties. Int. J. Mol. Sci. 2023, 24, 17285. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Li, V.; O’Mullane, A.P.; Bhargava, S.K. Shape Dependent Electrocatalytic Behaviour of Silver Nanoparticles. CrystEngComm 2010, 12, 4280–4286. [Google Scholar] [CrossRef]
- Yang, Y.; Matsubara, S.; Xiong, L.; Hayakawa, T.; Nogami, M. Solvothermal Synthesis of Multiple Shapes of Silver Nanoparticles and Their SERS Properties. J. Phys. Chem. C 2007, 111, 9095–9104. [Google Scholar] [CrossRef]
- Janik, M.; Khachatryan, K.; Khachatryan, G.; Krystyjan, M.; Oszczęda, Z. Comparison of Physicochemical Properties of Silver and Gold Nanocomposites Based on Potato Starch in Distilled and Cold Plasma-Treated Water. Int. J. Mol. Sci. 2023, 24, 2200. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.-K. Influence of Nitrogen Source and Growth Phase on Extracellular Biosynthesis of Silver Nanoparticles Using Cultural Filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Korzekwa, K.; Kędziora, A.; Stańczykiewicz, B.; Bugla-Płoskońska, G.; Wojnicz, D. Benefits of Usage of Immobilized Silver Nanoparticles as Pseudomonas aeruginosa Antibiofilm Factors. Int. J. Mol. Sci. 2022, 23, 284. [Google Scholar] [CrossRef]
- Pernas-Pleite, C.; Conejo-Martínez, A.M.; Marín, I.; Abad, J.P. Green Extracellular Synthesis of Silver Nanoparticles by Pseudomonas alloputida, Their Growth and Biofilm-Formation Inhibitory Activities and Synergic Behavior with Three Classical Antibiotics. Molecules 2022, 27, 7589. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Oćwieja, M.; Ciesielski, S.; Halecki, W.; Matras, E.; Gorczyca, A. Chemical Structure of Stabilizing Layers of Negatively Charged Silver Nanoparticles as an Effector of Shifts in Soil Bacterial Microbiome under Short-Term Exposure. Int. J. Environ. Res. Public Health 2022, 19, 14438. [Google Scholar] [CrossRef]
- Tymoszuk, A. Silver Nanoparticles Effects on In Vitro Germination, Growth, and Biochemical Activity of Tomato, Radish, and Kale Seedlings. Materials 2021, 14, 5340. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Lisiecki, K.; Pańka, D. Response of Rapeseed (Brassica napus L.) to Silver and Gold Nanoparticles as a Function of Concentration and Length of Exposure. Agronomy 2022, 12, 2885. [Google Scholar] [CrossRef]
- Hasan, M.; Mehmood, K.; Mustafa, G.; Zafar, A.; Tariq, T.; Hassan, S.G.; Loomba, S.; Zia, M.; Mazher, A.; Mahmood, N.; et al. Phytotoxic Evaluation of Phytosynthesized Silver Nanoparticles on Lettuce. Coatings 2021, 11, 225. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Abdel-Aziz, H.M.M.; Seleiman, M.F.; Tourky, S.M.N. How Can Biological and Chemical Silver Nanoparticles Positively Impact Physio-Chemical and Chloroplast Ultrastructural Characteristics of Vicia faba Seedlings? Plants 2023, 12, 2509. [Google Scholar] [CrossRef] [PubMed]
- Gruyer, N.; Dorais, M.; Bastien, C.; Dassylva, N.; Triffault-Bouchet, G. Interaction between silver nanoparticles and plant growth. Acta Hortic. 2014, 1037, 795–800. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Jarzembowski, Ł.; Izydorczyk, G.; Mikula, K.; Hoppe, V.; Mielko, K.A.; Pudełko-Malik, N.; Młynarz, P.; Chojnacka, K.; Witek-Krowiak, A. Hydrogel Alginate Seed Coating as an Innovative Method for Delivering Nutrients at the Early Stages of Plant Growth. Polymers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Peharec Štefanić, P.; Košpić, K.; Lyons, D.M.; Jurković, L.; Balen, B.; Tkalec, M. Phytotoxicity of Silver Nanoparticles on Tobacco Plants: Evaluation of Coating Effects on Photosynthetic Performance and Chloroplast Ultrastructure. Nanomaterials 2021, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.-M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Eliwa, N.E.; Safwat, G. Role of gamma-irradiated sodium alginate on growth, physiological and active components of iceberg lettuce (Lactuca sativa) plant. BMC Plant Biol. 2024, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Jurkow, R.; Pokluda, R.; Sękara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, M.; Wang, S.; Tang, J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT Food Sci. Technol. 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]


| Samples | Water [g] | Sodium Alginate [g] | Solution with Ag+ [g] | Glycerol [g] | Fructose Solution 4%, [g] | Total Mass of Gel [g] | AgNPs Concentration [mg/L] |
|---|---|---|---|---|---|---|---|
| AlgC | 144.02 | 1.50 | 0.00 | 0.75 | 0.00 | 146.27 | 0.00 |
| AlgAgNPs | 95.50 | 1.50 | 18.52 | 0.75 | 30.00 | 146.27 | 150.00 |
| Treatments | Escherichia coli [mm] | Pseudomonas aeruginosa [mm] | Bacillus cereus [mm] | Enterococcus faecalis [mm] |
|---|---|---|---|---|
| C | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Alg(20) | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Alg(60) | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 20 mg/L AgNPs | 2.44 ± 0.06 b | 3.86 ± 0.05 b | 6.63 ± 0.07 b | 5.54 ± 0.13 b |
| 60 mg/L AgNPs | 2.88 ± 0.13 c | 3.95 ± 0.10 b | 7.07 ± 0.09 c | 5.85 ± 0.11 c |
| Treatments | Carotenoids [mg/g FW] | Chlorophyll a [mg/g FW] | Chlorophyll b [mg/g FW] | Chl a + Chl b [mg/g FW] | Chl a : Chl b | Chl : Car | Anthocyanins [mg/g FW] |
|---|---|---|---|---|---|---|---|
| C | 0.107 ± 0.009 a | 0.397 ± 0.033 ab | 0.164 ± 0.016 ab | 0.530 ± 0.033 a | 2.468 ± 0.159 a | 5.251 ± 0.194 d | 60.852 ± 1.898 ab |
| Alg(20) | 0.143 ± 0.008 c | 0.447 ± 0.019 bc | 0.180 ± 0.007 b | 0.627 ± 0.026 b | 2.480 ± 0.031 a | 4.419 ± 0.093 c | 55.341 ± 1.500 a |
| Alg(60) | 0.151 ± 0.009 c | 0.460 ± 0.035 c | 0.179 ± 0.013 b | 0.639 ± 0.045 b | 2.578 ± 0.122 a | 4.229 ± 0.106 bc | 68.140 ± 1.808 c |
| 20 mg/L AgNPs | 0.117 ± 0.007 ab | 0.337 ± 0.022 a | 0.138 ± 0.010 a | 0.475 ± 0.032 a | 2.451 ± 0.034 a | 4.046 ± 0.042 b | 58.035 ± 3.353 a |
| 60 mg/L AgNPs | 0.134 ± 0.004 bc | 0.358 ± 0.014 a | 0.138 ± 0.006 a | 0.496 ± 0.020 a | 2.603 ± 0.037 a | 3.685 ± 0.051 a | 64.593 ± 1.897 bc |
| Treatments | DPPH * [mM Trolox/g FW] | FRAP [mM Trolox/g FW] | CUPRAC [mM Trolox/g FW] |
|---|---|---|---|
| C | 2.979 ± 0.074 ab | 0.968 ± 0.028 a | 2.132 ± 0.139 abc |
| Alg(20) | 2.869 ± 0.098 a | 0.976 ± 0.064 a | 1.945 ± 0.121 ab |
| Alg(60) | 2.922 ± 0.078 ab | 1.081 ± 0.037 a | 2.330 ± 0.134 c |
| 20 mg/L AgNPs | 2.739 ± 0.068 a | 0.998 ± 0.040 a | 1.878 ± 0.040 a |
| 60 mg/L AgNPs | 3.158 ± 0.091 b | 1.074 ± 0.022 a | 2.282 ± 0.155 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowski, M.; Makowski, W.; Krzemińska-Fiedorowicz, L.; Khachatryan, K.; Kalisz, A.; Malina, D.; Chwastowski, J.; Wzorek, Z.; Khachatryan, G.; Sękara, A.; et al. Silver Nanoparticles Embedded in Sodium Alginate: Antibacterial Efficacy and Effects on Red Cabbage Seedling Performance. Agronomy 2025, 15, 1640. https://doi.org/10.3390/agronomy15071640
Rutkowski M, Makowski W, Krzemińska-Fiedorowicz L, Khachatryan K, Kalisz A, Malina D, Chwastowski J, Wzorek Z, Khachatryan G, Sękara A, et al. Silver Nanoparticles Embedded in Sodium Alginate: Antibacterial Efficacy and Effects on Red Cabbage Seedling Performance. Agronomy. 2025; 15(7):1640. https://doi.org/10.3390/agronomy15071640
Chicago/Turabian StyleRutkowski, Miłosz, Wojciech Makowski, Lidia Krzemińska-Fiedorowicz, Karen Khachatryan, Andrzej Kalisz, Dagmara Malina, Jarosław Chwastowski, Zbigniew Wzorek, Gohar Khachatryan, Agnieszka Sękara, and et al. 2025. "Silver Nanoparticles Embedded in Sodium Alginate: Antibacterial Efficacy and Effects on Red Cabbage Seedling Performance" Agronomy 15, no. 7: 1640. https://doi.org/10.3390/agronomy15071640
APA StyleRutkowski, M., Makowski, W., Krzemińska-Fiedorowicz, L., Khachatryan, K., Kalisz, A., Malina, D., Chwastowski, J., Wzorek, Z., Khachatryan, G., Sękara, A., & Kołton, A. (2025). Silver Nanoparticles Embedded in Sodium Alginate: Antibacterial Efficacy and Effects on Red Cabbage Seedling Performance. Agronomy, 15(7), 1640. https://doi.org/10.3390/agronomy15071640












