Abstract
Mercury (Hg) pollution has led to a serious decline in crop yields. Methyl jasmonate (MJ), as a plant hormone, regulates plant responses to heavy metal stress. Nonetheless, the pathways by which MJ modulates Hg tolerance in plants are still not well elucidated. Our study aimed to elucidate the positive impacts of MJ in alleviating Hg-induced toxicity in maize (Zea mays L.) seedlings using an integrated approach combining physiological assessments and transcriptomic analysis. The findings indicated that exogenous MJ mitigated Hg-induced inhibition of photosynthetic performance by up-regulating photosynthesis-related and light-harvesting-related genes and increasing chlorophyll content. Under Hg stress, MJ enhances proline accumulation in maize seedlings by up-regulating essential genes in the proline biosynthesis pathway and down-regulating critical genes in the proline degradation pathway. MJ also elevates the expression of key enzymes involved in phenylpropanoid biosynthesis in maize seedlings, decreases malondialdehyde (MDA) content, and enhances root vitality. In addition, MJ may exert a detoxification effect on maize seedlings under Hg stress by regulating the expression of various genes linked to basic nutrient transport proteins, as well as those involved in the transport, influx, and distribution of metal ions. These findings indicate that MJ is essential for enhancing plant tolerance to Hg stress, thereby establishing a theoretical framework for the advancement and utilization of environmentally friendly agricultural methods involving plant hormones.
1. Introduction
As a highly hazardous heavy metal, mercury (Hg) contributes significantly to global environmental pollution [1]. With advancements in industrialization and urbanization, Hg pollution has emerged as a critical concern, jeopardizing ecological balance and human survival [2]. The primary contributors to Hg pollution in the environment include industrial activities, where wastewater and emissions from mining, metallurgy, and chemical industries are significant sources; coal combustion, particularly in thermal power plants, which release Hg during the burning process; and waste incineration, where burning Hg-containing waste emits Hg into the atmosphere [3]. Hg released into the environment can volatilize and enter the atmosphere, where it travels long distances and contributes to global pollution. Additionally, industrial wastewater discharge leads to varying levels of mercury contamination in rivers, lakes, and oceans. Hg can accumulate in soil through atmospheric deposition and irrigation with contaminated water. Once in the soil, Hg is readily absorbed by plant roots and moves into the edible parts, posing a risk to human health [4].
Maize occupies a crucial position and plays multiple roles in global agriculture and the economy [5]. It is a key food crop worldwide and ranks among the highest in terms of production [6]. Maize cultivation, processing, and sale have established a vast industrial chain, generating numerous employment opportunities and significant economic benefits. In many countries, maize is an essential component of the diet and has made substantial contributions to food security and economic growth [7]. However, with the advancement of society, pollution levels of Hg in the environment have become increasingly severe. The accumulation of Hg in plants can lead to abnormal growth and development, adversely affect chlorophyll biosynthesis, and reduce the photosynthetic capacity [8]. Hg can also interfere with the absorption and transportation of water and nutrients, disrupt the osmotic balance, result in osmotic stress, break redox homeostasis, and cause cellular oxidative damage in plants, especially within gramineous species [9,10,11,12]. Therefore, it is crucial to establish effective approaches to reduce Hg toxicity in maize plants.
Various technologies have been created to address environmental heavy metal contamination. These include methods such as the solidification and treatment of chemical reagents, centralized and unified management of heavy metal-containing wastewater, remediation of plants with high heavy metal accumulation, and application of certain macronutrients [12,13,14,15,16]. Nevertheless, these strategies have inherent drawbacks, including being resource-intensive and laborious, as well as presenting the potential risks of a unidimensional approach. Numerous studies conducted recently have shown the significant role of plant hormones in the response to various environmental stresses. Methyl jasmonate (MJ), an endogenous plant hormone, is nontoxic, environmentally friendly, and widely found in various higher plants. As a signaling molecule, it promotes the biosynthesis of specific metabolites and controls the expression of several defense genes, thereby enhancing plant tolerance to adverse conditions, such as drought, low temperatures, salt stress, high temperatures, heavy metal stress, and pest and disease attacks [17,18,19,20]. Studies have indicated that under cadmium (Cd) stress [21,22,23], copper (Cu) stress [24], arsenic (As) stress [25], and lead (Pb) stress [26], MJ contributes to the regulation of the photosynthetic system by promoting chlorophyll biosynthesis, and influences the antioxidant system by modulating antioxidant enzymes and the ascorbic acid–glutathione (AsA-GSH) cycle. Additionally, it regulates the plant osmotic system by affecting the synthesis of osmotic regulators such as proline [18]. In rice under Cd stress, MJ mitigates Cd toxicity by influencing the synthesis of root cell walls, controlling the expression of heavy metal transporters and altering the compartmentalized distribution of Cd [27]. Nonetheless, the exact mechanism by which exogenous MJ regulates mercury stress in maize seedlings is still unknown.
RNA sequencing (RNA-Seq) has emerged as a reliable and effective technique for analyzing transcriptomes and has become a powerful method for investigating gene expression changes in plants subjected to heavy metal stress [28]. RNA-Seq is vital for elucidating how plants respond to heavy metal stress, identifying key genes and regulatory networks, and enhancing plant resistance to heavy metals [29,30]. A transcriptomic analysis demonstrated that exogenous MJ improved the thermotolerance of perennial ryegrass by modifying the expression profiles of genes associated with chlorophyll biosynthesis and antioxidant pathways [31]. A transcriptomic investigation performed by Huang et al. (2024) demonstrated that sulfur (S) enhances rice tolerance to Hg by mediating pathways involved in biosynthetic metabolism, transport, stress response, redox processes, expression regulation, and cell wall biogenesis [8]. However, no studies have reported the molecular mechanisms by which MJ mediates Hg resistance in maize seedlings using transcriptome analysis.
This study examined how applying exogenous MJ affects the physiological and biochemical traits of maize seedlings under Hg stress. Orthogonal experiments were performed to determine the optimal concentrations of mercuric chloride (HgCl2) and MJ. Subsequently, the mechanism of MJ-mediated Hg resistance in maize seedlings was analyzed using RNA-Seq. Our findings enhance the understanding of how MJ mitigates Hg toxicity in plants and could be beneficial for breeding strategies.
2. Materials and Methods
2.1. Plant Materials and Mercury Stress Treatment
The seeds of maize utilized in this study are “Longrui 999”, which is commonly cultivated in southern China. After cleaning, the maize seeds were soaked in distilled water and incubated at 28 °C for 24 h. During the soaking period, ten layers of filter paper cut to a size of 15 × 22 cm were placed in a white tray for sterilization. After soaking for 24 h, each tray received an additional 100 mL of distilled water to ensure that the filter paper remained moist. Approximately 100 viable seeds were selected, evenly arranged in a covered white tray, and sprouted in darkness at 28 °C for 60 h. Each day, 25 mL of distilled water was added to stabilize the surface water layer. Seedlings of a uniform size, each approximately 4.0 cm tall and with 60 seedlings per tray, were carefully placed into a climate chamber (temperature conditions: 28 °C during the day and 25 °C at night, relative humidity at 70%, photoperiod of 16 h, and a photon flux density of 750 μmol·m2·s−1) and exposed to three different treatments for a duration of 96 h. These included: (1) CK group: control (1/2 Hoagland’s solution); (2) Hg stress group: 1/2 Hoagland’s solution + different concentrations of HgCl2 solution (30, 35, 40, 45, 50 mg/L HgCl2) treatment; (3) Hg + MJ treatment group: 1/2 Hoagland’s solution + combined solution of different concentrations of HgCl2 (30, 35, 40, 45, 50 mg/L HgCl2) and different concentrations of MJ (0, 50, 75, 100, 150 µmol/L MJ) treatment. To identify the appropriate concentrations of Hg and MJ, orthogonal experiments were designed and are presented in Table S1. To maintain the water layer in the trays, 30 mL 1/2 Hoagland nutrient solution was added daily. Maize seedlings were collected 96 h after the different treatments, and measurements of proline levels, chlorophyll content, malondialdehyde (MDA) content, and root activity were conducted. Every experiment included three biological replicates and was carried out a minimum of three times.
2.2. Determination of Physiological Traits
Epicotyl samples (0.2 g) of maize seedlings of uniform size were weighed and placed in 2 mL centrifuge tubes. The samples were then ground on ice, and the contents of chlorophyll, proline, MDA, and root activity of the maize seedlings were determined using plant chlorophyll content assay kits (chlorophyll was extracted with acetone and determined by spectrophotometry), proline (PRO) content assay kits (proline content was measured using the acid ninhydrin method), MDA content assay kits (MDA was determined by the thiobarbituric acid reaction), and plant root activity test kits (plant root activity was assayed by triphenyltetrazole chloride method) according to manufacturer’s recommendations, respectively. All kits were bought from Grace Biotechnology (Jiangsu Grace Biotechnology Co., Ltd., Suzhou, China; https://www.geruisi-bio.com/, accessed on 16 February 2024). Chlorophyll, proline, MDA, and root activity units were mg/g FW, μg/g FW, nmol/g FW, and μg formazan/h/g FW, respectively. Every experiment included three biological replicates and was carried out a minimum of three times. At least nine samples were collected at each treatment time point.
2.3. RNA Isolation and Sequencing
Based on the preliminary experimental findings, uniformly sized seedlings (roughly 4.0 cm tall, and 60 seedlings per tray) were exposed to three treatments for 96 h: (1) CK group: control (1/2 Hoagland’s solution); (2) HM group: 1/2 Hoagland’s solution+45 mg/L HgCl2; and (3) MJ-HM group: 1/2 Hoagland’s solution+45 mg/L HgCl2+75 µmol/L MeJA. Maize epicotyls were collected at 0, 48, and 96 h after the different treatments, and RNA-seq was performed. A total of 27 samples (1.0 g/sample) were collected during the experiment, which involved sampling at nine distinct treatment time points, each with three biological replicates. In the RNA-seq sample description, “CK” denotes the control group, “HM” represents the 45 mg/L Hg2+ stress group, and “MJ-HM” signifies the 45 mg/L HgCl2+75 μmol/L MeJA treatment group. Biological replicates were labeled as “I”, “II”, and “III”.
Following the manufacturer’s instructions, the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from the epicotyls of maize seedlings. DNase I (TaKaRa Bio, Kusatsu, Shiga, Japan) was used to eradicate genomic DNA contamination. An Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and a NanoDrop ND2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) were utilized to assess the integrity and purity of the RNA, respectively. High-quality RNA was used to create a transcriptome library using a TruSeq RNA Kit (Illumina, San Diego, CA, USA). For qualified RNA samples, cDNA libraries were prepared using a TruSeq Stranded mRNA Prep Kit (Illumina Technologies, San Diego, CA, USA). The Illumina NovaSeq 6000 platform was then used to sequence the created cDNA library, producing 150 bp paired-end reads (Note: Raw RNA-Seq data have been uploaded, and can be found in the Supplementary Materials section).
2.4. Assembly Standardization and Quality Assessment
Before assembly, low-quality reads and reads with adapter sequences were eliminated from the raw data of 27 samples using Trimmomatic software (version 0.39) [32]. FastQC (version 0.11.2) was used to assess the sequence quality for each base. Subsequently, FASTQ files were filtered. Next, the filtered reads were aligned with the reference genome (Ensembl_release56) using HISAT software (version 2-2.1.0) [33]. Finally, the genes were annotated using public databases including Swiss-Prot, KEGG, GO, homologous protein groups (KOG/COG/eggNOG), protein families (Pfam), and NCBI non-redundant protein sequences (NR).
2.5. Differentially Expressed Gene (DEG) Identification
Gene expression levels were evaluated using Transcripts Per Million (TPM) and read counts for each gene were quantified using Ident StringTie (v1.3.1) [34]. Differential expression evaluation between the two groups was performed using the DESeq2 program [35]. DEGs are defined as genes or transcripts with an absolute fold change of ≥2 and a false discovery rate (FDR) of less than 0.05 [36].
2.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
GO enrichment analysis filtered DEGs linked to particular biological functions by identifying all GO terms that were significantly enriched in DEGs relative to the genomic background. Initially, the GO database (https://geneontology.org/, accessed on 11 December 2023) was used to map all DEGs to GO terms. A hypergeometric test was subsequently employed to assess the significance of enrichment and to identify the number of genes associated with each GO term. GO terms are deemed highly enriched in DEGs if their false discovery rate (FDR) is less than 0.05. Additionally, Furthermore, the KEGG is a well-known public collection of data pertaining to pathways [37]. By applying an FDR threshold of ≤0.05, KEGG pathway analysis was conducted to identify enriched pathways.
2.7. Quantitative PCR (qRT-PCR) Verification of RNA-Seq Data
The DEGs (FDX1, PAL, PAO6, ABCG14, DTX45, AATL1, VATP-P1, pitC, and LHT1) found in the RNA-seq results were verified using qRT-PCR. Single-stranded cDNA was produced from RNA samples by utilizing the cDNA EcoDryTM Premix (TaKaRa Bio, Kusatsu, Shiga, Japan). qRT-PCR was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The qRT-PCR procedure was conducted following the instructions provided by the IQTM SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Each experiment included three biological replicates and was conducted at least three times. The relative abundance of transcripts was analyzed using the 2−ΔΔCT approach [38]. EF1α in maize served as the internal reference gene, and Supplementary Table S2 lists the primers used for each gene.
2.8. Statistical Analysis
One-way analysis of variance (ANOVA) was used for statistical analysis. SPSS 25 (IBM Inc., Chicago, IL, USA) was used to conduct the least significant difference (LSD) test at a significance level of p < 0.01. Every experiment included three biological replicates and was carried out a minimum of three times, and error bars represent the standard errors. Data were plotted using the Origin 2021 software (OriginLab Corp., Northampton, MA, USA). MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/home.xhtml, accessed on 21 June 2024) was performed for correlation analysis. The relevant metabolic heat maps were generated using an online tool (https://www.omicstudio.cn/tool, accessed on 26 June 2024).
3. Results
3.1. Effects of MJ Application on the Morphological and Physiological Traits of Maize Seedlings Under Hg Stress
When exposed to different concentrations of Hg for 96 h, the height of maize seedlings was notably lower than that of the control group (CK). As the concentration of Hg2+ increased, root browning in maize seedlings became more severe, whereas leaf expansion was markedly reduced. Conversely, in the Hg + MJ treatment group, both plant height and leaf unfolding showed notable improvements compared with those in the Hg stress group, and the physiological effects of Hg toxicity were considerably alleviated (Figure 1A–E). However, exogenous MeJA treatment had no effect on phenotypic appearance of maize seedlings under normal growth conditions (Figure S1A). These results imply that the exogenous MJ significantly alleviated the negative effects of Hg on maize seedlings exposed to Hg stress.
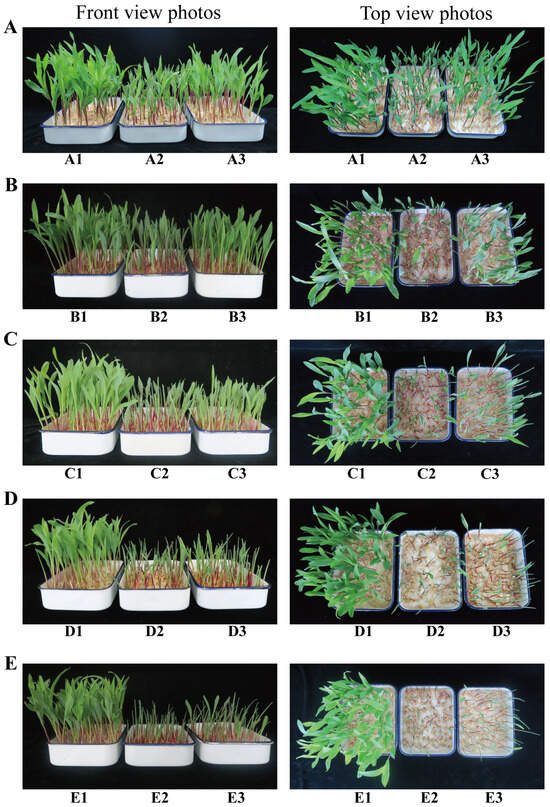
Figure 1.
Exogenous MJ’s effects on morphological traits of maize seedlings during Hg stress. (A1) Control 1, (A2) 30 mg/L HgCl2, (A3) 30 mg/L HgCl2+75 µmol/L MJ, (B1) Control 2, (B2) 35 mg/L HgCl2, (B3) 35 mg/L HgCl2+75 µmol/L MJ, (C1) Control 3, (C2) 40 mg/L HgCl2, (C3) 40 mg/L HgCl2+75 µmol/L MJ, (D1) Control 4, (D2) 45 mg/L HgCl2, (D3) 45 mg/L HgCl2+75 µmol/L MJ, (E1) Control 5, (E2) 50 mg/L HgCl2, (E3) 50 mg/L HgCl2+75 µmol/L MJ.
To investigate the physiological mechanisms by which MJ application enhances maize seedling growth under Hg stress, an L25 (25) orthogonal experiment was conducted. The variables included HgCl2 concentrations (30–50 mg/L), MJ concentrations (50–150 µmol/L), and a CK. The levels of proline, malondialdehyde (MDA), and chlorophyll, and the root vitality of the maize seedlings under various treatment conditions were measured (Table S1). The findings indicated that compared to the CK, different concentrations of Hg stress for 96 h notably decreased the chlorophyll levels in maize seedlings by 43.79%, 45.43%, 47.42%, 48.88%, and 54.75%, respectively (Figure 2A). In contrast, Hg stress significantly increased the MDA content in maize seedlings by 116.34%, 129.09%, 151.26%, 168.85%, and 179.33%, respectively (Figure 2B). Compared to the CK group, different concentrations of Hg stress increased the proline content in maize seedlings by 6.56%, 6.81%, 30.26%, 159.09%, and 103.77%, respectively (Figure 2C). However, these varying concentrations of Hg stress decreased the root vitality of maize seedlings by 22.49%, 52.21%, 53.45%, 54.85%, and 57.26%, respectively, compared to CK seedlings (Figure 2D). The exogenous application of different concentrations of MJ significantly increased the chlorophyll, proline, and root vitality of maize seedlings under Hg stress, while reducing the MDA content. Notably, the treatment with 75 μmol/L MJ exhibited the most pronounced effects. Under 45 mg/L HgCl2 stress, treatment with 75 μmol/L MJ led to a 28.67% increase in chlorophyll content, a 22.83% decrease in MDA content, a 42.98% increase in proline content, and a 34.94% increase in root vitality (Figure 2). However, exogenous MeJA treatment had no significant effect on the concentrations of proline, MDA, chlorophyll, or root vitality of maize seedlings under normal growth conditions (Figure S1B–E). These results indicate that this concentration of MJ significantly alleviated mercury toxicity in maize seedlings. Based on the results of orthogonal experiments, the effect of MJ on enhancing Hg tolerance in maize seedlings was further investigated using the combination of 45 mg/L HgCl2 and 75 μmol/L MJ.
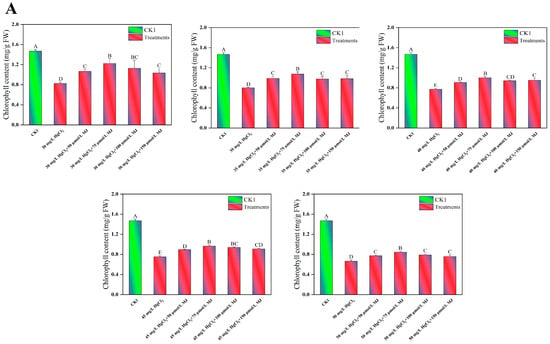
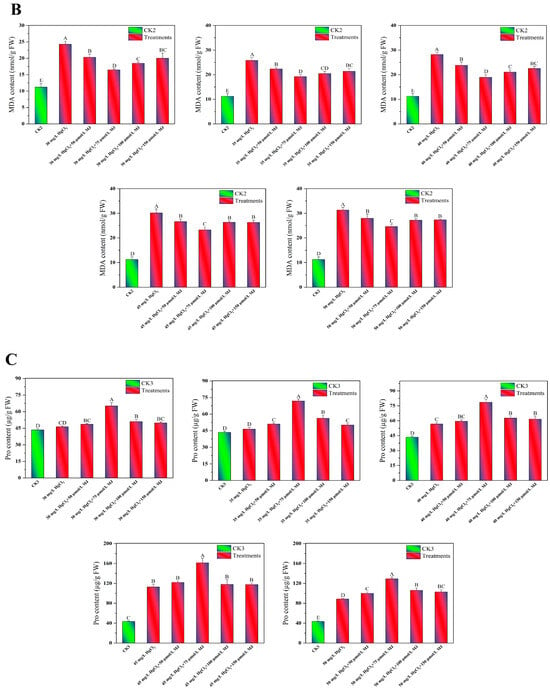
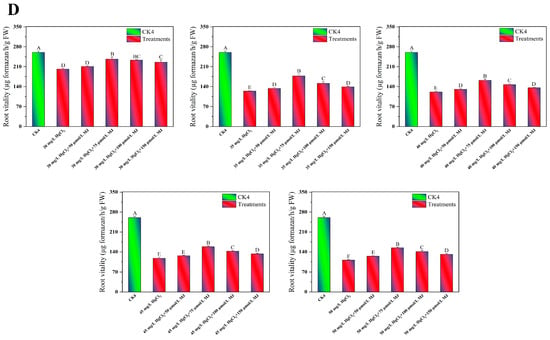
Figure 2.
Exogenous MJ’s effects on the physiological traits of maize seedlings during Hg stress. (A) Effects of various concentrations of MJ on chlorophyll content in maize seedlings under different levels of Hg Stress, (B) Effects of various concentrations of MJ on MDA content in maize seedlings under different levels of Hg Stress, (C) Effects of various concentrations of MJ on proline (Pro) content in maize seedlings under different levels of Hg Stress, (D) Effects of various concentrations of MJ on root vitality in maize seedlings under different levels of Hg Stress. Significant differences between treatments at p < 0.01 are indicated by different capital letters in the figures.
3.2. RNA Sequencing, Assembly, and Annotation
To comprehensively investigate how Hg stress affects maize seedlings’ gene expression and to elucidate the molecular mechanism by which MJ enhances Hg tolerance, RNA-seq analysis was conducted on 27 samples, which included three different treatments (CK, HM, and MJ-HM) and three time points (0, 48, and 96 h), and each treatment had three biological duplicates. Clean readings from the maize seedlings’ epicotyls totaling 195.9 GB were obtained using transcriptome sequencing, with an average of more than 7.25 GB per sample. The minimum proportion of bases that met the Q30 standard was ≥91.84%, and the range of the GC content was 52.76% to 56.01%. The minimum percentage of clean reads aligned with the reference genome was ≥86.73% (Table S3). Pearson correlation analysis revealed a high correlation between biological replicates within each group (R = 0.982–0.997) (Figure 3A), indicating the high quality and suitability of the transcriptome data used in this investigation for further bioinformatic research.
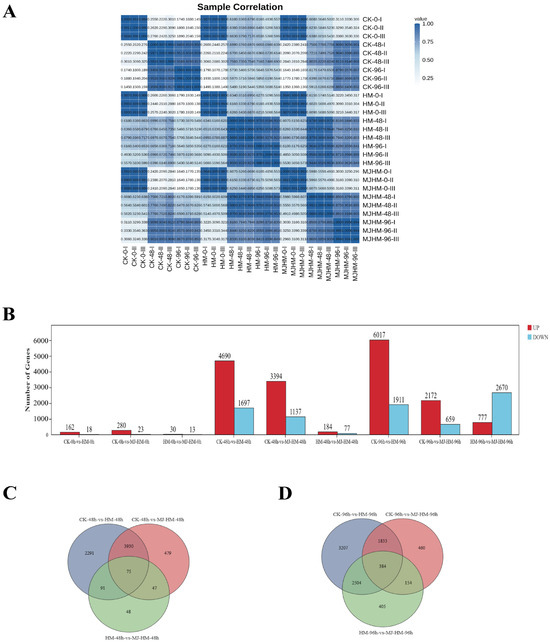
Figure 3.
Analysis of differentially expressed genes (DEGs) in maize seedlings under different treatments. (A) Pearson correlation analysis shows the existence of a correlation between biological replicates, and the correlation coefficient R is shown on the heat map. (B) The histogram shows the quantities of DEGs that are either up-regulated (in red) or down-regulated (in blue) across various treatment groups. Venn diagrams show the variations in gene expression within the maize seedlings’ epicotyls at 48 h (C) and 96 h (D) in three treatments. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.3. Analysis of Differentially Expressed Genes in Maize Seedlings Under Different Treatments
In order to ascertain how exogenous MJ affected Hg stress in maize seedlings, we screened for DEGs using a threshold of |log2 (fold change)| ≥ 1 and p < 0.05. In total, 20,770 DEGs were identified. After 48 h of treatment, we detected 6387 DEGs (4690 up-regulated and 1697 down-regulated) between CK-48 h and HM-48 h, 4531 DEGs (3394 up-regulated and 1137 down-regulated) between CK-48 h and MJ-HM-48 h, and 261 DEGs (184 up-regulated and 77 down-regulated) between HM-48 h and MJ-HM-48 h. After 96 h of treatment, we identified 7928 DEGs (6017 up-regulated and 1911 down-regulated) between CK-96 h and HM-96 h, 2831 DEGs (2172 up-regulated and 659 down-regulated) between CK-96 h and MJ-HM-96 h, and 3447 DEGs (777 up-regulated and 2670 down-regulated) between HM-96 h and MJ-HM-96 h (Figure 3B). The results indicated that MJ significantly influenced gene expression in maize seedlings under Hg stress, with a greater number of DEGs observed at 96 h than at 48 h. Furthermore, analysis of DEGs at 48 and 96 h using Venn diagrams identified 166 common genes through pairwise comparisons of CK-48 h vs. HM-48 h and HM-48 h vs. MJ-HM-48 h, whereas 2888 common DEGs were found in the comparisons of CK-96 h vs. HM-96 h and HM-96 h vs. MJ-HM-96 h. In the comparison of three different treatments (CK-48 h vs. HM-48 h, CK-48 h vs. MJ-HM-48 h, HM-48 h vs. MJ-HM-48 h; CK-96 h vs. HM-96 h, CK-96 h vs. MJ-HM-96 h, and HM-96 h vs. MJ-HM-96 h) at 48 and 96 h, 75 and 384 common DEGs were identified, respectively. This indicates that the MJ-induced regulation of gene expression under Hg stress plays a role in enhancing the Hg tolerance of maize seedlings (Figure 3C).
3.4. Gene Ontology and Kyoto Encyclopedia of Genes Assessment of DEGs in Maize Seedlings Under Different Treatments
To further understand the alterations in DEGs caused by MJ under Hg stress, we conducted GO and KEGG enrichment analyses, encompassing terms from molecular function (MF), cellular component (CC), and biological process (BP) ontologies. The findings revealed that in the comparison between CK-48 h and HM-48 h, the predominant terms in the biological process category were “cellular process” and “metabolic process”. Furthermore, the categories that showed the highest enrichment included “cellular anatomical entity” in the cellular component and “binding” and “catalytic activity” in molecular function (Figure 4A). In contrast, the comparison of HM-48 h vs. MJ-HM-48 h showed a lack of terms related to “locomotion”, “viral process”, and “nitrogen utilization” (Figure 4B). Additionally, when comparing CK-48 h vs. HM-48 h with CK-96 h vs. HM-96 h, the latter included terms for “detoxification” and “biological adhesion” (Figure 4C). Notably, the number of down-regulated genes in the HM-96 h vs. MJ-HM-96 h comparison exceeded that of the up-regulated genes (Figure 4D). The DEGs identified were significantly associated with the role of MJ in enhancing Hg tolerance. This effect was likely due to the ability of MJ to modify the expression profiles of specific genes that are detrimental to maize seedlings, thereby improving their tolerance to Hg exposure.
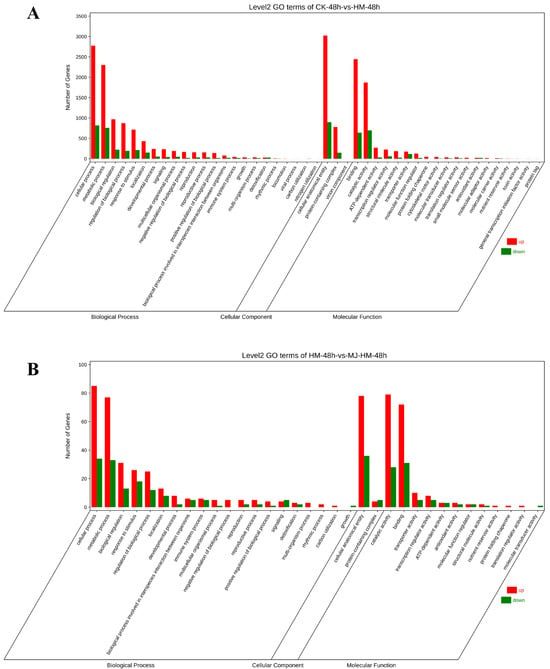
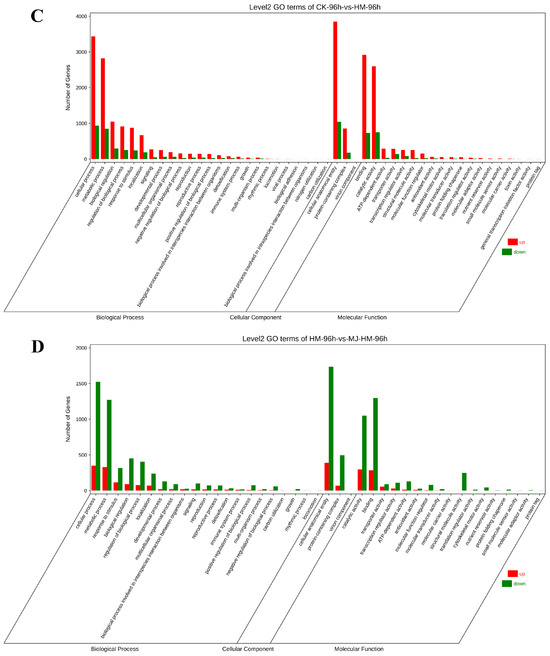
Figure 4.
Gene Ontology (GO) analysis of differentially expressed genes (DEGs) in different treatment groups in the epicotyls of maize seedlings. (A) CK-48 h vs. HM-48 h, (B) HM-48 h vs. HM-MJ-48 h, (C) CK-96 h vs. HM-96 h, (D) HM-96 h vs. HM-MJ-96 h. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
KEGG pathway analysis revealed that photosynthesis-antenna proteins and indole alkaloid biosynthesis were the most significantly enriched pathways when comparing CK-48 h with HM-48 h and HM-48 h with MJ-HM-48 h (Figure 5A,B). Benzoxazinoid biosynthesis was identified as the most prominently enriched pathway in the comparison between CK-96 h and HM-96 h (Figure 5C), whereas photosynthesis-antenna proteins were significantly enriched in the HM-96 h vs. MJ-HM-96 h comparison (Figure 5D). All four comparisons revealed pathways linked to carbon fixation in photosynthetic organisms, as well as those involved in glycolysis/gluconeogenesis and pyruvate metabolism.

Figure 5.
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of differentially expressed genes (DEGs) in different treatment groups in the epicotyls of maize seedlings. (A) CK-48 h vs. HM-48 h, (B) HM-48 h vs. HM-MJ-48 h, (C) CK-96 h vs. HM-96 h, (D) HM-96 h vs. HM-MJ-96 h. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.5. Effects of MJ Application on the Expression of Photosynthesis-Related Genes in Maize Seedlings Under Hg Stress
KEGG analysis indicated that genes associated with photosynthetic pathways were significantly affected by Hg exposure and exogenous MJ treatment (HgCl2+MeJA) (Figure 6). After a 96 h treatment period, Hg stress led to the down-regulation of genes encoding light-harvesting chlorophyll proteins, the cytochrome b6/f complex, photosystems I (PSI), photosystems II (PSII), F-type ATP synthase, and components of the photosynthetic electron transport chain, in comparison to the CK (Figure 6). However, when exogenous MJ was applied, these genes were up-regulated under Hg stress (Figure 6B,D). These findings suggest that MJ enhances Hg resistance in maize seedlings by modulating photosynthesis-related pathways.
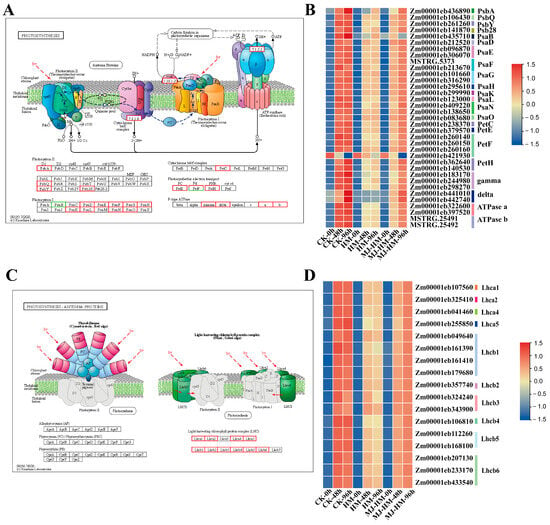
Figure 6.
Schematic diagrams and heat maps of differentially expressed genes (DEGs) involved in the “photosynthesis” and “photosynthetic antenna proteins” pathways in the epicotyls of maize seedlings. (A) “Photosynthesis” pathway, (B) expression profiles of DEGs in the “Photosynthesis” pathway, (C) “Photosynthetic antenna proteins” pathway, (D) expression profiles of DEGs in “Photosynthetic antenna proteins” pathway. High expression is represented by red, while low expression is shown in blue. In subfigures (A) and (C), red boxes indicate up-regulation of the gene expression coding for the related protein, and green boxes indicate down-regulation. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.6. Effects of MJ Application on the Expression of Phenylpropanoid Biosynthesis-Related Genes in Maize Seedlings Under Hg Stress
Phenylpropanoids (PPs) are vital secondary metabolites in plants. They enhance mechanical strength, contribute to vessel formation, help resist pathogen attacks, and aid in coping with drought, cold, and heavy metal stress [39]. In the present study, DEGs involved in phenylpropanoid biosynthesis were significantly enriched (Figure 7). Compared with the CK, the levels of expression for genes that encode phenylalanine ammonia-lyase (PAL), peroxidase (POD), shikimate-O-hydroxycinnamoyl transferase (HCT4) [40], peroxidase (HRPN), cinnamyl alcohol dehydrogenase (CAD), trans-cinnamic acid-4-monooxygenase (CYP), cinnamoyl-CoA reductase (CCR1), and coniferyl aldehyde dehydrogenase (ALDH2C4) [41], were continuously up-regulated under Hg stress (Figure 7). However, following MJ application, the degree of up-regulation of gene expression was further enhanced. After exogenous application of MJ, genes encoding spermidine hydroxycinnamoyl transferase (PHT) and peroxidase (PER) [42], whose expression patterns were restored to levels consistent with those in the CK (Figure 7B). These findings suggest that MJ enhances Hg tolerance in maize seedlings by regulating phenylpropanoid biosynthesis.
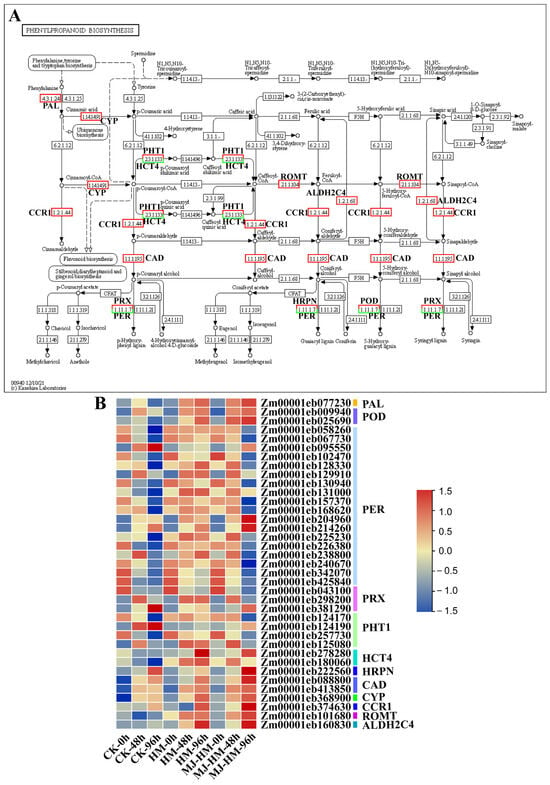
Figure 7.
Schematic diagrams and heat maps of differentially expressed genes (DEGs) involved in the “phenylpropanoid biosynthesis pathway” in the epicotyls of maize seedlings. (A) The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enriched by phenylpropanoid biosynthesis. Red boxes indicate up-regulation of the gene expression coding for the related protein, and green boxes indicate down-regulation. Solid lines represent confirmed research, whereas dotted lines represent areas requiring further research. (B) Heat map showing expression changes in phenylpropanoid biosynthesis. High expression is represented by red, while low expression is shown in blue. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.7. Effects of MJ Application on the Expression of Proline Metabolism-Related Genes in Maize Seedlings Under Hg Stress
Proline is a crucial stress response compound that assists plants in coping with adverse environmental conditions and sustaining normal growth and development [43]. As shown in Figure 8, the genes encoding polyamine oxidase (PAO), ornithine aminotransferase (OAT), Δ1-pyrroline-5-carboxylic acid reductase (P5CR), Δ1-pyrroline-5-carboxylic acid synthase (P5CS) [42], and aspartate aminotransferase (ASP5) [44], which are involved in proline metabolism of maize seedlings, were significantly up-regulated under Hg stress compared to the CK. Notably, treatment with exogenous MJ enhanced the expression of these genes (Figure 8). Under Hg stress, genes encoding aldehyde dehydrogenase (ALDH) [45], proline-4-hydroxylase (P4HA) [41], ornithine decarboxylase (ODC), glutamic-oxaloacetic transaminase 1 (GOT1) [44], spermidine synthase (SPMS) [46], and proline dehydrogenase (ProDH) [42] were up-regulated compared to the CK. However, in contrast to the treatment with Hg alone, the combination of Hg and MJ significantly down-regulated these genes’ expression levels, particularly at 96 h of treatment (Figure 8B). These findings indicate that MJ enhanced proline levels in maize seedlings under Hg stress by promoting proline biosynthesis and inhibiting proline degradation.
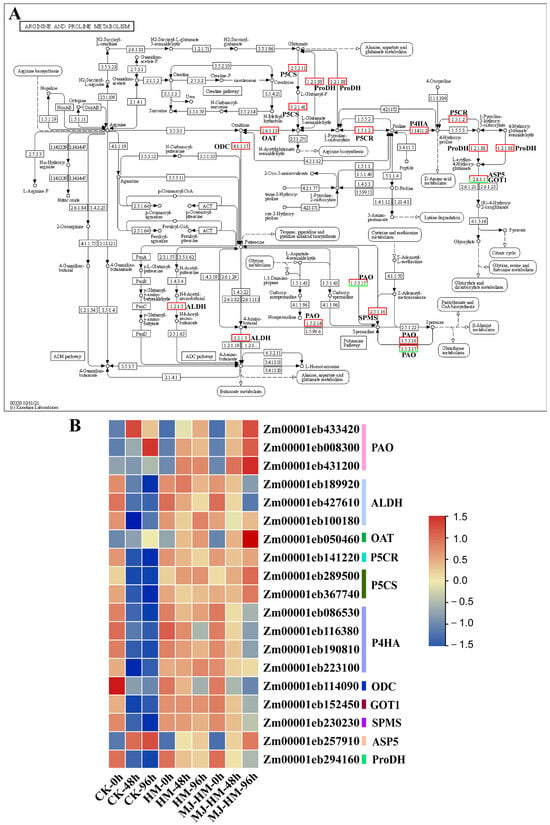
Figure 8.
Schematic diagrams and heat maps of differentially expressed genes (DEGs) involved in “proline metabolism” in the epicotyls of maize seedlings. (A) The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enriched by proline metabolism. Red boxes indicate up-regulation of the gene expression coding for the related protein, and green boxes indicate down-regulation. Solid lines represent confirmed research, whereas dotted lines represent areas requiring further research. (B) Heat map showing the variations in proline metabolism-related gene expression. High expression is represented by red, while low expression is shown in blue. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.8. Effects of MJ Application on the Expression of Transport Genes in Maize Seedlings Under Hg Stress
Plant adaptation to heavy metal stress is critically dependent on the transport and distribution of substances regulated by transporters [47]. In our study, GO analysis and KEGG enrichment analysis were conducted on DEGs that respond to MJ under Hg stress (Figure 9A–C). We successfully identified 15 transport protein genes that were significantly regulated by MJ. Among these, AATL1, which encodes a lysine–histidine transporter [48]; NAT6, which encodes a cation symporter [49]; VATP-P1, which encodes a proton transmembrane transporter [50]; ABCG3, which encodes a member of the ABC transporter G family [51]; FAX3, which encodes a fatty acid transporter [52]; URGT2, which encodes a UDP-galactose transmembrane transporter [53]; and NAT11, which encodes a nucleobase-ascorbic acid transporter [54], were up-regulated when exposed to Hg stress in comparison to the CK, while it exhibited down-regulation when exposed to Hg + MJ treatment relative to the Hg treatment alone. Additionally, PHT1, which encodes an inorganic phosphate transmembrane transporter [55]; DTX45, which encodes a xenobiotic transmembrane transporter [56]; ABCG14, which encodes another member of the ABC transporter G family [51]; AAP3, which encodes a transmembrane amino acid transporter [57]; LHT1, which encodes a lysine-histidine transporter [48]; pitC, which encodes an intermembrane phospholipid transporter [58]; and PLT5, which encodes a polyol transporter [59], were down-regulated under Hg stress. However, under Hg + MJ treatment, the expression levels of these genes increased (Figure 9D). These findings indicate that MJ modulates various transport proteins involved in nutrient uptake, transport, and cellular homeostasis in maize subjected to Hg stress.
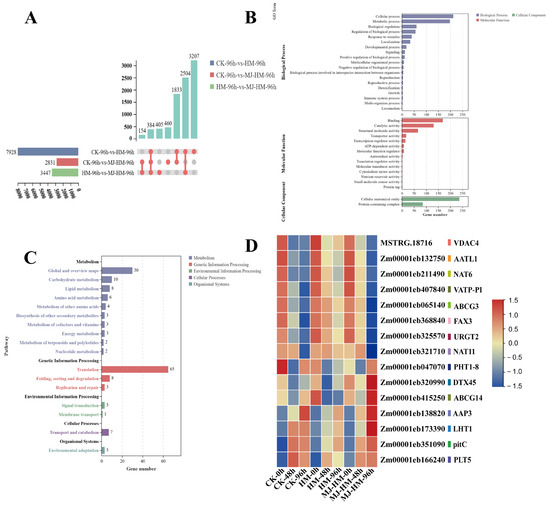
Figure 9.
Analysis of differentially expressed genes (DEGs) that only respond to Hg+MJ treatment. (A) The number of DEGs after different treatments for 96 h. (B) Gene Ontology (GO) analysis of 405 DEGs that only respond to Hg+MJ treatment. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of 405 DEGs that only respond to Hg+MJ treatment. (D) Heat map of transport protein DEGs that respond to Hg+MJ treatment. High expression is represented by red, while low expression is shown in blue. CK (Control), HM (45 mg/L HgCl2), and HM-MJ (45 mg/L HgCl2+75 µmol/L MJ).
3.9. Verification of RNA-Seq Results by qRT-PCR
In order to confirm the RNA-Seq findings, we chose nine genes for qRT-PCR, Zm00001eb260140 (FDX1), Zm00001eb077230 (PAL), Zm00001eb008300 (PAO6), Zm00001eb415250 (ABCG14), Zm00001eb130600 (DTX45), Zm00001eb132750 (AATL1), Zm00001eb407840 (VATP-P1), Zm00001eb351090 (pitC), and Zm00001eb173390 (LHT1). These genes are associated with the photosynthetic antenna proteins, phenylpropanoid biosynthesis, proline metabolism, and protein transport. The results indicated that, under Hg stress and Hg+MJ treatment, the trustworthiness of the RNA-Seq results was confirmed by the expression patterns of the nine chosen DEGs, which matched the observations from the RNA-Seq data (Figure 10).
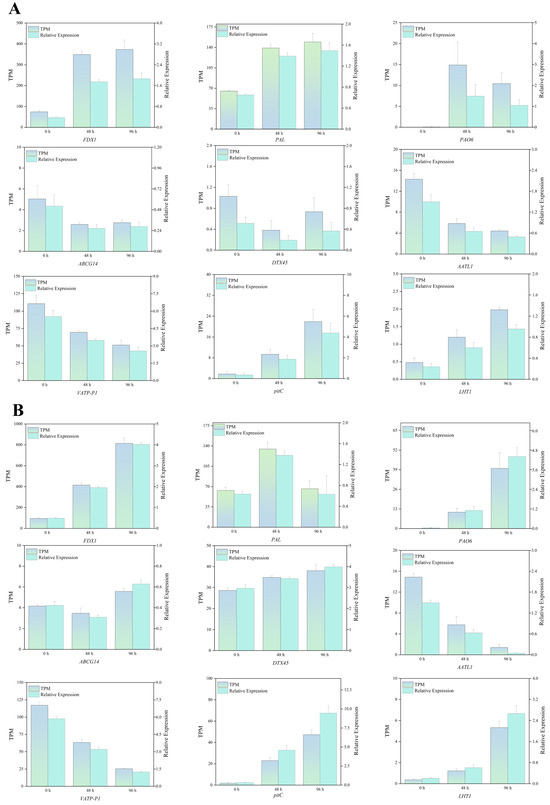
Figure 10.
Verification of the RNA-Seq expression profiles of nine genes through qRT-PCR. (A) The expression patterns of the selected nine differentially expressed genes (DEGs) under Hg stress and RNA-seq data. (B) The expression patterns of nine DEGs under Hg+MJ treatment and RNA-seq data. TPM and relative expression levels of the qRT-PCR products from three separate biological replicates are shown in the bar graphs. The relative expression level as determined by qRT-PCR is on the right y-axis; the TPM value as determined by RNA-Seq is on the left.
4. Discussion
Excessive Hg negatively affects plant growth, development, and physiological processes, ultimately affecting plant yield and quality. We found that Hg stress inhibited the development of maize seedlings, disrupted chlorophyll synthesis, reduced root activity, and increased malondialdehyde and proline levels, thereby damaging maize growth. In rice (Oryza sativa L.) [8], it has been observed that Hg disrupts the photosynthetic system, reduces chlorophyll synthesis, increases the MDA content, and disturbs the balance of the antioxidant system. These effects damage the cell membrane and adversely affect normal rice growth. Cd can also reduce the root activity of wheat and disrupt proline metabolism, thereby negatively affecting wheat seedlings [60]. Some studies have shown that MJ can increase plants’ tolerance to abiotic stress by significantly increasing the total carbohydrate, total soluble sugar, polysaccharide [33], free amino acid, total proline, protein [61,62], and chlorophyll contents [63]. Additionally, MJ has been found to activate enzymatic systems, notably increasing SOD, APX, POD, and CAT activities [64]. Our study demonstrated that MJ reduced MDA content, increased chlorophyll levels, enhanced photosynthesis, and enhanced the ability of maize seedlings to withstand Hg stress. Additionally, MJ elevated proline levels and boosted root activity in maize seedlings, aiding recovery from Hg-induced damage. However, the specific mechanisms through which MJ regulates Hg tolerance in maize remain unclear.
To elucidate the molecular mechanism by which MJ regulates Hg tolerance in maize seedlings, we identified DEGs after MJ treatment using RNA-Seq data. Compared to Hg treatment alone, KEGG enrichment analysis indicated that the combined Hg+MJ treatment significantly enriched pathways such as “photosynthesis”, “photosynthesis-antenna proteins”, “phenylpropanoid biosynthesis”, “proline metabolism”, and “transporters”. These results suggest that these pathways are crucial for MJ to induce Hg tolerance in maize seedlings.
Owing to their sessile nature, plants cannot avoid the adverse effects of environmental fluctuations. Consequently, they must develop intricate defense strategies to adapt to continuous changes in their surroundings [65]. The primary method of response involves the modulation of photosynthetic physiological functions. Drought, high temperature, and heavy metal stress diminish chlorophyll biosynthesis and induce photoinhibition and photooxidative stress within the photosynthetic apparatus in plants [66,67,68]. Our study demonstrated that MJ positively influenced photosynthesis. This regulation was mediated by key DEGs, including those associated with F-type ATPase (γ, δ, a, b), the cytochrome b6/f complex, PSI, PSII, photosynthetic electron transport (PetE, PetF, PetH), and light-harvesting chlorophyll protein complexes (Lhca1-Lhca5, Lhcb1-Lhcb6), as shown in Figure 6. PSI, which consists of an antenna complex and reaction center complex, generates NADPH to provide reducing power for dark reactions [69]. The peripheral PSI protein subunits include PsaD, PsaE, PsaF, PsaG, and PsaH. PsaD is integral to the structure and function of PSI, ensuring structural stability, positioning, and conformation of its components, in addition to providing a binding site for ferredoxin. PsaE stabilizes the complex structure and helps maintain its conformation [70]. PsaF regulates electron transfer [71]. However, the functions of PsaG are not well understood. It is hypothesized that it may play a role in the assembly, structural stability, or interaction with other components [72]. The role of PsaH remains unclear; however, it may contribute to the regulation of its assembly and stability. Both PsaG and PsaH cooperate and function in conjunction with the core PSI complex. These findings indicated that Hg stress reduced the expression of PsaD, PsaE, PsaF, and other genes, indicating potential damage to the PSI core complex [72,73]. However, MJ treatment alleviated this damage by up-regulating the expression of PSI-related genes. PSII, a water-splitting enzyme, pulls electrons from water to reduce the plastoquinone pool while simultaneously generating two oxygen molecules [74]. PSII protein subunits include PsbA, PsbO, PsbQ, PsbY, Psb28, and others [69]. During photosynthesis, the build-up of reactive oxygen species (ROS) within the chloroplasts disrupts the D1 protein encoded by PsbA, thereby interfering with PSII’s function [75]. PSII water oxidation is executed through the CaMn4O5 cluster, which is stabilized by the PsbO protein. Furthermore, the water-splitting reaction necessitates Ca2+ and Cl− and is regulated by the PsbQ protein [76]. The function of the PsbY protein remains unclear; however, it is believed to fine-tune electron transfer and water oxidation and could play a role in repairing PSII [77]. Our research demonstrated that Hg stress reduced the expression of PsbA, PsbQ, PsbY, and Psb28 (Figure 6B). This suggests that as a divalent cation, Hg can destabilize the CaMn4O5 cluster by competing with Ca2+ and inhibiting the D1 protein through the accumulation of ROS within the chloroplasts. The addition of MJ enhanced the expression of PsbA, PsbQ, PsbY, and Psb28, indicating that MJ improved the photosynthetic efficiency of maize seedlings by enhancing the function and activity of the PSII system. Our results also showed that Hg stress significantly reduced the chlorophyll content, impaired photosynthetic electron transport, limited LHC activity, disrupted the equilibrium of excitation between PSI and PSII, and decreased ATP production from photosynthesis. However, the application of MJ mitigated these adverse effects.
The phenylpropanoid biosynthesis pathway is crucial for plant resilience against stress by producing essential secondary metabolites, such as lignin and flavonoids [78]. Research indicates that, under Cd stress, this pathway enhances lignin production in wheat, strengthening the ability of cell walls to bind Cd, thereby minimizing its entry into cells and reducing cellular damage. Additionally, it supports the stability of cell walls, helping wheat maintain its cellular structure and function under Cd stress and thus lowering the risk of cell damage [79,80,81]. Similar findings have been observed in rice, where Cd triggers POD gene regulation within the phenylpropanoid pathway, leading to increased synthesis of antioxidant compounds that mitigate oxidative stress and protect cellular components [82]. However, the mechanism by which MJ regulates the phenylpropanoid pathway genes in maize seedlings under Hg stress remains unclear. Our findings revealed that both Hg stress and Hg+MJ treatment groups exhibited continuous up-regulation of the PAL gene, with a more pronounced effect in the Hg+MJ group (Figure 7B). As PAL is a crucial enzyme within the phenylalanine pathway [83,84], MJ treatment appears to activate the phenylpropanoid biosynthesis pathway. Additionally, POD, another crucial enzyme in this pathway [85], was up-regulated by MJ treatment. Similarly, MJ also regulated other lignin biosynthesis-related genes under Hg stress, including PRX, PHT, CAD, CYP, and CCR1 (Figure 7B). This suggests that MJ enhances the synthesis of flavonoids, lignin, and other phenylpropanoids, thereby improving Hg tolerance in maize seedlings.
Proline, a cyclic amino acid, serves as a fundamental building block of proteins and is essential for their structure and function [86]. Under stress conditions such as drought and high salinity, maize cells accumulate proline to decrease osmotic potential, thereby preserving turgor pressure and maintaining normal physiological activities [87,88]. In addition, proline interacts with phospholipids to stabilize cell membranes and reduce membrane fluidity at low temperatures [89]. In environments subjected to heavy metal stress, proline functions as an antioxidant by scavenging ROS that can cause oxidative damage. Under Cd stress, proline enhances the antioxidant capacity of wheat by modulating superoxide dismutase and catalase activities [90,91,92]. This study found that proline content increased under both Hg and Hg + MJ treatments, with the amount synthesized under the Hg + MJ treatment being higher than that under Hg treatment alone (Figure 2C). KEGG analysis indicated a notable enrichment of DEGs involved in proline metabolism following MJ treatment. Genes (PAO, P5CS, P5CR, P5CS) encoding PAO, P5CS, P5CR, and OAT were consistently up-regulated by MJ. PAO catalyzes the oxidation of proline to pyrroline-5-carboxylic acid (P5C), whereas P5CS converts glutamate to P5C, and OAT facilitates the ornithine pathway to produce P5C and glutamate-semialdehyde (GSA). P5C is subsequently reduced to proline by P5CR and enters various metabolic processes, thereby influencing the overall metabolism of plants [93,94]. Our results also indicate that the levels of expression for two proline-degrading enzyme genes, ALDH and ProDH, decreased over time following MJ treatment, suggesting that proline degradation was inhibited (Figure 2C and Figure 8B). Thus, MJ may enhance maize seedlings’ tolerance to Hg through promoting the expression of proline biosynthesis genes while simultaneously inhibiting proline degradation, leading to proline accumulation.
Transporters are vital for the growth and development of plants, as they control the intake of nutrients, such as sugars, lipids, and amino acids, and facilitate the transport of necessary materials for plant functions [95,96]. When subjected to heavy metal stress, the Nramp family of transporters in plants, particularly OsNramp5 in rice roots, is essential for the uptake of metals, such as lead [97]. Studies have shown that compartmentalization and detoxification are critical functions of transporters [98]. ABC transporters facilitate the transport of heavy metal chelates into the vacuoles, thereby mitigating cellular toxicity and safeguarding the normal physiological functions of plant cells [99]. Some transporters help attach heavy metals to the cell wall, limiting their entry into cells, and thereby reducing potential damage, which serves as a plant defense mechanism. P-type ATPases maintain intracellular heavy metal ion balance by pumping heavy metal ions out of the cell using the energy from ATP hydrolysis [100,101,102,103,104]. Our study demonstrated that MJ influenced the expression of several transporter genes (Figure 9D). Notably, under Hg stress, the expression levels of the heterologous transmembrane transporter (DTX45), transmembrane amino acid transporter (AAP3), phospholipid transporter (pitC), and polyol transporter (PLT5) were lower than those in the CK seedlings. This suggests that MJ enhances the expression of specific transporter genes related to nutrient regulation, thereby facilitating detoxification in maize seedlings and promoting their growth under Hg stress. In contrast, the expression of the cation transporter (NAT6) and ABC family transporter (ABCG3) increased under mercury stress compared to control seedlings but decreased with Hg+MJ treatment. This indicates that MJ may reduce the expression of genes associated with Hg2⁺ transport, potentially limiting the entry of mercury into cells and mitigating its detrimental effects. MJ appears to exert a detoxifying effect on maize seedlings subjected to Hg stress and facilitates their recovery from stress via regulating the expression of essential nutrients and metal ion transport genes.
5. Conclusions
Taken together, our study demonstrates that the exogenous application of methyl MJ alleviates the inhibitory effects of Hg on photosynthetic efficiency by up-regulating genes associated with photosynthesis and light harvesting, as well as by stimulating chlorophyll biosynthesis. Under Hg stress, MJ enhances proline accumulation in maize seedlings by up-regulating key enzyme genes in the proline biosynthesis pathway and down-regulating genes related to proline degradation. Furthermore, MJ increased the expression of key enzyme genes involved in phenylpropionic acid biosynthesis in maize seedlings, decreased MDA levels, and improved root activity. Remarkably, MJ may facilitate detoxification in maize seedlings subjected to Hg-induced stress by controlling the expression of various genes associated with essential nutrient transporters, as well as those implicated in the transport, influx, and distribution of metal ions. These results indicate that MJ is pivotal for augmenting plant tolerance to Hg-induced stress. The potential molecular mechanism by which MJ enhances Hg resistance in maize seedlings is illustrated in Figure 11.
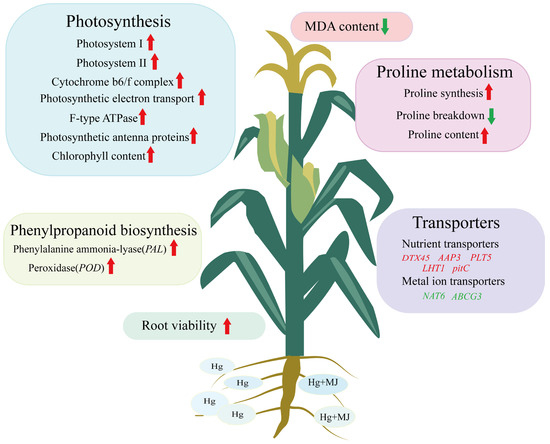
Figure 11.
MJ participates in the response of maize seedlings to Hg stress. Upward red arrows indicate that the relevant biological process is continuously up-regulated in Hg + MJ treatment compared with Hg stress treatment; downward green arrows indicate that the relevant biological process is continuously down-regulated in Hg + MJ treatment compared with Hg stress treatment. Genes in red font indicate up-regulated expression under the Hg + MJ treatment, and genes in green font indicate down-regulated expression under the Hg + MJ treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061369/s1, Figure S1: Effects of different treatments for 96 h on phenotypic appearance and physiological traits of maize seedlings; Table S1: Raw data from the orthogonal experiment conducted in this study.
Author Contributions
X.L.: Writing—original draft, Software, Methodology, Investigation, Data curation, Conceptualization. Y.C. (Yunliang Chen): Validation. S.L.: Software, Methodology, Investigation, Conceptualization. S.Y.: Writing—review and editing, Funding acquisition, Formal analysis, Conceptualization. J.S.: Validation. Z.Z.: Validation. Y.C. (Yanmei Chang): Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Nos. 31260169, 32160076) and the open research program of Yunnan Key Laboratory of Potato Biology, Yunnan Normal University (YNPKF202203).
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and lonomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Sobariu, D.L.; Fertu, D.I.T.; Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Dragoi, E.N.; Curteanu, S.; Lenz, M.; Corvinid, P.F.X.; Gavrilescu, M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol. 2017, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research progress of soil microorganisms in response to heavy metals in rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef] [PubMed]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Pollut. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Niu, L.; Hao, R.; Wu, X.; Wang, W. Maize mesocotyl: Role in response to stress and deep-sowing tolerance. Plant Breed. 2020, 139, 466–473. [Google Scholar] [CrossRef]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Influence of heat stress, variations in soil type, and soil amendment on the growth of three drought-tolerant maize varieties. Agronomy 2021, 11, 1485. [Google Scholar] [CrossRef]
- Jafari, F.; Wang, B.; Wang, H.; Zou, J. Breeding maize of ideal plant architecture for high-density planting tolerance through modulating shade avoidance response and beyond. J. Integr. Plant Biol. 2024, 66, 849–864. [Google Scholar] [CrossRef]
- Huang, Y.; Yi, J.; Li, X.; Li, F. Transcriptomics and physiological analyses reveal that sulfur alleviates mercury toxicity in rice (Oryza sativa L.). J. Environ. Sci. 2024, 135, 10–25. [Google Scholar] [CrossRef]
- Goix, S.; Leveque, T.; Xiong, T.T.; Schreck, E.; Baeza-Squiban, A.; Geret, F.; Uzu, G.; Austruy, A.; Dumat, C. Environmental and health impacts of fine and ultrafine metallic particles: Assessment of threat scores. Environ. Res. 2014, 133, 185–194. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Xu, K.; Wang, J. Cadmium in cereal crops: Uptake and transport mechanisms and minimizing strategies. J. Agric. Food Chem. 2022, 70, 5961–5974. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Q.; Guo, Z.; Fu, J.; Sun, Y.; Gu, C.; Xing, B.; Dhankher, O.P. Sulfur nanoparticles improved plant growth and reduced mercury toxicity via mitigating the oxidative stress in Brassica napus L. J. Clean. Prod. 2021, 318, 128589. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal. Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Safeer, R.; Liu, G.; Yousaf, B.; Ashraf, A.; Haider, M.I.S.; Cheema, A.I.; Ijaz, S.; Rashid, A.; Sikandar, A.; Pikon, K. Insights into the biogeochemical transformation, environmental impacts and biochar-based soil decontamination of antimony. Environ. Res. 2024, 251, 118645. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Liu, H.Q.; Guo, X.P. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 2021, 286, 124989. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, U. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Rasulov, B.; Niinemets, U. Role of stomatal conductance in modifying the dose response of stress-volatile emissions in methyl jasmonate treated leaves of cucumber (Cucumis sativa). Int. J. Mol. Sci. 2020, 21, 1018. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Li, X. Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 104, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Anwar, T.; Qureshi, H.; Jabeen, M.; Zaman, W.; Ali, H.M. Mitigation of cadmium-induced stress in maize via synergistic application of biochar and gibberellic acid to enhance morpho-physiological and biochemical traits. BMC Plant Biol. 2024, 24, 192. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, W.; Chen, J.; Li, X. Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol. Plant. 2015, 59, 373–381. [Google Scholar] [CrossRef]
- Kaushik, S.; Sharma, P.; Kaur, G.; Singh, A.K.; Al-Misned, F.A.; Shafik, H.M.; Sirhindi, G. Seed priming with methyl jasmonate mitigates copper and cadmium toxicity by modifying biochemical attributes and antioxidants in Cajanus cajan. Saudi J. Biol. Sci. 2022, 29, 721–729. [Google Scholar] [CrossRef]
- Nazir, F.; Jahan, B.; Iqbal, N.; Rajurkar, A.B.; Siddiqui, M.H.; Khan, M.I.R. Methyl jasmonate influences ethylene formation, defense systems, nutrient homeostasis and carbohydrate metabolism to alleviate arsenic-induced stress in rice (Oryza sativa). Plant Physiol. Biochem. 2023, 202, 107990. [Google Scholar] [CrossRef]
- Salavati, J.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol. Mol. Biol. Plants 2021, 27, 1089–1104. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Zhang, Y.; Su, Z.; Hu, T.; Liu, J.; Ding, Q.; Niu, N.; Ma, L. Methyl jasmonate enhances the safe production ability of Cd-stressed wheat by regulating the antioxidant capacity, Cd absorption, and distribution in wheat. Plant Physiol. Biochem. 2024, 212, 108788. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wang, R.; Zhang, D.; Chu, S.; Yang, X.; Hayat, K.; Fan, Z.; Cao, X.; Ok, Y.S.; et al. Insights into growth-promoting effect of nanomaterials: Using transcriptomics and metabolomics to reveal the molecular mechanisms of MWCNTs in enhancing hyperaccumulator under heavy metal(loid)s stress. J. Hazard. Mater. 2022, 439, 129640. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Ai, Q.; Han, J.; Wang, H.; Fu, Q. Transcriptomic and metabolomic analyses reveal that exogenous strigolactones alleviate the response of melon root to cadmium stress. Hortic. Plant J. 2022, 8, 637–649. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J.; Huo, H.; Wu, Z.; Yang, R.; Gong, H. Transcriptomic dynamics provide an insight into the mechanism for silicon mediated alleviation of salt stress in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 174, 245–254. [Google Scholar] [CrossRef]
- Nie, G.; Zhou, J.; Jiang, Y.; He, J.; Wang, Y.; Liao, Z.; Appiah, C.; Li, D.; Feng, G.; Huang, L.; et al. Transcriptome characterization of candidate genes for heat tolerance in perennial ryegrass after exogenous methyl Jasmonate application. BMC Plant Biol. 2022, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Rainer, J.; Gatto, L.; Weichenberger, C.X. ensembldb: An R package to create and use Ensembl-based annotation resources. Bioinformatics 2019, 35, 3151–3153. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Aubert, J.; Bar-Hen, A.; Daudin, J.J.; Robin, S. Determination of the differentially expressed genes in microarray experiments using local FDR. BMC Bioinform. 2004, 5, 125. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, C.; Lan, H.; Gao, S.; Liu, H.; Liu, J.; Cao, M.; Pan, G.; Rong, T.; Zhang, S. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS ONE 2014, 9, e95445. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Sun, F.L.; Wang, J.H.; Geng, S.W.; Liang, Y.J.; Gong, Z.L.; Yang, N.; Qian, S.S.; Zhang, N.L.; Li, X.Y.; Wang, J.D.; et al. Comprehensive transcriptomic and metabolomic analysis revealed drought tolerance regulatory pathways in upland cotton. Front. Plant Sci. 2025, 16, 1571944. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, X.R.; Meng, J.H.; Zhou, X.F.; Xu, H.W. Multi-omics research reveals the effects of the ABA-regulated phenylpropanoid biosynthesis pathway on the UV-B response in Rhododendron chrysanthum Pall. Plants 2025, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Mu, J.X.; Yang, T.; Shen, Q.; Wang, Y.K. Integrated transcriptome and metabolome analyses reveal candidate genes associate with phenolic compound biosynthesis in different varieties of Perilla frutescens. Int. J. Mol. Sci. 2025, 26, 2841. [Google Scholar] [CrossRef] [PubMed]
- Adamipour, N.; Nazari, F.; Nalousi, A.M.; da Silva, J.A.T. Evaluation of the molecular mechanism underlying proline metabolic and catabolic pathways and some morpho-physiological traits of tobacco (Nicotiana tabacum L.) plants under arsenic stress. BMC Plant Biol. 2025, 25, 258. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, R.; Bourdès, A.; Schuller, M.; Jorrin, B.; Ahel, I.; Poole, P.S. Aspartate aminotransferase of Rhizobium leguminosarum has extended substrate specificity and metabolizes aspartate to enable N2 fixation in pea nodules. Microbiol. Sgm 2024, 170, 001471. [Google Scholar] [CrossRef]
- Qiu, Z.T.; Liu, X.H.; Yu, J.; Zhao, Y.S.; Zhao, G.R.; Li, S.Y.; Liu, K.; Du, L.; Ma, L. Efficient conversion of aromatic and phenylpropanoid alcohols to acids by the cascade biocatalysis of alcohol and aldehyde dehydrogenases. Synth. Syst. Biotechnol. 2024, 9, 187–195. [Google Scholar] [CrossRef]
- Li, B.; Liang, J.; Baniasadi, H.R.; Kurihara, S.; Phillips, M.A.; Michael, A.J. Functional identification of bacterial spermine, thermospermine, norspermine, norspermidine, spermidine, and N1-aminopropylagmatine synthases. J. Biol. Chem. 2024, 300, 107281. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, Y.C.; Zhong, Y.Q.; Chen, J.J.; Qi, X. Deciphering the functional roles of transporter proteins in subcellular metal transportation of plants. Planta 2023, 258, 17. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Zhu, L.Z. Amino acid transporter as a potential carrier protein for the root-to-shoot translocation of polybrominated diphenyl ethers in rice. Environ. Sci. Technol. 2023, 57, 9722–9731. [Google Scholar] [CrossRef]
- Bittner, A.J.; Huntley, R.B.; Mourad, G.S.; Schultes, N.P. An Erwinia amylovora uracil transporter mutant retains virulence on immature apple and pear fruit. Microb. Pathog. 2020, 147, 104363. [Google Scholar] [CrossRef]
- Li, C.H.; Yue, Z.; Newstead, S.; Voth, G.A. Proton coupling and the multiscale kinetic mechanism of a peptide transporter. Biophys. J. 2022, 121, 2266–2278. [Google Scholar] [CrossRef]
- Elejalde-Palmett, C.; San Segundo, I.M.; Garroum, I.; Charrier, L.; De Bellis, D.; Mucciolo, A.; Guerault, A.; Liu, J.; Zeisler-Diehl, V.; Aharoni, A.; et al. ABCG transporters export cutin precursors for the formation of the plant cuticle. Curr. Biol. 2021, 31, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.T.; Chou, J.C.C.; Oo, H.; Dassama, L.M.K. Structures and mechanisms of a novel bacterial transport system for fatty acids. Chembiochem 2023, 24, e202300156. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Newstead, S. Gateway to the Golgi: Molecular mechanisms of nucleotide sugar transporters. Curr. Opin. Struct. Biol. 2019, 57, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kourkoulou, A.; Pittis, A.A.; Diallinas, G. Evolution of substrate specificity in the nucleobase-ascorbate transporter (NAT) protein family. Microb. Cell 2018, 5, 280–292. [Google Scholar] [CrossRef]
- Lee, Y.; Nishizawa, T.; Takemoto, M.; Kumazaki, K.; Yamashita, K.; Hirata, K.; Minoda, A.; Nagatoishi, S.; Tsumoto, K.; Ishitani, R.; et al. Structure of the triose-phosphate/phosphate translocator reveals the basis of substrate specificity. Nat. Plants 2017, 3, 825–832. [Google Scholar] [CrossRef]
- Liu, M.N.; He, J.F.; He, G.H.; Zhang, Y.; Zhang, M.P.; Wang, Y.; Wang, K.Y.; Zhao, M.Z. Genome-wide identification analysis of the ATP-binding cassette transporter family and expression analysis under methyl jasmonate treatment in Panax ginseng. BMC Plant Biol. 2025, 25, 565. [Google Scholar] [CrossRef]
- Yao, X.H.; Sui, X.L.; Zhang, Y.Y. Amino acid metabolism and transporters in plant-pathogen interactions: Mechanisms and implications. Plant Cell Environ. 2025, 5, 15594. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, L.K.; Lin, F.; Liao, Q.; Xiao, S.; Zhang, W.H. Anionic phospholipid-mediated transmembrane transport and intracellular membrane trafficking in plant cells. New Phytol. 2025, 245, 1386–1402. [Google Scholar] [CrossRef]
- Yoshino, K.; Yamamoto, K.; Hara, K.; Sonoda, M.; Yamamoto, Y.; Sakamoto, K. The conservation of polyol transporter proteins and their involvement in lichenized Ascomycota. Fungal Biol. 2019, 123, 318–329. [Google Scholar] [CrossRef]
- Liu, H.; Jiao, Q.; Fan, L.; Jiang, Y.; Alyemeni, M.N.; Ahmad, P.; Chen, Y.; Zhu, M.; Liu, H.; Zhao, Y.; et al. Integrated physio-biochemical and transcriptomic analysis revealed mechanism underlying of Si-mediated alleviation to cadmium toxicity in wheat. J. Hazard. Mater. 2023, 452, 131366. [Google Scholar] [CrossRef]
- Ozturk, B.; Yildiz, K.; Ozkan, Y. Effects of pre-harvest methyl jasmonate treatments on bioactive compounds and peel color development of “fuji” apples. Int. J. Food Prop. 2015, 18, 954–962. [Google Scholar] [CrossRef]
- Zamani, H.; Arvin, M.J.; Jahromi, A.A.; Abdossi, V.; Torkashvand, A.M. The effect of sodium silicate and methyl jasmonate on pigments and antioxidant activity of tomato (Solanum lycopersicum L.) under salinity stress. J. Agric. Sci. Tarim Bilim. Derg. 2020, 26, 479–487. [Google Scholar] [CrossRef]
- He, W.; Luo, H.; Xu, H.; Zhou, Z.; Li, D.; Bao, Y.; Fu, Q.; Song, J.; Jiao, Y.; Zhang, Z. Effect of exogenous methyl jasmonate on physiological and carotenoid composition of yellow maize sprouts under NaCl stress. Food Chem. 2021, 361, 130177. [Google Scholar] [CrossRef]
- Vukmirovic, A.; Skvorc, Z.; Bogdan, S.; Krstonosic, D.; Bogdan, I.K.; Karazija, T.; Bacurin, M.; Brener, M.; Sever, K. The role of phosphorus fertilization in antioxidant responses of drought-stressed common beech and sessile oak provenances. Int. J. Mol. Sci. 2025, 26, 3053. [Google Scholar] [CrossRef]
- Tavallali, V.; Karimi, S. Methyl jasmonate enhances salt tolerance of almond rootstocks by regulating endogenous phytohormones, antioxidant activity and gas-exchange. J. Plant Physiol. 2019, 234, 98–105. [Google Scholar] [CrossRef]
- Muller, M.; Munne-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Munoz, P.; Munne-Bosch, S. Photo-oxidative stress during leaf, flower and fruit development. Plant Physiol. 2018, 176, 1004–1014. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, N.; Li, K.; Li, C.Y.; Guo, J.P.; He, F.Y.; Liu, G.M.; Chen, X.L.; Gao, J.; Liu, L.N.; et al. Architecture of symbiotic dinoflagellate photosystem I-light-harvesting supercomplex in Symbiodinium. Nat. Commun. 2024, 15, 2392. [Google Scholar] [CrossRef]
- Rumbaugh, T.D.; Gorka, M.J.; Baker, C.S.; Golbeck, J.H.; Silakov, A. Light-induced H2 generation in a photosystem I-O2-tolerant FeFe hydrogenase nanoconstruct. Proc. Natl. Acad. Sci. USA 2024, 121, e2400267121. [Google Scholar] [CrossRef]
- Dai, G.Z.; Song, W.Y.; Xu, H.F.; Tu, M.; Yu, C.; Li, Z.K.; Shang, J.L.; Jin, C.L.; Ding, C.S.; Zuo, L.Z.; et al. Hypothetical chloroplast reading frame 51 encodes a photosystem I assembly factor in cyanobacteria. Plant Cell 2024, 36, 1844–1867. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lv, G. Nitraria sibirica adapts to long-term soil water deficit by reducing photosynthesis, stimulating antioxidant systems, and accumulating osmoregulators. Plant Physiol. Biochem. 2024, 206, 108265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Jing, T.; Liu, X.; Ai, X.; Bi, H. Melatonin promotes the chilling tolerance of cucumber seedlings by regulating antioxidant system and relieving photoinhibition. Front. Plant Sci. 2021, 12, 789617. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Redondo-Gomez, S.; Pagliano, C.; Figueroa Clemente, M.E.; Rascio, N.; La Rocca, N.; Antonacci, A.; Andreucci, F.; Barbato, R. Chloroplast ultrastructure and thylakoid polypeptide composition are affected by different salt concentrations in the halophytic plant Arthrocnemum macrostachyum. J. Plant Physiol. 2012, 169, 111–116. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, S.T.; He, N.Y.; Wang, Q.L.; Zhao, Y.; Gao, W.; Guo, F.Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020, 6, 570–580. [Google Scholar] [CrossRef]
- Semin, B.K.; Davletshina, L.N. High-efficiency oxygen evolution by photosystem II oxygen-evolving complex containing 3Mn per reaction center. J. Biol. Inorg. Chem. 2023, 28, 393–401. [Google Scholar] [CrossRef]
- Arshad, F.; Eaton-Rye, J.J. Indirect interactions involving the PsbM or PsbT subunits and the PsbO, PsbU and PsbV proteins stabilize assembly and activity of Photosystem II in Synechocystis sp. PCC 6803. Photosynth. Res. 2024, 160, 61–75. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef]
- Ali, S.; Ali, H.; Hussain, S. Influence of osmoprotectants and their different application methods on wheat (Triticumaestivum L.) cultivars under water deficiency in south punjab region of pakistan. Appl. Ecol. Environ. Res. 2023, 21, 3895–3910. [Google Scholar] [CrossRef]
- Dube, S.P.; Sibiya, J.; Kutu, F. Genetic diversity and population structure of maize inbred lines using phenotypic traits and single nucleotide polymorphism (SNP) markers. Sci. Rep. 2023, 13, 17851. [Google Scholar] [CrossRef]
- Turkoglu, A.; Haliloglu, K.; Mohammadi, S.A.; Ozturk, A.; Bolouri, P.; Ozkan, G.; Bocianowski, J.; Pour-Aboughadareh, A.; Jamshidi, B. Genetic diversity and population structure in turkiye bread wheat genotypes revealed by simple sequence repeats (SSR) markers. Genes 2023, 14, 1182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, L.; Wu, Z.; Chen, J.; Wang, T.; Zhang, X.; Mei, G.; Wang, J.; Lv, G. Classification and expression profile of the U-Box E3 ubiquitin ligase enzyme gene family in maize (Zea mays L.). Plants 2022, 11, 2459. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.C.; Yang, Y.T.; Wu, R.; Han, B.X.; Wu, D.L.; Ou, J.M. Transcriptome analysis of Akebia quinata (Thunb.) Decne. and discovery of key enzyme genes in the phenylpropanoid biosynthesis pathway. Acta Physiol. Plant. 2023, 45, 117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Wei, F.; Song, T.; Yu, Y.; Yu, M.; Fan, Q.; Yang, Y.; Xue, G.; Zhang, X. Nucleoredoxin gene TaNRX1 positively regulates drought tolerance in transgenic wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 756338. [Google Scholar] [CrossRef]
- Jin, S.; Ye, N.; Chen, X.; Wang, P.; Gu, M.; Hou, B.; Zhang, C.; Zheng, Y.; Sun, Y. Identification of PAL genes related to anthocyanin synthesis in tea plants and its correlation with anthocyanin content. Hortic. Plant J. 2022, 8, 381–394. [Google Scholar] [CrossRef]
- Tian, J.; Xu, R.; Chang, K.; Yuan, S.; Huang, C.; Wang, J.; Li, S.; Liu, F.; Zhong, F. Identification of PAL gene in purple cabbage and functional analysis related to anthocyanin synthesis. Horticulturae 2023, 9, 469. [Google Scholar] [CrossRef]
- Cui, X.; Tang, M.; Li, L.; Chang, J.; Yang, X.; Chang, H.; Zhou, J.; Liu, M.; Wang, Y.; Zhou, Y.; et al. Expression patterns and molecular mechanisms regulating drought tolerance of soybean Glycine max (L.) Merr. conferred by transcription factor gene GmNAC19. Int. J. Mol. Sci. 2024, 25, 2396. [Google Scholar] [CrossRef]
- Han, X.; Wu, Z.; Liu, F.; Wang, Y.; Wei, X.; Tian, P.; Ling, F. Transcriptomic analysis and salt-tolerance gene mining during rice germination. Genes 2023, 14, 1556. [Google Scholar] [CrossRef]
- Gu, L.; Chen, X.; Hou, Y.; Cao, Y.; Wang, H.; Zhu, B.; Du, X.; Wang, H. ZmWRKY30 modulates drought tolerance in maize by influencing myo-inositol and reactive oxygen species homeostasis. Physiol. Plant. 2024, 176, e14423. [Google Scholar] [CrossRef]
- Amist, N.; Khare, S.; Azim, Z.; Singh, N.B. Protective role of polyethylene glycol towards the damaging effects of cadmium. Appl. Biochem. Biotechnol. 2024, 197, 113–136. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Kadyrbaev, M.K.; Boyko, E.V. Protective role of melatonin and IAA in the regulation of resistance of potato regenerants to cold stress. Potato Res. 2024, 67, 421–449. [Google Scholar] [CrossRef]
- Wang, D.; Ni, Y.; Xie, K.; Li, Y.; Wu, W.; Shan, H.; Cheng, B.; Li, X. Aquaporin ZmTIP2;3 Promotes Drought Resistance of Maize through Symbiosis with Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2024, 25, 4205. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, F.; Okla, M.K.; Abbas, Z.K.; Qahtani, S.M.A.; Al-Harbi, N.A.; Abdel-Maksoud, M.A.; Olivan, L.M.G. Nanoparticles synergy: Enhancing wheat (Triticum aestivum L.) cadmium tolerance with iron oxide and selenium. Sci. Total Environ. 2024, 915, 169869. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Saleem, M.H.; Khalid, M.R.; Ali, B.; Fahad, S. Nitric oxide reduces cadmium uptake in wheat (Triticum aestivum L.) by modulating growth, mineral uptake, yield attributes, and antioxidant profile. Environ. Sci. Pollut. Res. 2024, 31, 9844–9856. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savoure, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Su, W.; Zhang, H.; Xu, W.; Chu, X. Metabolic engineering strategy for synthetizing trans-4-hydroxy-l-proline in microorganisms. Microb. Cell Factories 2021, 20, 87. [Google Scholar] [CrossRef]
- Kim, T.L.; Lim, H.; Denison, M.I.J.; Oh, C. Transcriptomic and physiological analysis reveals genes associated with drought stress responses in populus alba x populus glandulosa. Plants 2023, 12, 3238. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Rengel, Z.; Gao, W.; Nie, Z.; Li, C.; Hou, M.; Cheng, J.; Zhao, P. Boron inhibits cadmium uptake in wheat (Triticum aestivum) by regulating gene expression. Plant Sci. 2020, 297, 110522. [Google Scholar] [CrossRef]
- Bari, M.A.; Prity, S.A.; Das, U.; Akther, M.S.; Sajib, S.A.; Reza, M.A.; Kabir, A.H. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020, 22, 472–479. [Google Scholar] [CrossRef]
- Gonzalez, D.A.; de la Torre, V.S.G.; Fernandez, R.R.; Barreau, L.; Merlot, S. Divergent roles of IREG/Ferroportin transporters from the nickel hyperaccumulator Leucocroton havanensis. Physiol. Plant. 2024, 176, e14261. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Sankaranarayanan, K. In silico analysis of natural resistance-associated macrophage protein (NRAMP) family of transporters in rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Wentao, C.; Zhu, K.; Hasanuzzaman, M.; Li, G.; Liu, J. Phosphorus-induced restructuring of the ascorbate-glutathione cycle and lignin biosynthesis alleviates manganese toxicity in peach roots. Tree Physiol. 2024, 44, tpae098. [Google Scholar] [CrossRef] [PubMed]
- Rono, J.K.; Wang, L.L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a new function of metallothionein-like gene OsMT1e for cadmium detoxification and potential phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).