Melatonin Priming Increases the Tolerance of Tartary Buckwheat Seeds to Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographical Location of the Study Area
2.2. Seed Priming Treatment and Standard Germination Test
2.3. Seed Storage and Germination Test
2.4. Conductivity Measurement
2.5. Measurement of SOD and APX Activities
2.6. Statistical Analysis
3. Results
3.1. Determination of Melatonin Priming Concentration and Duration
3.1.1. Effects of Melatonin Treatment on Tartary Buckwheat Seeds Under Salt Stress
3.1.2. Effects of Melatonin Treatment on Tartary Buckwheat Seedlings Under Salt Stress
3.2. Seed Phenotypes Following Water Priming and Melatonin Priming for Storage Tolerance
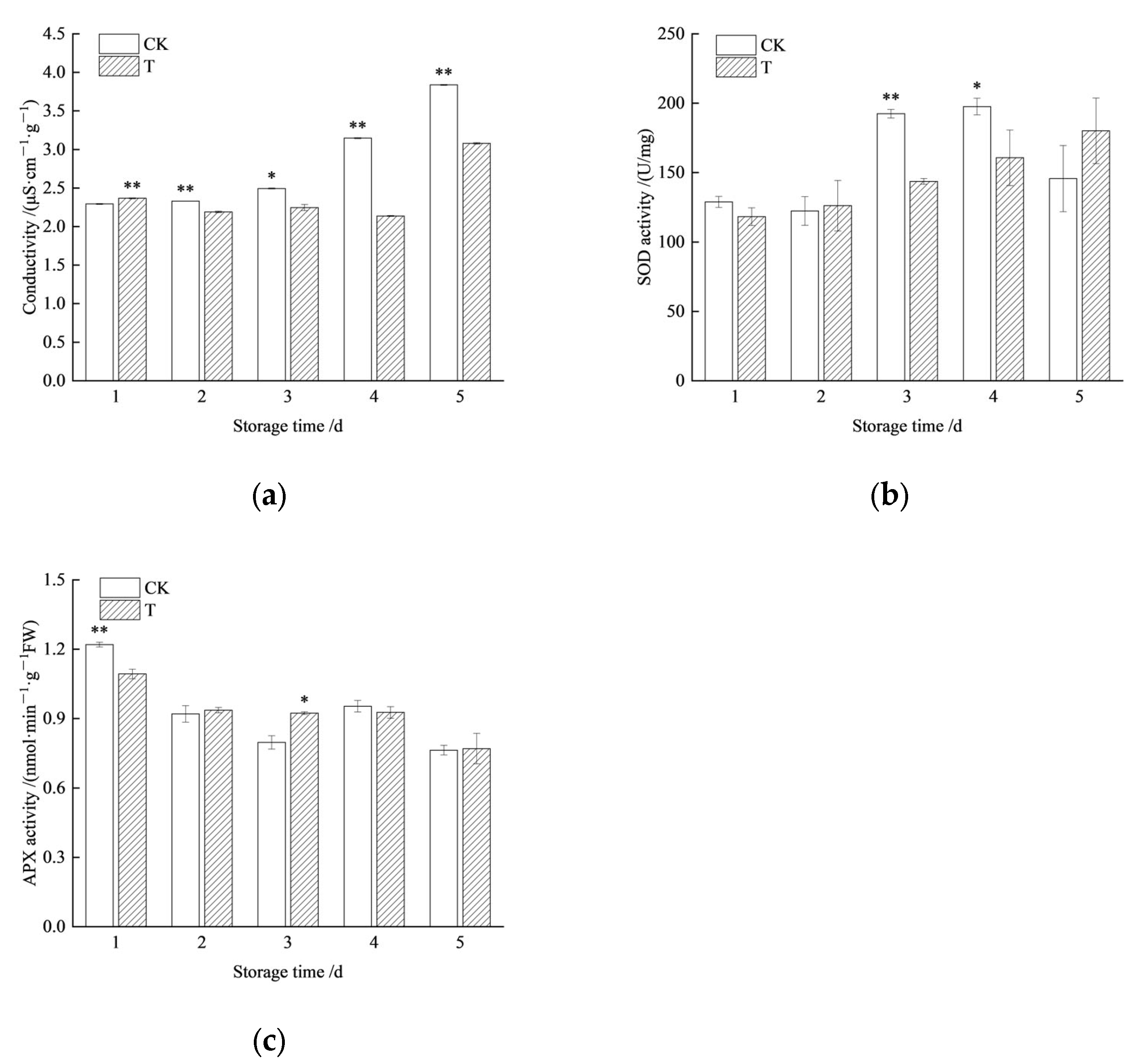
3.3. Storage Tolerance of Redrying Seeds Under High Temperature and Humidity Conditions
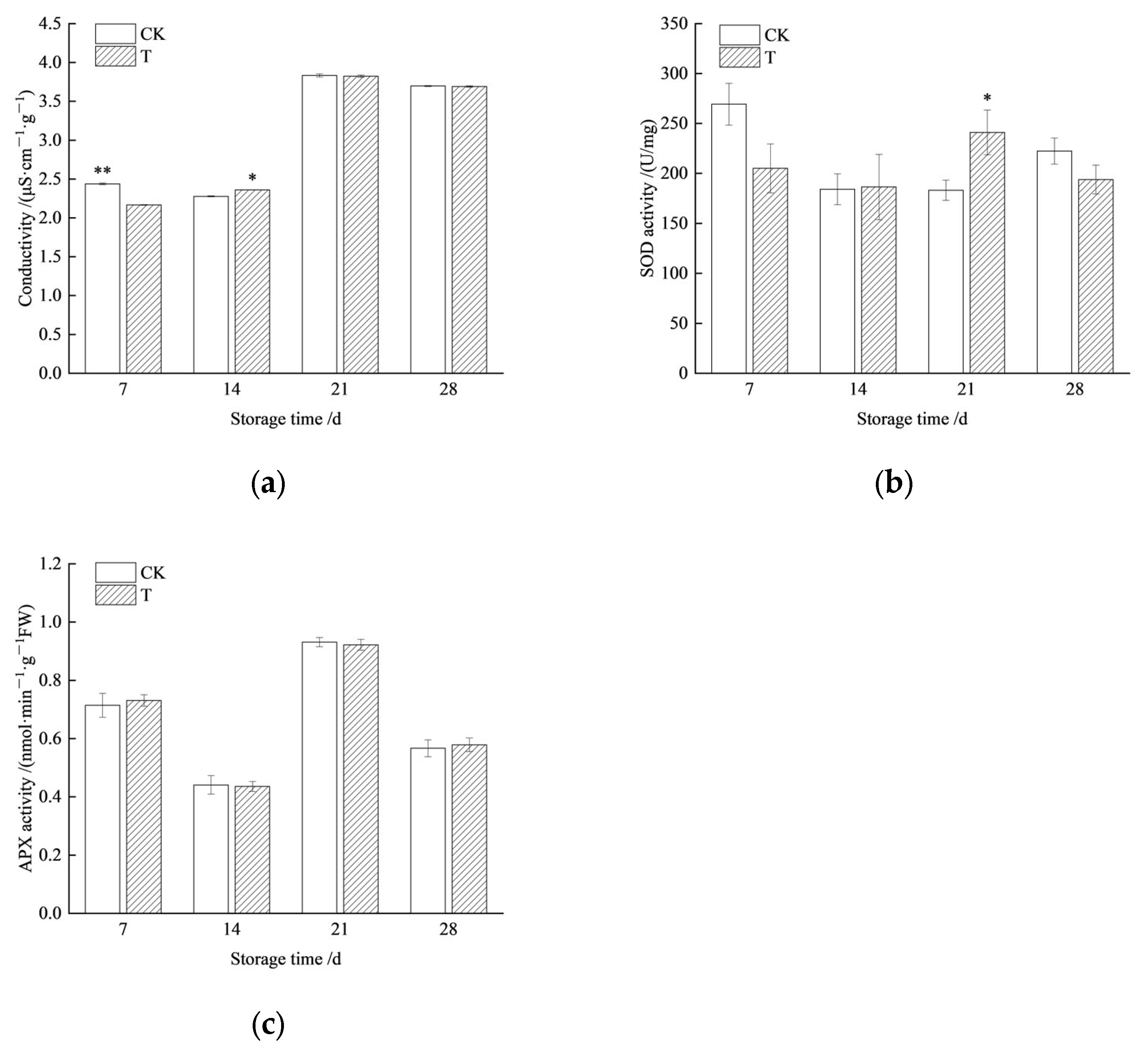
3.4. Storage Tolerance of Redrying Seeds at Room Temperature
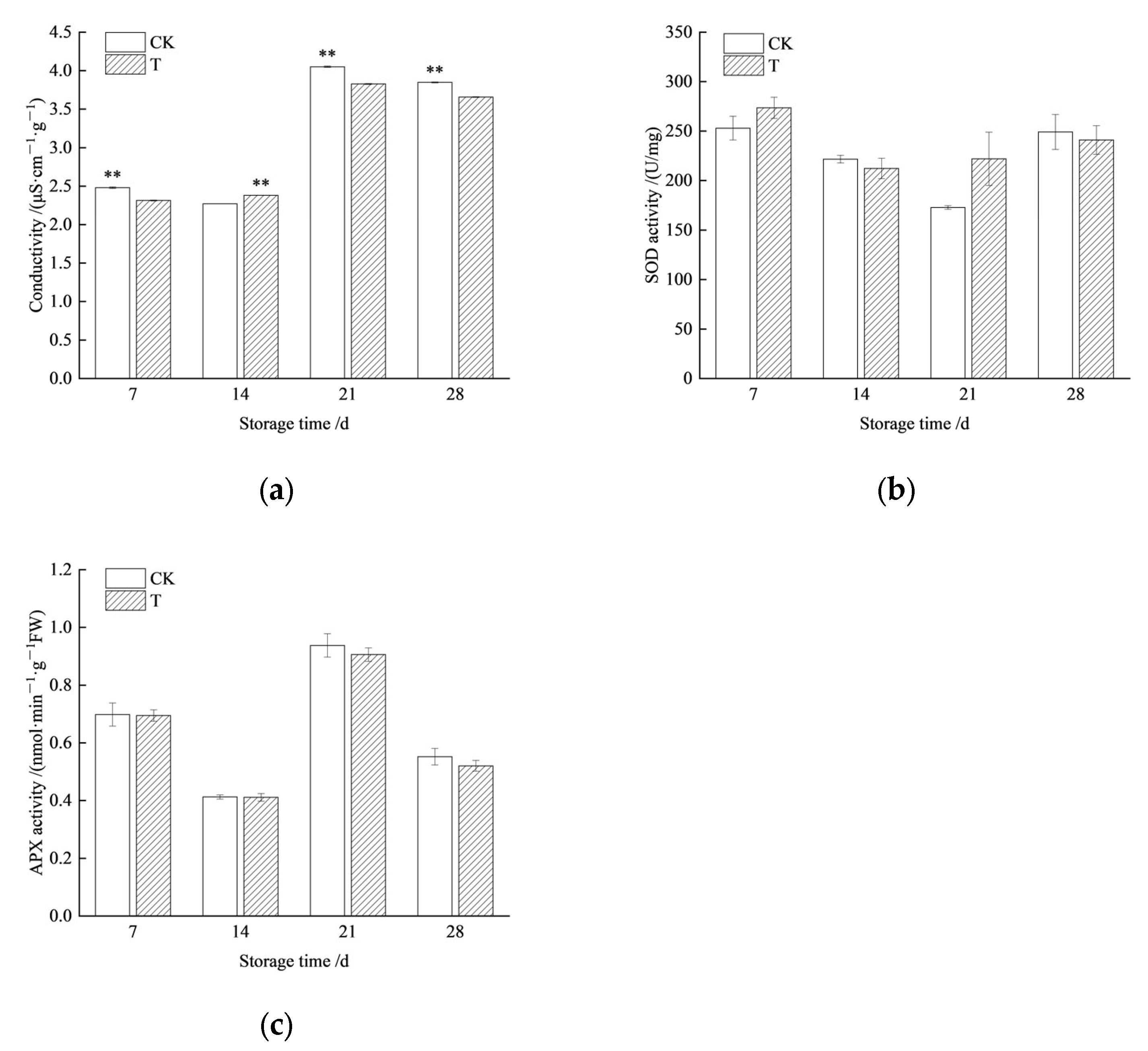
3.5. Storage Tolerance of Redrying Seeds at Low Temperature
4. Discussion
4.1. Melatonin Priming Increases Salt Tolerance of Seeds
4.2. Vigor and Storability of Redrying Seeds After Priming
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, C.; Zhao, G. Chinese Buckwheat Science; China Agriculture Press: Beijing, China, 2015. [Google Scholar]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lv, M.; Peng, Q.; Shan, F.; Wang, M. Physicochemical and textural properties of Tartary buckwheat starch after heat–moisture treatment at different moisture levels. Starch-Stärke 2015, 67, 276–284. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of global tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ding, M.; Zhang, K.; Yang, K.; Tang, Y.; Zhang, Z.; Fang, W.; Yan, J.; Zhou, M. Germplasm resource of the genus Fagopyrum Mill. J. Plant Genet. Resour. 2019, 20, 813–828. [Google Scholar]
- He, Y.; Zhang, K.; Shi, Y.; Lin, H.; Huang, X.; Lu, X.; Wang, Z.; Li, W.; Feng, X.; Shi, T.; et al. Genomic insight into the origin, domestication, dispersal, diversification and human selection of Tartary buckwheat. Genome Biol. 2024, 25, 61. [Google Scholar] [CrossRef]
- Tan, P.; Guo, W. Research progress of tartary buckwheat flavonoids on human body’s physiological function and mechanism. Med. Recapitul. 2018, 24, 1627–1632. [Google Scholar]
- Elias, S.G. The importance of using high quality seeds in agriculture systems. Agric. Res. Technol. 2018, 4, 555961. [Google Scholar] [CrossRef]
- Yao, D.; Wu, L.; Shen, H.; Tian, S.; Li, M. Advances in research and application on seed priming technology. Acta Agric. Shanghai 2020, 36, 153–160. [Google Scholar]
- Liu, Y.; Yuan, H.; Yan, X.; Yang, D. Advances in seed priming and the primed seeds’ treatment with pesticides. Seed 2024, 43, 76–83. [Google Scholar]
- Zha, Y.; Zhang, Z.; Xu, H.; Liu, X.; Li, Y.; Li, X. Effect of hydro-priming on seed germination and seedling growth of Elymus nutans. J. Northeast Agric. Sci. 2021, 46, 70–73. [Google Scholar]
- Luo, J.; Zhang, Z.; Sun, X.; Chen, W.; Peng, Y. Study on hydro-priming of cowpea seeds. J. Agric. 2019, 9, 45–48. [Google Scholar]
- Li, J.; Xun, J.; Lin, C.; Guan, Y.; Hu, J. Effects of priming on seed germination and seedling physiological characteristics of different maize types under low-temperature stress. Plant Physiol. J. 2016, 52, 157–166. [Google Scholar]
- Song, Z.; Guo, S.; Yang, W.; Zhao, Y. Effects of different initiators on seed germination of wheat. J. Hebei Agric. Sci. 2018, 22, 13–15, 21. [Google Scholar]
- Jiang, X.; Ren, C.; Hu, J.; Zeng, Q.; Zhao, Q.; Wang, J. Study of water priming effect on seed germination and vigor of spinach (Spinacia oleracea). J. China Agric. Univ. 2011, 16, 43–51. [Google Scholar]
- Wang, Y.; Zhang, J.; Liu, H.; Hu, X. Physiological and ecological responses of alfalfa and milkvetch seed to PEG priming. Acta Ecol. Sin. 2004, 24, 402–408. [Google Scholar]
- Chen, B.; Zhang, Q.; Dai, Z.; Zhou, X.; Liu, J. Physiological and molecular effects of salicylic acid on rice seed germination at low temperature. Sci. Agric. Sin. 2024, 57, 1220–1236. [Google Scholar]
- Khalequzzaman; Ullah, H.; Himanshu, S.K.; Islam, N.-E.-T.; Tisarum, R.; Cha-um, S.; Datta, A. Seed priming improves germination, yield, and water productivity of cotton under drought stress. J. Soil Sci. Plant Nutr. 2023, 23, 2418–2432. [Google Scholar] [CrossRef]
- Hassan, M.J.; Geng, W.; Zeng, W.; Raza, M.A.; Khan, I.; Iqbal, M.Z.; Peng, Y.; Zhu, Y.; Li, Z. Diethyl aminoethyl hexanoate priming ameliorates seed germination via involvement in hormonal changes, osmotic adjustment, and dehydrins accumulation in white clover under drought stress. Front. Plant Sci. 2021, 12, 709187. [Google Scholar] [CrossRef]
- Yan, X.; Chen, S.; Pan, Z.; Zhao, W.; Rui, Y.; Zhao, L. AgNPs-triggered seed metabolic and transcriptional reprogramming enhanced rice salt tolerance and blast resistance. ACS Nano 2023, 17, 492–504. [Google Scholar] [CrossRef]
- Qin, Z.; Lin, X.; Wei, Q.; Liang, L.; Li, Y. Effects of cerium oxide nanoparticles seed priming on seed germination and seedling growth of pepper under salt stress. Guihaia 2023, 43, 2300–2308. [Google Scholar]
- Song, S.; Liu, J.; Tang, C.; Cheng, H.; Wang, W.; Zhang, Q.; Zhang, W.; Gao, J. Research progress on the physiology and its molecular mechanism of seed desiccation tolerance. Sci. Agric. Sin. 2022, 55, 1047–1063. [Google Scholar]
- Zhao, Q.; Zhang, H.; Huang, X.; Liu, L.; Liu, X.; Xiao, H.; Liu, C. Study of breaking through a priming affecting seedling uniformity on Cucurbita ficifolia Seeds. Bot. Res. 2021, 10, 587. [Google Scholar]
- Liu, C.; Liu, J.; Huang, M.; Sun, J.; Liu, C. Study on re-germination and storage ability on dehydration wheat (Triticum aestivum L.) seeds after germination. Bot. Res. 2018, 7, 294. [Google Scholar]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Wang, W.; Shen, F.; Wu, Y.; Mei, Y.; Zu, Y.; Chen, C.; Wan, H.; Zheng, J. Biosynthesis of melatonin and its role in plant stress: A review. Jiangsu Agric. Sci. 2022, 50, 1–6. [Google Scholar]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Kumar, M.; Kesarwani, A.K.; Sonah, H.; Sharma, T.R.; et al. Seed priming with melatonin: A promising approach to combat abiotic stress in plants. Plant Stress 2022, 4, 100071. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, H.; Guo, Z.; Yu, H.; Yuan, Y.; Gong, Z.; Wang, Y. The melatonin and its resistance to stress in plants. Genom. Appl. Biol. 2013, 32, 260–266. [Google Scholar]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C.; Wu, Y.; Zhang, Y. The physiological regulation effect of exogenous melatonin relieving drought stress at seedling stage of spring soybean. Soybean Sci. 2020, 39, 742–750. [Google Scholar]
- Yao, R.; Liu, H.; Wang, J.; Shi, S.; Zhao, G.; Zhou, X. Cytological structures and physiological and biochemical characteristics of covered oat (Avena sativa L.) and naked oat (Avena nuda L.) seeds during high-temperature artificial aging. BMC Plant Biol. 2024, 24, 530. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, J.L.; Ramos Aquino, R.G.; Lorente González, G.Y.; González-Olmedo, J.L.; Martínez Montero, M.E. ROS production and antioxidant enzyme activity in relation to germination and vigor during tobacco seed development. Vegetos 2023, 36, 506–515. [Google Scholar] [CrossRef]
- Bicalho, E.M.; Gomes, M.P.; Rodrigues-Junior, A.G.; Oliveira, T.G.S.; Gonçalves, C.D.A.; Fonseca, M.B.; Garcia, Q.S. Integrative effects of zinc and temperature on germination in Dimorphandra Wilsonii rizz.: Implications of climate changes. Environ. Toxicol. Chem. 2017, 36, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, L.; Wang, H.; Huang, J.; Chen, Q. Effects of melatonin on germination and seedling growth of tartary buckwheat under salt stress. Hubei Agric. Sci. 2021, 60, 25–28. [Google Scholar]
- Chen, L.; Liu, L.; Ma, T.; Jiang, D.; Sun, H.; Zhang, Y.; Zhang, K.; Bai, Z.; Li, C. Effects of melatonin on the antioxidant enzyme activities and seed germination of cotton (Gossypium hirsutum L.) under salt-stress conditions. Cotton Sci. 2019, 31, 438–447. [Google Scholar]
- Castañares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Li, A.; Li, R.; Chen, Y.; Yu, S. Effects of exogenous salicylic acid or melatonin on seed germination and seedling growth of soybean under salt stress. Anhui Agric. Sci. Bull. 2024, 30, 31–36. [Google Scholar]
- García-Cánovas, I.; Giraldo-Acosta, M.; Cano, A.; Arnao, M.B.; Hernández-Ruiz, J. Effect of melatonin on germination and seedling growth in aging seeds or under drought conditions. Seeds 2024, 3, 341–356. [Google Scholar] [CrossRef]
- Lei, X.; Wan, C.; Tao, J.; Leng, J.; Wu, Y.; Wang, J.; Wang, P.; Yang, Q.; Feng, B.; Gao, J. Effects of soaking seeds with MT and EBR on germination and seedling growth in buckwheat under salt stress. Acta Agron. Sin. 2022, 48, 1210–1221. [Google Scholar] [CrossRef]
- Wang, N.; Luo, X.; Chen, M.; Guo, R.; Liu, J. Effects of exogenous melatonin on seed germination and seedling growth of Cyperus esculentus L. under salt and drought stress. J. Agric. Sci. Technol. 2025, 27, 51–61. [Google Scholar]
- Li, Z.; Wang, J. Advances in research of physiological and molecular mechanism in seed vigor and germination. Sci. Agric. Sin. 2015, 48, 646–660. [Google Scholar]
- Dong, S.; Chen, L.; Zhang, M.; Liu, Q.; Wu, Y.; Li, X. Effect of priming-redrying treatment and storage on wine sorghum seed germination. J. North. Agric. 2022, 50, 127–134. [Google Scholar]
- Copeland, L.; McDonald, M. Principles of Seed Science and Technology; Springer: Boston, MA, USA, 2001. [Google Scholar]
- Nie, M.; Ning, N.; Zhang, Y.; Li, S.; Fan, X.; Liu, Q.; Zhang, H. The physiological mechanisms and alleviating effect of melatonin on sorghum seed germination under salt stress. Seed 2023, 42, 31–40, 63. [Google Scholar]
- Huang, J.; Zhai, J.; Wang, Y.; Cen, H.; Zhu, H.; Xu, T.; Xia, F. Effect of melatonin on seed germination and physiological characteristics of alfalfa seedlings under drought dress. Acta Agrestia Sin. 2024, 32, 2575–2583. [Google Scholar]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Liu, J.; Gong, M. Advances in Antioxidant Systems of Plants. J. Yunnan Norm. Univ. 1999, 19, 1–11. [Google Scholar]
- He, H.; Huang, N.; Cao, R.; Meng, L. Structures, antioxidation mechanism, and antioxidation test of the common natural antioxidants in plants. Biophysics 2015, 3, 25. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Li, X.; Hu, L.; Sun, C.; Liu, X.; Wei, L.; Zhu, J. Brassinolide promotes the growth of zanthixylum schinifolium by improving photosynthetic efficiency and antioxidant capacity. Agronomy 2024, 14, 2892. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Zhong, C.; Zhang, Y.; Wang-Pruski, G.; Zhang, Z.; Wu, J. Alleviating effect of melatonin on melon seed germination under autotoxicity and saline-alkali combined stress. J. Plant Growth Regul. 2023, 42, 2474–2485. [Google Scholar] [CrossRef]
- Ismaeil, F.M.; Ahmed, E.E.B.M.; Zubeir, M.M.E.; Suleman, N.N. Salt stress mitigation on seedling establishment, antioxidant capacity, ion homeostasis of maize by seed priming with melatonin. Asian J. Res. Crop Sci. 2025, 10, 1–14. [Google Scholar] [CrossRef]
- Samadi, M.; Kazemeini, S.A.; Razzaghi, F.; Edalat, M.; Andersen, M.N.; Jacobsen, S.-E.; Mastinu, A. Melatonin priming manipulates antioxidant regulation and secondary metabolites production in favour of drought tolerance in Chenopodium quinoa Willd. S. Afr. J. Bot. 2024, 166, 272–286. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Yang, C.; Mei, Z. Effects of different priming and drying treatments on seed germination of gentiana rigescens. Lishizhen Med. Mater. Med. Res. 2014, 25, 1480–1484. [Google Scholar]
- Muhammad, I.; Khan, A.; Mustafa, A.E.-Z.M.A.; Elshikh, M.S.; Shen, W. Elucidating the Modulatory Effect of Melatonin on Enzyme Activity and Oxidative Stress in Wheat: A Global Meta-Analysis. Physiol. Plant. 2024, 176, e14294. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Taraban, D.A.; Kokorev, A.I.; Yastreb, T.O.; Pysarenko, V.M.; Sherstiuk, E.; Karpets, Y.V. Effect of Melatonin and Hydropriming on Germination of Aged Triticale and Rye Seeds. Botanica 2024, 30, 1–13. [Google Scholar] [CrossRef]

| Treatments (μmol·L−1) | Germination Percentage (%) | ||||
|---|---|---|---|---|---|
| 3 h | 6 h | 9 h | 12 h | 24 h | |
| CK (0) | 100.0 ± 0.0 a | 99.3 ± 1.2 a | 99.3 ± 1.2 a | 98.7 ± 1.2 a | 99.3 ± 1.2 ab |
| T1(1) | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 98.7 ± 1.2 ab |
| T2 (5) | 98.7 ± 2.3 a | 100.0 ± 0.0 a | 98.0 ± 3.5 a | 97.3 ± 1.2 a | 99.3 ± 1.2 ab |
| T3 (10) | 100.0 ± 0.0 a | 98.0 ± 3.5 a | 99.3 ± 1.2 a | 98.7 ± 2.3 a | 100.0 ± 0.0 a |
| T4 (50) | 98.7 ± 1.2 a | 99.3 ± 1.2 a | 98.7 ± 2.3 a | 97.3 ± 3.1 a | 98.7 ± 1.2 ab |
| T5 (100) | 100.0 ± 0.0 a | 99.3 ± 1.2 a | 98.7 ± 1.2 a | 98.7 ± 2.3 a | 98.0 ± 0.0 b |
| Treatments (μmol·L−1) | Seedling Length (cm) | Root Length (cm) | Root Fresh Weight (g) |
|---|---|---|---|
| CK (0) | 1.46 ± 0.13 c | 6.68 ± 1.51 c | 0.54 ± 0.03 bc |
| T1 (1) | 1.40 ± 0.10 c | 6.07 ± 0.53 c | 0.50 ± 0.01 c |
| T2 (5) | 1.45 ± 0.03 c | 6.16 ± 0.97 c | 0.51 ± 0.03 bc |
| T3 (10) | 1.47 ± 0.16 c | 6.49 ± 1.07 c | 0.54 ± 0.04 bc |
| T4 (50) | 2.36 ± 0.25 a | 10.64 ± 0.38 a | 0.75 ± 0.10 a |
| T5 (100) | 1.85 ± 0.25 b | 8.67 ± 1.12 b | 0.60 ± 0.04 b |
| Treatments | Germination Percentage (%) | Germination Energy (%) | Germination Index | Root Length (cm) | Shoot Length (cm) | SFW (g) | SDW (g) | |
|---|---|---|---|---|---|---|---|---|
| 1 d | CK | 98.0 ± 3.5 | 98.0 ± 3.5 | 86.9 ± 3.3 | 4.8 ± 0.3 | 0.82 ± 0.04 | 1.51 ± 0.04 | 0.092 ± 0.003 * |
| T | 99.3 ± 1.2 | 99.3 ± 1.2 | 85.4 ± 2.6 | 4.9 ± 0.3 | 0.82 ± 0.03 | 1.45 ± 0.08 | 0.085 ± 0.003 | |
| 2 d | CK | 98.7 ± 2.3 | 98.7 ± 2.3 | 83.9 ± 2.9 | 4.5 ± 0.3 | 0.80 ± 0.01 | 1.52 ± 0.02 | 0.094 ± 0.002 |
| T | 98.7 ± 2.3 | 98.7 ± 2.3 | 84.7 ± 2.1 | 4.4 ± 0.1 | 0.85 ± 0.01 ** | 1.56 ± 0.03 | 0.090 ± 0.002 | |
| 3 d | CK | 94.7 ± 3.1 | 94.0 ± 4.0 | 79.5 ± 3.7 | 4.3 ± 0.2 | 0.79 ± 0.01 | 1.38 ± 0.04 | 0.093 ± 0.002 |
| T | 98.0 ± 0.0 | 98.0 ± 0.0 | 82.2 ± 1.2 | 4.8 ± 0.4 | 0.87 ± 0.10 | 1.52 ± 0.07 * | 0.092 ± 0.003 | |
| 4 d | CK | 92.0 ± 7.2 | 91.3 ± 6.4 | 76.5 ± 5.4 | 1.5 ± 0.1 | 0.56 ± 0.05 | 1.40 ± 0.08 | 0.090 ± 0.004 |
| T | 94.0 ± 4.0 | 94.0 ± 4.0 | 78.4 ± 4.7 | 1.5 ± 0.2 | 0.53 ± 0.02 | 1.38 ± 0.18 | 0.089 ± 0.003 | |
| 5 d | CK | 86.7 ± 5.0 | 86.0 ± 5.3 | 66.8 ± 4.6 | 1.5 ± 0.1 * | 0.53 ± 0.04 * | 1.42 ± 0.09 | 0.085 ± 0.003 |
| T | 92.0 ± 2.0 | 92.0 ± 2.0 | 76.1 ± 1.3* | 1.2 ± 0.1 | 0.39 ± 0.05 | 1.37 ± 0.10 | 0.087 ± 0.002 | |
| Treatments | Germination Percentage (%) | Germination Energy (%) | Germination Index | Root Length (cm) | Shoot Length (cm) | SFW (g) | SDW (g) | |
|---|---|---|---|---|---|---|---|---|
| 7 d | CK | 100.0 ± 0.0 | 100.0 ± 0.0 | 92.7 ± 0.6 | 4.2 ± 0.3 | 0.85 ± 0.03 | 1.25 ± 0.12 | 0.093 ± 0.003 |
| T | 100.0 ± 0.0 | 100.0 ± 0.0 | 93.0 ± 1.0 | 4.6 ± 0.1 * | 0.92 ± 0.02 * | 1.35 ± 0.02 | 0.095 ± 0.002 | |
| 14 d | CK | 99.3 ± 1.2 | 99.3 ± 1.2 | 85.0 ± 1.9 | 2.6 ± 0.2 | 0.65 ± 0.04 | 1.18 ± 0.07 | 0.089 ± 0.002 |
| T | 100.0 ± 0.0 | 100.0 ± 0.0 | 87.8 ± 1.4 | 3.3 ± 0.2 * | 0.77 ± 0.06 * | 1.25 ± 0.03 | 0.089 ± 0.005 | |
| 21 d | CK | 99.3 ± 1.2 | 97.3 ± 1.2 | 92.2 ± 3.5 | 1.0 ± 0.1 | 0.44 ± 0.06 | 1.52 ± 0.09 | 0.086 ± 0.005 |
| T | 98.0 ± 2.0 | 98.0 ± 2.0 | 93.0 ± 2.9 | 1.0 ± 0.1 | 0.47 ± 0.04 | 1.65 ± 0.04 | 0.095 ± 0.011 | |
| 28 d | CK | 95.3 ± 3.1 | 92.7 ± 7.6 | 84.1 ± 6.5 | 0.7 ± 0.0 | 0.29 ± 0.02 * | 1.27 ± 0.05 | 0.091 ± 0.004 |
| T | 97.3 ± 1.2 | 96.7 ± 1.2 | 89.0 ± 0.7 | 0.4 ± 0.1 | 0.20 ± 0.05 | 1.39 ± 0.14 | 0.092 ± 0.005 | |
| Treatments | Germination Percentage (%) | Germination Energy (%) | Germination Index | Root Length (cm) | Shoot Length (cm) | SFW (g) | SDW (g) | |
|---|---|---|---|---|---|---|---|---|
| 7 d | CK | 98.7 ± 1.2 | 98.0 ± 2.0 | 87.7 ± 1.1 | 5.0 ± 0.2 | 0.80 ± 0.01 | 1.21 ± 0.05 | 0.086 ± 0.002 |
| T | 99.3 ± 1.2 | 99.3 ± 1.2 | 90.9 ± 1.7 | 4.6 ± 0.2 | 0.84 ± 0.03 | 1.28 ± 0.01 | 0.091 ± 0.003 | |
| 14 d | CK | 95.3 ± 1.2 | 95.3 ± 1.2 | 81.6 ± 1.9 | 2.8 ± 0.2 | 0.63 ± 0.02 | 1.14 ± 0.04 | 0.088 ± 0.002 |
| T | 96.7 ± 1.2 | 96.0 ± 0.0 | 81.0 ± 0.9 | 3.4 ± 0.3 | 0.82 ± 0.03 ** | 1.18 ± 0.02 | 0.091 ± 0.002 | |
| 21 d | CK | 98.0 ± 2.0 | 97.3 ± 1.2 | 83.1 ± 2.6 | 1.3 ± 0.1 | 0.53 ± 0.03 | 1.37 ± 0.12 | 0.093 ± 0.004 |
| T | 97.3 ± 1.2 | 96.7 ± 1.2 | 86.9 ± 1.6 | 1.2 ± 0.1 | 0.53 ± 0.04 | 1.50 ± 0.09 | 0.087 ± 0.003 | |
| 28 d | CK | 97.3 ± 1.2 | 96.0 ± 2.0 | 83.5 ± 2.7 | 0.5 ± 0.1 | 0.21 ± 0.01 | 1.34 ± 0.06 | 0.091 ± 0.006 |
| T | 96.7 ± 1.2 | 95.3 ± 3.1 | 83.4 ± 0.3 | 1.0 ± 0.2 ** | 0.40 ± 0.06 ** | 1.32 ± 0.13 | 0.091 ± 0.007 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Tang, G.; An, X.; Li, H.; Chen, Q. Melatonin Priming Increases the Tolerance of Tartary Buckwheat Seeds to Abiotic Stress. Agronomy 2025, 15, 1606. https://doi.org/10.3390/agronomy15071606
Zhu L, Tang G, An X, Li H, Chen Q. Melatonin Priming Increases the Tolerance of Tartary Buckwheat Seeds to Abiotic Stress. Agronomy. 2025; 15(7):1606. https://doi.org/10.3390/agronomy15071606
Chicago/Turabian StyleZhu, Liwei, Guohong Tang, Xiaoyu An, Hongyou Li, and Qingfu Chen. 2025. "Melatonin Priming Increases the Tolerance of Tartary Buckwheat Seeds to Abiotic Stress" Agronomy 15, no. 7: 1606. https://doi.org/10.3390/agronomy15071606
APA StyleZhu, L., Tang, G., An, X., Li, H., & Chen, Q. (2025). Melatonin Priming Increases the Tolerance of Tartary Buckwheat Seeds to Abiotic Stress. Agronomy, 15(7), 1606. https://doi.org/10.3390/agronomy15071606






