Evaluation of Major Soil Nutrients After the Application of Microbial-Inoculated Acidified Biochar Pellets Using a Sigmoid Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations of the Acidified Biochar Pellets Inoculated with a Microorganism (ABPM)
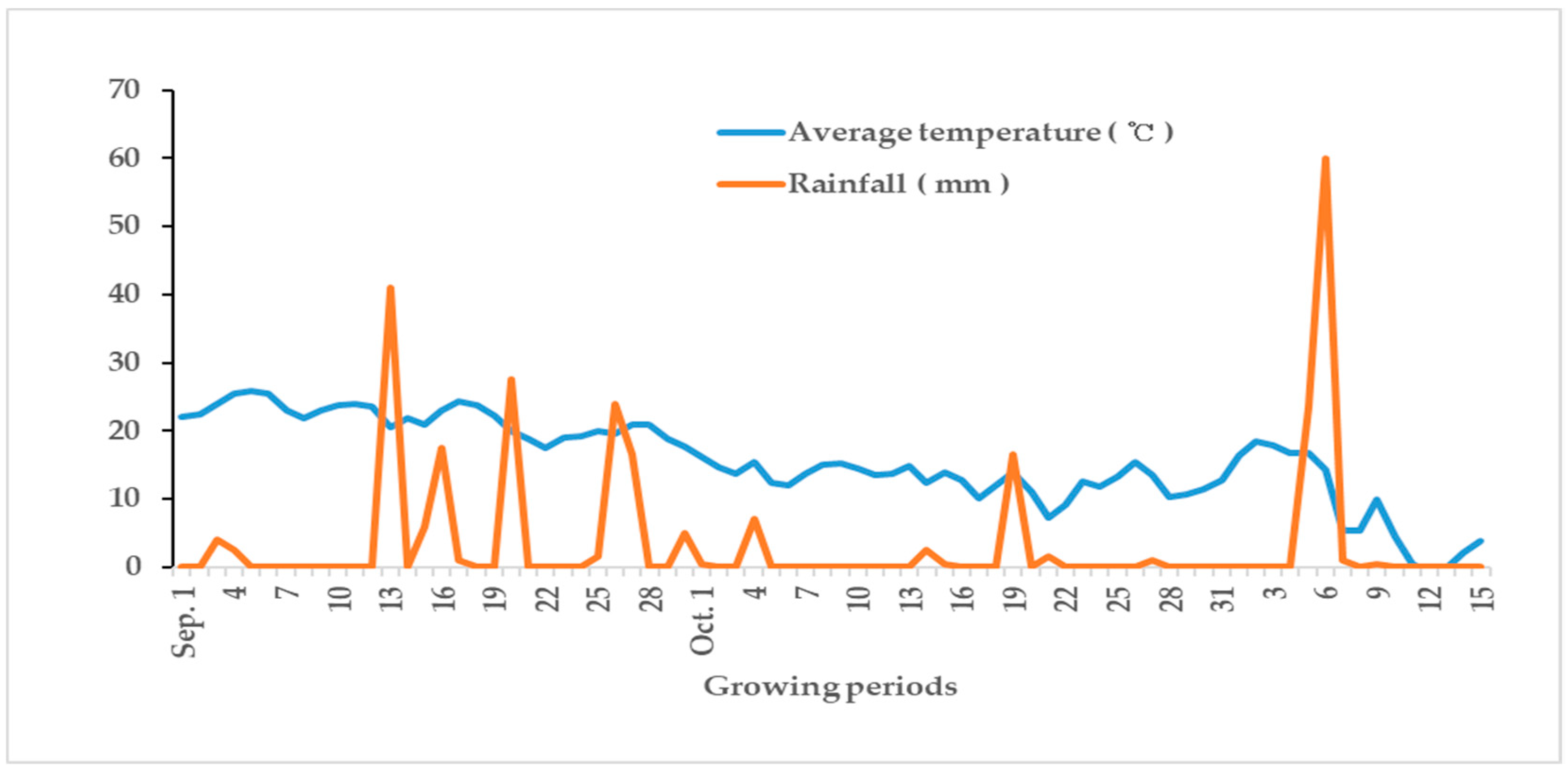
2.2. Cabbage Cultivation
2.3. Analysis of Physicochemical Properties
2.4. Statistical Analysis
3. Results and Discussions
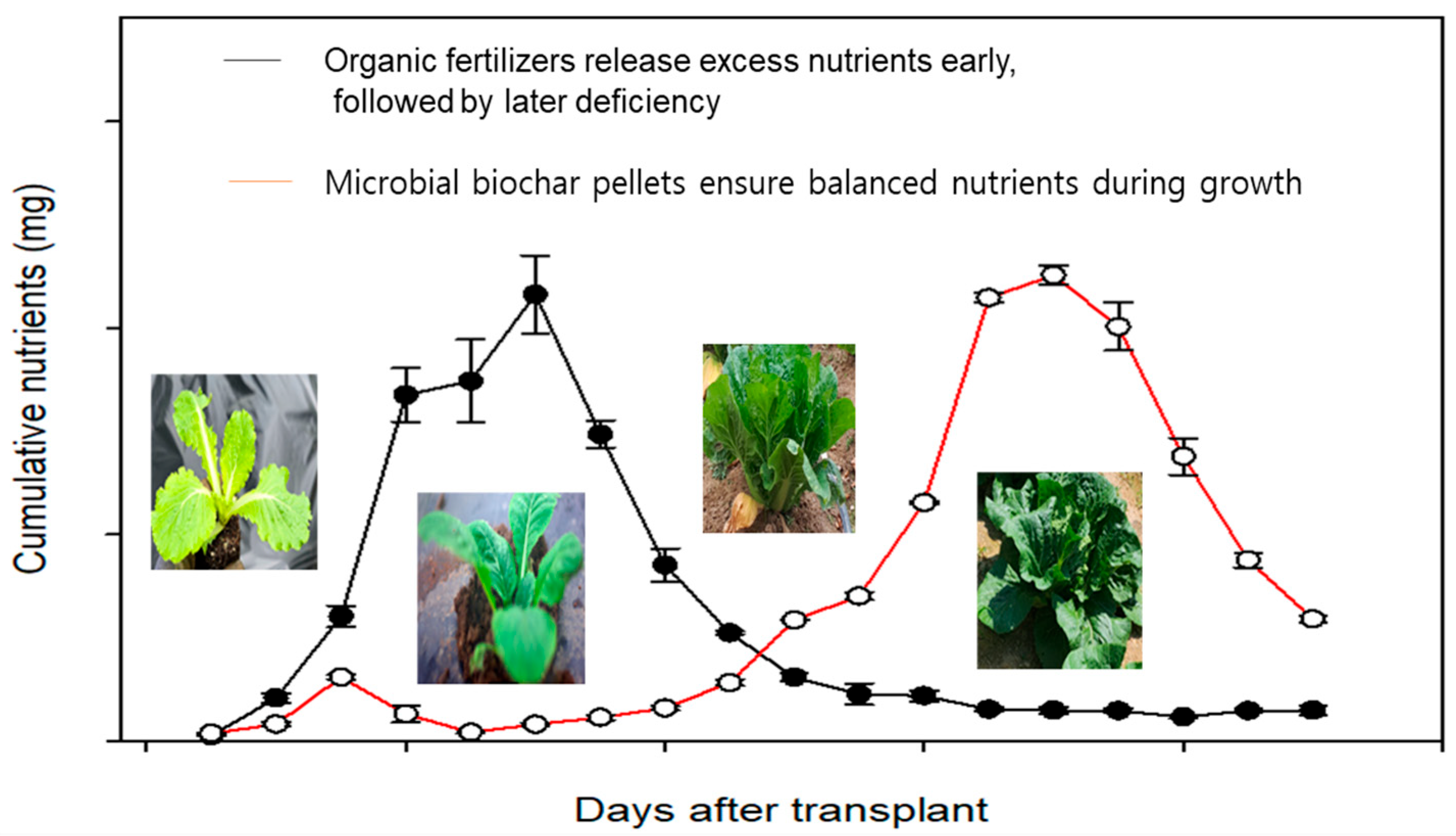
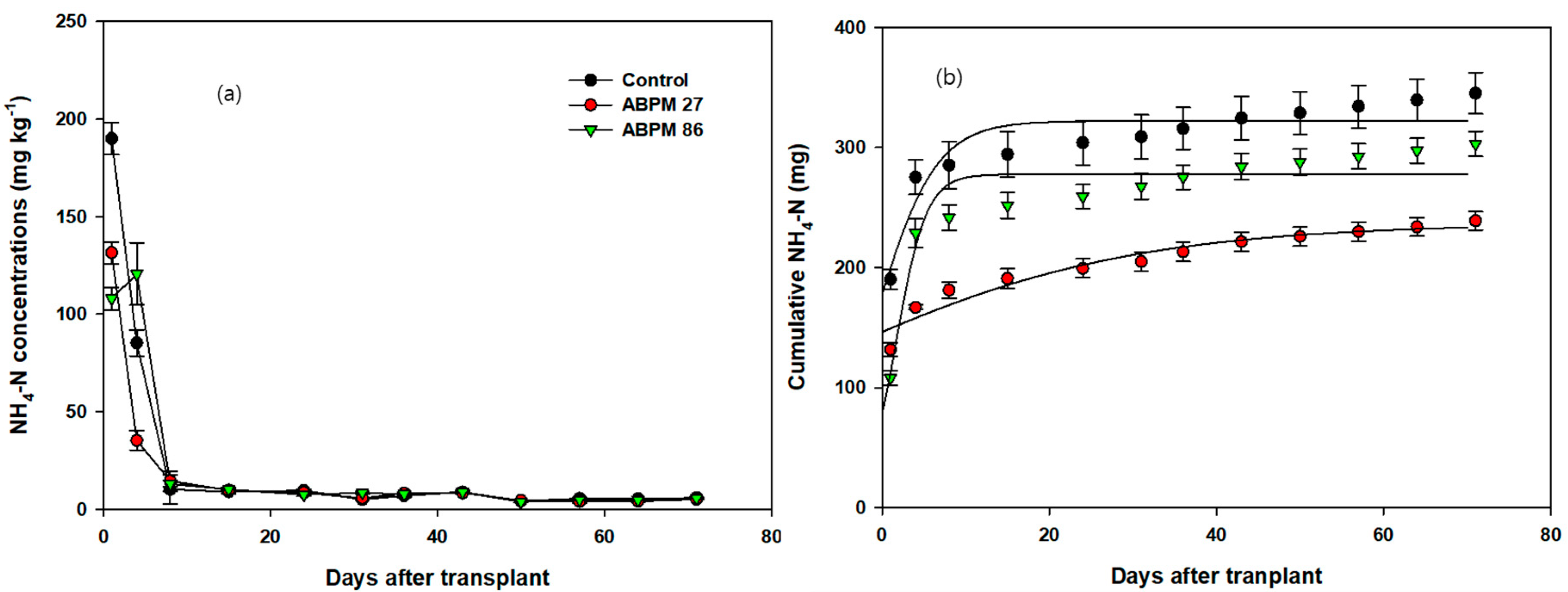
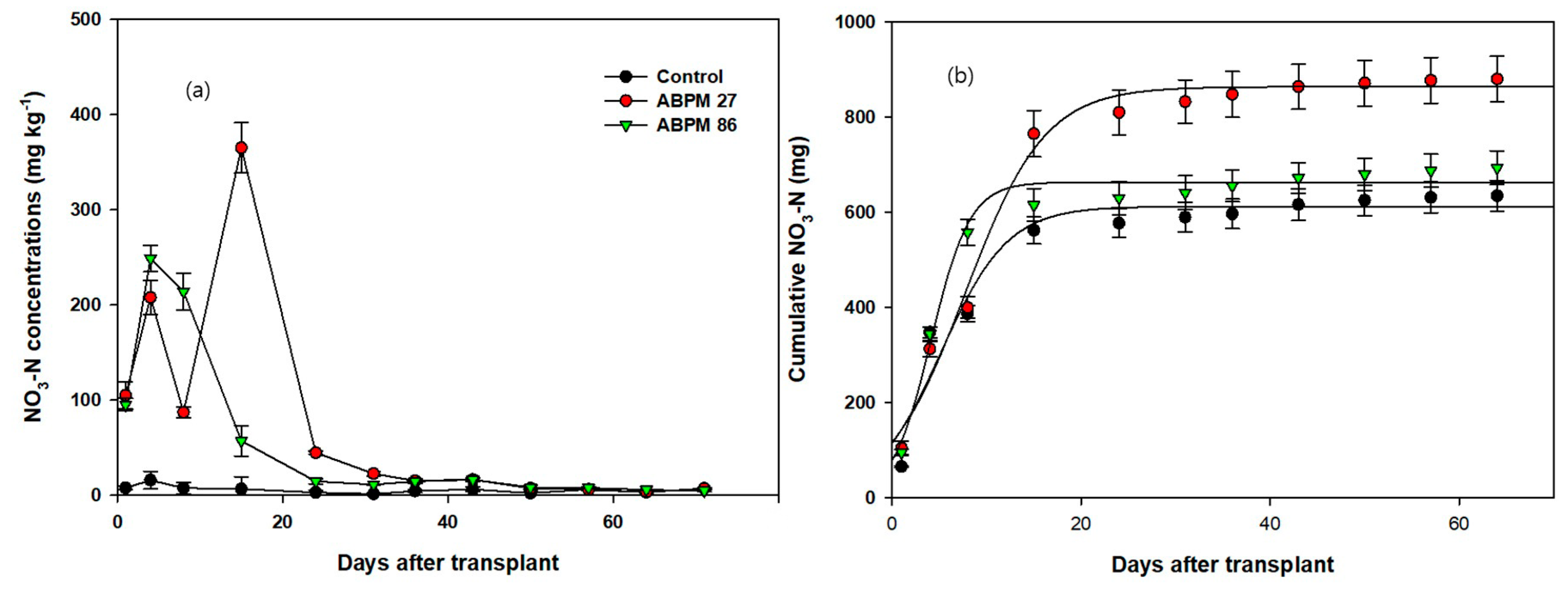
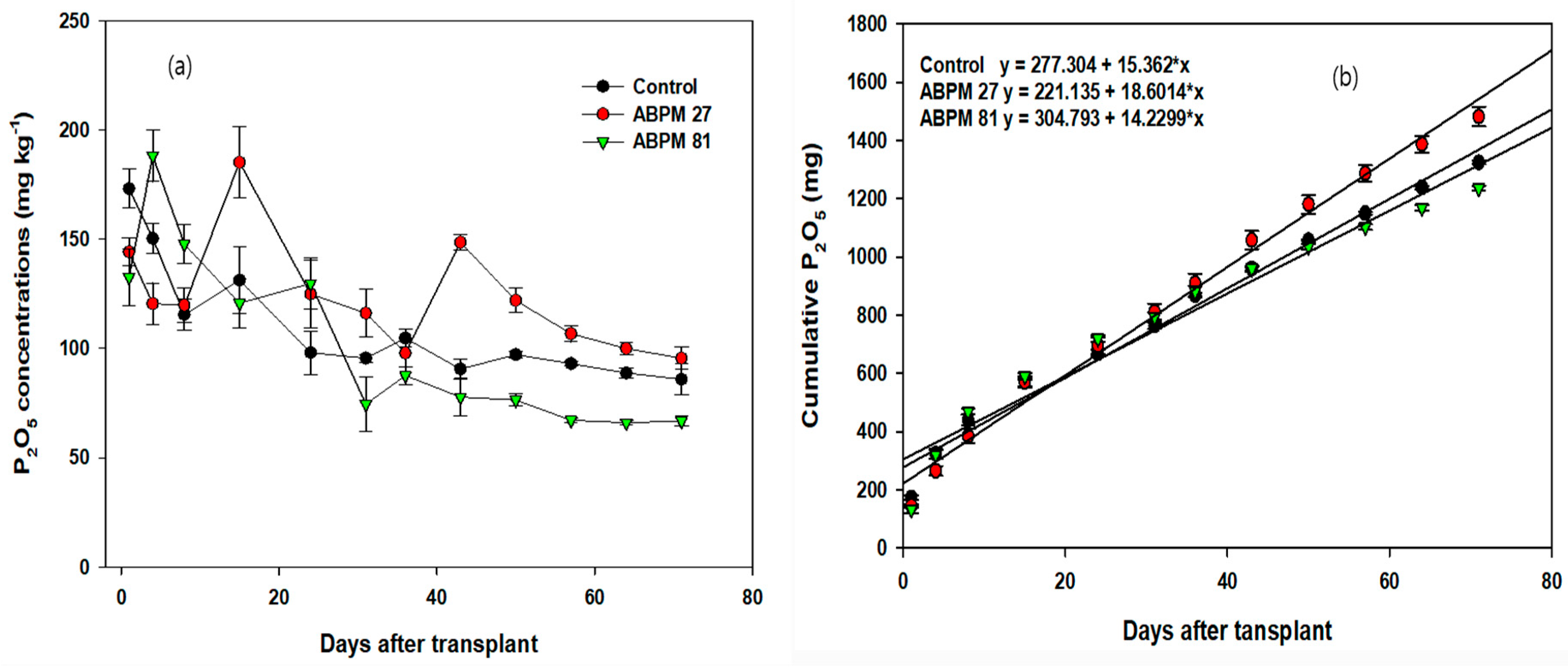
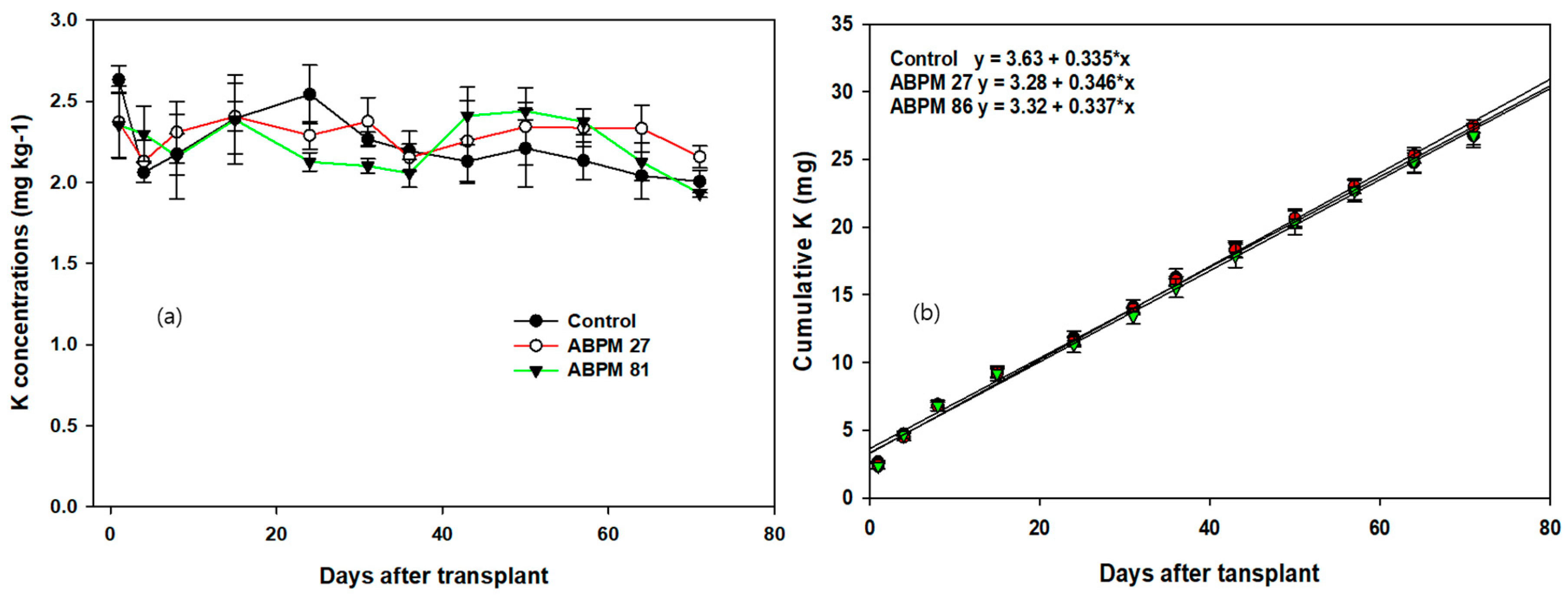
3.1. Cumulative Plant Nutrients in the Soil
3.2. Plant Growth Responses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Data. FAO. 2022. Available online: https://www.fao.org/faostat (accessed on 20 March 2023).
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health-improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997; pp. 234–318. [Google Scholar]
- Ombodi, A.; Saigusa, M. Broadcast application versus band application of polyolefin coated fertilizer on green peppers grown on andisol. J. Plant Nutr. 2000, 23, 1485–1493. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Zhang, K.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. Available online: http://www.nature.com/scientificreports (accessed on 1 February 2024). [CrossRef]
- Shin, J.D.; Park, D.G.; Hong, S.G.; Jeong, C.; Kim, H.; Chung, W. Influence of activated biochar pellet fertilizer application on greenhouse gas emissions and carbon sequestration in rice (Oryza sativa L.) production. Environ. Pollut. 2021, 285, 117457. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.D.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 147–203. [Google Scholar]
- Tan, K.; Radziah, O.; Halimi, M.; Khairuddin, A.; Shamsuddin, Z. Assessment of plant growth-promoting rhizobacteria (PGPR) and rhizobia as multi-strain biofertilizer on growth and N2 fixation of rice plant. Aust. J. Crop Sci. 2015, 9, 1257–1264. [Google Scholar]
- Myrold, D.D.; Posavatz, N.R. Potential importance of bacteria and fungi in nitrate assimilation in soil. Soil Biol. Biochem. 2007, 39, 1737–1743. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Jiang, Q.; Fei, Z.; Xiao, M. Genome analysis of plant growth-promoting rhizobacterium Pseudomonas chlororaphis subsp. aurantiaca JD37 and insights from comparison of genomics with three Pseudomonas strains. Microbiol. Res. 2020, 237, 126483. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, Y.; Wang, Z.; Yu, Y.; Zhang, N.; Yang, C.; Zeng, Q.; Wang, Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Micro Biotechnol. 2021, 14, 488–502. [Google Scholar] [CrossRef]
- Khan, H.; Akbar, W.A.; Shah, Z.; Rahim, H.U.; Taj, A.; Alatalo, J.M. Coupling phosphate-solubilizing bacteria (PSB) with inorganic phosphorus fertilizer improves mung bean (Vigna radiata) phosphorus acquisition, nitrogen fixation, and yield in alkaline-calcareous soil. Heliyon 2022, 8, 09081. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, K.; Zhu, W.; Xiao, J.; Zhu, C.; Zhang, N.; Yu, F.; Li, S.; Zhu, C.; Tu, Q.; et al. Large losses of ammonium-nitrogen from a rice ecosystem under elevated CO2. Sci. Adv. 2020, 6, 7433. [Google Scholar] [CrossRef]
- Bashir, S.; Bakhsh Gulshan, A.; Iqbal, J.; Husain, A.; Alwahibi, M.S.; Alkahtani, J.; Dwiningsih, Y.; Bakhsh, A.; Ahmed, N.; Jamal Khan, M.; et al. Comparative role of animal manure and vegetable waste induced compost for polluted soil restoration and maize growth. Saudi J. Biol. Sci. 2021, 28, 2534–2539. [Google Scholar] [CrossRef]
- Khalil, M.; Gutser, R.; Schmidhalter, U. Effects of urease and nitrification inhibitors added to urea on nitroxide emissions from loess soil. J. Plant Nutr. Soil Sci. 2009, 172, 651–660. [Google Scholar] [CrossRef]
- Ro, K.S.; Cantrell, K.B.; Hunt, P.G. High temperature pyrolysis of blended animal manures for producing renewable energy and value-added biochar. Int. Eng. Chem. Res. 2010, 49, 10125–10131. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Bhatnagar, A.; Jeyakumar, P.; Wang, H.; Wang, Y.; Li, X. Microorganisms-carbonaceous materials immobilized complexes: Synthesis, adaptability and environmental applications. J. Hazard. Mater. 2021, 416, 125915. [Google Scholar] [CrossRef]
- Dong, D.; Wang, C.; Van Zwieten, L.; Wang, H.; Jiang, P.; Zhou, M.; Wu, W. An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J. Soils Sediments 2020, 20, 3027–3040. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci. Rep. 2017, 7, 12114. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Yang, L.; Jing, H.; Mao, W.; Liu, J.; Zou, Y.; Wu, Y.; Zhou, H.; Yang, W.; et al. A Critical Review of Biochar Application for the Remediation of Greenhouse Gas Emissions and Nutrient Loss in Rice Paddies: Characteristics, Mechanisms, and Future Recommendations. Agronomy 2023, 13, 893. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Sultan, H.; Ahmed, N.; Mubashir, M.; Danish, S. Chemical production of acidified activated carbon and its influences on soil fertility comparative to thermo-pyrolyzed biochar. Sci. Rep. 2020, 10, 595. [Google Scholar] [CrossRef]
- Kerner, P.; Struhs, E.; Mirkouei, A.; Aho, K.; Lohse, K.A.; Dungan, R.S.; You, Y. Microbial Responses to Biochar Soil Amendment and Influential Factors: A Three-Level Meta-Analysis. Environ. Sci. Technol. 2023, 57, 19838–19848. [Google Scholar] [CrossRef]
- Choi, J.; Shin, J.D.; Cho, H.J.; Chung, W.; Lee, S.B.; Yun, S.I. Effects of biodegradable polymer coating urea to nitrogen release in the soil column. J. Korea Org. Resour. Recycl. Assoc. 2024, 32, 49–59. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2018, 654, 463–472. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gikes, R.J. Dissolution of K, Ca, and P from biochar grains in tropical soils. Geoderma 2018, 312, 139–150. [Google Scholar] [CrossRef]
- Subedi, R.; Taupe, N.; Ikoyi, I.; Bertora, C.; Zavattaro, L.; Schmalenberger, A.; Leahy, J.; Grignani, C. Chemically and biologically-mediated fertilizing value of manure-derived biochar. Sci. Total Environ. 2016, 550, 924–933. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef]
- Archontoulis, S.A.; Miguez, F.E. Nonlinear regression models and applications in agricultural research. Agron. J. 2015, 107, 786–798. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. 1825, 115, 513–585. [Google Scholar]
- Verhulst, P.F. A note on population growth. Corresp. Math. Phys. 1838, 10, 113–121. [Google Scholar]
- Lucio, A.D.; Nunes, L.F.; Rego, F. Nonlinear models to describe production of fruit in Cucurbita pepo and Capiscum annuum. Sci. Hortic. 2015, 193, 286–293. [Google Scholar] [CrossRef]
- Shin, J.D.; Park, S.W.; Jeong, C. Assessment of agri-environmental impacts for supplemented methods to biochar manure pellets during rice (Oryza sativa L.) cultivation. Energies 2020, 13, 2070. [Google Scholar] [CrossRef]
- Stuti, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- NAS. Recommended Application Amounts of Fertilizers for Crop Cultivation; Rural Administration; National Institute of Agricultural Sciences: Jeonbuk Innovation City, Republic of Korea, 2010; p. 16. [Google Scholar]
- MehLich, A. Mehlich III soil test extractant; Modification of the Mehlich II extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Shin, J.; Park, S.; Lee, S. Optimum method uploaded nutrient solution for blended biochar pellet with application of nutrient releasing model as slow release fertilizer. Appl. Sci. 2019, 9, 1899. [Google Scholar] [CrossRef]
- Qin, S.Q.; Quan, Z.; Ma, J.; Chen, X.; Shi, Y.; Huang, B. Regulating nitrate excess in lettuce-planted greenhouse soil with available carbon addition through irrigation, Environ. Sci. Pollut. Res. 2019, 26, 19241–19249. [Google Scholar] [CrossRef]
- Sarah, A.D.; Miguel, L.C.; Keshav, C.D.; Julia, W.G.; Leticia, S.S.; William, P.M. Release of Nitrogen and Phosphorus from Poultry Litter Amended with Acidified Biochar. Int. J. Environ. Res. Public Health 2011, 8, 1491–1502. [Google Scholar] [CrossRef]
- Khan, M.I.; Afzal, M.J.; Bashir, S.; Naveed, M.; Anum, S.; Cheema, S.A.; Wakeel, A.S.M.; Ali, M.H.; Chen, Z. Improving Nutrient Uptake, Growth, Yield and Protein Content in Chickpea by the Co-Addition of Phosphorus Fertilizers, Organic Manures, and Bacillus sp. MN-54. Agronomy 2021, 11, 436. [Google Scholar] [CrossRef]
- Biswakarma, B.; Verma, H.; Sarkar, N.C. Effect of phosphate solubilizing bacteria on yield of transplanted rice under lateritic belt of West Bengal. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3192–3204. [Google Scholar] [CrossRef]
- Ahmed, N.; Basit, A.; Bashir, S.; Bashir, S.; Bibi, I.; Haider, Z.; Ali, M.A.; Aslam, Z.; Aon, M.; Alotaibi, S.S.; et al. Effect of acidified biochar on soil phosphorus availability and fertilizer use efficiency of maize (Zea mays L.). J. King Saud Univ. Sci. 2021, 33, 101635. [Google Scholar] [CrossRef]
- Kopp, C.; Sica, P.; Lu, C.; Tobler, D.; Jensen, L.S.; Müller-Stover, D. Increasing phosphorus plant availability from P-rich ashes and biochars by acidification with sulfuric acid. J. Environ. Chem. Eng. 2023, 11, 111489. [Google Scholar] [CrossRef]
- Zheng, B.X.; Ding, K.; Yang, X.R.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.G. Straw biochar increases the abundance of inorganic phosphate solubilizing bacterial community for better rape (Brassica napus) growth and phosphate uptake. Sci. Total Environ. 2019, 647, 1113–1120. [Google Scholar] [CrossRef]
- Wang, Y.M.; Peng, S.; Hua, Q.Q.; Qiu, C.W.; Wu, P.; Liu, X.L.; Lin, X.G. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol. 2021, 12, 693535. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.J.; Su, J.Q.; Sun, G.X.; Wu, J.S.; Wei, W.X. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biotechnol. 2018, 102, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.F.; Li, K.J.; Zheng, B.X.; Liu, X.P.; Li, H.Z.; Jin, B.J.; Ding, K.; Yang, X.R.; Lin, X.Y.; Zhu, Y.G. Partial replacement of inorganic phosphorus (P) by organic manure reshapes phosphate mobilizing bacterial community and promotes P bioavailability in a paddy soil. Sci. Total Environ. 2020, 703, 134977. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Haider, G.; Raza, M.A.; Mohamed, A.K.S.H.; Rizwan, M.; El-Sheikh, M.A. Straw-based biochar mediated potassium availability and increased growth and yield of cotton (Gossypium hirsutum L). J. Saudi Chem. Soc. 2020, 24, 963–973. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2021, 144, 175–187. [Google Scholar] [CrossRef]
- Basak, B.B. Potassium-solubilizing microorganisms and their role in plant growth promotion. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–17. [Google Scholar]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Sun, H.; Shi, W.Q.; Zhou, M.; Ma, X.; Zhang, H. Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 2017, 65, 83–89. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mining drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, Q.; Liu, L. Modified biochar-immobilized Bacillus spp. for the release of nutrients and its response to soil microbial community activity and structure. Ind. Crops Prod. 2025, 225, 120466. [Google Scholar] [CrossRef]

| Soil Type | pH (1:10) | EC (mS m−1) | O.M (mg kg−1) | NH4-N | NO3-N | P2O5 | K |
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Silty loam | 6.7 | 0.03 | 14.0 | 14.3 | 45.9 | 116.1 | 12.2 |
| Biochar Pellets * | pH (1:20) | EC (dS m−1) | N | C | P2O5 | K |
|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | |||||
| Guano | 7.45 | 6.00 | 135.2 | 163.2 | 33.2 | 0.90 |
| Rice hull biochar | 10.68 | 0.88 | 2.2 | 562.7 | 0.1 | 0.02 |
| ABPM 27 | 7.54 | 6.00 | 43.5 | 192.6 | 0.6 | 0.20 |
| ABPM 86 | 7.34 | 5.93 | 43.6 | 192.6 | 0.6 | 1.10 |
| Treatments | Sigmoidal Equations | p-Values | R2 |
|---|---|---|---|
| Control | Y = 322.1/(1 + exp(−(t + 0.73)/3.6)) | <0.0001 | 0.825 |
| ABPM 27 | Y = 236.8/(1 + exp(−(t + 9.0)/18.7)) | <0.0001 | 0.924 |
| ABPM 86 | Y = 277.6/(1 + exp(−(t − 1.7)/1.8)) | <0.0001 | 0.862 |
| Treatments | Sigmoidal Equations | p-Values | R2 |
|---|---|---|---|
| Control | Y = 612.3/(1 + exp(−(t − 5.2)/3.5)) | <0.0002 | 0.927 |
| ABPM 27 | Y = 863.6/(1 + exp((t − 7.8)/4.2)) | <0.0001 | 0.982 |
| ABPM 86 | Y = 663.1/(1 + exp(−(t − 4.1)/2.1)) | <0.0001 | 0.972 |
| Treatments | Bulb Height (cm) | Bulb Width (cm) | Yield (tonnes ha−1) |
|---|---|---|---|
| Control | 35.8 a | 19.8 a | 71.3 b |
| ABPM 27 | 36.1 a | 19.0 ab | 77.4 a |
| ABPM 86 | 34.9 a | 15.8 c | 70.2 bc |
| F | 4.9 | 10.4 | 12.5 |
| p-values | <0.004 | <0.00002 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.; Shin, J.; Choi, J.-Y.; Park, S.; Chung, J.; Jeong, C. Evaluation of Major Soil Nutrients After the Application of Microbial-Inoculated Acidified Biochar Pellets Using a Sigmoid Function. Agronomy 2025, 15, 1607. https://doi.org/10.3390/agronomy15071607
Nam J, Shin J, Choi J-Y, Park S, Chung J, Jeong C. Evaluation of Major Soil Nutrients After the Application of Microbial-Inoculated Acidified Biochar Pellets Using a Sigmoid Function. Agronomy. 2025; 15(7):1607. https://doi.org/10.3390/agronomy15071607
Chicago/Turabian StyleNam, JooHee, JoungDu Shin, Jae-Yee Choi, SangWon Park, JaeWook Chung, and Changyoon Jeong. 2025. "Evaluation of Major Soil Nutrients After the Application of Microbial-Inoculated Acidified Biochar Pellets Using a Sigmoid Function" Agronomy 15, no. 7: 1607. https://doi.org/10.3390/agronomy15071607
APA StyleNam, J., Shin, J., Choi, J.-Y., Park, S., Chung, J., & Jeong, C. (2025). Evaluation of Major Soil Nutrients After the Application of Microbial-Inoculated Acidified Biochar Pellets Using a Sigmoid Function. Agronomy, 15(7), 1607. https://doi.org/10.3390/agronomy15071607






