Abstract
Root lesion nematodes (RLNs) are major plant parasites causing significant global yield losses in a wide range of crops. Current management strategies largely depend on synthetic nematicides, which raise environmental and human health concerns due to their broad-spectrum toxicity and persistence in the ecosystem. Volatile allelochemicals offer a promising, environmentally safer alternative due to their biodegradability and lower toxicity to mammals. In this study, we assessed the nematicidal activity of five allelochemical volatiles—dimethyl sulphide (DMS), dimethyl disulphide (DMDS), trans-cinnamaldehyde (TCA), trans-2-decenal (T2D), and trans-2-undecenal (T2U)—against Pratylenchus penetrans, using direct-contact bioassays, in comparison with the conventional nematicide oxamyl. Additionally, we assessed their environmental behaviour and toxicity profiles through in silico modelling. At 1 mg/mL, TCA, T2D, and T2U exhibited strong activity against P. penetrans, outperforming oxamyl by up to 1.6-fold, while DMS and DMDS showed reduced activity. The environmental risk assessment revealed that these compounds have a lower predicted persistence and bioaccumulation compared with oxamyl or fluopyram, a new generation nematicide. Though these findings boost the potential of these compounds as sustainable alternatives for RLN management, field validation and testing with non-target organisms remain necessary for the development of biopesticides. Nevertheless, this study emphasizes the need for an integrated risk-based assessment in the selection of nematicidal agents, warranting efficacy as well as environmental safety.
1. Introduction
Plant-parasitic nematodes (PPNs) are among the most widespread and destructive pests of agricultural crops, posing a significant threat to global food production. Root lesion nematodes (RLNs), of the genus Pratylenchus, are migratory endoparasites that cause substantial yield losses in a variety of economically important crops, including carrot, potato, coffee, corn, maize, alfalfa, and soybean [1]. Within the roots, RLNs feed on cortical and vascular cells, disrupting vital functions, while in the soil, they target epidermal cells and root hairs [2]. The progression of RLN infection leads to the formation of necrotic lesions, which impair water and nutrient uptake and compromise overall plant health [3]. Moreover, the migration of RLNs throughout the root system increases the dispersal of these lesions, predisposing plants to secondary infections by opportunistic pathogens [4,5]. Among the RLNs, P. penetrans (Cobb, 1917) Filipjev & Schuurmans-Stekhoven, 1941, is one of the most economically significant species, with a remarkably broad host range of over 400 plant species. P. penetrans reproduces sexually, and females deposit eggs either within host root tissues or in the surrounding rhizosphere, enabling persistent infection and spread. Its life cycle can be completed between 30 to 60 days, depending on environmental factors such as temperature and soil moisture, allowing a rapid population buildup under favourable conditions [2].
Pest management of the RLN is particularly difficult due to its broad host range and the ability to persist in the absence of a host, either in egg or juvenile form [3]. Conventional management approaches, including crop rotation, fallowing, and the use of non-host cover crops, have shown limited effectiveness in suppressing RLN populations under field conditions [6]. As a result, synthetic nematicides have historically been preferred over more sustainable methods. Among these, synthetic chemicals, such as the non-fumigant carbamate oxamyl, have been widely used to manage soil PPNs, including RLNs [7]. This chemical inhibits acetylcholinesterase (AChE), thereby disrupting cholinergic neurotransmission in the nematode. However, due to its non-specific mode of action, oxamyl affects a broad spectrum of organisms beyond its target pests, raising serious environmental and human health concerns. For example, beneficial soil organisms, which are critical for maintaining ecosystem functions such as nutrient cycling and soil fertility, are known to be negatively influenced by conventional nematicides [8,9]; additionally, the persistent residues of oxamyl, and similar first-generation nematicides, are frequently detected in soil, water, and food products [8]. The indiscriminate suppression of beneficial organisms not only reduces biodiversity but also impairs agricultural productivity and compromises food security [10]. Consequently, many of these traditional nematicides have been banned or severely restricted in several countries [11]. In response to these impositions, recent research has focused on developing new generation nematicides with lower environmental impacts and improved specificity when compared with traditional nematicides. One such example is fluopyram, which targets the mitochondrial electron transport chain (complex II) in nematodes [12,13]. Nonetheless, despite showing a safer environmental behaviour in comparison with more traditional nematicides, new generation nematicides can still impact agroecosystem biodiversity if used indiscriminately [8].
The increasingly strict legislation on conventional nematicides has driven a renewed interest in developing sustainable strategies to control RLNs. Among these, plant allelochemicals have emerged as promising alternatives due to their favourable ecological profiles. These natural products encompass a diverse array of bioactive molecules with extensive chemical diversity, high specificity, and biodegradability. Furthermore, they exhibit lower toxicity to mammals and benefit from a long history of safe use, making them an attractive option for sustainable pest management [14,15,16]. Several studies have reported on the use of plant allelochemicals, e.g., essential oils (EOs), extracts, volatiles or fractions, as prospective fungicides, antimicrobials, and nematicides [8,17,18,19]. Dimethyl sulphide (DMS) and dimethyl disulphide (DMDS), are both naturally occurring organosulfur compounds, commonly obtained from the Alliaceae or Brassicaceae families of plants [20]. These volatile compounds have shown promising nematicidal activities against other soil dwelling PPNs, such as the root-knot nematodes (Meloidogyne) or potato cyst nematodes (Globodera) [20]. DMDS and DMS appear to exert their nematicidal activity by inhibiting the enzyme cytochrome oxidase, thereby disrupting mitochondrial respiration. This interference compromises the nematode’s ability to effectively move, feed, and reproduce [21]. Additionally, these compounds are advantageous due to their high biodegradability and low environmental persistence, minimizing their ecological impact. Notably, low concentrations of DMDS (3 g per 10 L of soil) enhance saprophytic nematode populations, which in turn support plant growth by improving soil health and nutrient cycling [20]. Another volatile compound that has attracted significant attention for its nematicidal properties is trans-cinnamaldehyde (TCA), a phenylpropanoid identified as a major constituent of Cinnamomum cassia EO. Several studies have found that C. cassia EO shows strong nematicidal activity against Bursaphelenchus xylophilus, M. incognita and M. javanica [19,22,23,24]. More recently, a study which isolated TCA from C. cassia EO, concluded that it induced a high mortality, reduced J2 viability and egg hatchability in M. incognita, Xiphinema index and G. rostochiensis. Importantly, this compound’s low toxicity to mammals or other beneficial organisms further positions it as a candidate for safe, sustainable pest management strategies [24]. Also, trans-2-decenal (T2D) and trans-2-undecenal (T2U), medium carbon chain aldehydes commonly extracted from the EO of Coriandrum sativum, have demonstrated high nematicidal activities [25]. Medium carbon chain aldehydes have a unique advantage in their ability to act as both contact and vapor-phase toxicants, allowing for versatile application methods [26]. Despite this, little is known on the mode of action of T2D and T2U, which limits their use and commercialization as prospective nematicides. Interestingly, the EO of C. sativum shows a broad range of activities, e.g., insecticide, nematicide, anti-fungal and anti-bacterial [25].
In the present study, the biopesticide potential of DMS, DMDS, TCA, T2D, and T2U was evaluated, for the first time, against the RLN, P. penetrans, and compared with the activity of the conventional synthetic nematicide, oxamyl. We hypothesize that these natural plant products, due to their distinct modes of action and ecotoxicologically beneficial profiles, can offer a sustainable and effective alternative to conventional pesticides. Thus, the present study seeks to contribute to the development of more integrated pest management strategies, which are both environmentally safer and economically viable.
2. Materials and Methods
2.1. Chemicals
Pure chemical standards of dimethyl sulphide (purity ≥ 99%), dimethyl disulphide (purity ≥ 99%), trans-cinnamaldehyde (purity 99%), trans-2-decenal (purity 92%) and trans-2-undecenal (purity 90%) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Oxamyl was tested in its commercially available formulation, the traditional nematicide AFROMYL® (Epagro, Lisbon, Portugal) at 1 mg of active compound (oxamyl)/mL. The compound stock solutions were prepared with HPLC-grade solvent methanol, acquired from Fisher Chemicals (Hampton, NH, USA).
2.2. Obtaining Root Lesion Nematode Suspensions
A standardized carrot disk-based technique was used to obtain suspension of RLNs [27]. Briefly, medium sized carrots with no visible signs of external damage were acquired from a certified organic seller, thoroughly rinsed under running tap water, and then rinsed with a commercial detergent solution (1 drop per 40 mL of water), to remove leftover soil or debris. Undamaged carrots with organic certification were preferred in order to reduce the probability of microbial contamination or the presence of pesticides that can hinder RLN development [28]. After drying in a paper laboratory towel, carrots were surface sterilized in a laminar flow hood by dipping in ethanol at 96% (v/v) (LabChem, Lisbon, Portugal) and flame sterilizing. Under asepsis, a sterilized peeler was used to carefully remove the outer skin, and a sterile scalpel was used to discard the upper and lower 2 cm extremities. The middle portion was then sectioned into 0.5 cm thick disks, distributed in sterile Petri dishes, and subjected to UV radiation for 1 h, on each side. The sterility of the carrot disks was assessed by keeping the closed Petri dishes at 25 °C, in darkness, for one week. The carrot disks with no visible microbial contamination were parasitized with the RLN, P. penetrans. The initial inoculum was supplied by Nematolab at Coimbra University in the form of a parasitized carrot disk [29]. Aqueous suspensions of mixed life stage RLNs, isolate A44L4 [6], were filtered through a 20 µm mesh sieve and surface sterilized with a 20% hydrogen peroxide solution, in a flow hood. Sterilized nematodes were then washed 3× with sterilized tap water and resuspended [27]. Approximately 60 ± 5 sterilized RLNs were added to each carrot disk and maintained at 25 °C in darkness. After 3 months, RLNs were extracted from the carrot disks for 24 h with distilled water supplemented with carbenicillin and kanamycin, at 50 μg/mL each. Finally, a suspension was prepared with 600 to 800 mixed life stage RLNs per mL, to be used in the direct-contact bioassays. Experiments were initiated in September of 2024.
2.3. Determination of Mortality Through Direct Contact Bioassays
Direct contact bioassays were performed to determine the nematicidal activity of the volatile allelochemicals on the RLN P. penetrans in comparison with the pesticide oxamyl. Briefly, 95 μL of a suspension containing 60 to 80 mixed life stage RLNs was pipetted to each well of a flat bottom 96-well microtiter plate (Carl Roth GmbH & Co. KG, Karlsruhe, Germany). Afterwards, 5 μL of stock solution set to 20 mg compound/mL was added, to obtain a final concentration of 1 mg/mL. The microplate was sealed with plastic film to reduce compound volatilization and mixed in an orbital shaker (IKA labortechnik, Staufen, Germany) at 800 rpm for 1 min. In each microtiter plate, blank wells were performed to assess natural RLN mortality by adding 5 µL of ultrapure water instead of the stock solution and control wells were added with 5 µL of methanol to determine its contribution to RLN mortality. The plates were finally covered with aluminium foil to establish darkness, and incubated at 25 ± 1 °C in an orbital shaker at 50 rpm. After 24, 48 and 72 h, dead and live nematodes were counted under a binocular microscope Olympus SZX-12 (10x) (Olympus Corporation, Tokyo, Japan). For each treatment, three independent experiments were performed, each with at least three replicates. Physical prodding was performed to ascertain mortality of immobile nematodes with extended body shape.
2.4. Determination of Motility Inhibition Through Direct Contact Bioassays
Inhibition of RLN motility was assessed with a WMicrotracker MINI device (Phylumtech S.A., Santa Fe, Argentina), which can quantify nematode movement through the scattering of infrared light [30]. Movement was detected through the interference of a light beam passing through the aqueous suspension containing the RLNs, in each well of a microtiter plate. Motility was quantified as the number of continuous interferences, during a selected time interval. The experimental setup followed the description in the previous section with modifications. Briefly, 38 μL of a suspension with 25 to 35 mixed life stage RLNs was pipetted to each well of a U-bottom 96-well microtiter plate (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) and 2 μL of compound stock solution (at 20 mg/mL) was added to obtain a final compound concentration of 1 mg/mL. The microplate was covered and mixed in an orbital shaker (IKA labortechnik, Staufen, Germany) at 800 rpm for 1 min. In each microtiter plate, blank wells were performed to assess control RLN motility by adding 2 µL of ultrapure water instead of the stock solution and control wells were added with 2 µL of methanol to determine the contribution of methanol to RLN motility inhibition. Nematode movement in each well was quantified, for 60 min, after incubation at 25 ± 1 °C for 24, 48 or 72 h. For each treatment, three independent experiments were performed, each with at least eight replicates.
2.5. Assessment of Environmental Benefit
The environmental advantages of using dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal or trans-2-undecenal instead of the conventional pesticides oxamyl or fluopyram, old generation and new generation commercial nematicides, respectively, were assessed by using data obtained from predictive software combined with experimental toxicity data that are available online in freely accessible databases. To understand the potential dispersal into the air, water, soil, or sediment environmental compartments, their predicted environmental distribution (PED) percentages were determined through the equilibrium criterion model [31] by resorting to the Level I Mackay fugacity model (version 4.39), made available by Trent University (Peterborough, ON, Canada [32]. Predictions were set to a situation in which 100,000 kg of compound was introduced to a closed system under steady-state and equilibrium conditions at 25 °C. The experimental data on the chemical properties of the compounds were retrieved from PubChem [33] and from the Pesticide Properties Database (PPDB) [34] and fed into the fugacity model (Table 1). These were the molecular mass (g/mol), melting point (°C), vapour pressure (Pa) and solubility in water (mg/L); as well as the partition coefficient air/water partition, Henry’s law constant (Pa.m3/mol), n-octanol/water partition (log value of Kow), and soil organic carbon/water partition (Koc). Additionally, the EPISuite™ software (version 4.11) [35], which is freely available from the US Environmental Protection Agency, was used to predict the persistence of a compound (in h), its potential volatilization rate from a river or lake models (half-life in h), using the WVOLWIN™ module. We estimated the bioaccumulation factor (BAF) for generic fish species in the upper trophic level of aquatic food webs, the bioconcentration factor (BCF) for water exposure, and biotransformation (half-life in days) in an aquatic environment, based on the Arnot–Gobas method [36], using the BCFBAF™ module. Finally, we predicted the percentage removal of a chemical in a typical activated sludge-based sewage treatment plant, using the STPWIN™ module.

Table 1.
Physical and chemical properties of dimethyl sulphide (DMS), dimethyl disulphide (DMDS), trans-cinnamaldehyde (TCA), trans-2-decenal (T2D) or trans-2-undecenal (T2U) and the conventional pesticides oxamyl or fluopyram, required to perform the Level I Mackay fugacity model [32]. Data were retrieved from the PubChem database [33] and the Pesticide Properties Database (PPDB) [34].
For the assessment of the environmental risks to aquatic organisms, experimental data were retrieved from reputed databases that host these parameters, namely, the European Chemicals Agency (ECHA) database [37], the Pesticide Properties Database (PPDB) [34], and PubChem [33]. The toxicity thresholds—experimentally obtained from model organisms for fish, occupying the trophic level of secondary consumers; algae, which are primary producers; and invertebrates such as Daphnia spp., occupying the trophic level of primary consumers/secondary producers—were compiled in order to compare the toxicity of the allelochemical volatiles to that of the conventional pesticides oxamyl or fluopyram.
2.6. Estimation of Safety to Human Health
To estimate the safety to human health of dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal or trans-2-undecenal in comparison with the conventional pesticides oxamyl or fluopyram, experimental data that are freely available in online databases were combined with data estimated from predictive software on toxicity parameters that are commonly determined for human health. The experimental acute oral or dermal toxicity thresholds were retrieved from the European Chemicals Agency (ECHA) [32], the Pesticide Properties Database (PPDB) [33,34], and PubChem [35]. Toxicity to human organ functions was gauged by resorting to computational toxicity estimations using the specialised ADMETlab 3.0 [38] webtool, which can estimate the probability of a compound to induce several toxicity endpoints of interest. Using its ADMET evaluation module, each compound can be identified through the specific SMILES code and information on the probability of toxicity for the selected endpoints can be processed by the server using deep learning models. For the present work, toxicity probabilities for the following endpoints were retrieved:
- -
- the FDA medium daily dose (FDAMDD), that provides an estimate of the toxic dose threshold of chemicals in humans;
- -
- the hERG blockers, related to the voltage-gated potassium channel encoded by hERG whose blockade may cause illness or even death;
- -
- hematotoxicity, which refers to adverse effects of chemicals on blood-forming organs;
- -
- carcinogenicity, related to the ability to damage the genome or disrupt cellular metabolic processes;
- -
- RPMI-8226 immunotoxicity, which helps determine the toxicity of compounds to a type of multiple myeloma cell line;
- -
- genotoxicity, which refers to the ability of harmful substances to damage genetic information in cells;
- -
- respiratory toxicity, which has become the main cause of drug withdrawal;
- -
- A549 cytotoxicity, which helps determine the toxicity of compounds to a human non-small cell lung cancer cell line;
- -
- human hepatotoxicity, for compounds demonstrating adverse liver reactions;
- -
- drug-induced liver injury (DILI), for compounds that have well known associations with liver injury and have a significant number (>10) of independent clinical reports of hepatotoxicity;
- -
- drug-induced nephrotoxicity, which refers to the harmful effects that occur in the kidneys;
- -
- Hek293 cytotoxicity, which helps determine the toxicity of compounds to human embryonic kidney cells;
- -
- AMES toxicity, which determines a mutagenic effect that has a close relationship with the carcinogenicity;
- -
- skin sensitization, for potential adverse effects for dermally applied products;
- -
- eye irritation, which assesses the potential to affect cornea and conjunctiva tissues;
- -
- drug-induced neurotoxicity, for compounds that can harm both the central nervous system and the peripheral nervous system;
- -
- ototoxicity, for compounds that have the potential to harm the inner ear by either damaging the ear’s structures directly or the related nervous system.
2.7. Data Treatment and Statistical Analysis
To assess RLN mortality, live and dead nematode counts were used to determine mortality percentages according to Formula (1):
Mortality % = (dead RLNs/total no. of RLNs) × 100
For each compound, mortality percentages were corrected to exclude control (methanol) mortality through Formula (2):
Corrected mortality % = [(mortality % in treatment − mortality % in control)/(100 − mortality % in control)] × 100
The toxicological strength of the compounds was characterized as complete when mortality was 100%, strong when above 80%, moderate when between 80 and 61%, weak when between 60 and 40%, and low or inactive when below 40% [39].
To assess RLN motility inhibition, nematode movement counts were used to determine the inhibition of nematode movement according to Formula (3):
Motility inhibition % = [(movement counts in control well − counts in compound added well)/movement counts in control well] × 100
Statistical processing was performed with version 2019 of Origin Graphing & Analysis software (OriginLab, Northampton, MA, USA). Statistical significance of the data was determined with one-way ANOVA, and individual means were compared using the Tukey’s Post-Hoc test with p < 0.05 (Shapiro–Wilk Test ensured data normality, and Browns–Forsythe Test was used for homoscedasticity). The results are presented as average and standard error value of replicates.
3. Results
3.1. Nematotoxic Activity
The activity of the volatile allelochemicals was determined through direct-contact bioassays. Corrected mortality values were obtained for P. penetrans after 24, 48, and 72 h of direct contact. Control mortality, which allowed for the assessment of the impact of methanol on the RLN, varied between 3.8 ± 1.6 and 6.2 ± 2.0% during the time course study. These data were used to obtain corrected mortality values in the treatment groups. The analysed compounds induced different mortality percentages, with dead nematodes presenting the typical motionless extended body shape. From the sulphide volatiles analysed, DMDS exhibited a weak nematicidal activity, with corrected mortality values ranging from 39.6 ± 1.3% at 48 h to 53.4 ± 1.3% at 72 h (Figure 1). In comparison, DMS demonstrated a low activity, with values rising from 20.2 ± 3.0% at 24 h to 41.1 ± 3.1% at 72 h.
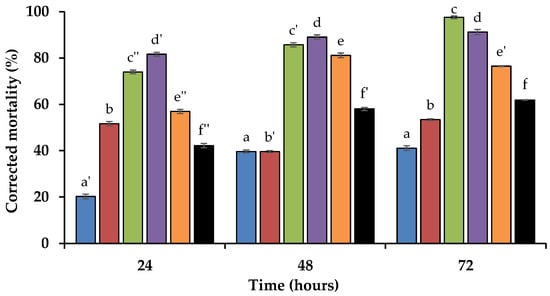
Figure 1.
Corrected mortality values (average ± standard error) for the root lesion nematode Pratylenchus penetrans after 24, 48, and 72 h of direct contact with 1 mg/mL of dimethyl sulphide (blue), dimethyl disulphide (red), trans-cinnamaldehyde (green), trans-2-decenal (purple), and trans-2-undecenal (orange), in comparison with the pesticide oxamyl (black). Different variations of the same letter indicate statistically significant differences (p < 0.05) based on Tukey’s test, between the different timepoints for each compound.
Among the tested aldehydes, the phenylpropanoid TCA displayed the highest nematicidal activity across all tested compounds. Corrected mortality values increased consistently, from 74.0 ± 2.5% after 24 h to 97.6 ± 1.8% after 72 h (Figure 1). The strong nematicidal activity positions it as a potential candidate for development as a prospective sustainable nematicide. T2D demonstrated a strong nematicidal activity with mortality values ranging from 81.6 ± 2.5% at 24 h to 91.2 ± 3.3% at 72 h (Figure 1). Similarly, T2U also showed moderate activity increasing from 57.0 ± 2.7% at 24 h to 81.1 ± 3.0% at 48 h, followed by a slight decrease to 76.5 ± 0.7% at 72 h (Figure 1). Although both aldehydes exhibited high activity, T2D maintained a higher mortality over time. In comparison, the synthetic pesticide oxamyl exhibited moderate nematicidal activity. Corrected mortality values increased from 42.2 ± 2.7% at 24 h to 61.9 ± 0.4% at 72 h (Figure 1). Throughout the time-course study, only TCA, T2D, and T2U showed higher corrected mortality values than oxamyl.
The inhibition of P. penetrans motility was assessed by quantifying nematode movement after 24, 48, and 72 h in direct contact with 1 mg/mL of the tested allelochemicals. The organosulfur compounds demonstrated variable levels of motility inhibition. DMS exhibited relatively low inhibition values, with a slight decrease over time, from 48.2 ± 1.9% at 24 h to 39.1 ± 1.0% at 48 h and 34.0 ± 2.2% at 72 h (Figure 2). The motility inhibition activity of DMDS can also be described as low or inactive, with values remaining consistently below the 40% motility inhibition, increasing from 28.7 ± 1.5% at 24 h to 35.2 ± 1.1% at 48 h, followed by a slight decrease to 34.5 ± 1.0% at 72 h (Figure 2).
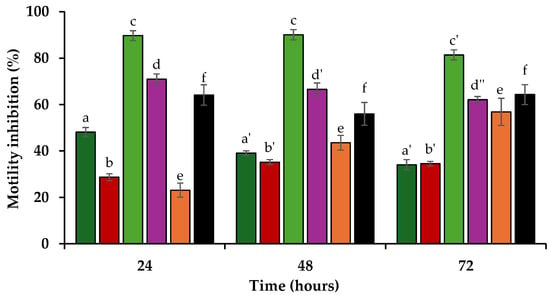
Figure 2.
Motility inhibition percentages (average ± standard error) for the root lesion nematode Pratylenchus penetrans after 24, 48, and 72 h of direct contact with 1 mg/mL of dimethyl sulphide (dark green), dimethyl disulphide (red), trans-cinnamaldehyde (light green), trans-2-decenal (purple), and trans-2-undecenal (orange), in comparison with the pesticide oxamyl (black). Different variations of the same letter indicate statistically significant differences (p < 0.05) based on Tukey’s test, between the different timepoints for each compound.
The phenylpropanoid TCA showed high motility inhibition throughout the 72 h period, achieving 89.7 ± 1.3% after 24 h, with a slight increase over time to 90.1 ± 2.1% at 48 h and a decrease to 81.4 ± 2.2% at 72 h (Figure 2). The tested aldehydes exhibited a contrasting trend. While T2D demonstrated low initial inhibition which increased over time, from 23.1 ± 2.2% at 24 h to 43.6 ± 2.8% at 48 h and 56.8 ± 1.4% after 72 h, T2U showed greater activity, with inhibition values decreasing over time from 71.0 ± 3.1% at 24 h to 66.6 ± 3.2% at 48 h and 62.0 ± 5.8% at 72 h (Figure 2). The synthetic nematicide oxamyl exhibited moderate motility inhibition, with no significant change in activity over the course of the 72 h, with motility inhibition ranging from 64.1 ± 4.4% at 24 h to 56.0 ± 4.9% at 48 h, before increasing to 64.3 ± 4.3% at 72 h (Figure 2).
3.2. Potential Environmental Safety
To assess the ecological risks of shifting from conventional pesticides to the analysed phytochemicals, predictive models were exploited to evaluate compound persistence, distribution and removal in different environmental compartments. The fugacity model, used to determine predicted environmental distribution, showed that, as expected for volatile compounds, most of the phytochemicals assayed had a strong predicted affinity to the air environmental compartment (Table 2). DMS, DMDS, and the aldehydes T2D and T2U were predicted to mainly volatilize to the air (97.0, 95.8, 76.4 and 87.9%, respectively) (Table 2). T2D was also predicted to show affinity to the water environmental compartment (20.6%). TCA showed a higher predicted distribution to the water environmental compartment (86.7%), in a similar manner to the conventional nematicide oxamyl (98.3%) (Table 2). On the other hand, fluopyram showed an almost complete predicted distribution to the soil environmental compartment (96.9%). The environmental persistence (in h) predicted for the nematicides oxamyl and fluopyram, was 12- to 100-fold higher than that predicted for the analysed allelochemicals (Table 2). Predictive models were also used to estimate the volatilization (half-life in h) of these compounds in river and lake models. As expected, volatilization of the tested phytochemical volatiles was higher than that of the synthetic chemical nematicides. Among the tested compounds, the highest predicted volatilization values were obtained for TCA, with 191 and 2183 h for the river and lake models, respectively (Table 2).

Table 2.
Predicted environmental distribution (%) to the environmental compartments of the air, water, soil, and sediments; compound persistence (h); and potential for volatilization from a model river or lake of dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal in comparison with the conventional pesticides oxamyl and fluopyram. Determined using the Mackay fugacity model and the US Environmental Protection Agency’s EPISuite™ software (v. 4.11) [35,40].
The dangers of exposure to aquatic environments and the toxicological effects of the compounds were further assessed through predictive modelling. The theoretical bioaccumulation factor (BAF), bioconcentration factor (BCF) and biotransformation of the plant allelochemicals were estimated and compared with oxamyl and fluopyram. DMS and DMDS were estimated to show low BAF and BCF values (1.7 and 1.0 L/kg FW, respectively), along with TCA (1.8 L/kg FW) (Table 3). Due to their chemical properties, the aldehydes T2D and T2U were predicted to have the highest BAF and BCF, at 203.2 and 400 L/kg FW, respectively, for both endpoints. In contrast, the synthetic nematicides, oxamyl and fluopyram, were predicted to have 0.9 and 61.8 L/kg FW for both endpoints, respectively (Table 3). The predicted biotransformation (half-life, days) of the analysed compounds was 0.1 days for DMDS, DMS and TCA, 1.1 and 1.5 days for the aldehydes, and 0.01 and 0.4 days for oxamyl and fluopyram, respectively.

Table 3.
Predicted bioaccumulation factor (BAF) for generic fish species in the upper trophic level of aquatic food webs, predicted bioconcentration factor (BCF) for water exposure (L/kg FW, fresh weight), and potential biotransformation (half-life in days), determined with the US Environmental Protection Agency’ EPISuite™ software (v. 4.11) [35,40], based on the Arnot–Gobas method [36], for dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal in comparison with the conventional pesticides oxamyl and fluopyram.
Predictive modelling was employed using the US EPA’s EPIsuite software, version 4.11), to evaluate the removal efficiency in a typical activated sludge-based sewage treatment plant. Amongst the compounds analysed, DMDS was predicted to show the highest total removal rate, i.e., 99.4%, followed by T2U (38.0%), DMS (34.5%), and T2D (20.7%) (Table 4). In contrast, the conventional nematicides were predicted to show significantly lower removal efficiencies. Oxamyl exhibited the lowest predicted total removal rate, compared with all other compounds examined, showing a total removal of 1.9% (Table 4). According to these models, fluopyram is predicted to exhibit a total removal of 5.7%.

Table 4.
Predicted percentage removal in a typical activated sludge-based sewage treatment plant of dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal and the conventional pesticides oxamyl and fluopyram. Determined using the US Environmental Protection Agency’s EPISuite™ software (v. 4.11) [35,40].
Typically, the use of chemical pesticides results in their bioaccumulation in the soil and subsequent distribution and retention in various aquatic ecosystems. As a result, biodiversity in groundwater and aquifers is generally negatively affected by pesticide misuse. Therefore, the evaluation of the acute effects that these chemicals have on various aquatic non-target organisms is crucial to recognize their potential threat to aquatic ecosystems. Publicly accessible online databases were utilized to review the acute toxicological thresholds, reported for both the allelochemical volatiles and synthetic pesticides, in various aquatic trophic groups (Table 5). In fish models, DMS and TCA stand out for their high LC50 values, being 100- and 10-fold higher than the values reported for oxamyl and fluopyram (3.1 and 1.0 mg/L, respectively). The remaining allelochemicals displayed toxicity thresholds, similar to those observed for the synthetic pesticides ranging from 0.16 to 0.97 mg/L. This trend is also seen in the algae model organisms, having DMS and TCA 30- and 100-fold higher EC50 values than those reported for oxamyl and fluopyram (0.9 and >1.1 mg/L, respectively). The remaining compounds, DMDS, T2D, and T2U, were reported to have between 2- and 6-fold higher EC50 values compared with oxamyl and fluopyram. In the model organism Daphnia magna (an invertebrate), the surveyed allelochemicals were predicted to have lower acute toxicity thresholds compared with oxamyl. Interestingly, fluopyram is reported to have a lower toxicity to invertebrates (>100 mg/L) than the phytochemicals reviewed, presenting values between 1.8 and 81 mg/L (Table 5).

Table 5.
The acute ecotoxicological thresholds (median lethal/effective concentration, LC50/EC50, mg/L) reported for dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal and the conventional pesticides oxamyl and fluopyram against non-target aquatic model organisms (fish, algae, and invertebrates), retrieved from ECHA [37], the Pesticide Properties Database (PPDB) [34], and PubChem [33]. When parameters were unavailable at the databases, data were computed using the US Environmental Protection Agency’s EPISuite™ software (v. 4.11) [35,40].
3.3. Toxicity to Mammals
The potential of the tested compounds for toxicity to mammals was estimated in order to assess the human health risks associated with their use. Regarding the threshold values for oral toxicity, the synthetic pesticides and organosulfur compounds were reported to present the highest values. The pesticide oxamyl displayed a low oral toxicity threshold of 3 mg/kg, which was three orders of magnitude lower than the other reviewed compounds, except for DMS, with a reported LD50 of 190 mg/kg. The new generation pesticide, fluopyram, showed a substantially higher oral toxicity threshold value, with a reported LD50 value > 2000 mg/kg (Table 6). Regarding the thresholds for dermal toxicity, TCA was reported to have the highest reported LD50 value at more than 5000 mg/kg. The remaining allelochemicals, including DMS, DMDS, and T2D, exhibited dermal LD50 values comparable to those of the synthetic nematicides oxamyl and fluopyram, ranging between 2000 and 5000 mg/kg (Table 6).

Table 6.
Experimental oral and dermal acute toxicity thresholds (mg/kg) of dimethyl sulphide, dimethyl disulphide, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal, in comparison with the conventional pesticides oxamyl and fluopyram. Data were retrieved from PubChem [33], the Pesticide Properties Database (PPDB) [34], and ECHA [37]. When the parameters were unavailable, data were computed using the US Environmental Protection Agency’s EPISuite™ software (v. 4.11) [35,40].
Predictive toxicology using computational modelling was conducted to profile the potential toxic effects of the tested compounds on endpoints of human health. The conventional pesticides were predicted to have a higher probability of showing toxicity (in five endpoints for oxamyl and eight endpoints for fluopyram) than the allelochemicals analysed (between three and four endpoints of toxicity) (Table 7).

Table 7.
Probability of toxicity for endpoints of concern for human health, predicted through the toxicity tool of ADMETlab 3.0 online software [38], for dimethyl sulphide (DMS), dimethyl disulphide (DMDS, trans-cinnamaldehyde (TCA), trans-2-decenal (T2D), and trans-2-undecenal (T2U) and for the conventional pesticides oxamyl and fluopyram.
While the aliphatic aldehydes T2D and T2U were predicted to have a high probability of showing immunotoxicity, respiratory toxicity, and cytotoxicity, the organosulphur compounds DMS and DMDS were predicted to additionally function as hERG blockers. TCA showed a high probability of inducing hematotoxicity, immunotoxicity, respiratory toxicity, and nephrotoxicity. Regarding the pesticides, oxamyl exhibited fewer endpoints with a high probability than fluopyram. For the first, its effects of functioning as an hERG blocker, inducing hematotoxicity and immunotoxicity, and with regard to drug-induced liver injury and skin sensitization were predicted to be important. For fluopyram the predicted effects were more extensive, namely, inducing carcinogenicity, cytotoxicity, hepatotoxicity, drug-induced liver injury, drug-induced nephrotoxicity, skin sensitization, and drug-induced neurotoxicity. Interestingly, despite being a newer-generation nematicide, and being considered safer for commercial use, fluopyram displayed the highest predicted toxicity, with medium-to-high toxicity in 13 out of 17 parameters.
4. Discussion
In vitro direct-contact bioassays were performed to assess the potential of five volatile allelochemicals as biopesticides against the RLN P. penetrans. At the concentration of 1 mg/mL, none of the tested compounds achieved complete mortality (100%), during the time-course study. Nonetheless, the highest nematicidal efficacy was observed for TCA, T2D, and T2U, which exhibited mortality percentages above those of the synthetic nematicide oxamyl at the evaluated time points. Among the tested compounds, T2D demonstrated the strongest nematicidal activity at 24 and 48 h, whereas TCA exhibited a progressive increase in efficacy over time, eventually surpassing T2D at 72 h. In contrast, both T2D and T2U showed a decline in activity after 48 h, while TCA maintained a steady increase in effectiveness throughout the time-course study. This suggests that TCA may exert a progressively increasing nematicidal effect over time, whereas T2D and T2U appear to act more rapidly but also begin to lose efficacy after two days. Motility inhibition assays were conducted to assess paralysis in P. penetrans caused by the tested compounds. The results followed a similar pattern to those observed in the mortality bioassays. While oxamyl exhibited strong motility inhibition, as expected due to its mode of action as a cholinesterase inhibitor and neurotoxicant [8], TCA demonstrated greater paralysis activity, which remained consistent after 48 h but slightly decreased at 72 h. The methodology employed to determine motility inhibition may provide greater sensitivity compared with visual mortality assessment, as loss of activity was detected earlier using this approach. Thus, TCA not only exhibited the highest nematicidal activity, reaching a maximum mortality percentage of 97.6 ± 1.8% at 72 h, but also demonstrated a strong motility inhibition effect, ranging from 81.4 ± 2.2% to 90.1 ± 2.1%. These findings are consistent with previous studies that highlight the potent nematicidal activity of TCA against phytoparasitic nematodes, such as M. incognita, G. rostochiensis, or X. index, that have diverse anatomical structures and feeding behaviours [22]. Notably, TCA was initially identified as a promising nematicidal compound due to the high nematicidal efficacy of Cinnamomum cassia EO. Screening of C. cassia EO and its constituent compounds revealed that TCA was the primary bioactive component (>90%), exhibiting mortality values comparable to or even higher than those of the EO [24].
Among the tested aliphatic aldehydes, T2D demonstrated superior overall performance compared with T2U, maintaining mortality levels above 80% over 72 h. A similar trend was observed in the motility inhibition bioassays, where T2D consistently outperformed T2U. Oxygen-containing aliphatic compounds are known for their strong nematicidal activity and distinct structure–activity relationships. In particular, medium carbon chain length aliphatic compounds (C8–C14) with oxygen-containing functional groups exhibited a direct correlation between chain length and nematicidal potency. This relationship was influenced by the type of oxygen-containing functional group (alcohol, aldehyde or carboxylic acid) as well as the presence and positioning of double bonds (saturated vs. unsaturated molecules) [43]. However, beyond a certain chain length, this correlation was disrupted, in a cut-off effect, which varied based on the C chain length and the oxygenated functional group. This type of structure–activity relationship suggests that nematicidal toxicity may be conveyed through the inhibition of specific biosynthetic pathways. Studies on aldehydes tested against the pinewood nematode have shown that, while C6 to C14 alkanals exhibited low activity, C6 to C14 trans-2-alkenals displayed a pronounced cut-off effect. For instance, at 0.5 mg/mL, trans-2-decenal (C10) achieved complete mortality, whereas trans-2-undecenal (C11) exhibited only a weak effect [43]. In the present study, the higher efficacy of T2D compared with T2U suggests that a similar structure–activity relationship may be at play. In fact, aliphatic aldehydes, such as T2D and T2U, are believed to act by disrupting plant parasitic nematode internal structures. For example, in M. incognita the application of T2D led to a degeneration of the pseudocoel cells and malformation of somatic muscles, but caused no harm to the cuticle, suggesting that they enter the nematode body via the digestive system [44].
In contrast, DMS and DMDS, both volatile sulphides, exhibited a relatively low nematicidal activity. This outcome was unexpected, given previous reports highlighting the high activities of sulphide volatiles against Meloidogyne spp. or Globodera spp. [18,45]. Between these volatiles, DMDS demonstrated a slightly greater nematicidal strength, achieving a mortality rate of 53.4 ± 1.3% at 72 h, compared with 41.1 ± 3.1% for DMS. This indicates that the number of sulphur atoms may influence nematicidal activity. While no studies have explicitly examined the structure–activity relationship of sulphide volatiles in nematode control, research on volatile organic compounds (VOCs) extracted from broccoli shoots (Brassica oleracea L.) has shown promising results [46,47]. These volatile compounds were found to reduce M. incognita J2 motility, induce nematode mortality, and suppress gall formation and egg production in tomato roots under greenhouse conditions. Notably, DMDS at 176 mg/L reduced egg hatching by 96.8%, whereas DMS exhibited the opposite effect, increasing egg hatching by 13% at concentrations of 500 mg/L and 1000 mg/L [48]. These results indicate that subtle structural differences between sulfur-containing volatiles may significantly impact their biological activity. The synthetic pesticide oxamyl exhibited moderate nematicidal activity (between 42 and 62%) and induced an equally moderate motility inhibition (varying from 56 to 64%). This suggests that TCA, T2D, and T2U have a greater nematicidal activity compared with oxamyl and may exhibit a potentially higher efficacy when applied in natural conditions.
The combination of visual assessment with automated techniques was demonstrated to be a very valuable approach to evaluate the nematicidal activity of a compound. Traditional visual assessment, performed by counting nematodes under a stereomicroscope, remains a widely used and reliable method. However, it is inherently subjective, as it depends on the observer’s manual counting and provides only a single timepoint observation, thereby disregarding temporal variability in nematode behaviour. To complement visual assessment, motility-based techniques, such as those using infrared beam disruption technologies, offer an automated and quantitative approach to nematode viability assessment. The use of microplate readers in such assays allows for continuous monitoring over extended experimental periods and eliminates observer bias. However, this method may introduce higher variability due to differences in nematode motility patterns, as nematodes do not exhibit synchronized movement. Despite these limitations, integrating multiple assessment methods enhances the accuracy and robustness of nematicidal activity evaluations by capturing both instantaneous and dynamic behavioural responses of nematodes to treatment.
To further explore the potential for incorporation into sustainable bionematicidal formulations, environmental modelling was conducted on the selected volatile allelochemicals. A fugacity-based modelling approach was applied to estimate the theoretical partitioning behaviour of the selected volatile compounds and two conventional nematicides—oxamyl and fluopyram—across key environmental compartments: air, soil, sediments, and water. The volatile allelochemicals DMS, DMDS, T2D, and T2U demonstrated a strong tendency to partition into the air environmental compartment (76–97%), indicating a potential for usage as fumigant biopesticides. Their rapid volatilization could enable an effective and targeted application while minimizing residue accumulation in soil and water. Importantly, their low predicted environmental persistence supports the goal of reducing long-term contamination risks often associated with nematicidal treatments. In contrast, fluopyram, a next-generation synthetic nematicide, exhibited the highest predicted retention in the soil environmental compartment, with approximately 97% of the compound estimated to persist in the soil. This strong affinity suggests a higher risk of long-term environmental accumulation and potential adverse effects on non-target organisms. Notably, fluopyram exposure has been linked to disruptions in surrounding microbial communities, raising concerns about its ecological impact [49]. Although fluopyram is generally considered safe for terrestrial organisms, studies have shown that it can still exhibit moderate to high toxicity in certain species, such as earthworms and birds [34]. Moreover, the ecotoxicological effects of many of its degradation products remain insufficiently studied [34]. Thus, the predicted environmental persistence of fluopyram raises concerns about its limited biodegradability and potential for bioaccumulation in terrestrial ecosystems and agricultural products, which could ultimately pose risks to human health [50].
Both TCA and oxamyl were predicted to predominantly partition into the aquatic compartment, indicating an increased toxicological pressure on aquatic ecosystems. However, both compounds exhibit low predicted bioaccumulation and bioconcentration factors, coupled with rapid biotransformation rates, which may mitigate their long-term ecological impact. Notably, TCA was predicted to have a significantly lower volatilization rate, quantified by its half-life, compared with oxamyl, suggesting reduced environmental persistence in aquatic systems. Oxamyl is associated with severe acute toxicity across various aquatic model organisms, including fish, algae, and invertebrates, highlighting a substantial ecological risk. In contrast, TCA exhibits a comparatively lower toxicity profile and reduced predicted persistence, indicating a potentially lower overall risk to aquatic organisms [51]. In a study on cucumber, the toxicity and persistence of TCA-derived metabolites were evaluated using gas chromatography (GC) and ultra-high-performance liquid chromatography (UHPLC) coupled with a quadrupole high-resolution mass spectrometer (Q-Orbitrap). The results demonstrate a significantly lower toxicity and environmental persistence of TCA compared with conventional pesticides [52]. These findings emphasize the importance of evaluating both persistence and toxicity when assessing the environmental impact of nematicidal compounds. From a risk assessment viewpoint, there is a critical need to consider environmental fate and transport mechanisms when determining the suitability of these compounds for bionematicidal applications. The low predicted persistence of TCA is advantageous, with a predicted total wastewater removal rate of just 2.1%, close to that of oxamyl (1.8%). Given the limited effectiveness of conventional wastewater treatment processes in removing some chemicals, a thorough evaluation of their environmental behaviour is also essential to ensure sustainable nematicide development. The bioaccumulation potential of the tested compounds revealed key differences between natural and synthetic nematicides. DMS, DMDS and TCA exhibited low predicted bioaccumulation factors, suggesting a lower risk of biomagnification in aquatic ecosystems. However, T2D and T2U displayed higher bioaccumulation potential, warranting further studies to assess their long-term ecological effects, despite being considered generally safe for human use [53,54]. Interestingly, fluopyram exhibited moderate bioaccumulation potential despite being developed as a safer alternative to older chemical nematicides [55]. Ultimately, integrated pest management strategies for RLNs that incorporate the application of these volatile allelochemicals have the potential to significantly enhance treatment efficacy. Nevertheless, comprehensive evaluations are imperative to ascertain their precise impact on non-target soil nematodes, particularly free-living species, given their critical role in maintaining soil microbial homeostasis and overall biodiversity.
To understand the risks to human health of using the analysed volatile allelochemicals, data from freely available databases were combined with predictive toxicity endpoints. The toxicity assessments suggested that phytochemicals pose lower risks to human health compared with synthetic nematicides. The toxicological risk of a given compound can be categorized using the global harmonized system (GHS) for the classification of hazardous substances [56]. Compounds are assigned to a category group from 1 to 5 (most to least, respectively) based on the acute oral and dermal toxicity thresholds reported. From the acute oral toxicity thresholds compiled, oxamyl is classified as a category 1 toxicant, for displaying a reported experimental LD50 value below 5 mg/kg, while all other analysed compounds are above category 3 compounds, given their LD50 values higher than 50 mg/kg. For acute dermal toxicity, the analysed compounds belonged to category 5, as the reported LD50 values were above 2000 mg/kg, and can therefore be considered to have a low acute dermal toxicity. Predictive toxicology based on computational methods was employed to evaluate the developmental risks associated with various conditions relevant to human health. Overall, the toxicity profile of the allelochemicals analysed remained substantially lower than those observed for the synthetic chemicals. The first demonstrated a high probability of toxicity in three to four endpoints, whereas the synthetic pesticides, oxamyl and fluopyram, were predicted to have more pronounced toxicological profiles. Oxamyl was associated with a high probability of toxicity in five endpoints, while fluopyram displayed the highest predicted toxicity, with eight highly probable toxicity endpoints [57,58]. Among the tested allelochemicals, TCA exhibited a higher predicted toxicity, with a high probability of inducing four out of the seventeen evaluated conditions.
For TCA, the toxicology and carcinogenesis potential has been previously assessed through experimental studies on the exposure of this microencapsulated compound on mice and rats [59]. At a 4100 ppm exposure level of TCA, only reduced appetite and non-neoplastic tissue changes were induced. Exposure to TCA, even at relatively high concentrations, was not found to be carcinogenic [59].
The findings of the present study underscore the potential of allelochemical volatiles as environmentally sustainable and effective alternatives to synthetic nematicides. However, further research is needed to optimize formulations of volatiles, assess long-term efficacy under field conditions, and evaluate potential non-target effects. Additionally, regulatory frameworks should consider integrating volatile-based nematicides into current pest management programs to promote sustainable agricultural practices, while minimizing environmental and human health risks.
5. Conclusions
Plant allelochemical volatiles are still a largely unused class of chemicals, which have the potential to be utilized as prospective candidates for sustainable pest management strategies. A risk assessment evaluation of these compounds found that they can exert high levels of bioactivity whilst having favourable ecotoxicological profiles owing to their favourable environmental compartmentalization, low ecological persistence, and low toxicity to mammals. From the allelochemical volatiles assessed, trans-cinnamaldehyde, trans-2-decenal, and trans-2-undecenal emerged as promising candidates for nematicidal applications, exhibiting strong bioactivity and promising environmental and toxicological profiles. At the concentration tested, these volatiles demonstrate significant efficacy, suggesting that they can actively reduce root lesion nematode populations in the field. Their direct application could target and suppress these phytoparasites within the soil and root systems, thereby mitigating their damaging effects. This approach would represent a more environmentally benign alternative to conventional synthetic nematicides, contributing to healthier soil ecosystems and supporting long-term sustainable agricultural practices for managing RLNs. However, field trials are warranted to optimize application methods and confirm the consistent efficacy of this concentration under diverse environmental conditions.
Author Contributions
Conceptualization, J.M.S.F.; methodology, G.P., P.B., C.S.L.V. and J.M.S.F.; software, J.M.S.F.; formal analysis, G.P. and J.M.S.F.; investigation, G.P. and J.M.S.F.; resources, P.B., C.S.L.V. and J.M.S.F.; data curation, G.P. and J.M.S.F.; writing—original draft preparation, G.P. and J.M.S.F.; writing—review and editing, G.P., P.B., C.S.L.V. and J.M.S.F.; funding acquisition, P.B., C.S.L.V. and J.M.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
Partly funded by Fundação para a Ciência e a Tecnologia (FCT/MCTES) through projects ref. 2022.00359.CEECIND, DOI: 10.54499/2022.00359.CEECIND/CP1737/CT0002 (NemAct, J.M.S.F.); ref. CEECIND/00040/2018, DOI: 10.54499/CEECIND/00040/2018/CP1560/CT0001 (C.V.); PratyOmics ref. PTDC/ASP-PLA/0197/2020 (10.54499/PTDC/ASP-PLA/0197/2020) and structural funding ref. UIDB/00329/2020, DOI: 10.54499/UIDB/00329/2020 (CE3C), ref. LA/P/0121/2020, DOI: 10.54499/LA/P/0121/2020 (CHANGE); ref. UIDB/05183/2020, DOI: 10.54499/UIDP/05183/2020 (MED); and GreenIT (DOIs 10.54499/UIDB/04551/2020 and 10.54499/UIDP/04551/2020).
Data Availability Statement
The raw data are available from the corresponding author (Jorge M. S. Faria) upon reasonable request.
Acknowledgments
The authors wish to thank Isabel Abrantes and Ivânia Esteves from Nemato-lab at Coimbra University, Manuel Mota from Universidade Lusófona, Ana Cristina Figueiredo from Faculdade de Ciências da Universidade de Lisboa, and Tomás Cavaco from the Systems Biology Lab at Vrije Universiteit in Amsterdam.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AChE | Acetylcholinesterase |
| BAF | Bioaccumulation factor |
| BCF | Bioconcentration factor |
| DMDS | Dimethyl disulphide |
| DMS | Dimethyl sulphide |
| ECHA | European Chemicals Agency |
| EO | Essential oil |
| PED | Predicted environmental distribution |
| PPDB | Pesticide Properties Database |
| PPN | Plant-parasitic nematode |
| RLN | Root lesion nematode |
| T2D | trans-2-decenal |
| T2U | trans-2-undecenal |
| TCA | trans-cinnamaldehyde |
References
- Jones, M.G.K.; Fosu-Nyarko, J. Molecular Biology of Root Lesion Nematodes (Pratylenchus spp.) and Their Interaction with Host Plants. Ann. Appl. Biol. 2014, 164, 163–181. [Google Scholar] [CrossRef]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Leiden, The Netherlands, 2007; Volume 6, ISBN 9789004155640. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- MacGuidwin, A.E.; Rouse, D.I. Role of Pratylenchus penetrans in the Potato Early Dying Disease of Russet Burbank Potato. Phytopathology 1990, 80, 1077. [Google Scholar] [CrossRef]
- Viketoft, M.; Flöhr, A.; Englund, J.-E.; Kardell, J.; Edin, E. Additive Effect of the Root-Lesion Nematode Pratylenchus penetrans and the Fungus Rhizoctonia Solani on Potato Yield and Damage. J. Plant Dis. Prot. 2020, 127, 821–829. [Google Scholar] [CrossRef]
- Barbosa, P.; Faria, J.M.S.; Cavaco, T.; Figueiredo, A.C.; Mota, M.; Vicente, C.S.L. Nematicidal Activity of Phytochemicals against the Root-Lesion Nematode Pratylenchus penetrans. Plants 2024, 13, 726. [Google Scholar] [CrossRef]
- Olthof, T.H.A.; Townshend, J.L. Effect of Oxamyl Treatment of Potato Seed Pieces on Pratylenchus penetrans and Yield. J. Nematol. 1991, 23, 699–705. [Google Scholar]
- Cavaco, T.; Faria, J.M.S. Phytochemical Volatiles as Potential Bionematicides with Safer Ecotoxicological Properties. Toxics 2024, 12, 406. [Google Scholar] [CrossRef]
- Taylor, G.E.; Schaller, K.B.; Geddes, J.D.; Gustin, M.S.; Lorson, G.B.; Miller, G.C. Microbial Ecology, Toxicology and Chemical Fate of Methyl Isothiocyanate in Riparian Soils from the Upper Sacramento River. Environ. Toxicol. Chem. 1996, 15, 1694–1701. [Google Scholar] [CrossRef]
- Goldman, L.R.; Beller, M.; Oregon, H.; Jackson, R.J. Aldicarb Food Poisonings in California, 1985–1988: Toxicity Estimates for Humans. Arch. Environ. Health 1990, 45, 141–147. [Google Scholar] [CrossRef]
- European Comission. EU Pesticides Database. Available online: https://ec.europa.eu/food/plants/pesticides/eu-pesticides-database_en (accessed on 29 March 2025).
- Liu, Y.; Zhang, W.; Wang, Y.; Liu, H.; Zhang, S.; Ji, X.; Qiao, K. Oxidative Stress, Intestinal Damage, and Cell Apoptosis: Toxicity Induced by Fluopyram in Caenorhabditis Elegans. Chemosphere 2022, 286, 131830. [Google Scholar] [CrossRef]
- Schleker, A.S.S.; Rist, M.; Matera, C.; Damijonaitis, A.; Collienne, U.; Matsuoka, K.; Habash, S.S.; Twelker, K.; Gutbrod, O.; Saalwächter, C.; et al. Mode of Action of Fluopyram in Plant-Parasitic Nematodes. Sci. Rep. 2022, 12, 11954. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Koltai, H.; Bar-Eyal, M.; Mor, M.; Sharon, E.; Chet, I.; Spiegel, Y. New Strategies for the Control of Plant-Parasitic Nematodes. Pest Manag. Sci. 2000, 56, 983–988. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus Essential Oil as a Natural Pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Sarri, K.; Mourouzidou, S.; Ntalli, N.; Monokrousos, N. Recent Advances and Developments in the Nematicidal Activity of Essential Oils and Their Components against Root-Knot Nematodes. Agronomy 2024, 14, 213. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef]
- Coosemans, J. Dimethyl Disulphide (DMDS): A Potential Novel Nematicide and Soil Disinfectant. Acta Hortic. 2005, 698, 57–64. [Google Scholar] [CrossRef]
- Gómez-Tenorio, M.A.; Zanón, M.J.; de Cara, M.; Lupión, B.; Tello, J.C. Efficacy of Dimethyl Disulfide (DMDS) against Meloidogyne Sp. and Three Formae Speciales of Fusarium Oxysporum under Controlled Conditions. Crop Prot. 2015, 78, 263–269. [Google Scholar] [CrossRef]
- Jardim, I.N.; Oliveira, D.F.; Silva, G.H.; Campos, V.P.; de Souza, P.E. (E)-Cinnamaldehyde from the Essential Oil of Cinnamomum Cassia Controls Meloidogyne Incognita in Soybean Plants. J. Pest Sci. 2018, 91, 479–487. [Google Scholar] [CrossRef]
- Oka, Y. Nematicidal Activity of Essential Oil Components against the Root-Knot Nematode Meloidogyne Javanica. Nematology 2001, 3, 159–164. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Veronico, P.; Avato, P.; Argentieri, M.P. Nematicidal Activity of the Essential Oil from Cinnamomum Cassia and (E)-Cinnamaldehyde against Phytoparasitic Nematodes. J. Pest Sci. 2024, 98, 521–533. [Google Scholar] [CrossRef]
- Oro, V.; Krnjajic, S.; Tabakovic, M.; Stanojevic, J.S.; Ilic-Stojanovic, S. Nematicidal Activity of Essential Oils on a Psychrophilic Panagrolaimus sp. (Nematoda: Panagrolaimidae). Plants 2020, 9, 1588. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Song, B. An Natural Nematicidal Active Compounds: Recent Research Progress and Outlook. J. Integr. Agric. 2021, 20, 2015–2031. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Figueiredo, A.C.; Mota, M.; Vicente, C.S.L. In Vivo and In Vitro Infection of Potato Roots with Plant Parasitic Nematodes for the Assessment of Induced Structural Changes. JoVE 2025, 216, e67756. [Google Scholar] [CrossRef]
- Coyne, D.; Adewuyi, O.; Mbiru, E. Protocol for In Vitro Culturing of Lesion Nematodes Radopholus Similis and Pratylenchus spp. on Carrot Disc on Carrot Discs; International Institute of Tropical Agriculture (IITA): Ibadan, Niger, 2014; Volume 15. [Google Scholar]
- Figueiredo, J.; Vieira, P.; Abrantes, I.; Esteves, I. Commercial Potato Cultivars Exhibit Distinct Susceptibility to the Root Lesion Nematode Pratylenchus penetrans. Horticulturae 2022, 8, 244. [Google Scholar] [CrossRef]
- Kadlecová, A.; Hendrychová, R.; Jirsa, T.; Čermák, V.; Huang, M.; Grundler, F.M.W.; Schleker, A.S.S. Advanced Screening Methods for Assessing Motility and Hatching in Plant-Parasitic Nematodes. Plant Methods 2024, 20, 108. [Google Scholar] [CrossRef]
- Parnis, J.M.; Mackay, D. Multimedia Environmental Models, 3rd ed.; Parnis, J.M., Mackay, D., Eds.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780367809829. [Google Scholar]
- Trent University Level I Model. Available online: https://www.trentu.ca/cemc/resources-and-models/level-i-model (accessed on 29 March 2024).
- National Library of Medicine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 March 2024).
- University of Hertfordshire PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 29 March 2024).
- US EPA. Estimation Programs Interface SuiteTM for Microsoft® Windows, v 4.11; US EPA: Washington, DC, USA, 2023. [Google Scholar]
- Arnot, J.A.; Gobas, F.A.P.C. A Generic QSAR for Assessing the Bioaccumulation Potential of Organic Chemicals in Aquatic Food Webs. QSAR Comb. Sci. 2003, 22, 337–345. [Google Scholar] [CrossRef]
- ECHA European Chemicals Agency. Available online: https://echa.europa.eu/pt/home (accessed on 29 March 2023).
- Computational Biology & Drug Design Group. ADMETlab 3.0. Available online: https://admetlab3.scbdd.com/ (accessed on 29 March 2024).
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal Activity of Plant Essential Oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). J. Asia. Pac. Entomol. 2006, 9, 173–178. [Google Scholar] [CrossRef]
- Mackay, D.; Di Guardo, A.; Paterson, S.; Cowan, C.E. Evaluating the Environmental Fate of a Variety of Types of Chemicals Using the EQC Model. Environ. Toxicol. Chem. 1996, 15, 1627–1637. [Google Scholar] [CrossRef]
- CCTE, EPA (2022). Toxicity Estimation Software Tool (TEST). The United States Environmental Protection Agency’s Center for Computational Toxicology and Exposure. Software. Available online: https://epa.figshare.com/articles/software/Toxicity_Estimation_Software_Tool_TEST_/21379365/3 (accessed on 29 March 2024). [CrossRef]
- Computational Biology & Drug Design Group. ADMETlab3.0 Explanation. Available online: https://admetlab3.scbdd.com/explanation/#/ (accessed on 29 March 2024).
- Seo, S.-M.; Kim, J.; Kim, E.; Park, H.-M.; Kim, Y.-J.; Park, I.-K. Structure−Activity Relationship of Aliphatic Compounds for Nematicidal Activity against Pine Wood Nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef]
- Ntalli, N.; Ratajczak, M.; Oplos, C.; Menkissoglu-Spiroudi, U.; Adamski, Z. Acetic Acid, 2-Undecanone, and (E)-2-Decenal Ultrastructural Malformations on Meloidogyne Incognita. J. Nematol. 2016, 48, 248–260. [Google Scholar] [CrossRef]
- Dutta, A.; Mandal, A.; Kundu, A.; Malik, M.; Chaudhary, A.; Khan, M.R.; Shanmugam, V.; Rao, U.; Saha, S.; Patanjali, N.; et al. Deciphering the Behavioral Response of Meloidogyne Incognita and Fusarium Oxysporum Toward Mustard Essential Oil. Front. Plant Sci. 2021, 12, 714730. [Google Scholar] [CrossRef]
- Silva, J.C.P.; Campos, V.P.; Barros, A.F.; Pedroso, M.P.; Terra, W.C.; Lopez, L.E.; de Souza, J.T. Plant Volatiles Reduce the Viability of the Root-Knot Nematode Meloidogyne Incognita Either Directly or When Retained in Water. Plant Dis. 2018, 102, 2170–2179. [Google Scholar] [CrossRef]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Multiple Modes of Nematode Control by Volatiles of Pseudomonas Putida 1A00316 from Antarctic Soil against Meloidogyne Incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef]
- da Silva, J.C.P.; Campos, V.P.; Barros, A.F.; Pedroso, L.A.; Silva, M.d.F.; de Souza, J.T.; Pedroso, M.P.; de Medeiros, F.H.V. Performance of Volatiles Emitted from Different Plant Species against Juveniles and Eggs of Meloidogyne Incognita. Crop Prot. 2019, 116, 196–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Dong, F.; Liu, X.; Wu, X.; Zheng, Y. Response of Microbial Community to a New Fungicide Fluopyram in the Silty-Loam Agricultural Soil. Ecotoxicol. Environ. Saf. 2014, 108, 273–280. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Y.; Zhang, S.; Lu, H.; Liang, X.; Wang, L.; Wang, M.; Zhang, C. Residue Behavior and Dietary Risk Assessment of Fluopyram in Cowpea and Determination in Nine Foodstuffs. Front. Environ. Sci. 2023, 11, 1105524. [Google Scholar] [CrossRef]
- Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Hematological and Hematopoietic Effects of Bactericidal Doses of Trans-Cinnamaldehyde and Thyme Oil on Cyprinus Carpio Juveniles. Front. Physiol. 2021, 12, 771243. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ávila, A.; Romero-González, R.; Garrido Frenich, A. Degradation Study of the Trans-Cinnamaldehyde and Limonene Biopesticides and Their Metabolites in Cucumber by GC and UHPLC-HRMS: Laboratory and Greenhouse Studies. Food Chem. 2024, 442, 138443. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, 2-Decenal, CAS Registry Number 3913-71-1. Food Chem. Toxicol. 2022, 163, 112958. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, Undecenal, CAS Registry Number 1337-83-3. Food Chem. Toxicol. 2022, 163, 113052. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.X.; Song, B. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- GHS (United Nations). Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (4th Edition). Available online: http://digitallibrary.un.org/record/660120 (accessed on 29 March 2025).
- Rouquié, D.; Tinwell, H.; Blanck, O.; Schorsch, F.; Geter, D.; Wason, S.; Bars, R. Thyroid Tumor Formation in the Male Mouse Induced by Fluopyram Is Mediated by Activation of Hepatic CAR/PXR Nuclear Receptors. Regul. Toxicol. Pharmacol. 2014, 70, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G. Acute Toxicity Studies with Oxamyl. Fundam. Appl. Toxicol. 1986, 6, 423–429. [Google Scholar] [CrossRef]
- Hooth, M.J.; Sills, R.C.; Burka, L.T.; Haseman, J.K.; Witt, K.L.; Orzech, D.P.; Fuciarelli, A.F.; Graves, S.W.; Johnson, J.D.; Bucher, J.R. Toxicology and Carcinogenesis Studies of Microencapsulated Trans-Cinnamaldehyde in Rats and Mice. Food Chem. Toxicol. 2004, 42, 1757–1768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).