Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
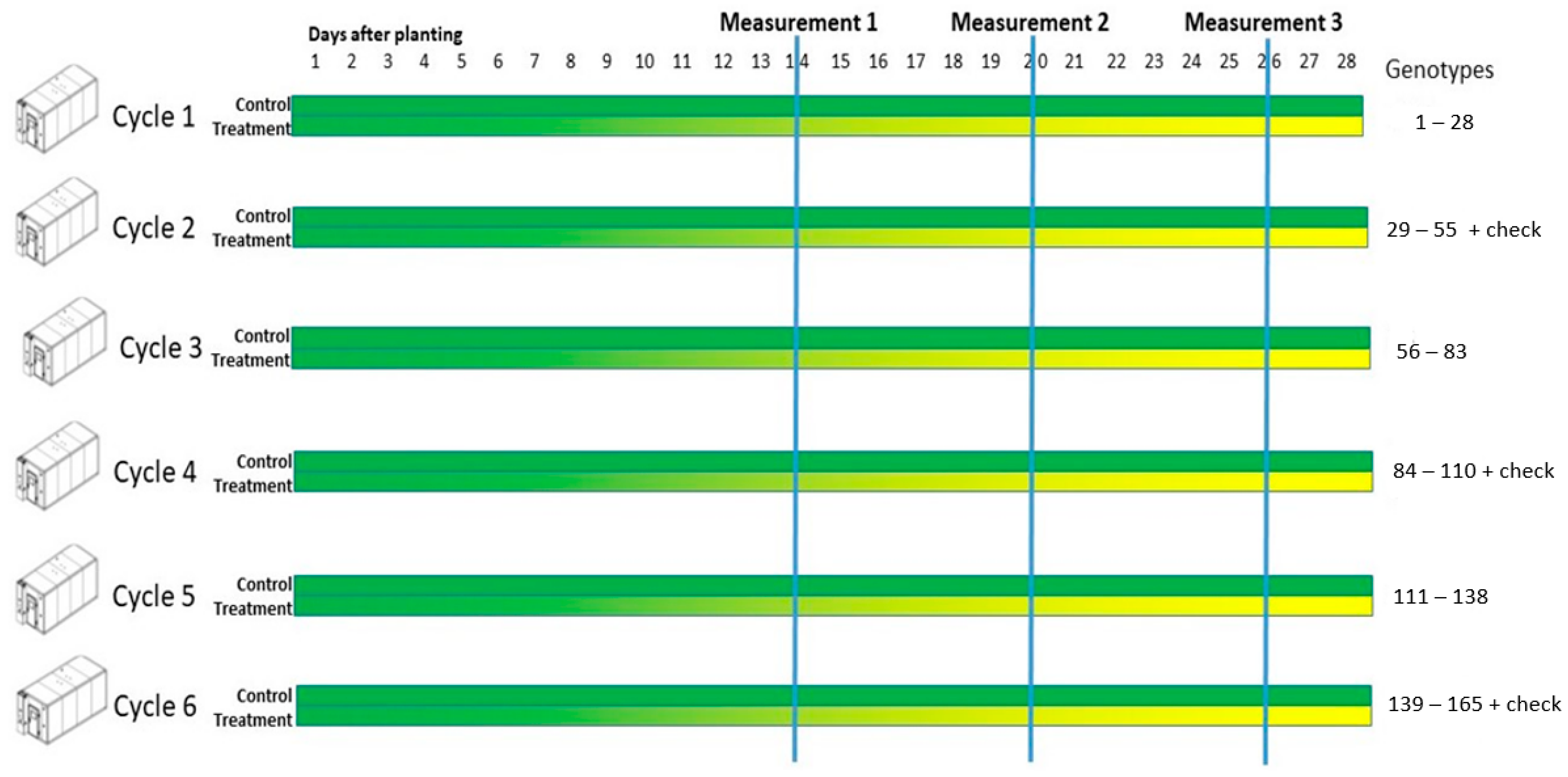
2.2. Preparation and Growing Conditions
2.3. Measurements
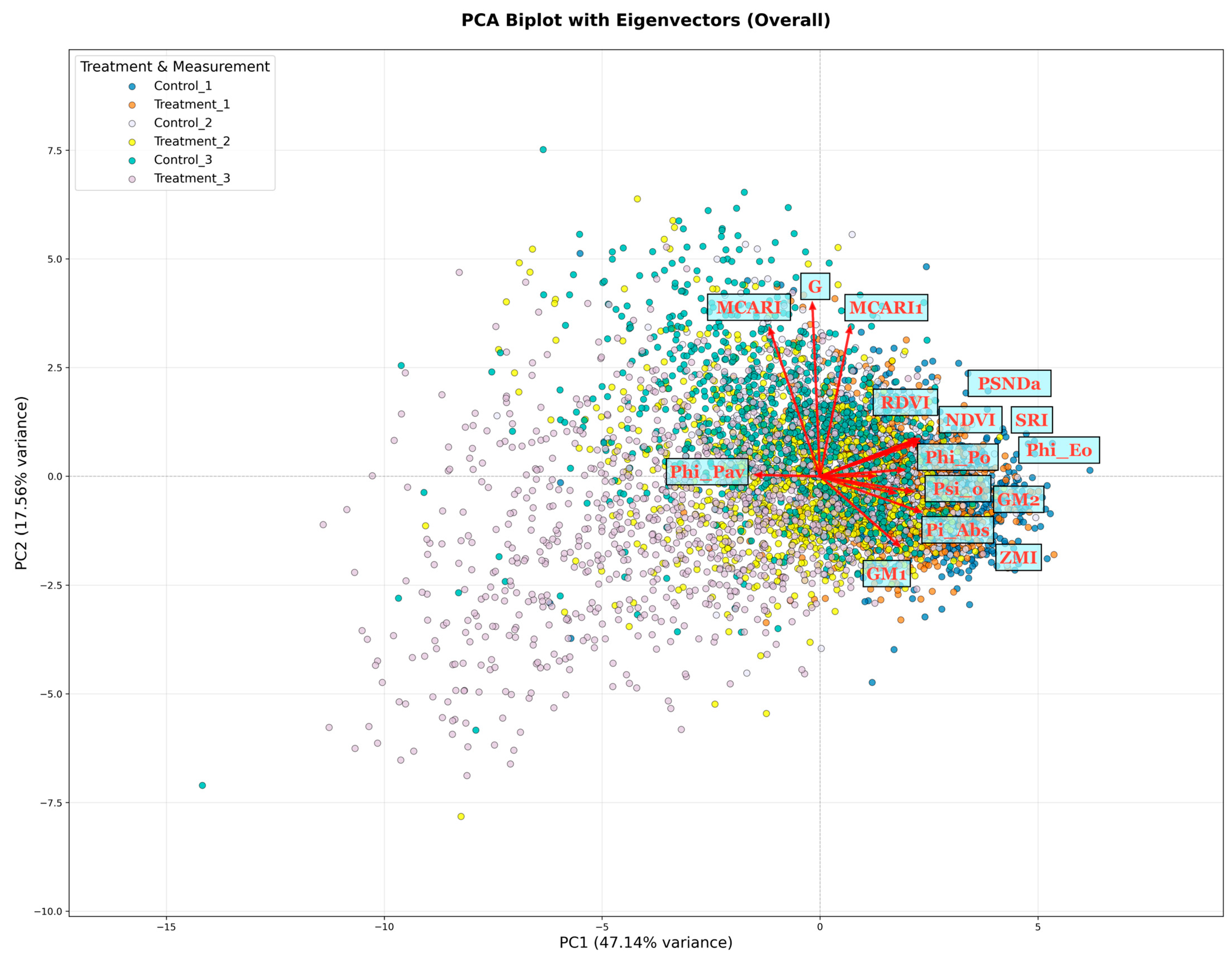
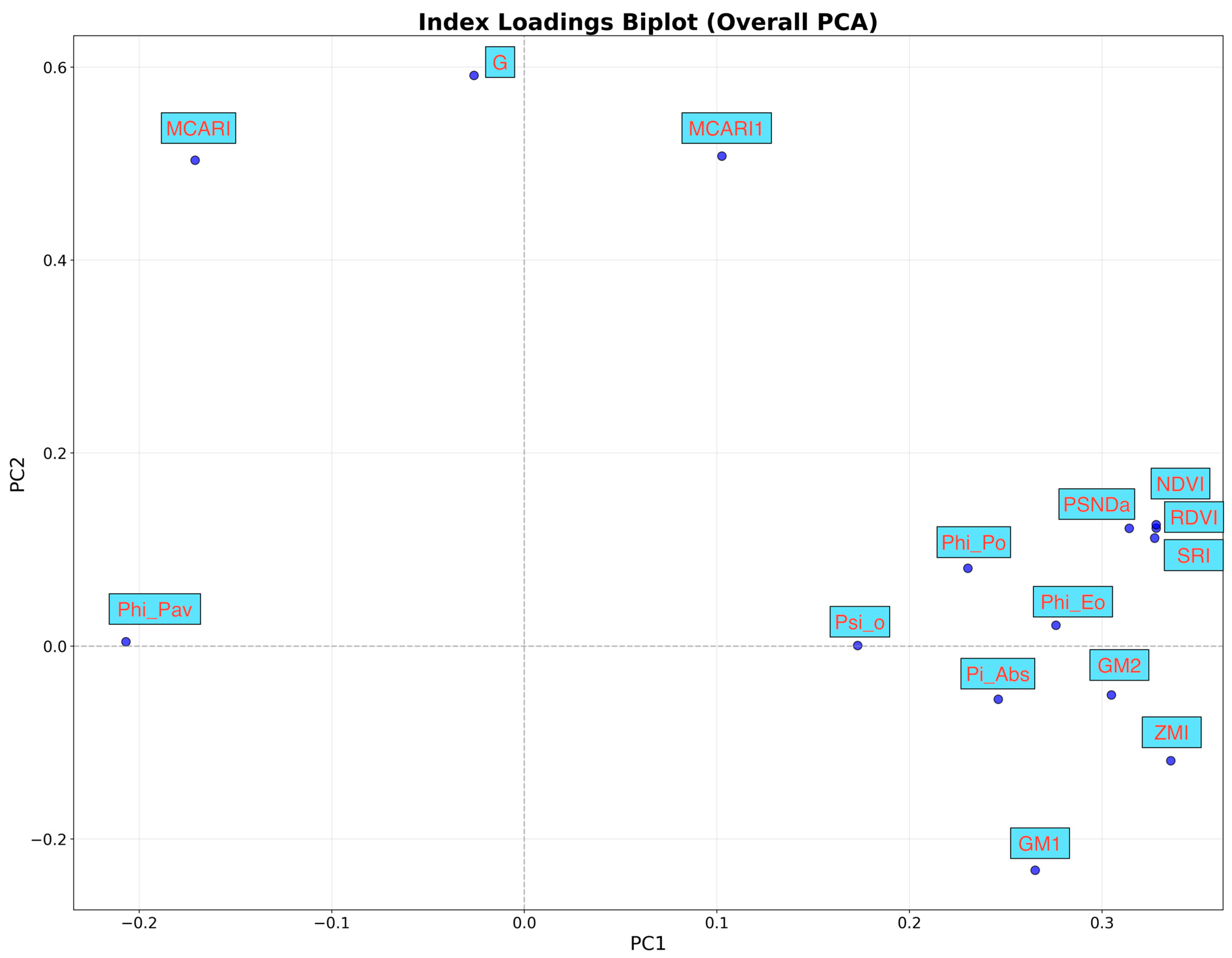
2.4. Data Analysis
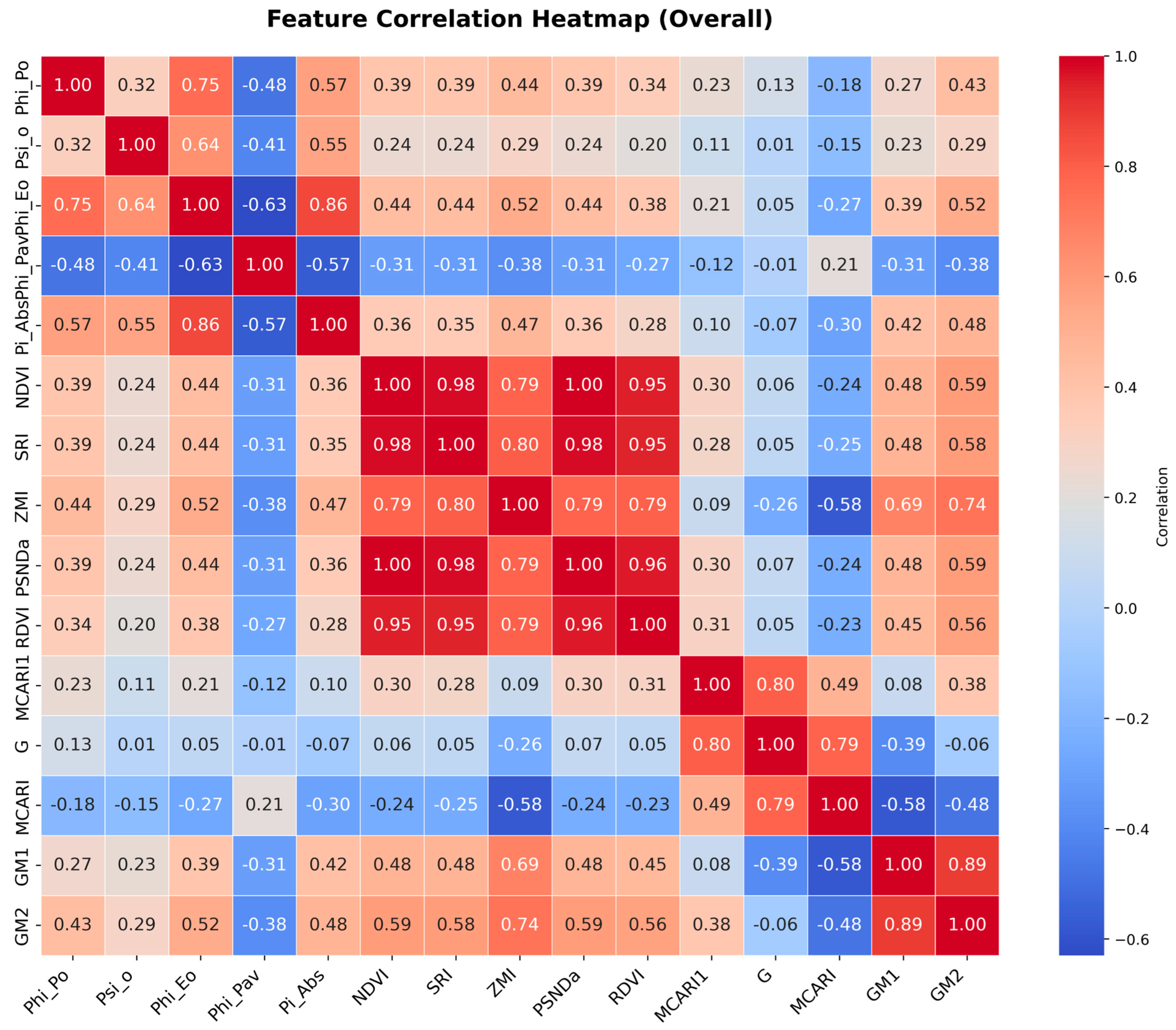
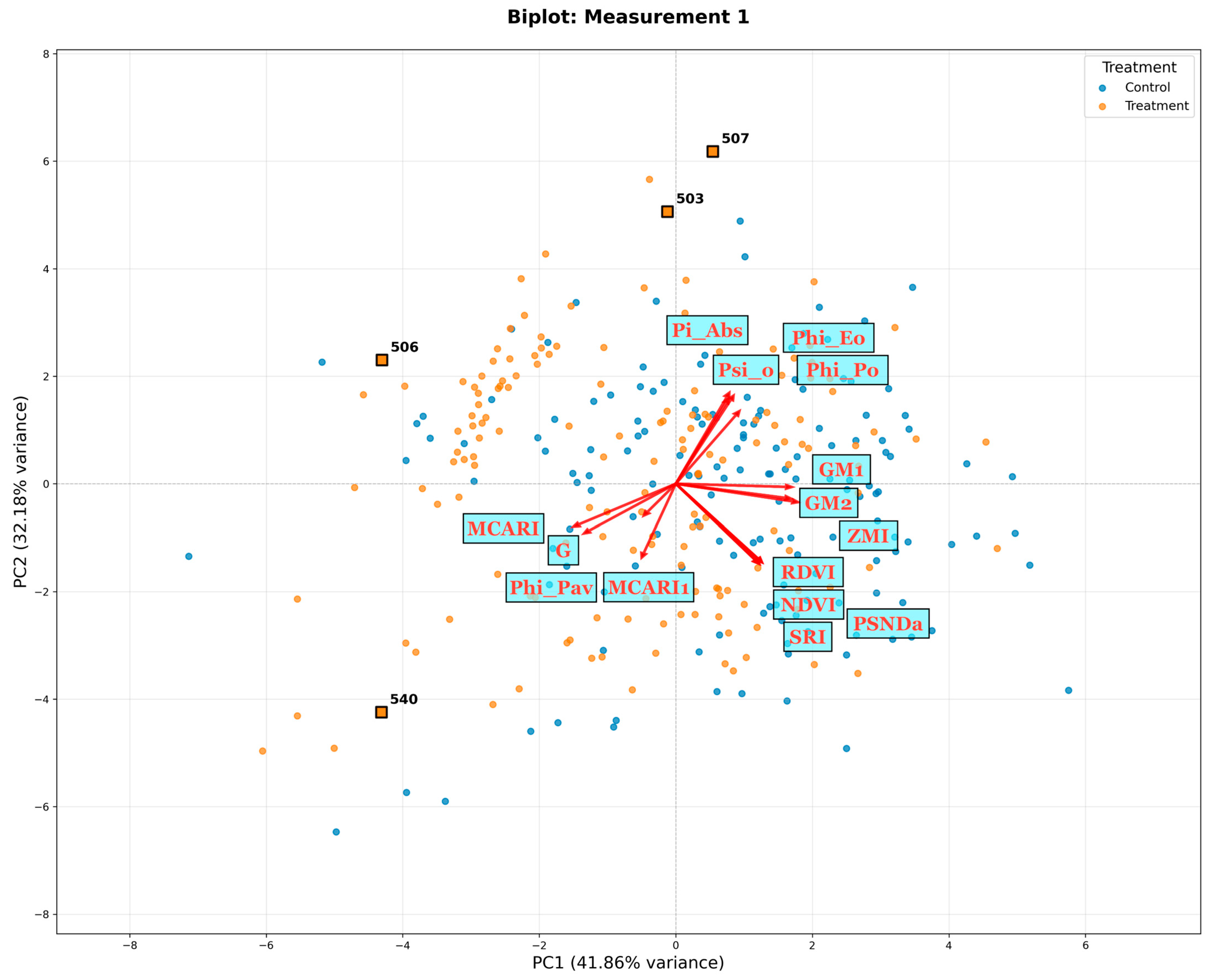
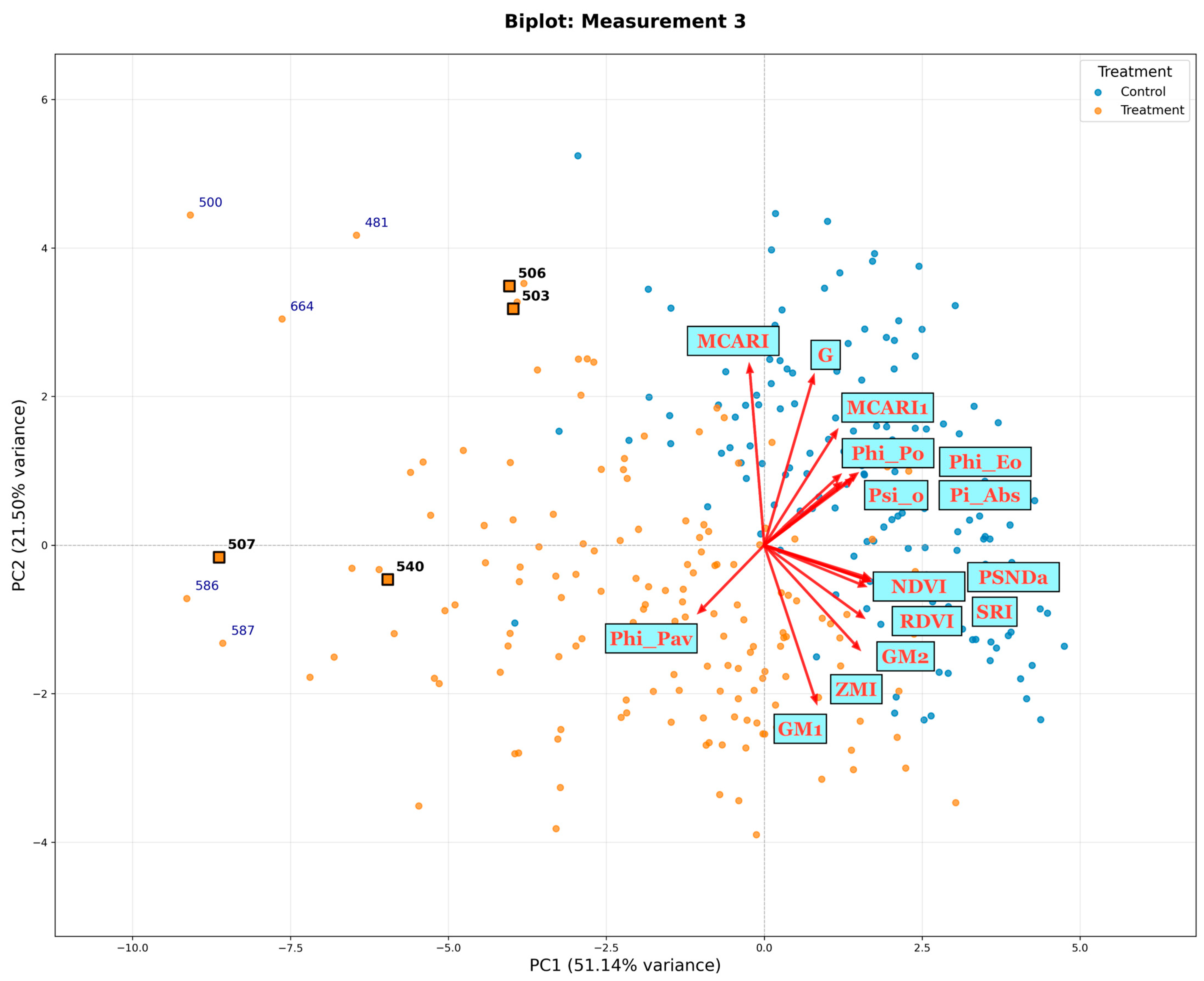
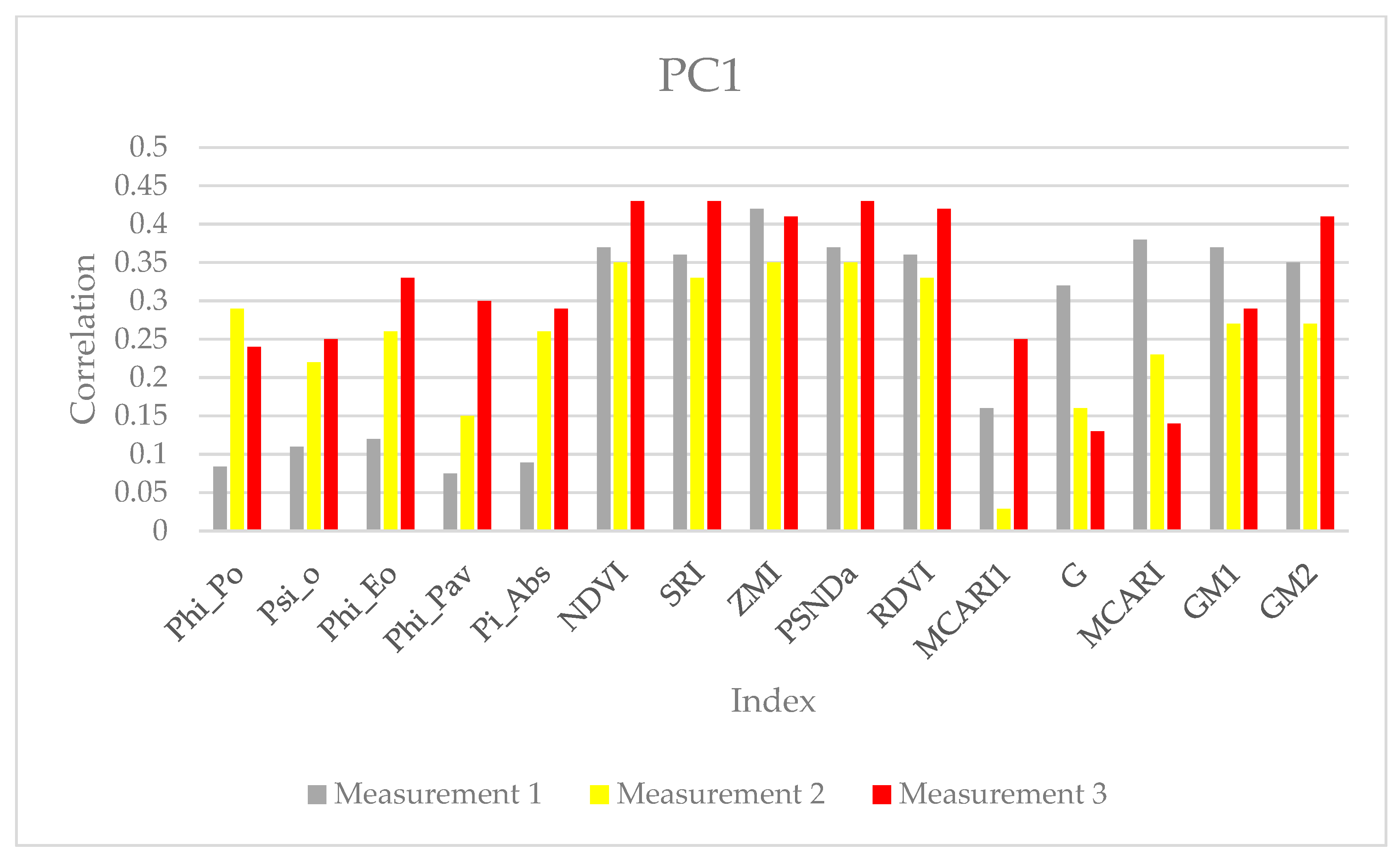
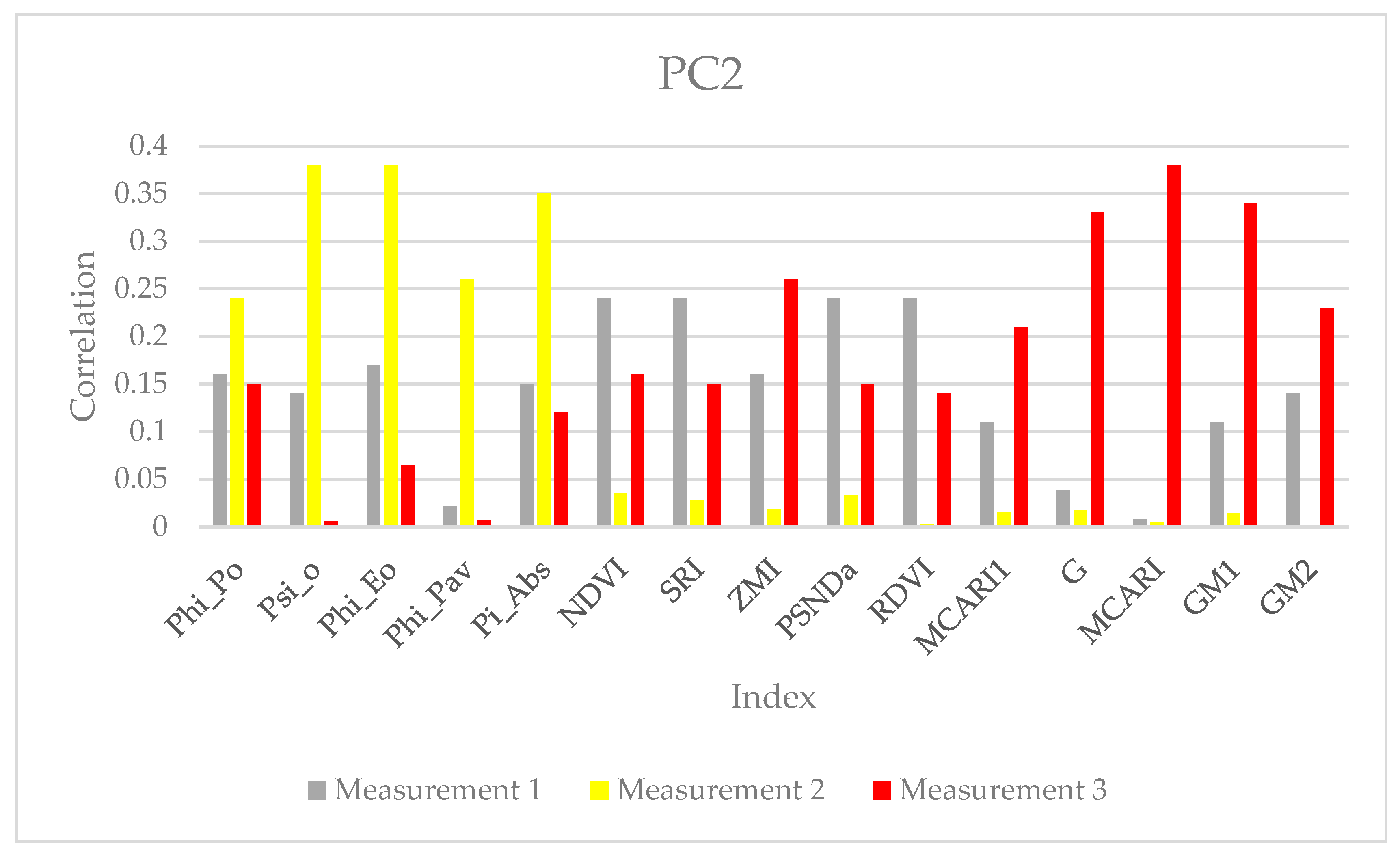
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Drought Stress in Maize (Zea mays L.); SpringerBriefs in Agriculture; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-25440-1. [Google Scholar]
- Galić, V.; Mlinarić, S.; Marelja, M.; Zdunić, Z.; Brkić, A.; Mazur, M.; Begović, L.; Šimić, D. Contrasting Water Withholding Responses of Young Maize Plants Reveal Link Between Lipid Peroxidation and Osmotic Regulation Corroborated by Genetic Analysis. Front. Plant Sci. 2022, 13, 804630. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Quan, C.; Song, Z.; Li, X.; Yu, G.; Li, C.; Muhammad, A. High-Throughput Plant Phenotyping Platform (HT3P) as a Novel Tool for Estimating Agronomic Traits from the Lab to the Field. Front. Bioeng. Biotechnol. 2021, 8, 623705. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Cardellicchio, A.; Nitti, M.; Lako, A.; Dimauro, G.; Renò, V. A Systematic Review of Effective Hardware and Software Factors Affecting High-Throughput Plant Phenotyping. Information 2023, 14, 214. [Google Scholar] [CrossRef]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Strasser, R.J. The Grouping Model of Plant Photosynthesis: Heterogeneity of photosynthetic units in thylakoids. In Photosynthesis. Vol. 3: Structure and Molecular Organization of the Photosynthetic Membrane; George, A., Ed.; Balaban International Science Services: Kalithea Kassandra, Halkidiki, Greece; Philadelphia, PA, USA, 1981; pp. 727–737. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Taylor & Francis: London, UK, 2000; p. 445. ISBN 978-0-7484-0821-4. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. ISBN 978-1-4020-3217-2. [Google Scholar]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP Test: A Tool to Screen the Capacity of Plant Adaptation to Climate Change. Scand. J. For. Res. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Matsubara, S.; Chen, Y.-C.; Caliandro, R.; Govindjee; Clegg, R.M. Photosystem II Fluorescence Lifetime Imaging in Avocado Leaves: Contributions of the Lutein-Epoxide and Violaxanthin Cycles to Fluorescence Quenching. J. Photochem. Photobiol. B 2011, 104, 271–284. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll Fluorescence Imaging for Early Detection of Drought and Heat Stress in Strawberry Plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Asaari, M.S.M.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. Analysis of Hyperspectral Images for Detection of Drought Stress and Recovery in Maize Plants in a High-Throughput Phenotyping Platform. Comput. Electron. Agric. 2019, 162, 749–758. [Google Scholar] [CrossRef]
- Mohd Asaari, M.S.; Mertens, S.; Verbraeken, L.; Dhondt, S.; Inzé, D.; Bikram, K.; Scheunders, P. Non-Destructive Analysis of Plant Physiological Traits Using Hyperspectral Imaging: A Case Study on Drought Stress. Comput. Electron. Agric. 2022, 195, 106806. [Google Scholar] [CrossRef]
- Ram, B.G.; Oduor, P.; Igathinathane, C.; Howatt, K.; Sun, X. A Systematic Review of Hyperspectral Imaging in Precision Agriculture: Analysis of Its Current State and Future Prospects. Comput. Electron. Agric. 2024, 222, 109037. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, Z.; Xie, C.; Crossa, J.; Araus, J.-L.; Gao, S.; Vivek, B.S.; Magorokosho, C.; Mugo, S.; Makumbi, D.; et al. Large-Scale Screening for Maize Drought Resistance Using Multiple Selection Criteria Evaluated under Water-Stressed and Well-Watered Environments. Field Crops Res. 2011, 124, 37–45. [Google Scholar] [CrossRef]
- Shuaibu, S.M.; Ogbodo, J.A.; Wasige, E.; Mashi, S.A. Assessing the Impact of Agricultural Drought on Maize Prices in Kenya with the Approach of the SPOT-VEGETATION NDVI Remote Sensing. Future Food J. Food Agric. Soc. 2016, 4, 8–18. [Google Scholar]
- Verma, B.; Prasad, R.; Srivastava, P.K.; Singh, P. Retrieval of Leaf Area Index Using Inversion Algorithm. In Proceedings of the 2022 12th Workshop on Hyperspectral Imaging and Signal Processing: Evolution in Remote Sensing (WHISPERS), Rome, Italy, 13–16 September 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Viña, A.; Arkebauer, T.; Rundquist, D. Green Leaf Area Index Estimation in Maize and Soybean: Combining Vegetation Indices to Achieve Maximal Sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and Model Inversion Methods with Narrowband Optical Indices for Chlorophyll Content Estimation in Closed Forest Canopies with Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Moorthy, I.; Miller, J.R.; Noland, T.L.; Nielsen, U.; Zarco-Tejada, P.J. Chlorophyll Content Estimation of Boreal Conifers Using Hyperspectral Remote Sensing. In Proceedings of the IGARSS 2003, 2003 IEEE International Geoscience and Remote Sensing Symposium, Proceedings (IEEE Cat. No. 03CH37477), Toulouse, France, 21–25 July 2003; Volume 4, pp. 2568–2570. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Adak, S.; Bandyopadhyay, K.K.; Sahoo, R.N.; Mridha, N.; Shrivastava, M.; Purakayastha, T.J. Prediction of Wheat Yield Using Spectral Reflectance Indices under Different Tillage, Residue and Nitrogen Management Practices. Curr. Sci. 2021, 121, 402–413. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Recent Advances in Crop Water Stress Detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Su, X.; Lu, J.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimating Leaf Area Index with a New Vegetation Index Considering the Influence of Rice Panicles. Remote Sens. 2019, 11, 1809. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping Maize Water Stress Based on UAV Multispectral Remote Sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef]
- Eddy, P.R.; Smith, A.M.; Hill, B.D.; Peddle, D.R.; Coburn, C.A.; Blackshaw, R.E. Hybrid Segmentation—Artificial Neural Network Classification of High Resolution Hyperspectral Imagery for Site-Specific Herbicide Management in Agriculture. Photogramm. Eng. Remote Sens. 2008, 74, 1249–1257. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Acharya, U.K.; Subedi, P.P.; Walsh, K.B.; McGlasson, W.B. Estimation of Fruit Maturation and Ripening Using Spectral Indices. Acta Hortic. 2016, 1119, 265–272. [Google Scholar] [CrossRef]
- Ardıç, M.; Işık, G.; Sezer, O.; Zeybek, Ş.; Gökçen, Ü.; Şahin, M.S.; Erkara, İ.P. Determination of Ecophysiological Responses to Temperature Shock in Different Wheat Genotypes. Int. J. Nat. Eng. Sci. 2021, 15, 36–42. [Google Scholar]
- Aliyeva, N.; Rustamova, S.; Huseynova, I. Non-Invasive Phenotyping of Drought Response in Bread Wheat Genotypes Using GM2 and MCARI1 Indices. Trans. Inst. Mol. Biol. Biotechnol. 2024, 8, 3–7. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Japper, G.; Chang, H.; Ma, Z.; Duan, Y. Hyperspectral Indices for Leaf and Pixel Chlorophyll Estimation in Open-Canopy Tree. In Proceedings of the Geoinformatics 2007: Remotely Sensed Data and Information, Nanjing, China, 10 June 2007; p. 675222. [Google Scholar]
- Liu, M.; Qi, H.; Zhang, Z.P.; Song, Z.W.; Kou, T.J.; Zhang, W.J.; Yu, J.L. Response of Photosynthesis and Chlorophyll Fluorescence to Drought Stress in Two Maize Cultivars. Afr. J. Agric. Res. 2012, 7, 4751–4760. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Leng, P.; Shang, G.-F.; Zhang, X.; Li, Z.-L. Sun-Induced Chlorophyll Fluorescence Is Superior to Satellite Vegetation Indices for Predicting Summer Maize Yield under Drought Conditions. Comput. Electron. Agric. 2023, 205, 107615. [Google Scholar] [CrossRef]
- Jan, M.F.; Li, M.; Liaqat, W.; Altaf, M.T.; Liu, C.; Ahmad, H.; Khan, E.H.; Ali, Z.; Barutçular, C.; Mohamed, H.I. Chlorophyll Fluorescence: A Smart Tool for Maize Improvement. CEREAL Res. Commun. 2024, 53, 617–648. [Google Scholar] [CrossRef]
- Römer, C.; Wahabzada, M.; Ballvora, A.; Pinto, F.; Rossini, M.; Panigada, C.; Behmann, J.; Léon, J.; Thurau, C.; Bauckhage, C.; et al. Early Drought Stress Detection in Cereals: Simplex Volume Maximisation for Hyperspectral Image Analysis. Funct. Plant Biol. 2012, 39, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; Demuynck, K.; Maleux, K.; Cannoot, B.; De Block, J.; Maere, S.; Nelissen, H.; Bonaventure, G.; et al. Proximal Hyperspectral Imaging Detects Diurnal and Drought-Induced Changes in Maize Physiology. Front. Plant Sci. 2021, 12, 640914. [Google Scholar] [CrossRef]
- Ganal, M.W.; Durstewitz, G.; Polley, A.; Bérard, A.; Buckler, E.S.; Charcosset, A.; Clarke, J.D.; Graner, E.-M.; Hansen, M.; Joets, J.; et al. A Large Maize (Zea mays L.) SNP Genotyping Array: Development and Germplasm Genotyping, and Genetic Mapping to Compare with the B73 Reference Genome. PLoS ONE 2011, 6, e28334. [Google Scholar] [CrossRef]
- Money, D.; Gardner, K.; Migicovsky, Z.; Schwaninger, H.; Zhong, G.-Y.; Myles, S. LinkImpute: Fast and Accurate Genotype Imputation for Nonmodel Organisms. G3 Genes|Genomes|Genet. 2015, 5, 2383–2390. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE Algorithm for Individual Ancestry Estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- PolyPen|Handheld Devices. Available online: https://handheld.psi.cz/products/polypen/#accessories (accessed on 17 April 2025).
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA: Washington, DC, USA, 1974.
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Kauth, R.; Thomas, G. The Tasselled Cap—A Graphic Description of the Spectral-Temporal Development of Agricultural Crops as Seen by LANDSAT. LARS Symp. 1976, 159. Available online: https://docs.lib.purdue.edu/cgi/viewcontent.cgi?article=1160&context=lars_symp (accessed on 17 April 2025).
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Gitelson, A.A.; and Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Visual Studio Code-Code Editing. Redefined. Available online: https://code.visualstudio.com/ (accessed on 17 April 2025).
- Welcome to Python.Org. Available online: https://www.python.org/ (accessed on 17 April 2025).
- IterativeImputer. Available online: https://scikit-learn.org/stable/modules/generated/sklearn.impute.IterativeImputer.html (accessed on 17 April 2025).
- StandardScaler. Available online: https://scikit-learn/stable/modules/generated/sklearn.preprocessing.StandardScaler.html (accessed on 17 April 2025).
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Mansoor, S.; Chung, Y.S. Functional Phenotyping: Understanding the Dynamic Response of Plants to Drought Stress. Curr. Plant Biol. 2024, 38, 100331. [Google Scholar] [CrossRef]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual Framework for Drought Phenotyping during Molecular Breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef]
- Morari, F.; Meggio, F.; Lunardon, A.; Scudiero, E.; Forestan, C.; Farinati, S.; Varotto, S. Time Course of Biochemical, Physiological, and Molecular Responses to Field-Mimicked Conditions of Drought, Salinity, and Recovery in Two Maize Lines. Front Plant Sci 2015, 6, 314. [Google Scholar] [CrossRef]
- Eskikoy, G.; Kutlu, I. Inter-Subspecies Diversity of Maize to Drought Stress with Physio-Biochemical, Enzymatic and Molecular Responses. PeerJ 2024, 12, e17931. [Google Scholar] [CrossRef]
- Huete, A. Vegetation Indices. In Encyclopedia of Remote Sensing; Njoku, E.G., Ed.; Springer: New York, NY, USA, 2014; pp. 883–886. ISBN 978-0-387-36699-9. [Google Scholar]
- Yang, Y.; Liu, X.; Zhao, Y.; Tang, G.; Nan, R.; Zhang, Y.; Sun, F.; Xi, Y.; Zhang, C. Evaluation of Wheat Drought Resistance Using Hyperspectral and Chlorophyll Fluorescence Imaging. Plant Physiol. Biochem. 2025, 219, 109415. [Google Scholar] [CrossRef]
- Gorai, T.; Yadav, P.; Choudhary, G.L.; Kumar, A. Site-Specific Crop Nutrient Management for Precision Agriculture—A Review. Curr. J. Appl. Sci. Technol. 2021, 40, 37–52. [Google Scholar] [CrossRef]
- Damian, J.M.; Pias, O.H.D.C.; Cherubin, M.R.; Fonseca, A.Z.D.; Fornari, E.Z.; Santi, A.L. Applying the NDVI from Satellite Images in Delimiting Management Zones for Annual Crops. Sci. Agric. 2019, 77, e20180055. [Google Scholar] [CrossRef]
- Gimenez, K.; Blanc, P.; Argillier, O.; Pierre, J.-B.; Le Gouis, J.; Paux, E. Dissecting Bread Wheat Heterosis through the Integration of Agronomic and Physiological Traits. Biology 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Tamás, A.; Kovács, E.; Horváth, É.; Juhász, C.; Radócz, L.; Rátonyi, T.; Ragán, P. Assessment of NDVI Dynamics of Maize (Zea mays L.) and Its Relation to Grain Yield in a Polyfactorial Experiment Based on Remote Sensing. Agriculture 2023, 13, 689. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The Role of Chlorophyll Fluorescence in The Detection of Stress Conditions in Plants. C R C Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Legendre, R.; Basinger, N.T.; van Iersel, M.W. Low-Cost Chlorophyll Fluorescence Imaging for Stress Detection. Sensors 2021, 21, 2055. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental Stress—What Can We Learn from Chlorophyll a Fluorescence Analysis in Woody Plants? A Review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef]
- Kim, Y.; Glenn, D.M.; Park, J.; Ngugi, H.K.; Lehman, B.L. Hyperspectral Image Analysis for Plant Stress Detection. In Proceedings of the 2010 ASABE Annual International Meeting, Pittsburgh, PA, USA, 20–23 June 2010. [Google Scholar]
- Behmann, J.; Steinrücken, J.; Plümer, L. Detection of Early Plant Stress Responses in Hyperspectral Images. ISPRS J. Photogramm. Remote Sens. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Zivcak, M.; Olsovska, K.; Kovar, M.; Shao, H.; He, X. Applying Hyperspectral Imaging to Explore Natural Plant Diversity towards Improving Salt Stress Tolerance. Sci. Total Environ. 2017, 578, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Karley, A.; Britten, A.; McCallum, S.; Graham, J. Raspberry Plant Stress Detection Using Hyperspectral Imaging. Plant Direct 2023, 7, e490. [Google Scholar] [CrossRef]
- Zhou, J.; Li, F.; Wang, X.; Yin, H.; Zhang, W.; Du, J.; Pu, H. Hyperspectral and Fluorescence Imaging Approaches for Nondestructive Detection of Rice Chlorophyll. Plants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Bauriegel, E.; Herppich, W.B. Hyperspectral and Chlorophyll Fluorescence Imaging for Early Detection of Plant Diseases, with Special Reference to Fusarium Spec. Infections on Wheat. Agriculture 2014, 4, 32–57. [Google Scholar] [CrossRef]
- Feng, X.; Yu, C.; Chen, Y.; Peng, J.; Ye, L.; Shen, T.; Wen, H.; He, Y. Non-Destructive Determination of Shikimic Acid Concentration in Transgenic Maize Exhibiting Glyphosate Tolerance Using Chlorophyll Fluorescence and Hyperspectral Imaging. Front. Plant Sci. 2018, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Sampson, P.H.; Zarco-Tejada, P.J.; Mohammed, G.H.; Miller, J.R.; Noland, T.L. Hyperspectral Remote Sensing of Forest Condition: Estimating Chlorophyll Content in Tolerant Hardwoods. For. Sci. 2003, 49, 381–391. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Trujillo, J.; de la Orden, M.S.; Hernández-Clemente, R. Hyperspectral and Multispectral Satellite Sensors for Mapping Chlorophyll Content in a Mediterranean Pinus sylvestris L. Plantation. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 88–96. [Google Scholar] [CrossRef]
- Noh, H.K.; Lu, R. Hyperspectral Laser-Induced Fluorescence Imaging for Assessing Apple Fruit Quality. Postharvest Biol. Technol. 2007, 43, 193–201. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Cybulska, J.; Szymańska-Chargot, M.; Siedliska, A.; Zdunek, A.; Nosalewicz, A.; Baranowski, P.; Kurenda, A. Early Detection of Fungal Infection of Stored Apple Fruit with Optical Sensors—Comparison of Biospeckle, Hyperspectral Imaging and Chlorophyll Fluorescence. Food Control 2018, 85, 327–338. [Google Scholar] [CrossRef]
- Kasampalis, D.S.; Tsouvaltzis, P.; Ntouros, K.; Gertsis, A.; Gitas, I.; Siomos, A.S. The Use of Digital Imaging, Chlorophyll Fluorescence and Vis/NIR Spectroscopy in Assessing the Ripening Stage and Freshness Status of Bell Pepper Fruit. Comput. Electron. Agric. 2021, 187, 106265. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Verstraeten, W.W.; van Aardt, J.A.N.; Keulemans, W.; Coppin, P. Hyperspectral Indices to Diagnose Leaf Biotic Stress of Apple Plants, Considering Leaf Phenology. Int. J. Remote Sens. 2009, 30, 1887–1912. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Park, E.; Kim, T.; Kim, M.S.; Baek, I.; Joshi, R.; Kim, J.; Cho, B.-K. Fluorescence Hyperspectral Imaging for Early Diagnosis of Heat-Stressed Ginseng Plants. Appl. Sci. 2023, 13, 31. [Google Scholar] [CrossRef]
- Praprotnik, E.; Vončina, A.; Žigon, P.; Knapič, M.; Susič, N.; Širca, S.; Vodnik, D.; Lenarčič, D.; Lapajne, J.; Žibrat, U.; et al. Early Detection of Wireworm (Coleoptera: Elateridae) Infestation and Drought Stress in Maize Using Hyperspectral Imaging. Agronomy 2023, 13, 178. [Google Scholar] [CrossRef]
- Ejaz, I.; Li, W.; Naseer, M.A.; Li, Y.; Qin, W.; Farooq, M.; Li, F.; Huang, S.; Zhang, Y.; Wang, Z.; et al. Detection of Combined Frost and Drought Stress in Wheat Using Hyperspectral and Chlorophyll Fluorescence Imaging. Environ. Technol. Innov. 2023, 30, 103051. [Google Scholar] [CrossRef]

| Index | Definition | Reference(s) |
|---|---|---|
| Maximum quantum yield of primary photochemistry of a dark-adapted leaf (Phi_Po) | Phi_Po = 1 − (F0/Fm) (or Fv/Fm) | [8] |
| Probability that a trapped exciton moves an electron into the electron transport chain further than QA (Psi_o) | Psi_o = 1 − VJ | [8] |
| Quantum yield of electron transport (Phi_Eo) | (1 − (Fo/FM)) × Psi_o | [8] |
| Time to reach maximum chlorophyll fluorescence level (Phi_Pav) | Phi_Po (SM/tFm) | [8] |
| Performance index (Pi_Abs) | (RC/ABS) [Phi_Po/(1 − Phi_Po)] [Psi_o/(1 − Phi_Po)] | [8] |
| NIR indices | ||
| Normalized Difference Vegetation (NDVI) | (RNIR − RRED)/(RNIR + RRED) | [46] |
| Simple Ratio Index (SRI) | RNIR/RRED | [22,46] |
| Zarco-Tejada and Miller Index (ZMI) | R750/R710 | [24] |
| Plant Senescence Reflectance Index a (PSNDa) | (R790 − R680)/(R790 + R680) | [26] |
| Renormalized Difference Vegetation Index (RDVI) | (R780 − R670)/((R780 + R670)0.5) | [47] |
| UVIS indices | ||
| Modified Chlorophyll Absorption in Reflectance Index 1 (MCARI1) | 1.2 × [2.5 × (R790 − R670) − 1.3 × (R790 − R550)] | [48] |
| Greenness Index (G) | R554/R677 | [49] |
| Modified Chlorophyll Absorption in Reflectance Index (MCARI) | [(R700 − R670) − 0.2 × (R700 − R550)] × (R700/R670) | [50] |
| Gitelson and Merzlyak Index 1 (GM1) | R750/R550 | [51] |
| Gitelson and Merzlyak Index 2 (GM2) | R750/R700 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukadinović, L.; Galić, V.; Brkić, A.; Jambrović, A.; Šimić, D. Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel. Agronomy 2025, 15, 1604. https://doi.org/10.3390/agronomy15071604
Vukadinović L, Galić V, Brkić A, Jambrović A, Šimić D. Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel. Agronomy. 2025; 15(7):1604. https://doi.org/10.3390/agronomy15071604
Chicago/Turabian StyleVukadinović, Lovro, Vlatko Galić, Andrija Brkić, Antun Jambrović, and Domagoj Šimić. 2025. "Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel" Agronomy 15, no. 7: 1604. https://doi.org/10.3390/agronomy15071604
APA StyleVukadinović, L., Galić, V., Brkić, A., Jambrović, A., & Šimić, D. (2025). Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel. Agronomy, 15(7), 1604. https://doi.org/10.3390/agronomy15071604







