Biocontrol Potential of Microfighter: A Zeolite-Based Product Enriched with Pseudomonas synxantha DSL65
Abstract
1. Introduction
2. Materials and Methods
2.1. Zeo-Biopesticide Description
2.2. Site Description, Product Applications and Sample Collections
2.2.1. Olive
2.2.2. Grapevine
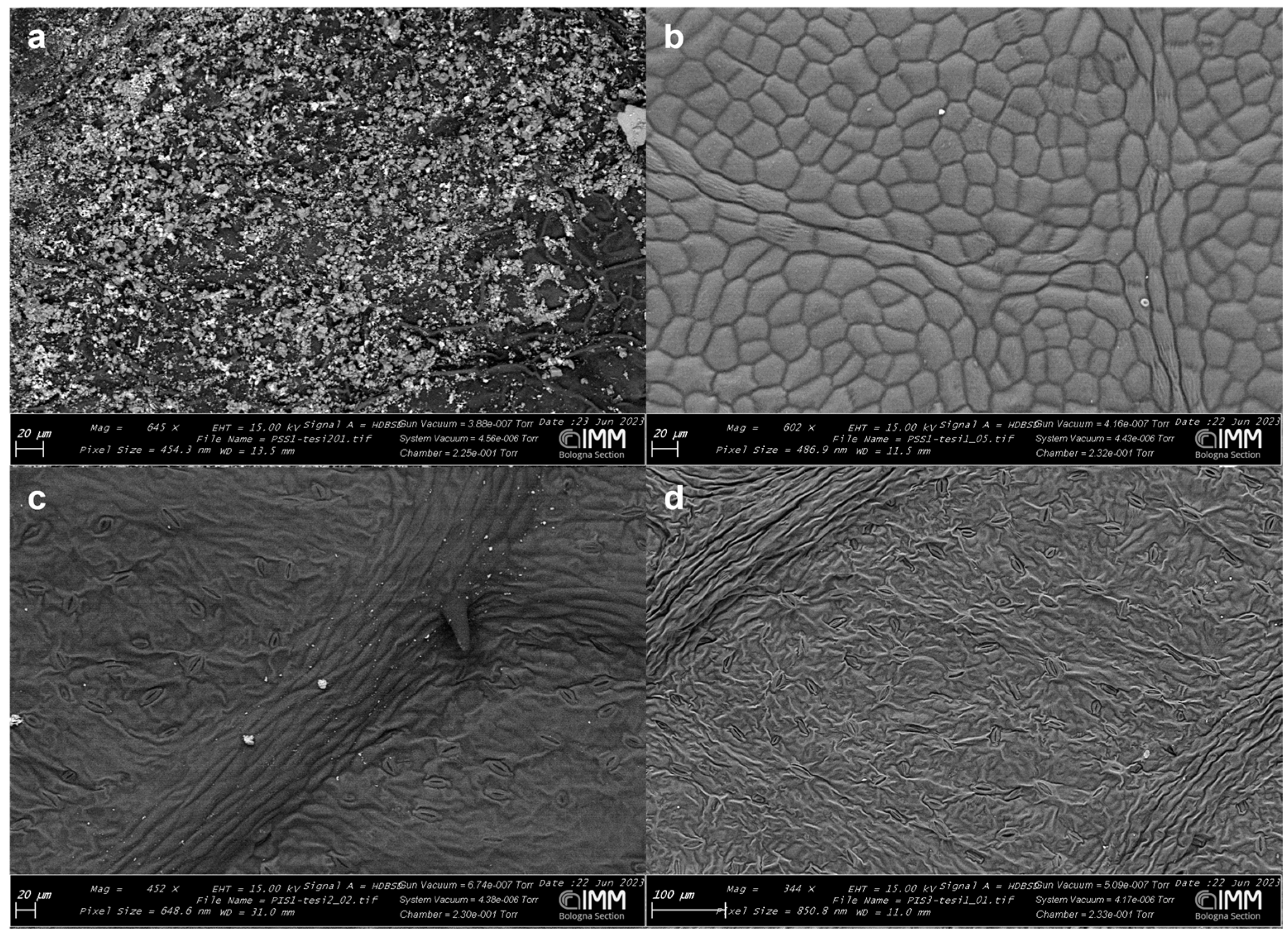
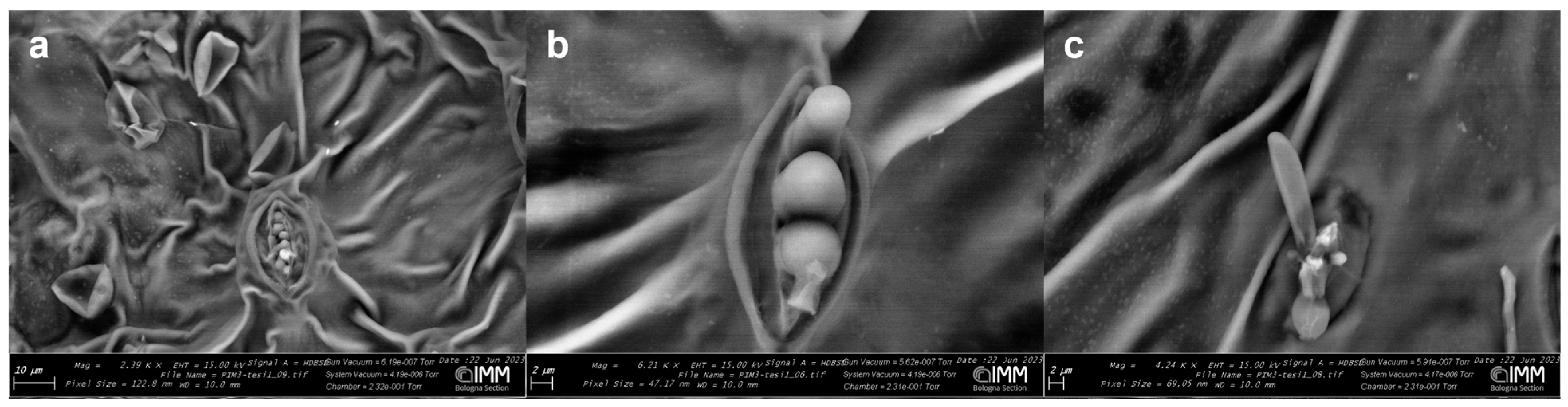
2.3. ESEM (Environmental Scanning Electron Microscopy) Analysis
Product Coverage Analyses and Its Leaching on Olive Leaf Surface
2.4. SEM Analysis
2.5. Phytopathometric Measures
2.6. Statistical Analysis
3. Results
3.1. Olive
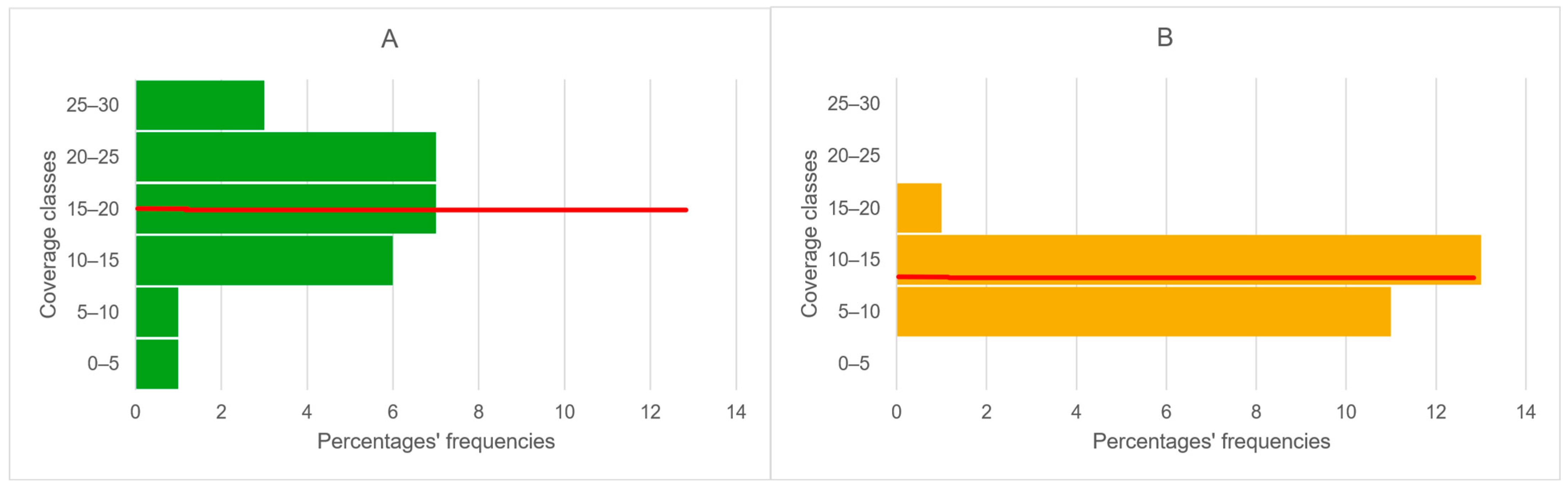
3.1.1. ESEM (Environmental Scanning Electron Microscopy)
3.1.2. Phytopathometric Measures
3.2. Grapevine
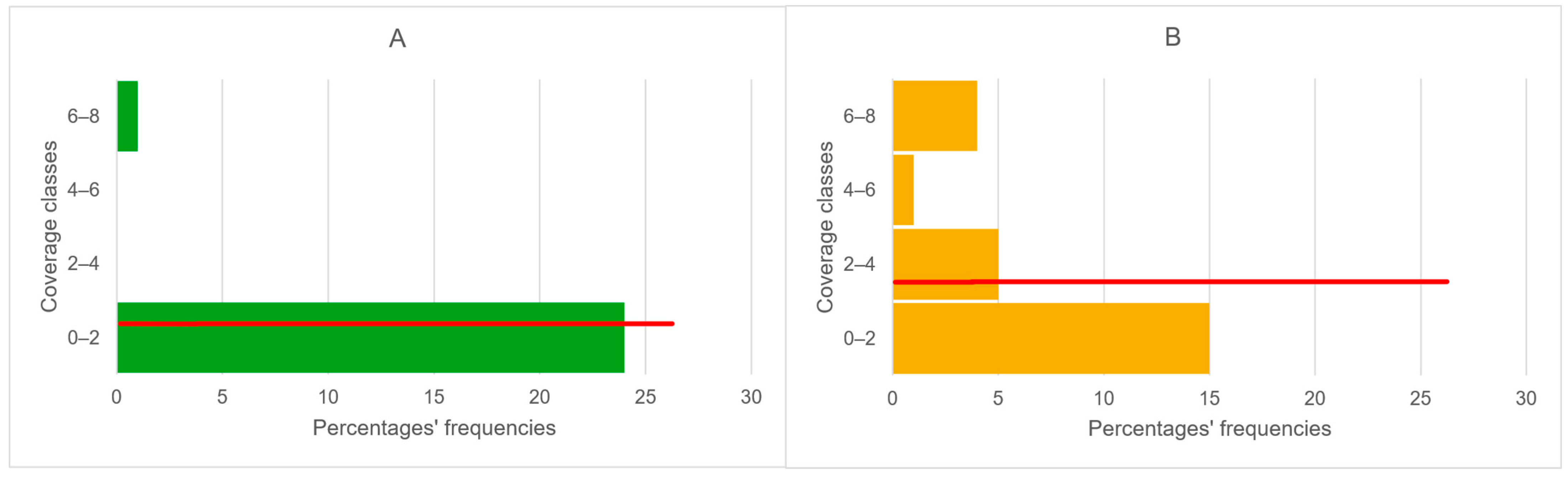
3.2.1. ESEM (Environmental Scanning Electron Microscopy)
3.2.2. Phytopathometric Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.R.; Reddy, S.V.R.; Datta, S.C. Particle Films and Their Applications in Horticultural Crops. Appl. Clay Sci. 2015, 116–117, 54–68. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle Films: A New Technology for Agriculture; CABI: Wallingford, UK, 2010; Volume 31, ISBN 978-0-470-65088-2. [Google Scholar]
- Bush, D.S.; Demkovich, M.; Aldunate, M.; Siegel, J.; Berenbaum, M.R. Kaolin as a Management Alternative for Insecticide-Resistant Navel Orangeworm (Lepidoptera: Pyralidae). J. Econ. Entomol. 2023, 116, 2095–2103. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Kovalev, A.; Gorb, E.; Gorb, S. Kaolin Nano-Powder Effect on Insect Attachment Ability. J. Pest Sci. 2020, 93, 315–327. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Sakka, M.; Berillis, P.; Athanassiou, C.G. Insecticidal Potential of Zeolite Formulations against Three Stored-Grain Insects, Particle Size Effect, Adherence to Kernels and Influence on Test Weight of Grains. J. Stored Prod. Res. 2016, 68, 93–101. [Google Scholar] [CrossRef]
- Walters, D.R. Disguising the Leaf Surface: The Use of Leaf Coatings for Plant Disease Control. Eur. J. Plant Pathol. 2006, 114, 255–260. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J.; Vanderzwet, T.; Byers, R.E.; Feldhake, C. Hydrophobic Particle Films: A New Paradigm for Suppression of Arthropod Pests and Plant Diseases. J. Econ. Entomol. 1999, 92, 759–771. [Google Scholar] [CrossRef]
- Daniel, C.; Pfammatter, W.; Kehrli, P.; Wyss, E. Processed Kaolin as an Alternative Insecticide against the European Pear Sucker, Cacopsylla pyri (L.). J. Appl. Entomol. 2005, 129, 363–367. [Google Scholar] [CrossRef]
- Andrić, G.G.; Marković, M.M.; Adamović, M.; Daković, A.; Golić, M.P.; Kljajić, P.J. Insecticidal Potential of Natural Zeolite and Diatomaceous Earth Formulations against Rice Weevil (Coleoptera: Curculionidae) and Red Flour Beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2012, 105, 670–678. [Google Scholar] [CrossRef]
- Percival, G.C.; Boyle, S. Evaluation of film forming polymers to control apple scab (Venturia inaequalis (Cooke) G. Wint.) under laboratory and field conditions. Crop Prot. 2009, 28, 30–35. [Google Scholar] [CrossRef]
- Kvachantiradze, M.; Tvalchrelidze, E.; Kotetishvili, M.; Tsitsishvili, T. Application of clinoptilolite as an additive for the photostabilization of the Bacillus thuringiensis formulation. In Studies in Surface Science and Catalysis; Kiricsi, I., Pál-Borbély, G., Nagy, J.B., Karge, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 125, pp. 731–735. ISBN 978-0-444-50244-5. [Google Scholar] [CrossRef]
- Kefalogianni, I.; Gkizi, D.; Pappa, E.; Dulaj, L.; Tjamos, S.E.; Chatzipavlidis, I. Combined Use of Biocontrol Agents and Zeolite as a Management Strategy against Fusarium and Verticillium Wilt. BioControl 2017, 62, 139–150. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and Actual Uses of Zeolites in Crop Protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Privé, J.-P.; Russell, L.; LeBlanc, A. Gas Exchange of Apple and Blackberry Leaves Treated with a Kaolin Particle Film on Adaxial, Abaxial, or Both Leaf Surfaces. HortScience 2007, 42, 1177–1182. [Google Scholar] [CrossRef]
- Gaskin, R.E.; Steele, K.D.; Forster, W.A. Characterising Plant Surfaces for Spray Adhesion and Retention. N. Z. Plant Prot. 2005, 58, 179–183. [Google Scholar] [CrossRef]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Council of the European Communities. Council Regulation (EEC) No 2092/91 of 24 June 1991 on Organic Production of Agricultural Products and Indications Referring Thereto on Agricultural Products and Foodstuffs. Off. J. Eur. Commun. 1991, 198, 7. [Google Scholar]
- Roda, R.; Prats-Llinàs, M.T.; Forcadell, S.; Mazzieri, M.; Calvo-Garrido, C.; Nadal, M.; de Lamo, S.; Ferrer-Gallego, R. The Effect of Copper Reduction on the Control of Downy Mildew in Mediterranean Grapevines. Eur. J. Plant Pathol. 2024, 169, 529–542. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Förster, H.; Adaskaveg, J.E. Efficacy of Copper and New Bactericides for Managing Olive Knot in California. Plant Dis. 2018, 102, 892–898. [Google Scholar] [CrossRef]
- Teviotdale, B.L.; Krueger, W.H. Effects of Timing of Copper Sprays, Defoliation, Rainfall, and Inoculum Concentration on Incidence of Olive Knot Disease. Plant Dis. 2004, 88, 131–135. [Google Scholar] [CrossRef][Green Version]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of Copper Compounds as Active Substances Under Regulation (EC) No 1107/2009 of the European Parliament and of the Council, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Off. J. Eur. Union. 2018, 317, 16–20. [Google Scholar]
- Tontou, R.; Gaggia, F.; Baffoni, L.; Devescovi, G.; Venturi, V.; Giovanardi, D.; Stefani, E. Molecular characterisation of an endophyte showing a strong antagonistic activity against Pseudomonas syringae pv. actinidiae. Plant Soil 2016, 109, 97–106. [Google Scholar] [CrossRef]
- Arpae Emilia-Romagna Tabelle Climatiche. Available online: https://www.arpae.it/it/temi-ambientali/clima/dati-e-indicatori/tabelle-climatiche (accessed on 18 February 2024).
- ARPAE Emilia-Romagna. DEXT3R—Dati Agrometeorologici in Emilia-Romagna. Available online: https://simc.arpae.it/dext3r/ (accessed on 27 June 2023).
- Danilatos, G.D. Foundations of Environmental Scanning Electron Microscopy. In Advances in Electronics and Electron Physics; Hawkes, P.W., Ed.; Academic Press: San Diego, CA, USA, 1988; Volume 71, pp. 109–250. [Google Scholar] [CrossRef]
- Donald, A.M. The Use of Environmental Scanning Electron Microscopy for Imaging Wet and Insulating Materials. Nat. Mater. 2003, 2, 511–516. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). EPPO Standard PP1/031(3): Plasmopara viticola; EPPO: Paris, France, 2000; Available online: https://pp1.eppo.int/standards/PP1-031-3 (accessed on 10 April 2024).
- Yin, X.; Liu, R.Q.; Su, H.; Su, L.; Guo, Y.R.; Wang, Z.J.; Du, W.; Li, M.J.; Zhang, X.; Wang, Y.J.; et al. Pathogen Development and Host Responses to Plasmopara viticola in Resistant and Susceptible Grapevines: An Ultrastructural Study. Hortic. Res. 2017, 4, 17033. [Google Scholar] [CrossRef]
- Díez-Navajas, A.M.; Wiedemann-Merdinoglu, S.; Greif, C.; Merdinoglu, D. Nonhost versus Host Resistance to the Grapevine Downy Mildew, Plasmopara viticola, studied at the tissue level. Phytopathology 2008, 98, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Bukovac, M.J.; Cooper, J.A.; Whitmoyer, R.E.; Brazee, R.D. Spray Application Plays a Determining Role in Performance of Systemic Compounds Applied to the Foliage of Fruit Plants. Acta Hortic. 2002, 594, 65–75. [Google Scholar] [CrossRef]
- Yuri, J.A.; Palma, M.; Sepúlveda, Á.; Moya, M. Water Retention on the Surface of Apples and Sweet Cherry Leaves and Fruits. J. Plant Prot. Res. 2022, 62, 136–144. [Google Scholar] [CrossRef]
- Fernández, V.; Almonte, L.; Bahamonde, H.A.; Galindo-Bernabeu, A.; Sáenz-Arce, G.; Colchero, J. Chemical and Structural Heterogeneity of Olive Leaves and Their Trichomes. Commun. Biol. 2024, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.; Rebora, M.; Piersanti, S.; Saitta, V.; Kovalev, A.; Gorb, E.; Gorb, S. Reduction in Insect Attachment Caused by Different Nanomaterials Used as Particle Films (Kaolin, Zeolite, Calcium Carbonate). Sustainability 2021, 13, 8250. [Google Scholar] [CrossRef]
- Arpae Emilia-Romagna. Rapporto IdroMeteoClima 2023—Online il Video Riepilogativo. Available online: https://www.arpae.it/it/notizie/rapporto-idro-meteo-clima-2023-online-il-video-riepilogativo (accessed on 31 May 2024).
- Scott, W.J. Water Relations of Staphylococcus aureus at 30 °C. Aust. J. Biol. Sci. 1953, 6, 549–564. [Google Scholar] [CrossRef]
- Scott, W.J. Water relations of food spoilage microorganisms. In Advances in Food Research; Mrak, E.M., Stewart, G.F., Eds.; Academic Press: New York, NY, USA, 1957; Volume 7, pp. 83–127. ISBN 978-0-12-016407-3. [Google Scholar] [CrossRef]
- Bellameche, F.; Modica, F.; Fagioli, L.; Giovanardi, D.; Stefani, E. Preliminary Characterization and Mode Action of Pseudomonas synxantha DLS65 as the active ingredient of Microfighter, an innovative biopesticide. J. Plant Pathol. 2024, 106, 1423–1534. [Google Scholar]
- Modica, F.; Fagioli, L.; Cortiello, M.; Giovanardi, D.; Reyes, F.; Stefani, E. Reduction of Copper Inputs in the Management of Key Diseases of Grapevine, Olive and Tomato by an Innovative Zeo-Biopesticide. In Proceedings of the XXVIII Congress of the Italian Phytopathological Society (SIPaV), Naples, Italy, 15 November 2023; Springer: Cham, Switzerland, 2023; Volume 105, p. 166. [Google Scholar]
- Lin, H.; Ma, R.; Lin, J.; Sun, S.; Liu, X.; Zhang, P. Positive Effects of Zeolite Powder on Aerobic Granulation: Nitrogen and Phosphorus Removal and Insights into the Interaction Mechanisms. Environ. Res. 2020, 191, 110098. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, R. Suitability of Amended Vermiculite as a Carrier for Bacterial inoculants. Res. Crops 2008, 9, 707–723. [Google Scholar]
- Holloway, P.J. Surface Factors Affecting the Wetting of Leaves. Pestic. Sci. 1970, 1, 156–163. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Alonso-Villaverde, V.; Santiago, J.L.; Mendez, J.; Pazos, I.; Martínez, M.C. Variability at the Electron Microscopic Level in Leaves of Members of the Genus Vitis. Sci. Hortic. 2011, 128, 228–238. [Google Scholar] [CrossRef]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Metruccio, E.G.; Di Marco, S. Control of Grapevine Downy Mildew by an Italian Copper Chabasite-Rich Zeolitite. Agronomy 2022, 12, 1528. [Google Scholar] [CrossRef]




| Galls Total Number | |||||
|---|---|---|---|---|---|
| Trt | 8 May 2023 | 18 July 2023 | 13 September 2023 | 17 October 2023 | 13 December 2023 |
| Cu0 | 0 | 0.10 a | 0.88 a | 0.94 a | 1.22 a |
| ZBp | 0 | 0.04 a | 0.10 b | 0.12 b | 0.30 b |
| Cu_100 | 0 | 0.08 b | 0.46 ab | 0.48 ab | 0.62 ab |
| Merlot Khorus Leaves Incidence | Merlot Khorus Leaves Severity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trt | 14 June 2023 | 30 June 2023 | 17 July 2023 | 2 August 2023 | Trt | 14 June 2023 | 30 June 2023 | 17 July 2023 | 2 August 2023 |
| Cu0 | 4.40 a | 39.80 a | 55.40 a | 73.40 a | Cu0 | 0.32 a | 9.41 a | 17.08 a | 25.78 a |
| ZBp | 4.00 a | 38.40 a | 54.40 a | 72.20 a | ZBp | 0.24 a | 8.90 a | 16.55 a | 23.13 a |
| Cu_100 | 0.40 b | 7.20 b | 23.20 b | 38.40 b | Cu_100 | 0.01 b | 0.34 b | 2.62 b | 6.86 b |
| Trebbiano Romagnolo Leaves Incidence | Trebbiano Romagnolo Leaves Severity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trt | 15 May 2023 | 1 June 2023 | 21 June 2023 | 10 July 2023 | 3 August 2023 | Trt | 15 May 2023 | 1 June 2023 | 21 June 2023 | 10 July 2023 | 3 August 2023 |
| Cu0 | 12.00 a | 52.40 a | 100.00 a | 80.20 a | 61.80 a | Cu0 | 1.61 a | 14.76 a | 49.36 a | 38.84 a | 34.43 a |
| ZBp | 3.00 b | 52.00 a | 100.00 a | 80.40 a | 61.60 a | ZBp | 0.34 b | 13.12 a | 48.70 b | 38.21 a | 32.64 a |
| Cu_100 | 0.00 c | 5.20 b | 67.80 b | 41.00 b | 22.60 b | Cu_100 | 0.00 c | 0.30 b | 8.29 c | 6.78 b | 5.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cudazzo, E.; Morrone, L.; Ferretti, G.; Faccini, B.; Mirandola, D.; Fagioli, L.; Rotondi, A. Biocontrol Potential of Microfighter: A Zeolite-Based Product Enriched with Pseudomonas synxantha DSL65. Agronomy 2025, 15, 1563. https://doi.org/10.3390/agronomy15071563
Cudazzo E, Morrone L, Ferretti G, Faccini B, Mirandola D, Fagioli L, Rotondi A. Biocontrol Potential of Microfighter: A Zeolite-Based Product Enriched with Pseudomonas synxantha DSL65. Agronomy. 2025; 15(7):1563. https://doi.org/10.3390/agronomy15071563
Chicago/Turabian StyleCudazzo, Elena, Lucia Morrone, Giacomo Ferretti, Barbara Faccini, Daniele Mirandola, Luca Fagioli, and Annalisa Rotondi. 2025. "Biocontrol Potential of Microfighter: A Zeolite-Based Product Enriched with Pseudomonas synxantha DSL65" Agronomy 15, no. 7: 1563. https://doi.org/10.3390/agronomy15071563
APA StyleCudazzo, E., Morrone, L., Ferretti, G., Faccini, B., Mirandola, D., Fagioli, L., & Rotondi, A. (2025). Biocontrol Potential of Microfighter: A Zeolite-Based Product Enriched with Pseudomonas synxantha DSL65. Agronomy, 15(7), 1563. https://doi.org/10.3390/agronomy15071563







